Mild traumatic brain injury in New Zealand: factors influencing post-concussion symptom recovery time in a specialised concussion service
Rachel H. J. Forrest 1 , Janis D. Henry 2 , Penelope J. McGarry 2 , Robert N. Marshall 31 Faculty of Education, Humanities and Health Science, Eastern Institute of Technology, Hawke’s Bay Campus, Taradale, Napier, New Zealand
2 National Service Managers, Auckland Concussion Service, Mt Eden, Auckland, New Zealand
3 Eastern Institute of Technology, Hawke’s Bay Campus, Taradale, Napier, New Zealand
Correspondence to: Rachel H. J. Forrest, Faculty of Education, Humanities and Health Science, Eastern Institute of Technology, Hawke’s Bay Campus, 501 Gloucester Street, Taradale, Napier 4112, New Zealand. Email: rforrest@eit.ac.nz
Journal of Primary Health Care 10(2) 159-166 https://doi.org/10.1071/HC17071
Published: 28 June 2018
Journal Compilation © Royal New Zealand College of General Practitioners 2018.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: By 2020, traumatic brain injuries (TBIs) are predicted to become the third largest cause of disease burden globally; 90% of these being mild traumatic brain injury (mTBI). Some patients will develop post-concussion syndrome.
AIM: To determine whether the time between sustaining a mTBI and the initial assessment by a specialised concussion service, along with the post-concussion symptoms reported at the assessment, affected recovery time.
METHODS: A retrospective medical record review of clients who had completed the Rivermead Post-Concussion Questionnaire (RPQ) at their initial assessment and were discharged from a large metropolitan concussion service in New Zealand was undertaken over a 6-month period in 2014 (n = 107). Using correlations, General Linear Mixed-effects Models (GLMM) and linear regressions, we explored associations between factors including ethnicity, gender and accident type, along with individual RPQ symptom scores and cluster scores, with time from injury to initial assessment by the specialised concussion service and initial assessment to discharge.
RESULTS: Time from injury to initial assessment by a specialist concussion service was correlated with proportionally more psychological symptoms present at initial assessments (r = 0.222, P = 0.024); in particular, feeling depressed or tearful (r = 0.292, P = 0.003). Time to discharge was correlated with individual RPQ symptom proportions present at initial assessment for headaches (r = –0.238, P = 0.015), sensitivity to noise (r = 0.220, P = 0.026), feeling depressed or tearful (r = 0.193, P = 0.051) and feeling frustrated or impatient (r = 0.252, P = 0.003), along with the psychological cluster proportion (r = 0.235, P = 0.017) and the total RPQ score (r = 0.425, P < 0.001).
CONCLUSION: Prompt diagnosis and treatment of mTBI may minimise the severity of post-concussion symptoms, especially symptoms associated with mental health and wellbeing.
KEYWORDS: Concussion; mild traumatic brain injury; mTBI; recovery; Rivermead Post-Concussion Questionnaire; Rivermead Symptom Checklist
| WHAT GAP THIS FILLS |
| What is already known: Mild traumatic brain injury (mTBI), also known as concussion, is referred to as the silent epidemic and is often associated with delayed diagnosis and referrals because it cannot be seen. Mild traumatic brain injury is predicted to become a major cause of disease burden globally by 2020. |
| What this study adds: This research demonstrates that delay in the time from injury to contact with a specialised concussion service is associated with an increase in the proportion of psychological post-concussion symptoms reported at the initial assessment by the service, which in turn is associated with longer recover time. This highlights that prompt diagnosis and treatment of mTBI may minimise the impact of mTBI on mental health and wellbeing. |
Introduction
Mild traumatic brain injury (mTBI), also known as concussion, is predicted to become a major cause of disease burden globally by 2020.1 Post-concussion symptoms typically fall into three clusters: physical (or somatic), including headache, dizziness, nausea and vomiting, poor sleep, fatigue, sensitivity to light, sensitivity to noise and visual disturbance (blurred or double vision); cognitive, which includes poor memory, poor concentration and slower thought processes; and psychological, including irritability, frustration, depression and restlessness.2–6 These symptoms can lead to people having difficulties with everyday activities such as maintaining family roles, managing financial responsibilities, working or studying, participating in recreational and hobby activities, keeping in contact with friends and driving.7 Typically, post-concussion symptoms resolve within the first month, but for 15–30% of mTBI cases, symptoms persist and may lead to post-concussion syndrome.8,9 Post-concussion syndrome is believed to be the result of both neurogenic damage caused by the impact, which resulted in the mTBI, and psychological factors related to the trauma of the impact event.10 The latter is similar to what people with post-traumatic stress disorder experience; psychological symptoms becoming more prevalent with time.11
In New Zealand (NZ), a regional study revealed the annual incidence of TBI to be 790 people per 100,000, with 95% classified as mild.1 This incident rate is substantially greater than TBI incident rates reported for other high-income countries in Europe (453 people per 100,000) and North America (618 cases per 100,000).1 The higher incident rate may be due, in part, to improved recruitment of cases for the purposes of the regional study. Many mTBI are undiagnosed due to quick resolution of acute signs and symptoms such as disorientation, confusion, loss of consciousness and post-traumatic amnesia before paramedics arrive.10
The non-fault insurer, Accident Compensation Corporation (ACC), has contracted 17 specialised concussion services throughout NZ to specifically diagnose mTBI (if a diagnosis has not already been made), evaluate and treat post-concussion symptoms and provide treatment, education and support to assist clients to return to their pre-injury activities as soon as possible.12 Delays in referral to specialised concussion services has been recognised as an issue internationally and can be due to many reasons including: the mTBI not being diagnosed or recognised at the time of injury; the client’s general practitioner (GP) not knowing about the service or choosing to manage the client themselves until they have exhausted their own resources and knowledge; or clients not seeking assistance until the symptoms prevent further appropriate functioning at home or at work.10 The aim of this study is to determine whether time between injury and the initial assessment by the concussion service was associated with the length of stay in that service, along with exploring whether there were associations between post-concussion symptoms reported at the initial assessment and the number of days from injury to initial assessment or in the service (ie from initial assessment to discharge).
Methods
A retrospective, descriptive, quantitative methodology that reviewed medical records was used in this study to determine whether statistically significant associations existed between the variables.13
Participants
We audited the files of all clients discharged from ACC’s largest contracted concussion service between 1 January and 30 June 2014. We included clients aged 16–65 years who had a mTBI, had completed a Rivermead Post-Concussion Questionnaire (RPQ) during initial assessment, had not been previously diagnosed with a mental illness and did not have a history of drug or alcohol abuse. The latter are known to complicate recovery from concussion.14
The RPQ was developed to measure the severity of various post-concussion symptoms compared to before the injury.15 A mTBI was defined as an injury to the brain resulting from extrinsic trauma for which the loss of consciousness is no more than 60 min, a Glasgow Coma Score of between 13 and 15 and the length of post-traumatic amnesia being no more than 24 h.16,17
Data
All data were extracted manually from information stored on the clients’ electronic file at the concussion service. The data collated included demographic information (age, gender and ethnicity), health information (previous mental health diagnosis or history of drug and alcohol abuse), injury information (mechanism of injury, date of injury and severity of injury) and concussion service information (date of referral, date of initial assessment, RPQ scores, date of discharge). Duration in the concussion service was calculated as the number of days from the date of initial assessment to the date of discharge.
The RPQs completed at initial assessment were either self-reported or completed by a clinician. The RPQ consists of 16 symptoms, which were grouped into three previously determined clusters:18,19
-
Physical – headaches, feelings of dizziness, nausea or vomiting, sensitivity to noise, easily upset by noise, poor sleep, tiring more easily, fatigue, blurred vision, upset by bright light and double vision.
-
Psychological – being irritable or easily angered, feeling depression or tearful, feeling frustrated or impatient, and restlessness.
-
Cognitive – forgetfulness, poor memory, poor concentration and taking longer to think.
The RPQ uses a Likert scale to determine severity: have not experienced the symptom (0), was a problem but no more (1), a mild problem (2), a moderate problem (3) and a severe problem (4). To assess symptoms still present at the initial assessment, the scale was recoded as follows: have not experienced the symptom or was a problem but no more (0); a mild problem (1); moderate problem (2); and a severe problem (3).
For each RPQ coding system (original and initial assessment symptoms only), a cluster score was calculated from the sum of the individual scores of each item; an overall total score was also calculated. Individual symptom and cluster scores were also expressed as a proportion of the total score to explore whether the proportion of an individual symptom or cluster contributed to the overall RPQ score, which is influenced by the number of days from injury to initial assessment or the duration of time in the concussion service, as opposed to the scale of the scores, as this is indicative of injury severity and therefore expected to be associated with duration in the concussion service.
Statistical analyses
For all statistical analyses, SPSS Statistics Version 2520 was used with a significance level of 0.05. Descriptive statistics (means, standard error of the mean and frequencies expressed as percentages) were used to describe the study sample. Differences between means were ascertained by using a one way ANOVA, while differences in proportions were assessed using z-tests (Bonferroni corrected where appropriate).
General Linear Mixed-effects Models (GLMM) were used to explore associations between the number of days from injury to initial assessment or initial assessment to discharge (dependent variable), with age at injury (co-variate), gender (factor) and ethnicity (factor). Only main effects were tested.
A Pearson’s correlation was used to determine the association between the number of days from injury to initial assessment and the number of days from initial assessment to discharge. Pearson’s correlations were also used to determine the association of individual symptom proportions and symptom cluster proportions with the number of days from injury to initial assessment and the number of days from initial assessment to discharge. Forward stepwise linear regressions were used to ascertain if the associations determined using the Pearson’s correlations were independent effects.
Ethical approval was obtained through the Eastern Institute of Technology (Hawke’s Bay, New Zealand) Research and Ethics Approvals Committee (ref# 11/15 and 12/15) and permission was also gained from the concussion service provider.
Results
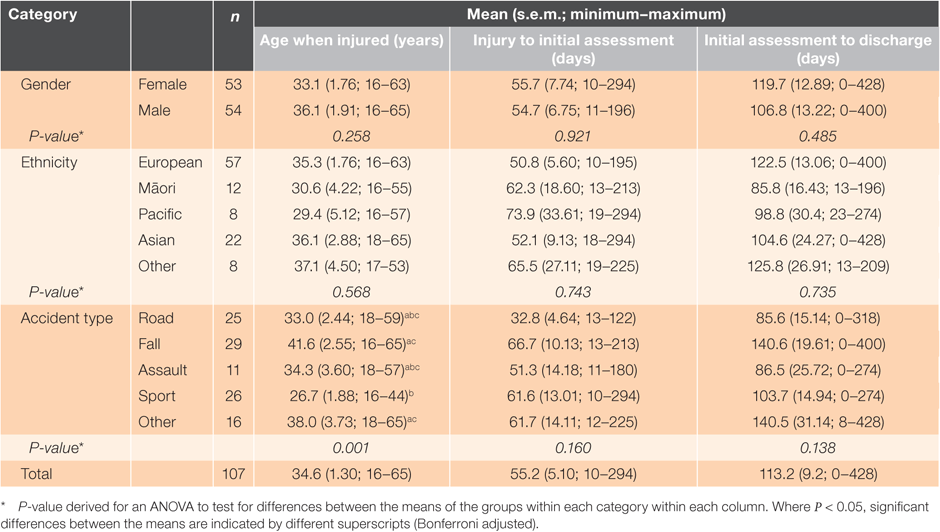
There were 255 clients discharged from the concussion service within the 6-month study period. Of these, 107 met the inclusion criteria and were included in the analyses. Each gender was equally represented, with 50.5% male and 49.5% female. Most were European (53.3%) and the main accident type was falls (27.1%), closely followed by sports injuries (24.3%) and road accident-related injuries (23.4%) (Table 1). More males than females had a mTBI caused by assault (Table 1; z-test, P < 0.05).

|
The mean age of clients at injury was 35 years for the sample, with an average of 55 days between injury and initial assessment and a further 113 days to discharge (Table 2). The average time from referral to the initial assessment was 9 days. The mean RPQ score for the cohort was 29.4 ± 1.32 (minimum score 2; maximum score 54). We detected a significant difference in the mean age at injury by accident type, with people experiencing sport accidents having a significantly lower mean age (27 years) than people experiencing falls (42 years) (Table 2). No significant differences were detected for the number of days between injury and initial assessment or the number of days between initial assessment and discharge due to accident type.
|
No association was detected between number of days from injury to initial assessment and length of stay in the concussion service (Pearson’s correlation, r = 0.076, n = 107, P = 0.437). Age, gender, ethnicity and mode of injury were not main effects on either the mean number of days from injury to initial assessment (GLMM, P = 0.320, P = 0.635, P = 0.494 and P = 0.133, respectively) or the mean number of days from initial assessment to discharge (GLMM, P = 0.936, P = 0.628, P = 0.872, and P = 0.257, respectively).
No correlation between the total RPQ score (original and initial assessment symptoms only) and the number of days between injury and initial assessment were detected (Pearson correlation, r = 0.142, n = 107, P = 0.144 and r = 0.144, n = 107, P = 0.140 respectively). The number of days from injury to initial assessment was positively correlated with the psychological symptom cluster proportion (Pearson correlation, all symptoms: r = 0.226, n = 107, P = 0.011; initial assessment symptoms only: r = 0.222, n = 103, P = 0.024). No associations between physical or cognitive symptom cluster proportions with the number of days between injury and initial assessment were detected. When each of the 16 individual symptom proportions were analysed separately, only Question 8 (feeling depressed or tearful) was associated with the number of days from injury to initial assessment (Pearson’s correlation, all symptoms: r = 0.304, n = 107, P = 0.001; initial assessment symptoms only: r = 0.292, n = 103, P = 0.003 respectively).
As expected, total RPQ score (original and initial assessment symptoms only) was correlated with number of days to discharge (Pearson correlation, all symptoms: r = 0.425, n = 107, P < 0.001; initial assessment symptoms only: r = 0. 391, n = 107, P < 0.001 respectively). When the symptom cluster proportions were analysed separately, a correlation existed between length of time between initial assessment to discharge and the psychological symptom cluster only (Pearson correlation, all symptoms: r = 0.289, n = 107, P = 0.003; initial assessment symptoms r = 0.235, n = 103, P = 0.017 respectively). When each of the 16 symptom proportions were analysed individually, the duration of time in the concussion service was negatively correlated with headaches (Question 1), and positively correlated with noise sensitivity (Question 4), feeling depressed or tearful (Question 8) and feeling frustrated (Question 9) (Pearson’s correlation, all symptoms, n = 107: Q1, r = –0.237, P = 0.014; Q4, r = 0.222, P = 0.021; Q8, r = 0.249, P = 0.010; Q9, r = 0.257, P = 0.010; initial assessment symptoms only, n = 103: Q1, r = –0.238, P = 0.015; Q4, r = 0.220, P = 0.026; Q8, r = 0.193, P = 0.051; Q9, r = 0.252, P = 0.003).
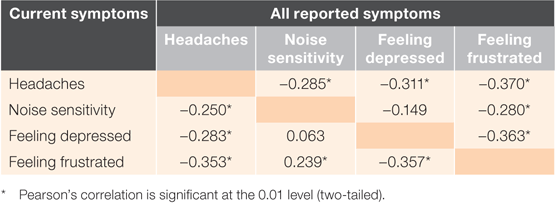
The symptom proportions for headaches, noise sensitivity, feeling depressed or tearful and feeling frustrated were all correlated with one another, with the exception of noise sensitivity and feeling depressed (Table 3). Forward stepwise linear regressions were used to explore the independent effects of these symptoms. When analysed together, only feeling frustrated was retained in the models (all symptoms: r = 0.257, P = 0.008; initial assessment symptoms only: r = 0.252, P = 0.010), indicating that the effect of this symptom was independent of any other symptom effects.
|
Discussion
The aim of this study was to identify factors (demographic, accident type and various symptom scores) associated with the number of days between injury and initial assessment, and the duration in the concussion service. Gender, ethnicity and age effects were not demonstrated in this study. The study by King,4 however, found that female gender and older age were vulnerability factors in the development of prolonged post-concussion syndrome, as did the studies by Dick,21 Lannsjö,22 McCauley et al.23 Dischinger et al.24 and Bazarian et al.25 In contrast, the study by Ganti et al.3 found no gender difference in developing post-concussion syndrome.
The severity of injury symptoms was not found to influence how quickly an individual was referred to and evaluated by the concussion service. More days between injury and initial assessment by the specialised concussion service was associated with more psychological post-concussion symptoms being reported, which, in turn, was associated with slower recovery and more time spent in the concussion service. In particular, ‘feeling depressed or tearful’ was positively correlated with the number of days from injury to initial assessment, and both ‘feeling depressed or tearful’ and ‘feeling frustrated or impatient’ was positively correlated with duration in the service.
Disturbances of mood and emotions including increased irritability and frustration, anxiety and depression are frequently reported after a mTBI.26,27 It has been proposed that early psychological post-concussion symptoms are more likely related to the actual injury (limbic system damage), whereas the later onset is more likely influenced by psychosocial or psychological responses to the injury.17,28 In regards to the latter, research suggests that often individuals self-manage their initial physical post-concussion symptoms (such as headaches and fatigue), but do so without realising the severity of their injury and the negative impact this can have on achieving tasks, such that they start to doubt their recovery and psychological symptoms become increasingly evident.8,9,29–31 As time passes with little or no improvement, and the reason for these ongoing difficulties is not understood by the individual and people associated with them, increased feelings of anxiety, frustration and depression may develop,32,33 which in turn can exacerbate cognitive difficulties.34,35 This can have a significant impact on an individual’s social, family and professional lives5,17 that may be offset if appropriate information is provided to explain and manage psychological symptoms.36 Thus, early diagnosis and intervention is important to minimise the development or escalation of psychological symptoms.
The physical symptoms of headaches and noise sensitivity were also found to be correlated with duration in the concussion service. Dischinger et al.24 suggest that noise sensitivity could be the most prominent factor in developing long-term post-concussion syndrome, and that early identification was paramount. However, noise sensitivity does not appear as one of the diagnostic symptoms of post-concussion syndrome in the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV)37 or the International Classification of Diseases (ICD-10),38 so may be overlooked by health professionals at the time of initial assessment. Increased noise sensitivity has been linked to decreased cognitive functioning and increased fatigue,33 along with increased frustration and lowered mood, thus contributing to an individual’s psychological status.26,33 Landon et al.33 concluded that early detection would reduce anxiety and stress in people who were noise intolerant post-concussion.
Headaches were negatively correlated with the duration in the service. A plausible explanation is that the physical symptom of headaches can be treated by several methods including medication, physiotherapy, chiropractic and osteopathy treatment, especially if a whiplash-type injury occurred. Obermann et al.39 stressed the importance of addressing and treating post-traumatic headache early to prevent the development of chronic headaches following a mTBI.
Once referred to the concussion service in this study, the average waiting time to the initial assessment was 9 days, indicating that there was adequate availability of appointments. The average time from injury to initial assessment in this study was nearly 2 months. Among other things, the delay in time to assessment by the specialised concussion service may, in part, reflect the advice given in the Concussion Service Operational Guidelines, whereby ongoing symptoms of more than 1 month are necessary for referral.40 Given that data regarding the number of mTBIs successfully treated without referral were not available for this study, it is difficult to determine whether these guidelines should be re-assessed and if all people with mTBI should be referred. Delay in referral does appear to reduce rather than facilitate overall recovery, given that new symptoms can often be reported months following the initial injury, and research suggests that these can be avoided if specialised intervention is put into action early.9,41 Key factors in minimising the likelihood of developing post-concussion syndrome are early interventions consisting of education, reassurance and support.39,41 Regardless of provider, there is a need for prompt diagnosis and treatment of mTBI. Additionally, the ACC may also see cost benefits with clients being less likely to develop post-concussion syndrome and returning to work or pre-injury function sooner.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
COMPETING INTERESTS
The authors declare that there are no competing or conflicts of interests.
References
[1] Feigin VL, Theadom A, Barker-Collo S, et al. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol. 2013; 12 53–64.| Incidence of traumatic brain injury in New Zealand: a population-based study.Crossref | GoogleScholarGoogle Scholar |
[2] Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil. 2001; 15 266–73.
| Base rate of post-concussion symptoms among normal people and its neuropsychological correlates.Crossref | GoogleScholarGoogle Scholar |
[3] Ganti L, Khalid H, Patel P, et al. Who gets post-concussion syndrome? An emergency department-based prospective analysis. Int J Emerg Med. 2014; 7 31–38.
| Who gets post-concussion syndrome? An emergency department-based prospective analysis.Crossref | GoogleScholarGoogle Scholar |
[4] King NS. A systematic review of age and gender factors in prolonged post-concussion symptoms after mild head injury. Brain Inj. 2014; 28 1639–45.
| A systematic review of age and gender factors in prolonged post-concussion symptoms after mild head injury.Crossref | GoogleScholarGoogle Scholar |
[5] Konrad C, Geburek AJ, Rist F, et al. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med. 2011; 41 1197–211.
| Long-term cognitive and emotional consequences of mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[6] Mittenberg W, Strauman S. Diagnosis of mild head injury and the postconcussion syndrome. J Head Trauma Rehabil. 2000; 15 783–91.
| Diagnosis of mild head injury and the postconcussion syndrome.Crossref | GoogleScholarGoogle Scholar |
[7] Bar-Haim Erez A, Rothschild E, Katz N, et al. Executive functioning, awareness, and participation in daily life after mild traumatic brain injury: a preliminary study. Am J Occup Ther. 2009; 63 634–40.
| Executive functioning, awareness, and participation in daily life after mild traumatic brain injury: a preliminary study.Crossref | GoogleScholarGoogle Scholar |
[8] King NS. Post-concussion syndrome: clarity amid the controversy? Br J Psychiatry. 2003; 183 276–8.
| Post-concussion syndrome: clarity amid the controversy?Crossref | GoogleScholarGoogle Scholar |
[9] Hou R, Moss-Morris R, Peveler R, et al. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012; 83 217–23.
| When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[10] Ruff RM, Iverson GL, Barth JT, et al. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009; 24 3–10.
| Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper.Crossref | GoogleScholarGoogle Scholar |
[11] King N, Kirwilliam S. The nature of permanent post-concussion symptoms after mild traumatic brain injury. Brain Impair. 2013; 14 235–42.
| The nature of permanent post-concussion symptoms after mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[12] Proactive. Auckland Concussion Service. Auckland: Proactive; 2015.
[13] Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013; 10 12–18.
| The retrospective chart review: important methodological considerations.Crossref | GoogleScholarGoogle Scholar |
[14] Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995; 76 302–9.
| Substance abuse as a mediating factor in outcome from traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[15] King NS, Crawford S, Wenden FJ, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995; 242 587–92.
| The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability.Crossref | GoogleScholarGoogle Scholar |
[16] New Zealand Guidelines Group. Traumatic brain injury: diagnosis, acute management and rehabilitation. Wellington, New Zealand: Accident Compensation Corporation; 2006.
[17] Silver JM, McAllister TW, Arciniegas DB. Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry. 2009; 166 653–61.
| Depression and cognitive complaints following mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[18] Røe C, Sveen U, Alvsåker K, Bautz-Holter E. Post-concussion symptoms after mild traumatic brain injury: influence of demographic factors and injury severity in a 1-year cohort study. Disabil Rehabil. 2009; 31 1235–43.
| Post-concussion symptoms after mild traumatic brain injury: influence of demographic factors and injury severity in a 1-year cohort study.Crossref | GoogleScholarGoogle Scholar |
[19] Smith-Seemiller L, Fow N, Kant R, Franzen M. Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Inj. 2003; 17 199–206.
| Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[20] IBM Corporation. IBM SPSS Software, Version 24. New York: IBM Corporation; 2016.
[21] Dick RW. Is there a gender difference in concussion incidence and outcomes? Br J Sports Med. 2009; 43 i46–50.
| Is there a gender difference in concussion incidence and outcomes?Crossref | GoogleScholarGoogle Scholar |
[22] Lannsjö M. Mild traumatic brain injury: studies on outcome and prognostic factors. Uppsala, Sweden: Uppsala University, Department of Neuroscience; 2012.
[23] McCauley SR, Wilde EA, Miller ER, et al. Preinjury resilience and mood as predictors of early outcome following mild traumatic brain injury. J Neurotrauma. 2013; 30 642–52.
| Preinjury resilience and mood as predictors of early outcome following mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[24] Dischinger PC, Ryb GE, Kufera JA, Auman KM. Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury. J Trauma Acute Care Surg. 2009; 66 289–97.
| Early predictors of postconcussive syndrome in a population of trauma patients with mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[25] Bazarian JJ, Wong T, Harris M, et al. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj 1999; 13 173–89.
| Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population.Crossref | GoogleScholarGoogle Scholar |
[26] Ashman TA, Spielman LA, Hibbard MR, et al. Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of Axis I disorders. Arch Phys Med Rehabil. 2004; 85 36–42.
| Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of Axis I disorders.Crossref | GoogleScholarGoogle Scholar |
[27] Yang CC, Tu YK, Hua MS, Huang SJ. The association between the postconcussion symptoms and clinical outcomes for patients with mild traumatic brain injury. J Trauma Acute Care Surg. 2007; 62 657–63.
| The association between the postconcussion symptoms and clinical outcomes for patients with mild traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[28] Draper K, Ponsford J. Long-term outcome following traumatic brain injury: a comparison of subjective reports by those injured and their relatives. Neuropsychol Rehabil. 2009; 19 645–61.
| Long-term outcome following traumatic brain injury: a comparison of subjective reports by those injured and their relatives.Crossref | GoogleScholarGoogle Scholar |
[29] Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006; 21 375–8.
| The epidemiology and impact of traumatic brain injury: a brief overview.Crossref | GoogleScholarGoogle Scholar |
[30] Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc. 2000; 6 568–79.
| Factors influencing outcome following mild traumatic brain injury in adults.Crossref | GoogleScholarGoogle Scholar |
[31] Rees RJ, Bellon ML. Post concussion syndrome ebb and flow: longitudinal effects and management. NeuroRehabilitation. 2007; 22 229–42.
[32] Buck PW. Mild traumatic brain injury: a silent epidemic in our practices. Health Soc Work. 2011; 36 299–302.
| Mild traumatic brain injury: a silent epidemic in our practices.Crossref | GoogleScholarGoogle Scholar |
[33] Landon J, Shepherd D, Stuart S, et al. Hearing every footstep: noise sensitivity in individuals following traumatic brain injury. Neuropsychol Rehabil. 2012; 22 391–407.
| Hearing every footstep: noise sensitivity in individuals following traumatic brain injury.Crossref | GoogleScholarGoogle Scholar |
[34] Bowen A, Neumann V, Conner M, et al. Mood disorders following traumatic brain injury: identifying the extent of the problem and the people at risk. Brain Inj. 1998; 12 177–90.
| Mood disorders following traumatic brain injury: identifying the extent of the problem and the people at risk.Crossref | GoogleScholarGoogle Scholar |
[35] van Zomeren AH, van den Burg W. Residual complaints of patients two years after severe head injury. J Neurol Neurosurg Psychiatry. 1985; 48 21–8.
| Residual complaints of patients two years after severe head injury.Crossref | GoogleScholarGoogle Scholar |
[36] Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002; 73 330–2.
| Impact of early intervention on outcome following mild head injury in adults.Crossref | GoogleScholarGoogle Scholar |
[37] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th edn. Washington, DC: American Psychiatric Association; 1994.
[38] World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993.
[39] Obermann M, Keidel M, Diener HC. Post-traumatic headache: is it for real? Crossfire debates on headache: Pro. Headache 2010; 50 710–5.
| Post-traumatic headache: is it for real? Crossfire debates on headache: Pro.Crossref | GoogleScholarGoogle Scholar |
[40] McCauley SR, Boake C, Levin HS, et al. Postconcussional disorder following mild to moderate traumatic brain injury: anxiety, depression, and social support as risk factors and comorbidities. J Clin Exp Neuropsychol. 2001; 23 792–808.
| Postconcussional disorder following mild to moderate traumatic brain injury: anxiety, depression, and social support as risk factors and comorbidities.Crossref | GoogleScholarGoogle Scholar |
[41] Accident Compensation Corporation. Concussion Service Operational Guidelines - October 2016. Wellington: New Zealand Government. Available from https://www.acc.co.nz/assets/contracts/concussion-og.pdf


