Deprescribing in a family health team: a study of chronic proton pump inhibitor use
Kate Walsh 1 4 , Debbie Kwan 2 , Patricia Marr 2 , Christine Papoushek 2 , W. Kirk Lyon 31 Pharmacist, Toronto Central Community Care Access Centre
2 Pharmacist, Toronto Western Family Health Team
3 Family Physician, Toronto Western Family Health Team
4 Correspondence to: Kate Walsh, 250 Dundas St West, Toronto, ON M5T 2Z6, Canada. Email: katewalsh478@gmail.com
Journal of Primary Health Care 8(2) 164-171 https://doi.org/10.1071/HC15946
Published: 30 June 2016
Journal Compilation © Royal New Zealand College of General Practitioners 2016.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
BACKGROUND: Proton pump inhibitors (PPIs) are often used inappropriately, without an indication, or for longer durations than recommended. Few tools exist to guide reassessment of their continued use and deprescribing if required. We aimed to reduce inappropriate drug use by developing and implementing a PPI deprescribing tool and process in a family medicine unit.
ASSESSMENT OF PROBLEM: Primary care providers of adults taking a PPI for 8 weeks with an upcoming periodic health examination were reminded to reassess therapy via electronic medical record (EMR) messaging. A PPI Deprescribing Tool was uploaded into the EMR as a second reminder and to guide reassessment and deprescribing where indicated. Ten weeks after the examination a chart review assessed changes to PPI use. A follow up survey of providers assessed the utility and barriers to implementing the Deprescribing Tool.
RESULTS: Forty-three of 46 patients on PPIs (93%) had their PPI reassessed, resulting in 11 patients (26%) having their PPI deprescribed.
Strategies for Improvement: Routine reassessment of long-term medications is often overlooked because of extensive demands on primary care providers’ time. Deprescribing likely improved because potentially eligible patients were identified to the provider and a tool was provided at the time of the encounter to guide the deprescribing process.
LESSONS: Reassessment and deprescribing of PPIs can be supported by implementing a standardised process and use of guidance tools for clinicians. Providers found the timely and selective reminder message to deprescribe the most useful component of the intervention.
KEYWORDS: proton pump inhibitor; deprescribing; reassessment; primary care; medication therapy management; gastroesophageal reflux disease
Background
In 2012, proton pump inhibitors (PPIs) accounted for nine of the top 100 prescribed drugs in Canada.1 This medication class was initially thought to have an excellent safety profile. More recent evidence suggests that long-term PPI use may be linked to increased risk of incident and recurrent Clostridium difficile infection, community and hospital acquired pneumonia, bone fractures, and nutrient malabsorption.2–6 The risk appears to be smaller with H2 receptor antagonists or lower PPI doses, suggesting that the degree of acid suppression plays a role in the risk of adverse effects.2–4
Gastroesophageal reflux disease (GERD) is a common indication for PPI use. Canadian Consensus Guidelines recommend that PPIs be discontinued after 4–8 weeks of initial treatment.7 If symptoms recur, reintroduction of PPIs at the lowest dose and frequency to control symptoms is recommended.7
Despite definitive recommendations for appropriate PPI use, evidence consistently shows they are not being used appropriately. A retrospective chart review performed in Australia found that only 37.1% of inpatients prescribed a PPI were taking it for an appropriate indication.8 Only 58.5% of these patients had tried an H2-receptor antagonist for mild/moderate oesophagitis, which is a recommended practice.8 As well, research recently conducted in our family medicine unit determined that many of our elderly patients who frequently visit the emergency department were taking a PPI inappropriately, according to the STOPP criteria (Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions).9,10
Deprescribing is the complex process of tapering or stopping medications to manage polypharmacy and improve patient outcomes.11 Interventions to reduce unnecessary PPI use have proven successful in previous studies but these interventions are often resource heavy and may be difficult to implement in some practices.12–14 A review of interventions to support appropriate PPI prescribing suggested that combining educational, multifaceted interventions that involve both health care providers and patients may be useful.15
Electronic medical records (EMR) make it relatively easy to identify patients taking potentially inappropriate medications. However, primary care providers (PCPs) often lack the time and knowledge to intervene. The aim of this project was to develop, implement and evaluate a tool and process to guide reassessment and deprescribing of PPIs where appropriate. Additional objectives included improving documentation of indications for PPI use in the EMR and assessing the utility and barriers to implementing the deprescribing process.
Assessment of Problem
This prospective, descriptive quality improvement project was approved by University Health Network’s research ethics board. It was conducted at the Toronto Western Family Health Team; a large, interdisciplinary, academic primary care clinic serving ~14 000 patients in downtown west Toronto. Family medicine residents, nurse practitioners, and staff physicians see patients by appointment. Residents review their cases with a supervising staff physician before implementing a care plan with patients.
Two documents were created for the project. A PPI Deprescribing Tool was developed from a review of current guidelines (Figure 1).8,16–22 The information was consolidated into a single document and the content was reviewed by a local gastroenterologist. Standard daily doses of PPIs for GERD and endoscopic negative reflux disease were defined as: omeprazole 20 mg, lansoprazole 20 mg, pantoprazole 40 mg, rabeprazole 20 mg and esomeprazole 20 mg.17,23 A usability pilot was conducted with several PCPs to ensure the tool would be easy to use. A handout was created to help patients understand the harms associated with long-term PPI use. It also guided patients through the taper process, including management of potential rebound symptoms. The handout was reviewed by a plain language editor in the Patient Education Department at the hospital.
A trial of weekly screening of the PCP’s schedules took ~30 min which demonstrated the feasibility of implementing this intervention in a resource limited environment. Eligible patients were aged 18 years or older who had an upcoming periodic health examination and were taking a PPI (for any indication) for 8 weeks. A standard EMR reminder message was sent to the PCP of these patients advising that the upcoming appointment would be an ideal opportunity to reassess therapy (Figure 2). The PPI Deprescribing Tool was uploaded into patients’ EMRs to serve as a second reminder at the time of the appointment and to assist with the reassessment and deprescribing process. The Tool encouraged detailed documentation of the indication for chronic PPI therapy when deemed appropriate. The patient handout was saved in the EMR for printing and distribution at the visit if required.
The intervention used PCPs to perform the reassessment because their existing relationship and knowledge of their patients helped to facilitate the efficiency and success of performing this task. The periodic health examination was deliberately chosen as the opportune time to perform the intervention. These appointments are longer than usual clinic visits and the concerns regarding PPI associated harms align with the visit’s objective of preventing health-related problems.24
Baseline data collected included patient age, PPI agent, dose, duration and indication. A chart review occurred 10 weeks after the periodic health examination to determine if the PPI had been reassessed and if changes to therapy or documentation of the indication had occurred. Reassessment of the PPI was defined as any documentation in the EMR chart notes demonstrating that the indication and intended duration of therapy were reviewed. At the conclusion of the project, an electronic survey was distributed to participating PCPs to determine the utility of the tools and process, as well as any perceived barriers to implementing the intervention.
Results of Assessment/Measurement
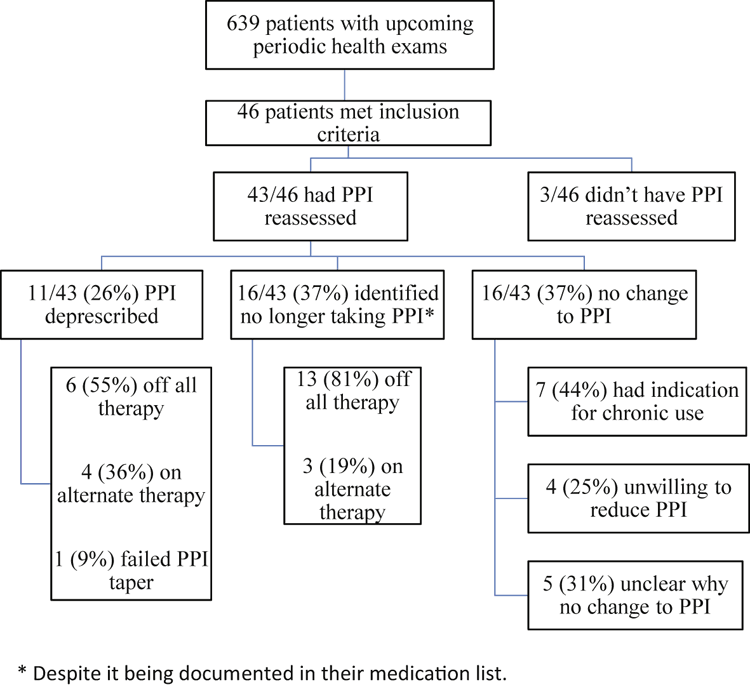
Nurse practitioners, family medicine residents, and staff physicians had 639 periodic health examinations booked over the 10-week study period: 46 patients met the inclusion criteria. The average patient age was 59 years (range 28–89).
Baseline data collection
The most common PPIs prescribed were omeprazole (n = 13 (28%)), pantoprazole (n = 9 (20%)), and rabeprazole (n = 9 (20%)). Of the 46 participants, 34 (74%) were on a standard dose PPI, six were on a high dose, two were on a half dose and four were on a different regimen, including ‘prn’ use. Forty two (91%) had been on the drug for more than a year. Only 34 of the 46 patients (74%) had a documented indication which included: GERD (n = 24), dyspepsia (n = 4), history of gastrointestinal ulcer (n = 4), and ‘other indications’ such as esophageal carcinoma (n = 2).
Follow up data collection
Re-assessment and changes to PPI use
Of the 46 patients, 43 (93%) had their PPI reassessed (Figure 3), resulting in one of the following outcomes:
No change to PPI use: Of the 43 patients, 16 (37%) had no change to their PPI use.
The patient was no longer taking the PPI: Of the 43 patients, 16 (37%) were identified as no longer taking the PPI despite it being documented in their medication list. Thirteen of these patients were off all therapy and three were taking an alternate therapy.
The PPI was deprescribed: Of the 43 patients, 11 (26%) had their PPI deprescribed, resulting in six patients taking no therapy and 4 patients taking an alternate therapy. One patient attempted a PPI taper but resumed the original dose due to worsening gastrointestinal symptoms.
Documentation
Documentation of indications for PPI use improved during the project. The number of patients taking a PPI without an indication for it documented in their problem list decreased from 12 at baseline to 4 at the end of the project. During reassessment, an indication for chronic therapy was identified in seven patients but only three of them had this indication documented in their problem list.
Prescriber Feedback Survey
The survey response rate was 67%. Among the different intervention components, respondents stated the EMR reminder message was the most useful, followed by the PPI Deprescribing Tool. The patient handout was ranked least useful. This was perhaps because only one patient received the handout, according to the EMR chart notes. Common themes on how the intervention components facilitated practice were identified by two co-authors independently. The themes were: (1) the tools served as a reminder to review the indication and duration for use, (2) the Deprescribing Tool helped guide the reassessment and taper process, and (3) the tools guided discussion with patients and implementation of recommendations. The first two themes are validated by the fact that 93% of patients had their PPI reassessed. Patients’ unwillingness to stop their PPI and lack of time were the most frequently cited barriers to deprescribing.
Survey respondents identified several ideas for future deprescribing initiatives including non-steroidal anti-inflammatories, opioids, acetylsalicylic acid for primary prevention, statins for primary prevention and antidepressants.
Strategies for Quality Improvement and Change
Most study patients were taking a PPI at the standard dose for GERD for more than one year. This suggests that reassessment and an attempt to reduce to the lowest effective dose was not occurring on a consistent basis. Routine reassessment of chronic medications is often overlooked during appointments because of extensive demands on PCPs’ time. It was proposed that deprescribing rates could improve if eligible patients were pre-identified, and a tool was provided at the time of the encounter to guide the deprescribing process.
Another deprescribing trial that specifically targeted patients on chronic PPI therapy for symptom controlled GERD eliminated medication use in 15% of patients.25 In our study, six of the 11 patients (55%) who had their PPI deprescribed were able to discontinue all acid-suppressive therapy. These results suggest that many patients who take a PPI chronically for GERD can successfully eliminate medication use.
At the end of the follow up period, four patients remained on a PPI without a documented indication. Incomplete documentation can lead to important downstream events including unintentional continuation of therapy. For example, one study patient had their PPI reassessed and it was found that they did not have any indication to be on the drug. The PPI was stopped but the medication list was not updated. Later, when the patient was hospitalised, the PPI was resumed because the inaccurate medication list was used. This resulted in continuation of a PPI without an indication.
Incomplete documentation was also seen in patients who did not change their PPI use. When reassessment identified an indication for long-term therapy it was rarely updated in patients’ problem lists. Problem lists are cumulative lists of the patients’ medical conditions that are readily located in the EMR. If they are not updated, this may lead to unnecessary work for other clinicians who would need to sort through chart notes to determine the PPI indication (as opposed to just looking in the problem list).
An unexpected finding from this study revealed that 37% of patients had a PPI in their EMR medication list but reassessment showed they were no longer using it. Perhaps this in part due to the inclusion of relatively ‘healthy’ patients who see their primary care provider only on an annual basis (and therefore their medication lists are not updated frequently). Even so, this is concerning from a broader research perspective in that EMRs are increasingly being used for data extraction and this study indicates that these records may be far from accurate.
Project results were presented to all staff during grand rounds as well as to a larger audience of pharmacists at a national conference. Individuals outside our institution repeatedly asked for access to the Deprescribing Tool. This validates that such a tool was not previously available and that clinicians felt it would be a useful addition to their practice. As well, the investigators of a national deprescribing guideline project approached the study team about using the Deprescribing Tool as part of the guideline resources.
LESSONS AND MESSAGES
As a result of our intervention, 93% of patients had their PPI reassessed and 26% had their PPI deprescribed. The positive uptake and usefulness of the intervention was also demonstrated through a ‘ripple effect’ whereby PCPs frequently informed the study team that they were using the tool and process for non-study patients.
Deprescribing has the potential to reduce pill burden, reduce medication costs and decrease the risks of medication related adverse effects. In addition, patients likely benefited from an increased knowledge about their medical condition, the harms associated with long-term PPI use, and non-pharmacological methods to manage gastrointestinal symptoms.
Other organisations could consider using technology to identify eligible patients as well as to support tools that assist prescribers through a clinical decision pathway. To correctly assess the impact of such an intervention, it is critical that EMR records be kept up to date with respect to current medications and medical conditions (problem list). As a result of the inaccurate EMR used in this study, unnecessary work was completed to identify and reassess therapy in patients who were not taking a PPI. A reduction in future workload may serve as a motivational factor for all clinicians to keep records up to date.
The notable uptake for the project (as evidenced through the reassessment rate) was the result of an inter-professional project team that engaged the various disciplines impacted by the project (physician, pharmacist, gastroenterologist). The project also had the support of the clinic’s administration (Chief of Staff). Having an evidence-based Deprescribing Tool that was reviewed by a gastroenterologist likely increased the PCPs’ confidence in applying the recommendations to their patient encounters.
In the months following the project’s cessation, it was observed that PPI reassessment was not being routinely conducted despite having the Deprescribing Tool and patient handout accessible in the EMR. This is likely because prescribers were no longer being reminded to reassess therapy in specific patients before an encounter. PCPs also identified the importance of this reminder in the prescriber feedback survey. In response to this, a follow up project will determine if discussing the expected duration of treatment when PPIs are first prescribed would be a more sustainable way to ensure patients do not remain on medications they do not need. This proactive intervention may also be more efficient as some patients will not continue unnecessary therapy, therefore eliminating the need for a dedicated reassessment appointment.
Competing Interests
No authors had any competing interests to declare.
References
[1] Top Rx Drugs of 2012. Pharm Pract (Granada) 2013; 37–39 41.[2] Kwok CS, Arthur AK, Anibueze CI, . et al. Risk of Clostridium difficile infection with acid supressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 2012; 107 1011–9.
| Risk of Clostridium difficile infection with acid supressing drugs and antibiotics: meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVSgtrjE&md5=87ae3b6955dd14ebcde539be8cc866c1CAS | 22525304PubMed |
[3] Eom CS, Jeon CY, Lim JW, . et al. Use of acid suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011; 183 310–9.
| Use of acid suppressive drugs and risk of pneumonia: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 21173070PubMed |
[4] Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med 2011; 124 519–26.
| Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtl2mtLs%3D&md5=c5a03847bf75f83c88d31cea342e841bCAS | 21605729PubMed |
[5] Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012; 36 405–13.
| Systematic review: hypomagnesaemia induced by proton pump inhibition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVCis7nE&md5=055f3bd3b7191c8c9a64551ecf6b55dbCAS | 22762246PubMed |
[6] Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J 2004; 97 887–9.
| Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia.Crossref | GoogleScholarGoogle Scholar | 15455980PubMed |
[7] Armstrong D, Marshall JK, Chiba N, . et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - Update 2004. Can J Gastroenterol 2005; 19 15–35.
| Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults - Update 2004.Crossref | GoogleScholarGoogle Scholar | 15685294PubMed |
[8] Naunton M, Peterson GM, Bleasel MD. Overuse of proton pump inhibitors. J Clin Pharm Ther 2000; 25 333–40.
| Overuse of proton pump inhibitors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktlWrsQ%3D%3D&md5=6ac27198216ba6e2ae6d13a4fcc03951CAS | 11123484PubMed |
[9] Wong J, Marr P, Kwan D, . et al. Identification of inappropriate medication use in elderly patients with frequent emergency department visits. Can Pharm J 2014; 147 248–56.
| Identification of inappropriate medication use in elderly patients with frequent emergency department visits.Crossref | GoogleScholarGoogle Scholar |
[10] Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing 2008; 37 673–9.
| STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria.Crossref | GoogleScholarGoogle Scholar | 18829684PubMed |
[11] Thompson W, Farrell B. Deprescribing: what is it and what does the evidence tell us? Can J Hosp Pharm 2013; 66(3):201–2. [cited 2013 Dec 15] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3694945&tool=pmcentrez&rendertype=abstract
[12] Bundeff AW, Zaiken K. Impact of clinical pharmacists’ recommendations on a proton pump inhibitor taper protocol in ambulatory care practice. J Manag Care Pharm 2013; 19 325–33.
| Impact of clinical pharmacists’ recommendations on a proton pump inhibitor taper protocol in ambulatory care practice.Crossref | GoogleScholarGoogle Scholar | 23627578PubMed |
[13] Lucas LM, Gerrity MS, Anderson T. A practice-based approach for converting from proton pump inhibitors to less costly therapy. Eff Clin Pract 2001; 4 263–70.
| 1:STN:280:DC%2BD38%2Fkt12hsA%3D%3D&md5=d165d620e56a42df02c3ef38a9e3a0dcCAS | 11769299PubMed |
[14] Murie J, Allen J, Simmonds R, de Wet C. Glad you brought it up: a patient-centred programme to reduce proton-pump inhibitor prescribing in general practice. Qual Prim Care 2012; 20 141–8.
| 22824567PubMed |
[15] Canadian Agency for Drugs and Technologies in Health Interventions for appropriate prescribing of proton pump inhibitors: a literature review. COMPUS 2007; 1
[16] Bhatt DL, Scheiman J, Abraham NS, . et al. ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risk of Antiplatelet Therapy and NSAID Use: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2008; 52 1502–17.
| ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risk of Antiplatelet Therapy and NSAID Use: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents.Crossref | GoogleScholarGoogle Scholar | 19017521PubMed |
[17] Canadian Agency for Drugs and Technologies in Health. Optimal Therapy Report: Proton Pump Inhibitor Project Overview Summaries. 2007:1(1)
[18] Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute Technical Review on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135 1392–1413.
| American Gastroenterological Association Institute Technical Review on the management of gastroesophageal reflux disease.Crossref | GoogleScholarGoogle Scholar | 18801365PubMed |
[19] Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107 345–60.
| Management of patients with ulcer bleeding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtlGru7w%3D&md5=d4e20caef21af9b102e388b1069cb220CAS | 22310222PubMed |
[20] Lanza FL, Chan FKL, Quigley EMM., Practice Parameters Commitee of the American College of Gastroenterology Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009; 104 728–38.
| Guidelines for prevention of NSAID-related ulcer complications.Crossref | GoogleScholarGoogle Scholar | 19240698PubMed |
[21] Talley NJ, Vakil N. , Practice Parameters Commitee of the American College of Gastroenterology Guidelines for the management of dyspepsia. Am J Gastroenterol 2005; 100 2324–37.
| Guidelines for the management of dyspepsia.Crossref | GoogleScholarGoogle Scholar | 16181387PubMed |
[22] Veldhuyzen van Zanten SJO, Bradette M, Chiba N, . et al. Evidence-based recommendations for short- and long-term management of uninvestigated dyspepsia in primary care: An update of the Canadian Dyspepsia Working Group (CanDys) clinical management tool. Can J Gastroenterol 2005; 19 285–303.
| Evidence-based recommendations for short- and long-term management of uninvestigated dyspepsia in primary care: An update of the Canadian Dyspepsia Working Group (CanDys) clinical management tool.Crossref | GoogleScholarGoogle Scholar |
[23] AstraZeneca Canada Inc. Nexium Product Monograph August 29; 2013.
[24] Medical Council of Canada. Periodic Health Examination (PHE) 2013. Available at: http://apps.mcc.ca/Objectives_Online/objectives.pl?lang=english&role=expert&id=74
[25] Inadomi JM, Jamal R, Murata GH, . et al. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001; 121 1095–100.
| Step-down management of gastroesophageal reflux disease.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mnkt1Crtg%3D%3D&md5=8a67d96419881b2d92da2005493485b5CAS | 11677201PubMed |