Impact of anions on the surface organisation of lipid monolayers at the air–water interface
Siyang Li A , Lin Du A B and Wenxing Wang AA Environment Research Institute, Shandong University, Shanda South Road 27, 250100 Shandong, China.
B Corresponding author: Email: lindu@sdu.edu.cn
Environmental Chemistry 14(7) 407-416 https://doi.org/10.1071/EN17147
Submitted: 14 August 2017 Accepted: 14 October 2017 Published: 31 January 2018
Journal Compilation © CSIRO 2017 Open Access CC BY-NC-ND
Environmental context. Lipids released from lysis of phytoplankton cells are enriched in the sea surface microlayer. Such surface-active organics can be transferred through bursting bubbles to sea-spray aerosols where they can influence atmospheric chemistry. The results presented here suggest that phospholipids combine more readily with SO42− than with Br−, leading to enrichment of organic-coated sulfate salts in marine aerosols.
Abstract. Inorganic salts and organic matter are known to be present at higher levels in the sea surface microlayer and marine aerosols; however, the impact of common anions on their surface properties is not well understood. Here, a 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayer was enriched with the sodium and ammonium salts of different anions (Br−, Cl−, NO3−, SO42−, CH3COO−, and HCO3−), and the effects on the surface properties of the monolayer were investigated. The monolayer phase behaviour and the structure of the lipid phases were studied by surface pressure–area (π–A) isotherms and infrared reflection-absorption spectroscopy (IRRAS). The presence of salts in the subphase was found to increase the surface pressure of the DPPC monolayer at a fixed area per molecule. The effect of the anions follows the order of the Hofmeister series. The higher concentration of salt solution caused the π–A isotherm to shift to larger area. The IRRAS spectra demonstrate that the ordering of the DPPC molecules in the liquid condensed phase remains essentially unaffected, even at higher electrolyte concentrations. DPPC molecules combined with SO42− could be transferred from the ocean to sea spray aerosol. The present study finds that the anions have significant influence on the surface organisation and, consequently, the interfacial properties, of the surface-active species at the air–water interface, a finding that has further implications for atmospheric aerosol nucleation.
Additional keywords: anions, Hofmeister series, sea spray aerosol, sea surface microlayer.
Introduction
The oceans, atmosphere and clouds are all interconnected through the release and deposition of chemical species.[1] Breaking waves formed through wind-driven mechanisms generate air bubbles that scavenge organic matter from the surrounding sea water. When ejected into the atmosphere, these bubbles burst, yielding sea spray aerosols (SSA) enriched in organic matter, relative to the sea water.[2–4]
The ocean represents a major source of primary aerosols; an estimated 2–100 × 1015 g of SSA is emitted from the ocean each year.[5] SSA is a globally important source of particulate matter, although its effect on the atmosphere is largely undetermined.[6] The composition of aerosols in the marine boundary layer has been extensively studied.[7] During phytoplankton bloom periods, the organic fraction dominates, contributing 63 % to the submicrometer aerosol mass, of which ~45 % is water-insoluble.[8,9] Film drops (<1 μm in diameter) produced by bursting of the bubble film efficiently transfer surface-active species that reside at the air–water interface into the atmosphere; this explains the presence of surfactants in fine SSA particles (<2.5 μm in diameter).[10] Given that the relative concentration of organic matter to inorganic salts in the ocean is extremely low (60–90 μM organic matter compared with approximately 460 000 μM Na+),[3] the high relative fraction of organic matter in SSA shows that the transfer of organic species from the ocean to SSA proceeds through selective processes. To better understand the biogeochemical connections between the ocean and atmosphere, it is critical to improve our understanding of the processes that control SSA composition in order to determine their effects on atmospheric chemistry and climate.[1]
The sea surface microlayer, defined as the uppermost tens to hundreds of micrometres of the ocean surface, is a thin film between the ocean and the atmosphere. It covers more than 70 % of the Earth’s surface.[11] The sea surface microlayer is mainly composed of surface-active, biogenically derived organics, such as carbohydrates, fatty acids, lipids and proteinaceous materials.[12] The most important source of surface-active substances is the sunlight-promoted production of organic substances by phytoplankton and bacteria. Phospholipids, glycolipids and triacylglycerides are mainly released during cell lysis of the phytoplankton.[13,14] Coastal and oligotrophic slicks are typically rich in these and other lipids.[12] There is evidence to show that naturally derived surfactants, such as fatty acids and their derivatives, may accumulate in the sea surface microlayer, showing a greater affinity than other soluble organic molecules for the air–water interface.[13] The air–water interface, having different chemical, physical and biological properties from subsurface waters, plays a significant role in biogeochemical processes on a global scale, a role that needs to be better understood for fundamental research on marine chemistry.[15]
Sea spray aerosol has been shown to comprise more than just sodium chloride; it contains other inorganic salts as well as biologically produced organic species.[16] While the majority of submicrometre SSA particles contain mostly sea salts (i.e. NaCl, KCl, MgCl2 and CaCl2), SSA particles contain increasing levels of organic species (relative to inorganic species) as particle diameter decreases to submicrometre sizes. Mean enrichment factors for major ions demonstrate significant enrichment in fine SSA for K+ (1.3), Mg2+ (1.4), and Ca2+ (1.7), likely because of their interactions with organic matter.[5] The significant enrichment of Ca2+ was observed in submicrometre SSA particles when particles were generated both from seawater sources in the laboratory and from ambient aerosol samples.[17]
Investigations into the role of ions in SSA indicate that cations such as Mg2+ and Ca2+ can enhance organic species at the interface, which has implications for the surface packing of organic molecules and subsequent interfacial reactivity.[18,19] Further, various chemical properties (e.g. surface activity, solubility, interfacial molecular structure) have been observed to play a role in defining the transfer of chemical matter from the ocean to SSA.[1] Most of these studies have used cations (e.g. Na+, Ca2+ and Mg2+) as the main inorganic salts, thus neglecting the importance of marine-relevant anions, such as Br−, Cl−, NO3−, and SO42−. Few studies have investigated how these anions affect surface films and the formation of SSA. Nonetheless, SO42−, NH4+, Cl− and NO3− are the dominant ions for marine cloud water collected over the eastern Pacific Ocean.[20–24] They also contribute 24.5 % of PM2.5 mass from continental sources.[25]
Based on the Hofmeister series, ions can be divided into kosmotropes and chaotropes, which have opposing affinities towards the interface.[26] Numerous experiments over several decades have shown that the Hofmeister series plays a significant role in a dramatic range of biological and physicochemical phenomena, affecting the solubility of hydrophobic solutes in water, the diffusion behaviour and interfacial molecular packing of surfactants, the activities of various enzymes, and the surface tension of electrolyte solutions.[26,27] In addition, the ion concentration becomes an important parameter since the relative concentration of ions will change as the marine aerosol loses or adsorbs water in the atmosphere.[19,28]
Although the chemical composition of the sea surface microlayer has been extensively studied, there is still a lack of knowledge about the interfacial properties of the sea surface microlayer and the physicochemical processes governing the formation of SSA. In attempts to explore the interaction between anion and surfactants at the air–water interface, the Langmuir method and infrared reflection-absorption spectroscopy (IRRAS) have been commonly used for the investigation of surface-active substances in different solutions. Langmuir monolayers of zwitterionic phospholipids are used as model systems to understand anion effects in physicochemical and biological systems. 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) is a representative lipid at the air–seawater interface. The sea surface microlayer is known to be enriched in DPPC , and DPPC is therefore likely to be found in marine aerosols.[29,30] The sodium and ammonium salt concentrations used here varied from 0.001–1.0 M to represent different stages of marine aerosol evaporation and different degrees of ion enrichment at the interface. The question we wish to address here is to how the Hofmeister and concentration effects of sodium and ammonium salts with selected anions influence the ordering of DPPC Langmuir monolayers.
Experimental
Materials and sample preparation
1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine (DPPC) (98 % purity, Sigma-Aldrich) was dissolved in chloroform at 1 mM without additional purification and sealed from the ambient environment. All isotherms were collected within three weeks of sample preparation to minimise the effect of solvent evaporation and lipid oxidation. Na2SO4, NaNO3, NaHCO3, NH4Br, (NH4)2SO4 were purchased from Alfa Aesar (purity >99 %). NaCl, NaBr, NaCH3COO, NH4Cl, NH4HCO3, NH4CH3COO were purchased from Acros (purity >99 %). Sodium and ammonium salts were dissolved in ultrapure water (specific resistivity of 18.2 MΩ cm) produced by Millipore filtration device to prepare stock solutions. The concentrations of salts used here are below the threshold of saturation. To keep the stability of solutions, all the solutions were prepared immediately before use. The salts of strong acids, such as NaCl, NaBr, NaNO3 and Na2SO4, were maintained at nearly constant pH values. It would have been possible to precisely control the pH of NaCH3COO, NaHCO3 and ammonium salts solutions through the addition of buffers; however, the resulting mixture of ions would have complicated the interpretation of the experiments. Therefore, the pH values of all salt solutions were not adjusted with buffer: pH 8.4 for 0.001 M NaHCO3, pH 8.2 for 0.001 M NaCH3COO, pH 5.5 for 0.001 M (NH4)2SO4, pH 5.8 for 0.001 M NH4Cl, pH 6.3 for 0.001 M NH4Br, pH 7.0 for 0.001 M NH4CH3COO, pH 8.0 for 0.001 M NH4HCO3. All experiments were conducted at ambient temperature (25 ± 2°C) and relative humidity (45 ± 2 %).
Monolayer spreading and surface pressure–area (π–A) isotherms
Surface pressure–molecular area (π–A) compression isotherms were measured on a computer-controlled Langmuir trough. It has a maximum trough area of 210 cm2 and two barriers that move symmetrically from both ends towards the trough centre.
In the Teflon-coated film trough, a known volume of a 1 mM solution of DPPC in chloroform was spread dropwise onto the surface of the water subphase or aqueous salt solution using a 50 µL microsyringe with the trough barriers initially in the fully expanded position. After 10–15 min to allow for complete solvent evaporation, the compression of the two hydrophobic barriers was initiated. The surface pressure was measured during compression by using a Wilhelmy plate made of filter paper hung to the surface pressure sensor. The films were compressed at a constant speed of 3 mm/min. The trough was cleaned by repeatedly rinsing with ethanol and Millipore water before use. The procedure followed to obtain the π–A isotherms has been described previously.[31] The measurements were performed three times to ensure consistent results.
Infrared reflection–absorption spectroscopy (IRRAS)
All spectra were obtained using a Vertex 70 FTIR spectrometer (Bruker, Germany) with a liquid-nitrogen cooled HgCdTe (MCT) detector for a single-beam measurement. The external reflection-absorption spectrum of ultrapure water was used as a reference. Sample (film-covered surface) and reference (film-free surface) troughs were fixed on a shuttle device driven by a computer-controlled stepper motor for allowing spectral collections from the two troughs in an alternating fashion. A time delay of 60 s was allowed for film equilibrium between trough movement and data collection. Spectra covered a range from 400 to 4000 cm−1 and were averaged over 2000 scans with a resolution of 8 cm−1. Surface pressure changed slightly for the monolayers (≤ 0.2 mN/m) during the data collection. Measurements were performed at least three times to ensure reproducibility.
Results and discussion
Phase behaviour and packing of DPPC monolayer
Surface pressure–area isotherms of Langmuir DPPC monolayers on pure water and sodium salt solution subphases are shown in Fig. 1. The isotherm of DPPC on pure water reveals distinct phases that have been previously identified.[32] Four phases of DPPC monolayer on pure water can be observed: a gas phase (G, >105 Å2 /molecule), a liquid-expanded phase (LE, 75–105 Å2 /molecule), a liquid-condensed phase (LC, <55 Å2 /molecule), and a typical LE–LC transition phase (LE–LC, 55–75 Å2 /molecule) defined by a plateau at ~13 mN/m, which is in agreement with the literature.[19] The DPPC monolayer on pure water collapsed at 45 Å2/molecule and 55 mN/m, following a sharp surface-pressure increase. This indicates that DPPC forms a rigid and condensed monolayer at the air–water interface.

|
All phases described above are also present for DPPC monolayers on sodium salt solutions. For sodium salts, anions have been ordered into a Hofmeister or lyotropic series, which is a classification of ions in order of their ability to salt out or salt in proteins.[33] The Hofmeister series orders anions with decreasing salting-out potency from left to right, as follows: SO42−, HPO42−, OH−, F−, HCOO−, CH3COO−, Cl−, Br−, NO3−, I−, SCN−, ClO4−. An underlying molecular-level description of the Hofmeister effect is still far from complete.[26,34,35] Two basic hypotheses on how Hofmeister ions affect the aggregation and self-assembly behaviour of macromolecular solutes in aqueous solution have been put forward: (1) indirect mechanism of action – by interacting with water around the macromolecules; (2) direct mechanism of action – by interacting directly with the macromolecules.[36] Anions were found to have a much greater impact on protein solubility than cations.[37] The indirect mechanism concerns the various ions’ ability to make or break hydrogen bonds (known as kosmotropic or chaotropic behaviour, respectively). Kosmotropic anions cause water molecules to favourably interact, which also in effect stabilises intramolecular interactions in macromolecules such as proteins. Chaotropic anions have the opposite effect, disrupting water structure, increasing the solubility of nonpolar solvent particles, and destabilising solute aggregates.[38] Another theory suggests that ions have little effect on the overall hydrogen-bonding of water in bulk solution and do not display the thermodynamic behaviour predicted by recent experiments.[39] A strong nonspecific penetration of anions into the lipid phases has been found in recent molecular dynamics simulations by Sachs and Woolf.[40] Adsorption through strong local binding or dispersion forces plays a role in the interaction of anions with lipid interfaces.[41] Our results, based on increasing area for the DPPC monolayer from left to right, show the order of the series as follows: SO42−, Cl−, Br−, NO3−. This sequence is in good agreement with Hofmeister.[42]
With 0.001 M or 0.1 M Na2SO4 as subphase, the isotherm (Fig. 1.) shows that the DPPC molecules are able to pack more tightly because the compression of the air–water interface enabled the surface area to reach 52 Å2/molecule at 20 mN/m. In contrast, the DPPC monolayers on NaBr and NaNO3 subphases were much less compressed. Chloride was found to have only a marginal interaction with DPPC monolayers at relatively low concentration (0.001 M). This is not inconsistent with previous work, which has shown chloride to exhibit ‘indifferent’ behaviour, locating it on the border between kosmotropic and chaotropic anions.[33,43] Given the little change in the π–A isotherms between 0.001 M and 0.1 M Na2SO4, we further measured the π–A isotherms for DPPC monolayers on NaCl and Na2SO4 solutions with varying concentrations (Fig. 2) to investigate the concentration effect.

|
The phase behaviour of DPPC on NaCl solutions has been studied extensively. As reported in previous work, it was concluded that Cl− ions do not affect the surface pressure of DPPC monolayer in any significant way, with very low electrolyte concentrations (0.001–0.01 M) in the subphase.[37,44] As can be seen from Fig. 2, the π–A isotherms of DPPC monolayers at various NaCl and Na2SO4 concentrations changed quite substantially with salt concentration. The concentration of NaCl giving rise to the change of lifting area where the surface pressure starts to increase is larger than 0.001 M, while the analogous concentration of Na2SO4 appeared to be larger than 0.1 M. Relative to water, an increase in mean molecular area occurs in the low surface-pressure region, and the LE–LC transition phase at ~10 mN/m is lower for 0.1 M NaCl. As the DPPC monolayer is compressed to higher surface pressures (>20 mN/m), DPPC molecules occupy the mean molecular area as on water, suggesting that excess NaCl ions are squeezed out of the monolayer. The isotherm obtained for 0.5 M NaCl has a similar form but with a less well-defined transition. A total expansion of the monolayer occurred on the ≥ 0.5 M NaCl solution, where a larger mean molecular area relative to water occurred at all surface pressures. Similar to the NaCl solutions, 0.5 and 1 M Na2SO4 solutions caused the DPPC monolayer to expand at all surface pressures compared with water. It should be noted that all salt solutions had identical ionic strength with the exception of the Na2SO4 solution, the ionic strength of which was three times higher than the others because of the divalent nature of the anion. Control experiments at a third of the Na2SO4 concentration used for the main experiment showed that the higher ionic strength did not change the ordering of any of the results with respect to the other ions.[39] The high film-collapse pressures of DPPC monolayers at various Na2SO4 concentrations reveal that the SO42− ions appear to be ejected out of the DPPC monolayers near the collapse point. Results shown in Fig. 2 indicate that, at high concentrations, Cl− and SO42− interact similarly with DPPC molecules, where expansion of the monolayer is likely due to increased electrostatic repulsions in the interfacial region.
The impacts of HCO3− and CH3COO− on the phase behaviour of DPPC in general have been less studied than those of SO42−, Cl−, Br− and NO3−. More specifically, the role of the HCO3− ion may include: (1) participation in the hydrogen-bonding network, invoking enhanced condensation, (2) ion-pair formation to mitigate like-charge repulsion, and (3) Hofmeister related HCO3−/water interactions.[45,46] As shown in Fig. 3, the extent of the mean molecular-area shift appears to be concentration-dependent; it increases by 8–10 Å2/molecule for 0.1 M solution and by 18–20 Å2/molecule for 1 M solution. Despite the strength of the interfacial interaction, the DPPC monolayer was very unstable on the 1 M NaHCO3 subphase, exhibiting relatively low collapse pressure. The π–A isotherms shown in Fig. S1 (available as the Supplementary material to this paper) indicate that CH3COO− behaves similarly to HCO3−. In conclusion, the main transition pressure depends on the salt concentration and the type of anion in the subphase.

|
Sodium is thought to be an indifferent cation in the Hofmeister series.[47,48] The effect of cations follows the order of the Hofmeister series, with NH4+ appearing in front of Na+.[49] It can be observed that the increase in surface pressure in Fig. 4 is different for different anions. The magnitude of the increase follows the order: SO42− < HCO3− < CH3COO− < Cl− < Br−.[50] The LE–LC phase-transition pressure in the DPPC monolayer also increases according to the Hofmeister sequence. Along with sodium salts, ammonium salts – which are major components of tropospheric aerosols – have been extensively studied in atmospheric aerosol nucleation.[51–53] The addition of ammonium salts (except ammonium sulfate) in the subphase of DPPC monolayer leads to a general increase in the surface pressure at a fixed molecular area. Ammonium sulfate is the traditional kosmotropic salt for the salting out of protein from an aqueous solution.[54] The general increase in the surface pressure for all electrolytes implies that the salts adsorb at the DPPC monolayer in some way. It is worthy to note that the sequences at higher concentrations of ammonium salt solutions behave differently from the Hofmeister sequence in this work (Fig. S2). At a fixed surface area in the LC phase, the surface pressure of the DPPC monolayer on 0.1 M ammonium salts increases according to the Hofmeister sequence. The difference in the sequence of ammonium salts between low and high concentrations implies that anions affect the ordering of the lipid chain and their tilt angle in the LC phase.[55] The expansions of the monolayers at fixed surface pressure follow the Hofmeister sequence at very low ammonium salt concentrations.

|
Conformation of the alkyl chain in the DPPC monolayer
Besides using π–A isotherms to observe anion effects on the phase behaviour of DPPC monolayers, we have examined the alkyl chain packing and conformation by using IRRAS. The IRRAS spectra of the methyl (CH3) and methylene (CH2) region (2800–3000 cm−1) for DPPC monolayers on water and sodium salt solutions are shown in Fig. 5a. IRRAS spectra were collected in the LC phase (at 30 mN/m) to provide information about the effect of anions on a DPPC monolayer. IRRAS is sensitive to the alkyl chain conformation of the monolayer. Peak positions of CH2 vibrational modes are sensitive to the conformational order of the alkyl chains, and can provide insight into the relative number of trans and gauche conformations in the monolayer; i.e., a shift to lower wavenumbers indicates that the alkyl chains have more trans bonds.[19,56] It was observed that, during the transition from the LE–LC phase to the LC phase in the DPPC monolayer on pure water, the symmetric CH2 stretching frequency decreased from 2855 to 2851 cm−1 and the asymmetric CH2 stretch decreased from 2924 to 2919 cm−1, indicating an increase in trans relative to gauche conformations and a higher degree of order.[19,37] Such a trend can be observed in Table 1, where the vibration modes of CH3 asymmetric (υa(CH3)), CH2 asymmetric (υa(CH2)), CH3 symmetric (υs(CH3)) and CH2 symmetric (υs(CH2)) at 2955, 2919, 2880 and 2850 cm−1, respectively, are shifted to lower wavenumbers when DPPC is compressed on the Na2SO4 solution. The υa(CH2) values below 2920 cm−1 are typical for all-trans conformations (Fig. S3).[55] It can be seen that the alkyl chains are slightly more ordered in the presence of SO42−. The CH3 symmetric stretch at 2888 cm−1 on pure water shifted to higher frequency (2968 cm−1) in the presence of NaBr. This observation confirms that gauche defects exist within the alkyl chain, as the existing IRRAS polarisation is sensitive to the orientation of the terminal methyl, which indirectly reports on gauche versus trans bonds in the alkyl chains.[19] The frequencies of the CH3 and CH2 groups show that, in the presence of NaBr, the order of the hydrocarbon chains is decreased with respect to DPPC molecules on pure water, indicating a higher content of gauche conformations. These findings are in accordance with results obtained in the π–A isotherms. Previous experimental studies have found that concentrated NaI solutions disrupt chain ordering at low surface pressures,[37] which demonstrates that chaotropic anion’s ability to disorder the alkyl chain region, as is typical for Br−. In the case of ammonium salt solutions, the intensities of the υa(CH2) and υs(CH2) bands of DPPC monolayers are lower than those on the pure water (Fig. 5b). Only in the presence of (NH4)2SO4 is the intensity of the υa(CH2) band a little stronger than that on pure water, as the DPPC molecules are packed closer together.

|

|
A novel Hofmeister effect was demonstrated by Gurau et al. in an octadecylamine monolayer spread on salt solutions by using vibrational sum frequency spectroscopy. The ratios of oscillator strengths from the CH3 symmetric stretch to the CH2 symmetric stretch for the anions follow a series from most ordered to least ordered monolayer, as is consistent with the Hofmeister series.[39] The peak-height intensity ratio between the antisymmetric and symmetric bands of the CH2 groups (Ias/Is) has been demonstrated to be a measure of the order/disorder parameter of the phospholipid chains in the lipid layer.[57–59] The shifts in the peak position of the DPPC alkyl chain CH2-asymmetric mode are less than 1 cm−1 from the value measured on water (Fig. S4). The Ias/Is ratio for the DPPC monolayer at the different concentrations of NaCl solutions remains practically constant. Similar to the previous IRRAS study of NaI, [37] I− anions do not affect the LC phase, which is recovered unchanged at high pressures. However, the LE–LC phase-transition pressure of the DPPC monolayer increases with I− concentration.[37] The LE phase appears to be favoured in the presence of anions. The grazing incidence X-ray diffraction results also imply that Br− ions do not penetrate into or bind to the LC phase.[37] At higher concentrations of NaHCO3, the positions of the methylene peaks do not change (Fig. S5), indicating that the shape of the LC phase domains is not strongly influenced by the concentrations of anions. This observation suggests that the LC phase appears to be less impacted by anions than the LE–LC phase.
The region between 1300 and 900 cm−1 gives information about the vibrations of the phosphate (PO2−) headgroup. Spectra of the phosphate vibrational modes of DPPC on pure water and salt solutions are shown in Fig. 6. The asymmetric and symmetric PO2− stretching (υa(PO2−) and υs(PO2−)) modes of DPPC on pure water were observed at 1233 and 1090 cm−1, respectively. Peaks at 1171 and 1053 cm−1 can be attributed to the CO-O-C asymmetric and symmetric stretches (υa(CO-O-C) and υs(CO-O-C)) of the carbonyl esters, respectively. The feature at 978 cm−1 is attributed to N-(CH3)3 asymmetric stretches of the choline group (υa(N-(CH3)3)). Comparison of the spectra in the presence as well as in the absence of salts shows a marked difference in the absorption frequencies of the phosphate groups (Table 2). These adsorption bands undergo shifts to diminished peak frequencies and intensities in the presence of salt solutions. The asymmetric υa(PO2−) stretching frequency at 1230 cm−1 decreases slightly in the presence of anions, indicating an increase in the average hydration of the phosphate group.[39]

|

|
A much weaker Na+/DPPC interaction has been proposed recently, as found by molecular simulations involving monolayers and bilayers.[26] The influence of concentrated ammonium nitrate on monolayers of some carboxylic acids and their derivatives at the air–water interface has been studied earlier.[60,61] NH4+ and NO3− participate as bridging components between the headgroups of dicarboxylic acid at the interface.[60,62] The separation between the υa(PO2−) and υs(PO2−) modes (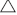 = υa(PO2−) – υs(PO2−)) increases according to the Hofmeister series. In the presence of Na2SO4, the separation between the υa(PO2−) and υs(PO2−) frequencies (
= υa(PO2−) – υs(PO2−)) increases according to the Hofmeister series. In the presence of Na2SO4, the separation between the υa(PO2−) and υs(PO2−) frequencies (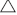 = 143 cm−1) is smaller than the presence of NaBr (
= 143 cm−1) is smaller than the presence of NaBr (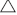 = 180 cm−1). The smaller separation suggests that the interaction of SO42− with DPPC is stronger.[28] For the ammonium salt solutions, the separations in the range of 139 to 144 cm−1 are much smaller than the sodium salt solutions. The interactions of NH4+ and SO42− with the polar headgroups of DPPC shift the peaks to lower frequencies (1227 and 1086 cm−1). The separation between the υa(PO2−) and υs(PO2−) for (NH4)2SO4 solution (
= 180 cm−1). The smaller separation suggests that the interaction of SO42− with DPPC is stronger.[28] For the ammonium salt solutions, the separations in the range of 139 to 144 cm−1 are much smaller than the sodium salt solutions. The interactions of NH4+ and SO42− with the polar headgroups of DPPC shift the peaks to lower frequencies (1227 and 1086 cm−1). The separation between the υa(PO2−) and υs(PO2−) for (NH4)2SO4 solution (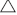 = 139 cm−1) is smaller than that for Na2SO4 (
= 139 cm−1) is smaller than that for Na2SO4 (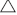 = 144 cm−1). The separation for NH4Br (
= 144 cm−1). The separation for NH4Br (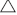 = 144 cm−1) is also smaller than that for NaBr (
= 144 cm−1) is also smaller than that for NaBr (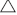 = 180 cm−1). A possible explanation for this difference is the position of NH4+ (to the right of Na+) in Hofmeister sequences for a fixed anion.[34] NH4+ adsorption on the surface is stronger than the other monovalent cations.[63] The introduction of those ions with relatively strong Hofmeister effects, such as ammonium and sulfate, can enhance the stabilities of the monolayers.
= 180 cm−1). A possible explanation for this difference is the position of NH4+ (to the right of Na+) in Hofmeister sequences for a fixed anion.[34] NH4+ adsorption on the surface is stronger than the other monovalent cations.[63] The introduction of those ions with relatively strong Hofmeister effects, such as ammonium and sulfate, can enhance the stabilities of the monolayers.
Atmospheric implications
Lipids such as DPPC molecules released from cell lysis of phytoplankton can spread on the sea surface microlayer, and then be transferred into the atmospheric aerosols through bursting bubbles. The formation of organic coatings can affect the surface properties of aerosols. In particular, highly ordered organic films coated on aerosols can disrupt the transport of volatile species and reactive free radicals such as OH and HO2 from the gas phase into particles. They could also reduce the evaporation of water from aerosol surfaces.[53] In addition, they could reduce the scavenging efficiency of particles by formation of larger cloud and rain droplets.[64] In the last case, organic films would enhance the atmospheric lifetime of aerosol relative to that expected if organic films were not present.[65] In general, the properties of organic-coated aerosols depend on the nature and concentration of the ions present.
Thus, the results presented here have implications for organic-coated aerosols, as several of the ions studied here have been found at higher levels in marine aerosols, especially SO42−, which has been found in large quantities.[22,66] SO42− is considered a much stronger kosmotropic anion than other anions;[34] its π–A isotherms show that it can facilitate the formation of highly ordered organic films. As discussed above, the formation of highly ordered organic films on aqueous aerosol surfaces could hinder the transport of volatile species through the interface. The IRRAS spectra suggest that NH4+ and SO42− are likely be more attracted to – and have a higher binding affinity for – DPPC headgroups. As illustrated in Fig. 7, preferential adsorption of SO42− with the DPPC monolayer may result in the transport of DPPC monolayer into the atmosphere. These results obtained in the present study could also explain the enrichment of these ions in marine aerosols.[67] The presence of Br− in the subphase disordered the packing of the DPPC alkyl chains; i.e., it would lower the stability of organic films on an aerosol surface, resulting in defect sites in the DPPC monolayer. Experimental studies of water uptake by hydrophobic surfaces suggest that water uptake does indeed occur on these surfaces, preferentially at the defect sites.[68–70] If a small amount of water does adsorb to the surface, it can then diffuse through the coating or through coating defects and activate the inorganic core.[51]

|
Inorganic and organic acids also have received attention recently in terms of their roles in Cl−/Br− depletion reactions in marine aerosols.[71] SO42− has been shown to substitute for Cl− more effectively than NO3− in fine marine particles.[72,73] Surface-active species in marine aerosols are suggested to combine with SO42− more tightly than NO3−. Organic acids, such as CH3COO−, have been shown to be more active in the Cl−/Br− depletion reactions for the smallest marine particle size (<1 μm).[74] Based on the Hofmeister sequence, our results also show that the interactions of CH3COO− ions with DPPC molecules are stronger than those of Cl− and Br−. These selective processes can alter the chemical composition of marine aerosols and, consequently, their hygroscopic and radiative properties.
Furthermore, the increased concentration of salt solution causes destabilisation of the surface film, as the π–A isotherms of DPPC monolayers are shifted to large areas. Concentrated sodium and ammonium solutions impact the surface morphology of lipid films, which will influence the rate of water exchange with the atmosphere. Surface tension also depends on solution concentration and the presence of complex aqueous mixtures in aerosols, including both surface-active organic solutes and inorganic electrolytes.[75,76] The thickness of a lipid monolayer depends on the concentration of ions in solution.[28] The refractive index of organic-coated aerosols will change with the concentration of ions as the particle experiences loss or uptake of water from the environment.[19] The water retention combined with the organic shell on the particles can potentially impact light scattering by these particles. It could also affect their activity as cloud condensation nuclei, as well as their heterogeneous chemistry and photochemistry on the particle surfaces.[77] The results found here provide some insight into the interfacial properties of marine aerosols and their atmospheric implications.
Conclusions
The goal of this study was to ascertain how anions in marine aerosols impact the interfacial organisation and structure of organic lipid films, and to understand the effects of concentration and of the Hofmeister series. The π–A isotherms and IRRAS measurements reveal that sodium and ammonium salts expand the DPPC monolayer and destroy the ordering of the lipid packing at a certain concentration. The increase in surface pressure follows the Hofmeister series for different anions with a fixed cation. The shape of the LC phase domains is independent of salt concentration, which indicates that the anions do not penetrate into or bind to the domains of the LC phase.
The presence of Br− reduces the stability of the DPPC monolayer, and suggests that defect sites may exist at the interface. The condensed monolayer and lower separation of phosphate vibrational modes imply that aerosols are enriched in SO42− ions, but not significantly in other anions. The concentration and nature of anions impact the surface morphology of lipid films, which will influence the air–water exchange, the evaporation of water and the refractive index of organic-coated aerosols in the atmosphere.
Supplementary material
The following information is available on the Journal’s website: surface pressure–area isotherms of DPPC monolayers on NaCH3COO solutions with varying salt concentrations (Fig. S1); surface pressure–area isotherms of DPPC monolayers on 0.1 M ammonium salt solutions (Fig. S2); the all-trans structure of DPPC molecule (Fig. S3); IRRAS spectra of the CH-stretching region of DPPC monolayers on NaCl (Fig. S4) and NaHCO3 (Fig. S5) solutions with varying salt concentrations.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (91644214 and 21577080) and Shandong Natural Science Fund for Distinguished Young Scholars (JQ201705).
References
[1] R. E. Cochran, O. S. Ryder, V. H. Grassian, K. A. Prather, Sea spray aerosol: The chemical link between the oceans, atmosphere, and climate Acc. Chem. Res. 2017, 50, 599.| Sea spray aerosol: The chemical link between the oceans, atmosphere, and climateCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXkt1WktLs%3D&md5=de1ca669f7b7b890571287b8bd8e1eb3CAS |
[2] D. J. Erickson, R. A. Duce, On the global flux of atmospheric sea salt J. Geophys. Res. 1988, 93, 14079.
| On the global flux of atmospheric sea saltCrossref | GoogleScholarGoogle Scholar |
[3] P. K. Quinn, D. B. Collins, V. H. Grassian, K. A. Prather, T. S. Bates, Chemistry and related properties of freshly emitted sea spray aerosol Chem. Rev. 2015, 115, 4383.
| Chemistry and related properties of freshly emitted sea spray aerosolCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXmtVWrsL8%3D&md5=e66ac5d5da56d781c9692a2e380d44b7CAS |
[4] P. K. Quinn, T. S. Bates, K. S. Schulz, D. J. Coffman, A. A. Frossard, L. M. Russell, W. C. Keene, D. J. Kieber, Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol Nat. Geosci. 2014, 7, 228.
| Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosolCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjt12nsbo%3D&md5=aa0b6df3ab01dc667f34bbb3c4fb03f6CAS |
[5] T. Jayarathne, C. M. Sultana, C. Lee, F. Malfatti, J. L. Cox, M. A. Pendergraft, K. A. Moore, F Azam, A. V. Tivanski, C. D. Cappa, T. H. Bertram, V. H. Grassian, K. A. Prather, E. A. Stone, Enrichment of saccharides and divalent cations in sea spray aerosol during two phytoplankton blooms Environ. Sci. Technol. 2016, 50, 11511.
| Enrichment of saccharides and divalent cations in sea spray aerosol during two phytoplankton bloomsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xhs1altrnJ&md5=d98c8b188b5a6a74baf79055553fdf5bCAS |
[6] C. D. O’Dowd, M. H. Smith, I. E. Consterdine, J. A. Lowe, Marine aerosol, sea-salt, and the marine sulphur cycle: A short review Atmos. Environ. 1997, 31, 73.
| Marine aerosol, sea-salt, and the marine sulphur cycle: A short reviewCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XntVOnt74%3D&md5=7e5f5d52323bf46b4f6a53a25b1352c9CAS |
[7] K. A. Prather, T. H. Bertram, V. H. Grassian, G. B. Deane, M. D. Stokes, P. J. DeMott, L. I. Aluwihare, B. P. Palenik, F. Azam, J. H. Seinfeld, R. C. Moffet, M. J. Molina, C. D. Cappa, F. M. Geiger, G. C. Roberts, L. M. Russell, A. P. Ault, J. Baltrusaitis, D. B. Collins, C. E. Corrigan, L. A. Cuadra-Rodriguez, C. J. Ebben, S. D. Forestieri, T. L. Guasco, S. P. Hersey, M. J. Kim, W. F. Lambert, R. L. Modini, W. Mui, B. E. Pedler, M. J. Ruppel, O. S. Ryder, N. G. Schoepp, R. C. Sullivan, D. Zhao, Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol Proc. Natl. Acad. Sci. USA 2013, 110, 7550.
| Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosolCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptFGrtro%3D&md5=057fc2ae73601dfeb81272a88774f32fCAS |
[8] C. D. O’Dowd, M. C. Facchini, F. Cavalli, D. Ceburnis, M. Mircea, S. Decesari, S Fuzzi, Y. J. Yoon, J.-P. Putaud, Biogenically driven organic contribution to marine aerosol Nature 2004, 431, 676.
| Biogenically driven organic contribution to marine aerosolCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXotFGrurk%3D&md5=6a76612d651b8a00e37f638c3b23821eCAS |
[9] D. B. Collins, T. H. Bertram, C. M. Sultana, C. Lee, J. L. Axson, K. A. Prather, Phytoplankton blooms weakly influence the cloud forming ability of sea spray aerosol Geophys. Res. Lett. 2016, 43, 9975.
| Phytoplankton blooms weakly influence the cloud forming ability of sea spray aerosolCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xhs1Ojt7jI&md5=2d3e72902df4e954439170999edfccbcCAS |
[10] S. Elliott, S. M. Burrows, C. Deal, X. Liu, M. Long, O. Ogunro, L. M. Russell, O Wingenter, Prospects for simulating macromolecular surfactant chemistry at the ocean-atmosphere boundary Environ. Res. Lett. 2014, 9,
| Prospects for simulating macromolecular surfactant chemistry at the ocean-atmosphere boundaryCrossref | GoogleScholarGoogle Scholar |
[11] D. J. Donaldson, C. George, Sea-surface chemistry and its impact on the marine boundary layer Environ. Sci. Technol. 2012, 46, 10385.
| Sea-surface chemistry and its impact on the marine boundary layerCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptVWmsLs%3D&md5=aa1bac7293b4f848dd6b69faa7c2ec0bCAS |
[12] M. Cunliffe, A. Engel, S. Frka, B. Gasparovic, C. Guitart, J. C. Murrell, M. Salter, C. Stolle, R. Upstill-Goddard, O. Wurl, Sea surface microlayers: A unified physicochemical and biological perspective of the air-ocean interface Prog. Oceanogr. 2013, 109, 104.
| Sea surface microlayers: A unified physicochemical and biological perspective of the air-ocean interfaceCrossref | GoogleScholarGoogle Scholar |
[13] R. E. Cochran, O. Laskina, T. Jayarathne, A. Laskin, J. Laskin, P. Lin, C. Sultana, C. Lee, K. A. Moore, C. D. Cappa, T. H. Bertram, K. A. Prather, V. H. Grassian, E. A. Stone, Analysis of organic anionic surfactants in fine and coarse fractions of freshly emitted sea spray aerosol Environ. Sci. Technol. 2016, 50, 2477.
| Analysis of organic anionic surfactants in fine and coarse fractions of freshly emitted sea spray aerosolCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhslShur8%3D&md5=c6ddf29f0bc8516dcd7d2008fd3f2059CAS |
[14] K. Hayakawa, N. Handa, K. Kawanobe, C. S. Wong, Factors controlling the temporal variation of fatty acids in piculate matter during a phytoplankton bloom in a marine mesocosm Mar. Chem. 1996, 52, 233.
| Factors controlling the temporal variation of fatty acids in piculate matter during a phytoplankton bloom in a marine mesocosmCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjsFOntb4%3D&md5=4cab71190f03313b393bb75dcf8b96f3CAS |
[15] D. J. Donaldson, V. Vaida, The influence of organic films at the air-aqueous boundary on atmospheric processes Chem. Rev. 2006, 106, 1445.
| The influence of organic films at the air-aqueous boundary on atmospheric processesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsVOmt7o%3D&md5=d607d344c425e5730e9037b9ee93efc4CAS |
[16] J. Y. Park, S. Lim, K. Park, Mixing state of submicrometer sea spray particles enriched by insoluble species in bubble-bursting experiments J. Atmos. Ocean. Technol. 2014, 31, 93.
| Mixing state of submicrometer sea spray particles enriched by insoluble species in bubble-bursting experimentsCrossref | GoogleScholarGoogle Scholar |
[17] M. E. Salter, E. Hamacher-Barth, C. Leck, J. Werner, C. M. Johnson, I. Riipinen, E. D. Nilsson, P Zieger, Calcium enrichment in sea spray aerosol particles Geophys. Res. Lett. 2016, 43, 8277.
| Calcium enrichment in sea spray aerosol particlesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xhtl2msbnE&md5=18ad248319bd08bac58eb9914e7449b8CAS |
[18] M. A. Shaloski, T. B. Sobyra, G. M. Nathanson, DCI transport through dodecyl sulfate films on salty glycerol: Effects of seawater ions on gas entry J. Phys. Chem. A 2015, 119, 12357.
| DCI transport through dodecyl sulfate films on salty glycerol: Effects of seawater ions on gas entryCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhsVWntrzK&md5=87d5a47b9b35e3f8e078b53dac24afe7CAS |
[19] E. M. Adams, C. B. Casper, H. C. Allen, Effect of cation enrichment on dipalmitoylphosphatidylcholine (DPPC) monolayers at the air-water interface J. Colloid Interface Sci. 2016, 478, 353.
| Effect of cation enrichment on dipalmitoylphosphatidylcholine (DPPC) monolayers at the air-water interfaceCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtVagsL%2FF&md5=83a4bd589570b07b054914f542f52183CAS |
[20] D. J. Straub, T. Lee, J. L. Collett, Chemical composition of marine stratocumulus clouds over the eastern Pacific Ocean J. Geophys. Res. Atmos. 2007, 112, D04307.
| Chemical composition of marine stratocumulus clouds over the eastern Pacific OceanCrossref | GoogleScholarGoogle Scholar |
[21] K. Ho, S. Lee, J. C. Chow, J. G. Watson, Characterization of PM 10 and PM 2.5 source profiles for fugitive dust in Hong Kong Atmos. Environ. 2003, 37, 1023.
| Characterization of PM 10 and PM 2.5 source profiles for fugitive dust in Hong KongCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtlGrtLk%3D&md5=b8b8b59adb6727ad9d78ad9b44a94ff2CAS |
[22] X. Querol, A. Alastuey, S. Rodriguez, F. Plana, C. R. Ruiz, N. Cots, G. Massagué, O. Puig, PM10 and PM2. 5 source apportionment in the Barcelona Metropolitan area, Catalonia, Spain Atmos. Environ. 2001, 35, 6407.
| PM10 and PM2. 5 source apportionment in the Barcelona Metropolitan area, Catalonia, SpainCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXoslOhsrc%3D&md5=2b5fa9ca6a64eaee60bda7fa03ec2e71CAS |
[23] K. Ito, N. Xue, G. Thurston, Spatial variation of PM 2.5 chemical species and source-apportioned mass concentrations in New York City Atmos. Environ. 2004, 38, 5269.
| Spatial variation of PM 2.5 chemical species and source-apportioned mass concentrations in New York CityCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVCquro%3D&md5=834067584cf28bcd8f77ef65fdf23b46CAS |
[24] N. N. Maykut, J. Lewtas, E. Kim, T. V. Larson, Source apportionment of PM2.5 at an urban IMPROVE site in Seattle, Washington Environ. Sci. Technol. 2003, 37, 5135.
| Source apportionment of PM2.5 at an urban IMPROVE site in Seattle, WashingtonCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotFCgtb8%3D&md5=728f8dc105cc2c37fcd9d8c8d3ccc949CAS |
[25] Q. Zhou, L. Wang, Z. Cao, X. Zhou, F. Yang, P. Fu, Z. Wang, J. Hu, L. Ding, W. Jiang, Dispersion of atmospheric fine particulate matters in simulated lung fluid and their effects on model cell membranes Sci. Total Environ. 2016, 542, 36.
| Dispersion of atmospheric fine particulate matters in simulated lung fluid and their effects on model cell membranesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhslCnu73J&md5=c26f03ec3a09efae1933d5848fb4a5bfCAS |
[26] E. Leontidis, Investigations of the Hofmeister series and other specific ion effects using lipid model systems Adv. Colloid Interface Sci. 2017, 243, 8.
| Investigations of the Hofmeister series and other specific ion effects using lipid model systemsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXlslymurs%3D&md5=ae830bcd44d818663f5974d08b4eda24CAS |
[27] B. Wen, C. Sun, B. Bai, E. Y. Gatapova, O. A. Kabov, Ionic hydration-induced evolution of decane-water interfacial tension Phys. Chem. Chem. Phys. 2017, 19, 14606.
| Ionic hydration-induced evolution of decane-water interfacial tensionCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXntFOhtL8%3D&md5=a108f43004ba40f8b5330c40badc4f85CAS |
[28] E. M. Adams, D. Verreault, T. Jayarathne, R. E. Cochran, E. A. Stone, H. C. Allen, Surface organization of a DPPC monolayer on concentrated SrCl2 and ZnCl2 solutions Phys. Chem. Chem. Phys. 2016, 18, 32345.
| Surface organization of a DPPC monolayer on concentrated SrCl2 and ZnCl2 solutionsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhvVOjsb3J&md5=c9b169b3e65a4648401a4428410a2027CAS |
[29] J. Brandsma, E. C. Hopmans, C. P. D. Brussaard, H. J. Witte, S. Schouten, J. S. S. Damste, Spatial distribution of intact polar lipids in North Sea surface waters: Relationship with environmental conditions and microbial community composition Limnol. Oceanogr. 2012, 57, 959.
| Spatial distribution of intact polar lipids in North Sea surface waters: Relationship with environmental conditions and microbial community compositionCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlCqs7bI&md5=6da9756f369d7dd50fa6dac0827a596cCAS |
[30] L. F. Espinosa, S. Pantoja, L. A. Pinto, J. Rullkoetter, Water column distribution of phospholipid-derived fatty acids of marine microorganisms in the Humboldt Current system off northern Chile Deep Sea Res. Part II: Top. Stud. Oceanogr. 2009, 56, 1063.
| Water column distribution of phospholipid-derived fatty acids of marine microorganisms in the Humboldt Current system off northern ChileCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnt1ymur8%3D&md5=5673d8b3d39c07cfa2bf1b1a4ed51e2bCAS |
[31] S. Li, L. Du, Z. Wei, W. Wang, Aqueous-phase aerosols on the air-water interface: Response of fatty acid Langmuir monolayers to atmospheric inorganic ions Sci. Total Environ. 2017, 580, 1155.
| Aqueous-phase aerosols on the air-water interface: Response of fatty acid Langmuir monolayers to atmospheric inorganic ionsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XitFehsrnM&md5=ad7366cbec50732a3a0d938f35a0c4c4CAS |
[32] M. C. Phillips, D. Chapman, Monolayer characteristics of saturated 1,2-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface Biochim. Biophys. Acta 1968, 163, 301.
| Monolayer characteristics of saturated 1,2-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interfaceCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXovVKq&md5=5d61da4288ab13b32dc89dc6e7785ef8CAS |
[33] K. D. Collins, M. W. Washabaugh, The Hofmeister effect and the behaviour of water at interfaces Q. Rev. Biophys. 1985, 18, 323.
| The Hofmeister effect and the behaviour of water at interfacesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXhsFWlurs%3D&md5=794c2c8584fc2c97c28e43ea38f448bdCAS |
[34] D. F. Parsons, M. Bostroem, P. Lo Nostro, B. W. Ninham, Hofmeister effects: interplay of hydration, nonelectrostatic potentials, and ion size Phys. Chem. Chem. Phys. 2011, 13, 12352.
| Hofmeister effects: interplay of hydration, nonelectrostatic potentials, and ion sizeCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXot1altrc%3D&md5=6b887f3355431e16a6d3036774878718CAS |
[35] X. Li, K. Huang, J. Lin, Y. Xu, H. Liu, Hofmeister ion series and its mechanism of action on affecting the behavior of macromolecular solutes in aqueous solution Prog. Chem. 2014, 26, 1285.
| 1:CAS:528:DC%2BC2MXhvVKltLjJ&md5=db4908412c1c61ce7f470a2136cbd66eCAS |
[36] Z. Yang, Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis J. Biotechnol. 2009, 144, 12.
| Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysisCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCntr7N&md5=659672dbcfbd167e2606a42ea295ab2cCAS |
[37] A. Aroti, E. Leontidis, E. Maltseva, G. Brezesinski, Effects of Hofmeister anions on DPPC Langmuir monolayers at the air-water interface J. Phys. Chem. B 2004, 108, 15238.
| Effects of Hofmeister anions on DPPC Langmuir monolayers at the air-water interfaceCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVyju7s%3D&md5=0d99786a4459d38141470f7218a12d50CAS |
[38] Y. J. Zhang, P. S. Cremer, Chemistry of Hofmeister anions and osmolytes Annu. Rev. Phys. Chem. 2010, 61, 63.
| Chemistry of Hofmeister anions and osmolytesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmt1eqtLc%3D&md5=6a301fd66fa196c0f42d8206b1614be4CAS |
[39] M. C. Gurau, S. M. Lim, E. T. Castellana, F. Albertorio, S. Kataoka, P. S. Cremer, On the mechanism of the Hofmeister effect J. Am. Chem. Soc. 2004, 126, 10522.
| On the mechanism of the Hofmeister effectCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmsVWjtb8%3D&md5=d533f863db2dd4e1308489b66aa9e322CAS |
[40] J. N. Sachs, T. B. Woolf, Understanding the Hofmeister effect in interactions between chaotropic anions and lipid bilayers: Molecular dynamics simulations J. Am. Chem. Soc. 2003, 125, 8742.
| Understanding the Hofmeister effect in interactions between chaotropic anions and lipid bilayers: Molecular dynamics simulationsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvFWlsrY%3D&md5=57b1e5f41cb30edc1d04924e1349784eCAS |
[41] M. Bostrom, D. R. M. Williams, B. W. Ninham, Specific ion effects: Why DLVO theory fails for biology and colloid systems Phys. Rev. Lett. 2001, 87, 168103.
| Specific ion effects: Why DLVO theory fails for biology and colloid systemsCrossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnjslGktQ%3D%3D&md5=f5e9c857477761c5d72fa0604b6fe045CAS |
[42] A. Aroti, E. Leontidis, M. Dubois, T. Zemb, Effects of monovalent anions of the Hofmeister series on DPPC lipid Bilayers part I: Swelling and in-plane equations of state Biophys. J. 2007, 93, 1580.
| Effects of monovalent anions of the Hofmeister series on DPPC lipid Bilayers part I: Swelling and in-plane equations of stateCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVKis7s%3D&md5=931b81515b40cfa1c262de2127f161e4CAS |
[43] M. G. Cacace, E. M. Landau, J. J. Ramsden, The Hofmeister series: salt and solvent effects on interfacial phenomena Q. Rev. Biophys. 1997, 30, 241.
| The Hofmeister series: salt and solvent effects on interfacial phenomenaCrossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c%2Fls1alug%3D%3D&md5=d7d8235f32451a0b4e66a7ba8cbd1dc1CAS |
[44] D. O. Shah, J. H. Schulman, Binding of metal ions to monolayers of lecithins plasmalogen cardiolipin and dicetyl phosphate J. Lipid Res. 1965, 6, 341.
| 1:CAS:528:DyaF2MXkt1Ggtrk%3D&md5=6549a1d7a5aff8a0af96df7ec96bcbacCAS |
[45] C. Lendrum, K. M. McGrath, The role of subphase chemistry in controlling monolayer behaviour J. Colloid Interface Sci. 2009, 331, 206.
| The role of subphase chemistry in controlling monolayer behaviourCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjs1Sksw%3D%3D&md5=6a079e7979e82f29f2b1482540c01971CAS |
[46] C. D. Lendrum, B. Ingham, B. Lin, M. Meron, M. F. Toney, K. M. McGrath, Nonequilibrium 2-hydroxyoctadecanoic acid monolayers: Effect of electrolytes Langmuir 2011, 27, 4430.
| Nonequilibrium 2-hydroxyoctadecanoic acid monolayers: Effect of electrolytesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVCqs78%3D&md5=6b23fdd3c41720687cbe6bb6f9874b90CAS |
[47] K. D. Collins, Sticky ions in biological systems Proc. Natl. Acad. Sci. USA 1995, 92, 5553.
| Sticky ions in biological systemsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmtFOgsLg%3D&md5=729fe9e70a4cb74e23c62bca391d2e11CAS |
[48] K. D. Collins, Charge density-dependent strength of hydration and biological structure Biophys. J. 1997, 72, 65.
| Charge density-dependent strength of hydration and biological structureCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmtFGltw%3D%3D&md5=4ed1263a749d3473cde1863b0b978e28CAS |
[49] P. H. von Hippel, K. Y. Wong, Neutral salts: the generality of their effects on the stability of macromolecular conformations Science 1964, 145, 577.
| Neutral salts: the generality of their effects on the stability of macromolecular conformationsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXks1anu7k%3D&md5=03be0173c0aa5207ee535cfcbd375902CAS |
[50] L. M. Pegram, M. T. Record, Hofmeister salt effects on surface tension arise from partitioning of anions and cations between bulk water and the air-water interface J. Phys. Chem. B 2007, 111, 5411.
| Hofmeister salt effects on surface tension arise from partitioning of anions and cations between bulk water and the air-water interfaceCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkt1Siur0%3D&md5=c494c55bd766efb0fddeebada994700aCAS |
[51] R. M. Garland, M. E. Wise, M. R. Beaver, H. L. DeWitt, A. C. Aiken, J. L. Jimenez, M. A. Tolbert, Impact of palmitic acid coating on the water uptake and loss of ammonium sulfate particles Atmos. Chem. Phys. 2005, 5, 1951.
| Impact of palmitic acid coating on the water uptake and loss of ammonium sulfate particlesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVejs7rM&md5=fba9a46ca0dbc6b79b50d5ad5e4abba8CAS |
[52] T. M. Raymond, S. N. Pandis, Formation of cloud droplets by multicomponent organic particles J. Geophys. Res. Atmos. 2003, 108, 4469.
| Formation of cloud droplets by multicomponent organic particlesCrossref | GoogleScholarGoogle Scholar |
[53] Q. T. Nguyen, K. H. Kjaer, K. I. Kling, T. Boesen, M. Bilde, Impact of fatty acid coating on the CCN activity of sea salt particles Tellus B Chem. Phys. Meterol. 2017, 69, 1304064.
| Impact of fatty acid coating on the CCN activity of sea salt particlesCrossref | GoogleScholarGoogle Scholar |
[54] K. D. Collins, Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process Methods 2004, 34, 300.
| Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization processCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvFCrs7o%3D&md5=84b949ba8d9d68015e69281322271978CAS |
[55] M. Christoforou, E. Leontidis, G. Brezesinski, Effects of sodium salts of lyotropic anions on low-temperature, ordered lipid monolayers J. Phys. Chem. B 2012, 116, 14602.
| Effects of sodium salts of lyotropic anions on low-temperature, ordered lipid monolayersCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslKhsbnM&md5=4ba215b24d20a74f3b5c113196452b73CAS |
[56] R. Mendelsohn, J. W. Brauner, A. Gericke, External infrared reflection absorption spectrometry of monolayer films at the air-water interface Annu. Rev. Phys. Chem. 1995, 46, 305.
| External infrared reflection absorption spectrometry of monolayer films at the air-water interfaceCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXptlOju78%3D&md5=b0a18279f724dd9504212cf8cdb583a9CAS |
[57] C. H. Huang, J. R. Lapides, I. W. Levin, Phase-transition behavior of saturated, symmetric chain phospholipid bilayer dispersions determined by Raman spectroscopy: correlation between spectral and thermodynamic parameters J. Am. Chem. Soc. 1982, 104, 5926.
| Phase-transition behavior of saturated, symmetric chain phospholipid bilayer dispersions determined by Raman spectroscopy: correlation between spectral and thermodynamic parametersCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XlslOgsL0%3D&md5=1da1107571430770aae51ebacd53dafeCAS |
[58] P. H. B. Aoki, L. F. C. Morato, F. J. Pavinatto, T. M. Nobre, C. J. L. Constantino, O. N. Oliveira, Molecular-level modifications induced by photo-oxidation of lipid monolayers interacting with erythrosin Langmuir 2016, 32, 3766.
| Molecular-level modifications induced by photo-oxidation of lipid monolayers interacting with erythrosinCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XkvVOrtbo%3D&md5=ffd7cca7f09d701ad4c16e361a6f4168CAS |
[59] I. W. Levin, T. E. Thompson, Y. Barenholz, C. Huang, Two types of hydrocarbon chain interdigitation in sphingomyelin bilayers Biochemistry 1985, 24, 6282.
| Two types of hydrocarbon chain interdigitation in sphingomyelin bilayersCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXlslCgtro%3D&md5=a9d1c3fbaa26e681aaf358517cbd71a4CAS |
[60] L. Ghaicha, R. M. Leblanc, A. K. Chattopadhyay, Influence of concentrated ammonium nitrate solution on monolayers of some dicarboxylic acid derivatives at the air/water interface Langmuir 1993, 9, 288.
| Influence of concentrated ammonium nitrate solution on monolayers of some dicarboxylic acid derivatives at the air/water interfaceCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXks1aqug%3D%3D&md5=fb33a4d7b64420b2dc454a9f166b31d4CAS |
[61] R. Maheshwari, A. Dhathathreyan, Influence of ammonium nitrate in phase transitions of Langmuir and Langmuir-Blodgett films at air/solution and solid/solution interfaces J. Colloid Interface Sci. 2004, 275, 270.
| Influence of ammonium nitrate in phase transitions of Langmuir and Langmuir-Blodgett films at air/solution and solid/solution interfacesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXktFKktbo%3D&md5=f2e0f53375f243899a7a83f15a9c580cCAS |
[62] I. Masalova, K. Kovalchuk, A. Y. Malkin, IR studies of interfacial interaction of the succinic surfactants with different head groups in highly concentrated W/O emulsions J. Dispers. Sci. Technol. 2011, 32, 1547.
| IR studies of interfacial interaction of the succinic surfactants with different head groups in highly concentrated W/O emulsionsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlGlsbrF&md5=845b00b50ff16b4b04e45adb9e927c2eCAS |
[63] J. Xia, L. X. Song, W. Liu, Y. Teng, Leveling effects of ammonium salts on thermal stabilities of polyethylene glycols Soft Matter 2013, 9, 9714.
| Leveling effects of ammonium salts on thermal stabilities of polyethylene glycolsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFSmtrnF&md5=ec243aaa56a28eca610fcbde95d7630aCAS |
[64] P. S. Gill, T. E. Graedel, C. J. Weschler, Organic films on atmospheric aerosol particles, fog droplets, cloud droplets, raindrops, and snowflakes Rev. Geophys. 1983, 21, 903.
| Organic films on atmospheric aerosol particles, fog droplets, cloud droplets, raindrops, and snowflakesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXkslequ7Y%3D&md5=b52723ec4475bbe539fa16ddf3ed3d28CAS |
[65] J. F. Davies, R. E. H. Miles, A. E. Haddrell, J. P. Reid, Influence of organic films on the evaporation and condensation of water in aerosol Proc. Natl. Acad. Sci. USA 2013, 110, 8807.
| Influence of organic films on the evaporation and condensation of water in aerosolCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFait77E&md5=6ffb8809e3bc455ffe8d35c157809e79CAS |
[66] Y. L. Sun, G. S. Zhuang, A. H. Tang, Y. Wang, Z. S. An, Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing Environ. Sci. Technol. 2006, 40, 3148.
| Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in BeijingCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtlSisbs%3D&md5=ab204b75bd6b1ab21ffe1dd121fa5042CAS |
[67] M. O. Andreae, R. J. Charlson, F. Bruynseels, H. Storms, R. Vangrieken, W. Maenhaut, Internal mixture of sea salt, silicates, and excess sulfate in marine aerosols Science 1986, 232, 1620.
| Internal mixture of sea salt, silicates, and excess sulfate in marine aerosolsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XksVKlsLY%3D&md5=f8b6a6e7f01d4ee260643d49f7858856CAS |
[68] E. Weingartner, H. Burtscher, U. Baltensperger, Hygroscopic properties of carbon and diesel soot particles Atmos. Environ. 1997, 31, 2311.
| Hygroscopic properties of carbon and diesel soot particlesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjslygs7c%3D&md5=929109e5bd987592884aaa06a9aebecbCAS |
[69] E. Thomas, Y. Rudich, S. Trakhtenberg, R. Ussyshkin, Water adsorption by hydrophobic organic surfaces: Experimental evidence and implications to the atmospheric properties of organic aerosols J. Geophys. Res. Atmos. 1999, 104, 16053.
| Water adsorption by hydrophobic organic surfaces: Experimental evidence and implications to the atmospheric properties of organic aerosolsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlt1Sgu7s%3D&md5=9c61f8c106c814df874e99f11bd1572aCAS |
[70] N. M. Persiantseva, O. B. Popovicheva, N. K. Shonija, Wetting and hydration of insoluble soot particles in the upper troposphere J. Environ. Monit. 2004, 6, 939.
| Wetting and hydration of insoluble soot particles in the upper troposphereCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVais7fN&md5=0e09b12a8eec399890584fe1ca73076cCAS |
[71] R. A. Braun, H. Dadashazar, A. B. MacDonald, A. M. Aldhaif, L. C. Maudlin, E. Crosbie, M. A. Aghdam, A. H. Mardi, A. Sorooshian, Impact of wildfire emissions on chloride and bromide depletion in marine aerosol particles Environ. Sci. Technol. 2017, 51, 9013.
| Impact of wildfire emissions on chloride and bromide depletion in marine aerosol particlesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtFGnsbzI&md5=d816315320e771b6b691e0e04635caa9CAS |
[72] R. Sander, P. J. Crutzen, Model study indicating halogen activation and ozone destruction in polluted air masses transported to the sea J. Geophys. Res. Atmos. 1996, 101, 9121.
| Model study indicating halogen activation and ozone destruction in polluted air masses transported to the seaCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XjtlCjt7s%3D&md5=1f0e193bbac3a99d5616e73e5e62d006CAS |
[73] X. H. Yao, M. Fang, C. K. Chan, The size dependence of chloride depletion in fine and coarse sea-salt particles Atmos. Environ. 2003, 37, 743.
| The size dependence of chloride depletion in fine and coarse sea-salt particlesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotlKhtw%3D%3D&md5=e6be98299ec6f32b186c7af07ee46ac4CAS |
[74] V. M. Kerminen, K. Teinila, R. Hillamo, T. Pakkanen, Substitution of chloride in sea-salt particles by inorganic and organic anions J. Aerosol Sci. 1998, 29, 929.
| Substitution of chloride in sea-salt particles by inorganic and organic anionsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkvFeisbY%3D&md5=2c21a31bffa7361ff6e7dbee91f6996bCAS |
[75] H. C. Boyer, C. S. Dutcher, Atmospheric aqueous aerosol surface tensions: Isotherm-based modeling and biphasic microfluidic measurements J. Phys. Chem. A 2017, 121, 4733.
| Atmospheric aqueous aerosol surface tensions: Isotherm-based modeling and biphasic microfluidic measurementsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXns1Ontr0%3D&md5=7d4ab01a57c417e2da763d687430ed92CAS |
[76] J. Ovadnevaite, A. Zuend, A. Laaksonen, K. J. Sanchez, G. Roberts, D. Ceburnis, S. Decesari, M. Rinaldi, N. Hodas, M. C. Facchini, J. H. Seinfeld, C. O’Dowd, Surface tension prevails over solute effect in organic-influenced cloud droplet activation Nature 2017, 546, 637.
| Surface tension prevails over solute effect in organic-influenced cloud droplet activationCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtVequrnE&md5=a9e5a51bd3dccc12aaff4696afc0f344CAS |
[77] A. Zelenyuk, M. J. Ezell, V. Perraud, S. N. Johnson, E. A. Bruns, Y. Yu, D. Imre, M. L. Alexander, B. J. Finlayson-Pitts, Characterization of organic coatings on hygroscopic salt particles and their atmospheric impacts Atmos. Environ. 2010, 44, 1209.
| Characterization of organic coatings on hygroscopic salt particles and their atmospheric impactsCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitF2lt7o%3D&md5=56bc4e6b1c6a85d296dda2c927c38d9dCAS |


