Breeding progress in the pasta-making quality of durum wheat cultivars released in Italy and Spain during the 20th Century
Joan Subira A , Roberto Javier Peña B , Fanny Álvaro A , Karim Ammar B , Abdelhamid Ramdani A and Conxita Royo A CA IRTA (Institute for Food and Agricultural Research and Technology), Field Crops Section, 25198 Lleida, Spain.
B CIMMYT (International Maize and Wheat Improvement Center), 06600, Mexico DF, Mexico.
C Corresponding author. Email: conxita.royo@irta.es
Crop and Pasture Science 65(1) 16-26 https://doi.org/10.1071/CP13238
Submitted: 4 July 2013 Accepted: 8 October 2013 Published: 2 January 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Genetic improvement of quality traits of durum wheat achieved in Italy and Spain during the 20th Century was investigated using an historical series of 12 cultivars from each country. The European Union durum wheat quality index increased by 6.25% (0.13% year–1 in Italian and 0.06% year–1 in Spanish cultivars). Protein content decreased by ~10% (–0.14% year–1 in Italian and –0.19% year–1 in Spanish cultivars) but protein per ha increased at a rate of 0.35% year–1 (0.41% year–1 in Spanish and 0.26% year–1 in Italian cultivars). Yellow colour index increased by 9.9% (0.15% year–1 in Italian and 0.10% year–1 in Spanish cultivars). Test weight and vitreousness did not suffer significant changes over time. Gluten strength increased by 32.1% or 0.54% year–1 in Italian, and 27.9% or 0.33% year–1 in Spanish cultivars. Much larger genetic control on gluten strength was found in Italian than in Spanish cultivars. Changes in sedimentation index (41.1% or 0.64% year–1 in Italy, and 41.6% or 0.49% year–1 in Spain) were the consequence of the progressive incorporation into recent cultivars of favourable low molecular weight glutenin subunits (LMW-GS). Breeding increased the frequency of the LMW-GS combination aaa, which was present in 75% of all intermediate cultivars and in 100% of the modern Italian cultivars. A LMW-GS combination not previously reported (d?b) was identified in two modern Spanish cultivars. Breeding programs were also successful in increasing the stability of gluten strength and the sedimentation index.
Additional keywords: genetic gain, genetic improvement, glutenin subunits, historical series, old to modern cultivars, quality stability.
Introduction
Italy and Spain are among the main durum wheat (Triticum turgidum L. var. durum) producers of the European Union (EU), where most of the grain is devoted to pasta manufacturing (Di Fonzo et al. 2005; Royo 2005). In Mediterranean environments, durum wheat is mostly grown under rainfed conditions, where the crop is frequently exposed to environmental stresses, with high temperatures and water scarcity common during the grain-filling period. This usually limits the achievement of high yields, but in most years has low or nil negative effects on grain attributes determining pasta-making quality. The release of durum cultivars with high quality standards has been a major breeding concern during the last half of the past century (Pagnotta et al. 2005) and is still one of the main goals of breeding programs in the region.
Pasta cooking properties are mostly related to high grain protein content and to the quality of its gluten protein. These traits, together with vitreousness and yellow semolina colour, are of great importance for durum wheat quality (Di Fonzo et al. 2005). Gluten, which constitutes around the 80% of the endosperm protein, is composed of gliadins and glutenins (Peña et al. 2002; Sissons 2008) and its properties depend on its protein subunit composition and resulting polymeric structure. It is generally accepted that glutenins confer elasticity and gliadins are responsible for the viscosity and extensibility of the gluten, with the interactions between the two protein fractions determining the ultimate gluten quality of one cultivar (Weegels et al. 1996). It is also accepted that, of the two fractions, the glutenin is still the main one responsible of the gluten strength (Peña et al. 2002).
Glutenin subunits can be separated according to their relative mobility into high molecular weight (HMW-GS) and low molecular weight (LMW-GS) using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Singh et al. 1991). The LMW-GS comprise 80% of the total glutenin (Peña et al. 2002; Ruiz et al. 2005; Sissons 2008). In durum wheat the synthesis of the HMW-GS is controlled by the Glu-A1 and Glu-B1 loci, whereas for the LMW-GS, the Glu-A3, Glu-B3 and Glu-B2 loci are responsible (Carrillo et al. 2000). Both HMW-GS and LMW-GS, but mainly the latter, have a great influence on gluten strength and on the pasta-making quality of durum wheat (Peña 2000; Ruiz et al. 2005), stressing the need to obtain cultivars with the optimal HMW-GS and LMW-GS combinations to boost gluten quality.
Contrasting breeding strategies were reportedly adopted in Italy and Spain during the 20th Century. Whereas ancient Italian cultivars were incorporated and their alleles recombined during the process of genetic improvement and modern cultivar development, Spanish breeding efforts did not involve the use of traditional local germplasm (Martos et al. 2005; Royo et al. 2007). Breeding programs conducted in Italy since the beginning of the 20th Century (Maliani 1979) are considered as pioneering in the world of durum wheat and, as a result, the Italian durum wheat pool currently represents the most outstanding and differentiated pool in the Mediterranean Basin compared with the dominating CIMMYT (International Maize and Wheat Improvement Center) derived germplasm (Royo et al. 2009). These early programs largely relied on local landraces. Old cultivars, such as ‘Senatore Cappelli’, were widely cultivated until the end of the 1960s and were broadly used as parents in Italian breeding programs, being present in the genetic background of many modern cultivars (Martos et al. 2005). Italian cultivars released from the late 1970s and the 1980s originated from crosses between local accessions and CIMMYT germplasm, with more recent ones, such as ‘Simeto’ and ‘Zenit’, still broadly used by many Italian farmers (Di Fonzo et al. 2005). On the other hand, the lack of continuity in Spanish local breeding programs during the first half of the 20th Century became a constraint for the use of the genetic background of local landraces into modern cultivar development (Royo and Briceño-Félix 2011). From the 1970s onwards, introduced CIMMYT germplasm had a great impact on cultivar releases in the country, where ‘Mexa’ (a cultivar derived from the CIMMYT hallmark ‘Mexicali 75’) covered ~90% of the durum wheat area during the mid-1980s (Martos et al. 2005; Royo 2005). Similar widespread impact was achieved with the release and extensive adoption, during the mid-1980s and 1990s and until very recently, of the ‘Yavaros’-derived lines such as ‘Yavaros 79’ itself, ‘Vitron’, ‘Nuño’ and others.
References to the genetic gains in durum wheat quality during the last Century in Mediterranean countries are scarce. De Vita et al. (2007) showed an increase in pasta-making quality during the 20th Century in durum wheat cultivars released in Italy, due to the incorporation of favourable alleles in modern cultivars, such as the 7+8 subunit of the Glu-B1 locus, which increased gluten strength. However, no similar studies have been conducted to assess the breeding progress in quality during the 20th Century in Spain.
The present study was conducted using an historical series of 24 durum wheat cultivars released in different periods during the 20th Century in Italy and Spain to: (i) ascertain the changes achieved by breeding on the most relevant grain quality traits, and (ii) to assess the relationship between allelic variations associated with HMW-GS and LMW-GS composition and the changes in gluten strength observed during the same period.
Materials and methods
Plant material
Twenty-four durum wheat (Triticum turgidum L. var. durum) cultivars, 12 Italian and 12 Spanish, were selected to represent the germplasm grown in Italy and Spain during the 20th Century. Based on the year of release, the cultivars were assigned to three periods: old (mainly landraces, released before 1945), intermediate (released between 1950 and 1985) and modern (released from 1988 to 2000) (Table 1). The intermediate group included early semi-dwarf cultivars derived from CIMMYT germplasm, such as cv. Mexa, and landmark early European cultivars such as cv. Creso. The modern set included cultivars released by local breeding programs during the last decade of the Century in both countries. In the selection of Spanish modern cultivars we avoided the inclusion of cultivars of foreign origin (derived from Italian, French or CIMMYT germplasm), despite the fact that these are dominant in the Spanish market. This was done to enable assessment of the impact of modern efforts conducted locally in Spain. Phylogenetic relationships have been previously ascertained in this historical series (Martos et al. 2005), which has also been used to assess changes in yield formation (Royo et al. 2007, 2008), and biomass production and allocation (Álvaro et al. 2008a, 2008b, 2008c).
Experimental
Five experiments were conducted in Lleida (north-eastern Spain) involving four growing seasons (2001, 2002, 2004 and 2005) and two locations: Gimenells (41°40ʹN, 0°20ʹE) under irrigated conditions (2001, 2002, 2004, 2005); and Foradada (41°88ʹN, 0°76ʹE), a rainfed site (2002). Soils were mesic Calcixerolic Xerochrept in Gimenells and Xerofluvent Oxiaquic in Foradada (Soil Survey Staff 1999), both with a fine-loamy texture. The experimental design was a randomised complete block with four replicates and plots of 12 m2 (8 rows 0.15 m apart). Planting time was between 31 October and 16 December in all cases, at a sowing rate of 400 fully viable seeds m–2. Plots were fertilised following the recommendations for maximising yields while preventing lodging, and were kept disease- and insect- free with preventive pesticide applications. Weather stations located near the experimental sites provided daily meteorological data. Water input (rainfall + irrigation) ranged from 275 to 322 mm from sowing to anthesis and from 36.1 to 137 mm from anthesis to maturity. Average daily mean temperatures ranged from 7.12 to 9.18°C from sowing to anthesis, and from 16.9 to 21.1°C from anthesis to maturity. Mean length of the grain filling period, expressed as thermal time (growing degree-days, GDD), ranged from 311 to 390 GDD.
Quality analyses
Plots were mechanically harvested at commercial maturity and grain yield was expressed at 12% moisture basis. A sample of ~250 g of whole grain from each plot was cleaned and used for quality analysis. Grain protein content (%) was determined by a near-infrared spectroscope (NIT, Infratec® 1241 grain analyser; Foss, Hilleroed, Denmark) previously calibrated for protein content against the standard Kjeldahl method. Whole-grain flour samples were obtained with a whole-meal grinder; fine particle size was ensured by attaching a 0.5-mm screen to the grinder. Gluten strength was determined on 1 g of whole-grain flour samples using the SDS sedimentation test, following the methodology of Axford et al. (1978) as modified by Peña et al. (1990), and using stoppered, 25-mL graduated cylinders. The sedimentation index was computed as the ratio between gluten strength and protein content, expressed as mL per % protein unit. Yellow colour index (b, CIE L*a*b* colour system) was estimated on whole-grain flour using a portable reflectance colourimeter (CR-400; Konica-Minolta Sensing, Inc., Tokyo) equipped with a filter tri-stimulate system. Test weight (TW, kg hL–1) was determined by the GAC2100 analyser (Dickey-John Co., Auburn, IL, USA). These four quality traits were used to calculate the EU quality index (QI) for durum (European Commission Regulation No. 2237/2003, 23 December 2003), using cv. Simeto as reference check. Each quality trait was expressed for each cultivar as a percentage of the mean value of cv. Simeto (assumed to be 100%), and the QI was calculated by weighting each trait according to the following percentages: protein content (40%), gluten strength (30%), yellow index (20%), TW (10%). Grain vitreousness (%) was determined by counting the number of vitreous grains after cutting a random sample of 100 grains per plot.
Allelic composition of HMW-GS and LMW-GS
Electrophoretic analyses were performed to identify HMW- and LMW-GS composition at five loci: Glu-A1, Glu-B1, Glu-A3, Glu-B3, Glu-B2. Electrophoresis was run on a bulk of 10 seeds from each cultivar. For the old cultivars, a spike of the dominant type was previously selected and its seeds were planted in the subsequent growing season on an individual row. This row was harvested at ripening, and 10 seeds from the bulk were taken for electrophoresis. Electrophoretic analysis (1D SDS-PAGE) was conducted according to the protein extraction process of Singh et al. (1991) and the protocols implemented at CIMMYT by Peña et al. (2004). The nomenclature followed was that proposed by Payne and Lawrence (1983) for the HMW-GS and that of Nieto-Taladriz et al. (1997) for the LMW-GS.
Statistical analyses
Combined analysis of variance (ANOVA), in which the cultivar effect was partitioned into its components (period, country, period × country interaction, and cultivar within period and country), were performed for all quality traits. Additional ANOVAs were conducted for the sedimentation index considering as factors the HMW-GS and LMW-GS allelic combinations identified in the cultivars from each country. Means were compared by Tukey test at P = 0.05. Absolute (AGG, trait unit year–1) and relative (RGG, % year–1) genetic gains were computed for each quality trait as the slope of the linear regression model fitted to the relationship between the absolute or relative value of the trait and the year of cultivar release. Relative values were computed for each cultivar as percentage irrespective of the average value of all the cultivars for a given country. The stability of each quality trait was determined for each cultivar as the slope (b) of the joint regression analysis (Finlay and Wilkinson 1963), and slopes were compared by using PROC GLM of SAS statistical package (SAS Institute Inc. 2009a). In order to assess the changes produced in the stability of the quality traits across time, the relationship between b and the year of release was studied for those traits in which the regression slopes differed significantly between cultivars. All analyses were performed with the JMP ver. 8 software (SAS Institute Inc. 2009b) and Genstat ver. 13 (Genstat 2010).
Results
Genetic changes on grain quality
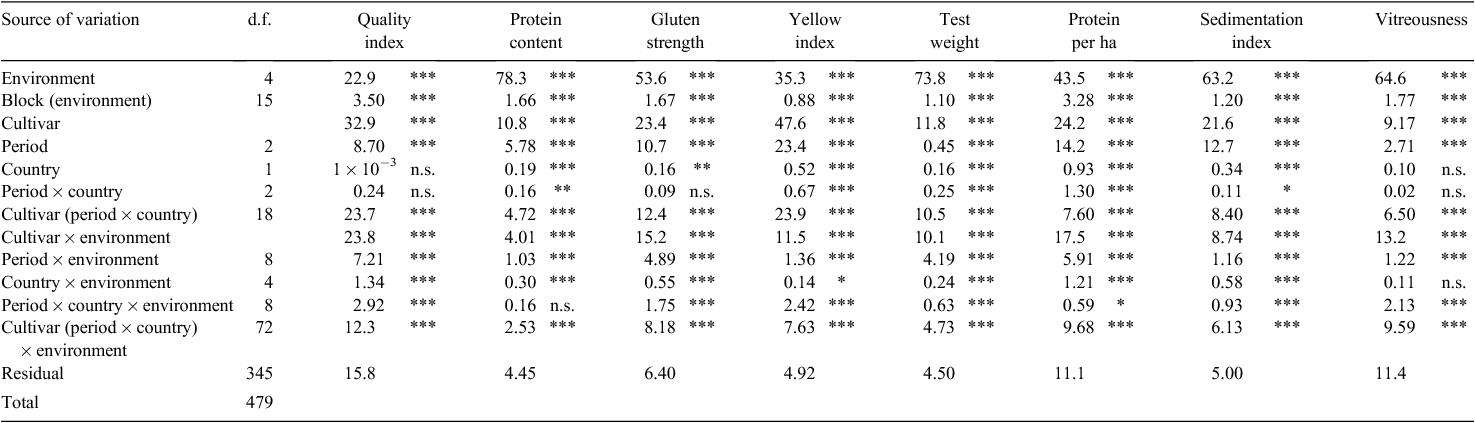
The combined ANOVA for grain quality traits revealed that the percentage of total variance explained by the cultivar effect was very large for yellow index and quality index (48% and 33%, respectively), somewhat lower but still substantial for protein per ha, gluten strength and sedimentation index (21–24%), and low for test weight, protein content and vitreousness (<12%) (Table 2). The partitioning of the cultivar effect into its components showed that differences between breeding periods explained 0.45–23.4% of total variance corresponding to 3.8% (for test weight) to 49.2% (for yellow index) of the cultivar effect. The country effect was significant for all traits except for quality index and vitreousness. Even in the case of statistical significance of the country effect, the percentage of variation explained was always ≤1%.
Comparison of the mean values of grain quality traits for the cultivars released in different periods in Italy and Spain showed a steady increase in all but protein content, test weight and vitreousness (Table 3). Positive changes in the quality index over time were due to substantial increases in gluten strength and yellow index, compensating for the significant decrease in protein content and test weight. Albeit significant statistically, this decrease in protein content was relative and did not result in values below 14% in any of the historical or country groups considered. Test weight did not suffer significant changes over time. The largest improvements from old to modern cultivars occurred in protein per unit area, gluten strength and, consequently, in the sedimentation index. Vitreousness suffered a significant decrease in cultivars from the intermediate period compared with the old cultivars, but this reduction was partially compensated for in the modern cultivars (Table 3).
The overall rate of genetic change in the quality index was 0.09% year–1, and the improvement was more than double for Italian than for Spanish cultivars (Table 4). Relative genetic gains for gluten strength, yellow index and sedimentation index were also greater in the Italian germplasm. Protein content decreased at a higher rate in Spanish than in Italian cultivars, but the protein yield per ha increased from old to modern cultivars by 0.41% year–1 in Spanish and 0.26% year–1 in Italian cultivars. The rates of genetic change in test weight and vitreousness were not significant for either country (Table 4).

|
The stability of the quality traits was assessed by comparing the cultivar slopes of the regression models (b) fitted to the relationship between the genotype and the environmental means. The results revealed differences between slopes (P < 0.05) only for gluten strength, yellow index, sedimentation index and vitreousness (data not shown). For these traits the slopes of the joint regression analyses were plotted against the year of cultivar release, with the results showing that for gluten strength and sedimentation index, the b values decreased over the 20th Century, at a rate of 0.01 year–1 and 0.004 year–1, respectively (Fig. 1a, c), but for yellow index and vitreousness changes were not statistically significant (Fig. 1b, d).

|
Allelic composition of HMW-GS and LMW-GS
Twelve and 18 alleles, encoded by Glu-1, Glu-3 or Glu-2, were identified in the Italian and Spanish cultivars, respectively (Table 1). The null allele was the most frequent at Glu-A1, with only two old cultivars—Italian cv. Balilla Falso and Spanish cv. Rubio de Belalcázar— having the alternative allele 1 at this locus (Table 1). Four and six different alleles were found at Glu-B1 in Italian and Spanish cultivars, respectively, with the old Italian cultivars included in this study being monomorphic for allele 20 at this locus. Greater allelic diversity was found at Glu-B1 in the modern cultivars of both countries than in the groups of intermediate or old cultivars. Band 6 was the most frequent at Glu-A3, but the null allele at this locus was present in some old and intermediate cultivars from both countries (Table 1). The most frequent banding pattern at Glu-B3 was 2+4+15+19 (Glu-B3a), which was common to 11 Italian and eight Spanish cultivars, in all three breeding periods considered together. The two alleles described previously at Glu-B2, Glu-B2b (null) and Glu-B2a (band 12) were present in the collection, but band 12 predominated, especially within the Italian germplasm.
Five and seven allelic combinations at the Glu-1 loci and three and five combinations specific to Glu-2/Glu-3 loci were detected in Italian and Spanish cultivars, respectively (Table 1). Four previously described LMW-GS combinations associated with the LMW models—LMW-2 (combination aaa), LMW-2– (combination haa), LMW-1 (efb) and LMW-1 (hab)—were identified in the historical series. Combination aaa at LMW-2 (allele 6 at Glu-A3, bands 2+4+15+19 at Glu-B3, and band 12 at Glu-B2) was found in eight Italian and seven Spanish cultivars (Table 1). Combination haa (null allele at Glu-A3, bands 2+4+15+19 at Glu-B3, and band 12 at Glu-B2) was not present in Spanish germplasm, but was in three Italian cultivars. Combinations efb (allele 11 at Glu-A3, bands 2+4+15+17 at Glu-B3, and the null allele at Glu-B2) and hab (null allele at Glu-A3, bands 2+4+15+19 at Glu-B3, and the null allele at Glu-B2) were found in old and intermediate Spanish cvv. Blanco Verdeal and Camacho, respectively. In addition, three allelic combinations, so far unclassified, were found in Italian cv. Balilla Falso and Spanish cvv. Pinet, Astigi and Boabdil (Table 1). The total number of different allelic combinations for HMW/LMW-GS loci found in the whole collection was 13, i.e. six in Italian and 10 in Spanish cultivars, only three of them being shared by both sets of genotypes.
In order to identify the environmental and genetic effects on gluten quality associated with specific allelic combinations, ANOVAs were conducted separately for Italian and Spanish cultivars with the mean sedimentation index values of the cultivars sharing a common HMW or LMW allelic combination, by considering these combinations as factors of the ANOVA. The results revealed much greater genetic control and less environmental effect on sedimentation index in Italian than in Spanish cultivars for both loci (Table 5). Comparison of the sedimentation index values of the cultivars from each country sharing a common allelic combination for HMW- or LMW-GS loci showed a larger range of variation within Italian than Spanish germplasm. The most favourable combination at HMW-GS loci was that identified in modern Spanish cv. Boabdil (null allele at Glu-A1 and bands 7+17 at Glu-B1) (Table 1) with a mean sedimentation index of 0.53 (Table 6). In the Spanish germplasm, cultivars carrying the LMW models hab and the unclassified d?b—none of them found in Italian cultivars—had the greatest sedimentation index values, 0.50 and 0.52, respectively. In the Italian germplasm, combination LMW-2 aaa was the most favourable for a high sedimentation index, with an average value of 0.49. Two unclassified LMW combinations (h?a and h?b), identified in Spanish and Italian germplasm, respectively, led to the poorest sedimentation index values, 0.37 and 0.22, respectively (Table 6).
Table 6 shows the mean sedimentation index values of each of the 13 allelic combinations found in the collection for HMW- and LMW-GS loci, and the number of cultivars carrying each of them. The combination formed by the null allele at Glu-A1, bands 6+8 at Glu-B1, and LMW-2 combination aaa, shared by Italian cvv. Creso and Zenit and Spanish cv. Senadur, resulted in the highest sedimentation index (Table 6). However, combination aaa of the LMW-2 model was present not only in cultivars with high gluten quality, but also in some with low sedimentation index (0.36 in Table 6), such as Spanish cv. Rubio de Belalcázar, which suggests a large interaction between HMW-GS and LMW-GS loci. Combination aaa of model LMW-2 (band 6 at Glu-A3, bands 2+4+15+19 at Glu-B3, and band 12 at Glu-B2) resulted in a high sedimentation index when the null allele was present at Glu-A1, interacting with bands 6+8, 7+8 or 15+16 at Glu-B1, but it resulted in reduced sedimentation index when combined with bands 20 or 13+16 at Glu-B1 (Table 6).
The distribution of HMW- and LMW-GS combinations in cultivars from different historical periods, shown in the right columns of Table 6, demonstrates that those resulting in low sedimentation index values were more frequent in the old cultivars, whereas the highest sedimentation index was associated with specific combinations with a high frequency in the intermediate and, particularly, in the modern cultivars. The most successful HMW- and LMW-GS combinations found in cultivars from both countries included combination aaa of LMW-2, but the presence at LMW locus of band 6+11 at Glu-A3, bands 4+15+19 at Glu-B3, and the null allele at Glu-B2 in modern Spanish cvv. Boabdil and Astigi also resulted in high sedimentation index values (Tables 1 and 6).
Discussion
Genetic gains in grain quality traits
Quality is one of the primary goals of durum wheat breeding programs in the EU, where premiums have been established to promote the cultivation of high-quality cultivars (Royo and Briceño-Félix 2011). With the aim of regulating these premiums, the quality index (QI) was defined to consider in an integrative way the most important durum wheat grain characteristics for pasta making. The relatively low environmental effect and large genetic control of this index observed in this study confirmed the value of QI for the quality classification of EU durum cultivars, as recently stated by Nazco et al. (2012). This study demonstrated a steady QI increase during the 20th Century in both Italian and Spanish durum wheat cultivars, with a larger genetic gain recorded in the former (7.13% from old to modern cultivars and a RGG of 0.13% year–1 in Italy, 5.37% with a RGG of 0.06% year–1 in Spain). However, the larger genetic gain obtained for Italian cultivars was due to the slightly lower quality of the old Italian genotypes compared with the old Spanish ones, since modern cultivars from both countries reached a similar values for QI. The greater efforts made by Italian breeders to improve the global quality of their durum wheats may be a consequence of the longer breeding tradition for this crop in this country (Royo et al. 2009), likely linked to the economic importance of durum wheat in Italy regarding its production and the land surface devoted to it (MPAAF 2011).
Protein content is the quality trait with a largest weight in the balanced QI. The large environmental effect obtained in this study for protein content confirms the findings of previous studies conducted with durum wheat in Mediterranean environments (Rharrabti et al. 2003). Nevertheless, our results suggest that cultivars of different periods had a similar response to environmental variations in terms of protein content, thus suggesting that breeding has not contributed to the stability of this trait. Despite the global decrease recorded in this study in grain protein content (0.17% year–1), total protein production per ha increased at a rate of 0.35% year–1, which is likely a consequence of large grain yield increases accompanied by increased nitrogen uptake and/or translocation capacity. A previous study with the same historical series used here reported an average yield gain of 0.61% year–1 (Royo et al. 2008), which indicates that the yield increases achieved during the 20th Century largely compensated for the decreases in the protein content of the newly released cultivars. Several studies have reported increases in grain yield accompanied by significant decreases in grain protein content (Motzo et al. 2004; De Vita et al. 2007; Dotlačil et al. 2010; Nazco et al. 2012). The negative relationship between yield and protein content (Rharrabti et al. 2001) has been associated with a dilution effect of nitrogen compounds when carbohydrate deposition increases through photosynthesis (Lawlor 2002; Martre et al. 2003). Even with the loss of grain protein content resulting from past breeding activities, the grain of modern durum wheats from Italy and Spain contained, on average, 14.7% and 14.2% protein, respectively, levels that exceed the minimum values required by the pasta-processing industry set at ~12.5% (Peña et al. 2002).
Of the four grain quality traits considered in the calculation of the QI index, gluten strength was the most important for explaining global quality increases in both countries, as it increased across periods by 32.1% and 27.9% in Italy and Spain, respectively, with RGG of 0.54% year–1 and 0.33% year–1. Gluten strength improvements largely compensated for the decreases in protein content, thus leading to gains in sedimentation index of ~41% on average. However, the rate of increase in sedimentation index in Italy was superior to that in Spain due to the larger rate of improvement of gluten strength and the lower rate of protein content decrease. Consequently, as sedimentation index increased (0.64% year–1 in Italy and 0.49% year–1 in Spain), as explained by the changes in the allelic composition of the glutenins, the quality of grain proteins was improved through breeding. Moreover, breeding programs were also successful in increasing the stability of gluten strength, and consequently, but with a lower intensity, that of the sedimentation index.
Yellow pigment concentration is mandatory to attain the bright yellow coloured pasta products demanded by the consumers, thus becoming an important goal in the EU (Di Fonzo et al. 2005). As expected, genotypic effects accounted for a large percentage (47.6%) of the phenotypic variability for yellow colour index. However, despite the large heritability of this trait (Clarke et al. 2006), the attained RGGs in both countries were less than one-third of those recorded for gluten strength. The largest changes in yellow colour index occurred between intermediate and modern cultivars. Rapid methods for semolina colour assessment, such as reflectance colourimeters, were already documented in the mid 20th Century (Matz and Larsen 1954; Walsh et al. 1969), but the largest changes in yellow colour were not recorded in that period because yellow colour has only been considered a relevant criterion for pasta making in recent decades (Digesù et al. 2009). Our results revealed that the stability of yellow colour was not improved in modern cultivars, which showed a wide range of response to environmental changes.
In agreement with the findings of other authors (Rharrabti et al. 2003; Taghouti et al. 2010), test weight and vitreousness were two of the traits with larger environmental effect, and any significant rate of variation over time was not observed. The lack of improvement of test weight may be related to the positive phenotypic and genotypic correlation between test weight and plant height reported in durum wheat (Clarke et al. 2009). As the old cultivars of this historical series do not carry dwarfing genes, the plant height reduction observed in some intermediate and all modern cultivars due to the introduction of dwarfing genes (Álvaro et al. 2008a) probably limited the attainment of grains with improved test weight. The fact that the cultivar slopes of the regression models (b) fitted to the relationship between the genotype and the environmental means did not differ between genotypes for test weight, and were maintained across time, is in agreement with this statement. Vitreousness showed a distinct behaviour; despite remaining unchanged through time, the stability of vitreousness differed between cultivars, but it remained unchanged over years, partially due to the wide variability detected within the most recently released cultivars.
Changes in glutenin allelic composition and its effects on gluten strength
Spanish cultivars showed high allelic variability in both their HMW- and LMW-GS loci, with 18 alleles encoded by Glu-1, Glu-3 or Glu-2 loci, compared with the 12 observed in the Italian germplasm. A previous study dealing with the phylogenetic relationships of the same historical series used here demonstrated closer genetic relationships within the Italian cultivars than within the Spanish ones (Martos et al. 2005), in agreement with the breeding histories of this crop in both countries. Durum wheat germplasm usually grown in Italy until 1970 seemed to be structured around a few, well-identified breeding groups with a relatively narrow genetic basis, which was dominated by a few hallmark, founder genotypes (Bozzini et al. 1998; Pecetti and Annicchiarico 1998; Di Fonzo et al. 2005; De Vita et al. 2007; Royo et al. 2010), whereas breeding in Spain largely relied on the introduction of germplasm of foreign origin (Royo and Briceño-Félix 2011). Despite the different breeding histories prevailing in both countries, the largest improvements in gluten strength were produced with the introduction and release of the first improved cultivars in both cases. Gluten strength increased 23.0% and 16.2% between old and intermediate cultivars in Italy and Spain, respectively, whereas increments between intermediate and modern cultivars were smaller at 7.2% and 10.0%.
Within our set of germplasm, changes in the allelic composition at the Glu-A1 locus during the whole period were characterised by the loss of the subunit 1—which was only identified in one old cultivar of each country—replaced by the null. The null allele has been found in very high frequencies in other durum collections (Vallega 1988; Branlard et al. 1989; Nazco et al. 2013), and it has been found practically fixed in modern germplasm worldwide. Despite studies reporting the positive effect on durum wheat quality of allele 1 at the Glu-A1 locus (Martínez et al. 2005), the two old cultivars carrying it in this study had weak gluten.
The largest number of alleles in the set of cultivars from both countries was found at the Glu-B1 locus. Subunit 20, which was monomorphic in locus Glu-B1 in Italian old cultivars seems to have been progressively replaced by subunits 6+8 and 7+8, which have been widely associated with strong gluten and, therefore, good pasta-making quality (Liu and Rathjen 1996; Sissons et al. 2005; Gregová et al. 2012). On the other hand, subunit 20 was not present in the old Spanish cultivars, one of which already had subunit 6+8 at Glu-B1, thus probably causing a slightly stronger gluten strength in old Spanish cultivars than in the Italian ones (Table 3). Previous studies have reported a high frequency of allele 6+8 in landraces from the Iberian Peninsula (Moragues et al. 2006). Subunit 7+17 at Glu-B1, found in modern cv. Boabdil, seems to have positively affected gluten strength.
Only two allelic variants (null and band 6 or Glu-A3a) were found in Italian germplasm at Glu-A3 locus, while subunits 11 and 6+11 were also found in Spanish germplasm. In cultivars from both countries the null allele, common in old and in a few intermediate cultivars, was replaced by band 6 (Glu-A3a) in the modern cultivars. Band 6 has been found to be the most frequent both in landraces and modern cultivars (Nieto-Taladriz et al. 1997; Carrillo et al. 2000; Moragues et al. 2006; Nazco et al. 2013). The two modern Spanish cultivars carrying subunit 6+11 at Glu-A3 had good gluten strength, but this result could not be attributed to this band exclusively, rather it is more likely due to the interaction with the alleles present at other loci.
Only two allelic variants were found at the Glu-B3 locus in both Italian and Spanish cultivars. One of the most important changes observed between old and intermediate cultivars in the allelic composition of LMW-GS in cultivars from both countries was the loss of diversity at Glu-B3, which became monomorphic for the banding pattern 2+4+15+19 in intermediate cultivars of both countries. This locus has a large influence on durum wheat gluten strength, but its effect may depend on its interaction with other LMW loci (Martínez et al. 2005). In this context, combination aaa of model LMW-2 (subunit 6 at Glu-A3, subunit 2+4+15+19 at Glu-B3, and subunit 12 at Glu-B2) has been largely recommended to be used in breeding programs (Sissons et al. 2005). Breeding increased the frequency of this combination, which was present in 75% of the intermediate cultivars of both countries and in 100% of the modern Italian ones. Greater allelic variability was observed within Spanish modern cultivars, with a previously unreported LMW-GS combination (d?b) identified, in addition to the combination aaa of the LMW-2 model. The uniformity in the banding pattern of LMW-GS of Italian intermediate and modern germplasm may have resulted from a breeding strategy based on few founder cultivars such as cv. Senatore Cappelli. This cultivar, an ‘africanum’ type selected from the North African population ‘Jennah Khortifa’, carries the combination aaa, and has probably given it to its descendants, cv. Capeiti 8 (derived from the cross Cappelli/Eiti), cv. Simeto (derived from the cross Capeiti 8/Valnova) and cv. Flavio (derived from the cross Latino/Cappelli), among others. However, combination aaa of model LMW-2 was present not only in cultivars with high gluten strength, such as cvv. Creso, Zenit and Senadur, but also in others with very weak gluten, such as cv. Clarofino. In addition, the two most successful allelic combinations in terms of gluten strength found in this study (resulting in sedimentation index values of 0.56 and 0.53, and SDS-sedimentation test values of ~7.86) had in common the null allele at the Glu-A1 locus. All of these results confirm the important interaction between HMW- and LMW-GS on gluten strength, as reported by previous studies (Payne et al. 1984; Pogna et al. 1990; Ruiz and Carrillo 1995; Nazco et al. 2013).
The results of the ANOVA conducted to quantify the effect of the allelic combinations at the HMW- and LMW-GS loci on gluten quality showed that the portion of the variance for the sedimentation index explained by these combinations was about four times higher in Italian than in Spanish cultivars. These results reveal much larger genetic control on gluten strength in Italian than in Spanish cultivars, probably an outcome of breeding efforts devoted in Italy to pyramid favourable alleles and allelic combinations that enhance gluten quality and its stability.
None of the six most favourable allelic combinations in terms sedimentation index—all resulting in average SDS-sedimentation values >0.50 (Table 6)—was present in either Italian or Spanish old cultivars, whereas they were more frequent in modern (87.5%) than in intermediate (62.5%) sets of germplasm. These results demonstrate that improvements in gluten quality were a consequence of the replacement of alleles and allelic combinations at HMW- and LMW-GS loci, which could also have provided a higher stability for gluten quality in recent cultivars.
Conclusions
The results of this study showed the significant improvements achieved in durum wheat quality in Italy and Spain during the 20th Century. Despite the fact that protein content was reduced, protein yield per ha increased very significantly. Yellow colour index and gluten strength also increased significantly. However, no significant changes were observed in test weight and vitreousness. Because the European quality index integrates several criteria, the lack of progress in test weight and vitreousness and the net loss of protein content was more than compensated for with the substantial progress in gluten strength and yellow colour. Breeding activities conducted during the 20th Century in Italy and Spain were successful in improving the traits related to pasta-making quality, as well as obtaining very favourable HMW- and LMW-GS combinations for gluten strength. However, past gluten strength enhancement relied on the use of very few allelic combinations, particularly in the case of Italy, where combination aaa of the LMW-2 model predominated in intermediate and modern Italian cultivars. Although the introgression of this LMW-GS model was useful to release cultivars with enhanced gluten strength, the narrowing of glutenin subunit diversity may constrain future breeding progress, thus making necessary the search for more favourable diversity to allow future quality improvements.
Acknowledgements
J. Subira was the recipient of a PhD grant from INIA, Spain. This study was partially funded by CICYT under projects AGL2009-11187, RTA2009-00061-C03-02 and AGL-2012-37217 and was developed within the framework of the agreement between INIA Spain and CIMMYT. The Centre UdL-IRTA is part of the Centre CONSOLIDER INGENIO 2010 on Agrigenomics funded by the Spanish Ministry of Education and Science.
References
Álvaro F, Isidro J, Villegas D, García del Moral LF, Royo C (2008a) Breeding effects on grain filling, biomass partitioning, and remobilization in Mediterranean durum wheat. Agronomy Journal 100, 361–370.| Breeding effects on grain filling, biomass partitioning, and remobilization in Mediterranean durum wheat.Crossref | GoogleScholarGoogle Scholar |
Álvaro F, Isidro J, Villegas D, García del Moral LF, Royo C (2008b) Old and modern durum wheat varieties from Italy and Spain differ in main spike components. Field Crops Research 106, 86–93.
| Old and modern durum wheat varieties from Italy and Spain differ in main spike components.Crossref | GoogleScholarGoogle Scholar |
Álvaro F, Royo C, García del Moral LF, Villegas D (2008c) Grain filling and dry matter translocation responses to source-sink modifications in a historical series of durum wheat. Crop Science 48, 1523–1531.
| Grain filling and dry matter translocation responses to source-sink modifications in a historical series of durum wheat.Crossref | GoogleScholarGoogle Scholar |
Axford DWE, McDemott EE, Redman DG (1978) Small scale test of bread making quality. Milling Feed and Fertiliser 161, 18–20.
Bozzini A, Corazza L, D’Egidio MG, Di Fonzo N, La Fiandra D, Pogna NE, Poma I (1998) Durum wheat (Triticum turgidum spp. durum). In ‘Italian contribution to plant genetics and breeding’. (Eds GT Scarascia Mugnozza, MA Pagnotta) pp. 181–194. (Tipolitografia Quatrini A.& F. Snc: Viterbo, Italy)
Branlard G, Autran JC, Monneveux P (1989) High molecular weight glutenin subunit in durum wheat (T. durum). Theoretical and Applied Genetics 78, 353–358.
| High molecular weight glutenin subunit in durum wheat (T. durum).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkslOk&md5=bbb76bea6055250bf8680e187a2b6e54CAS | 24227241PubMed |
Carrillo JM, Martínez MC, Moita Brites C, Nieto-Taladriz MT, Vázquez JF (2000) Relationship between endosperm protein and quality in durum wheat (Triticum turgidum L. var. durum). In ‘Durum wheat improvement in the Mediterranean region: New challenges. Zaragoza (Spain)’. (Eds C Royo, MM Nachit, N Di Fonzo, JL Araus). Options Méditerranéennes: Série A. Séminaires Méditerranéens No. 40, pp. 463–467. (CIHEAM: Paris)
Clarke FR, Clarke JM, McCaig TN, Knox RE, DePauw RM (2006) Inheritance of yellow pigment concentration in seven durum wheat crosses. Canadian Journal of Plant Science 86, 133–141.
| Inheritance of yellow pigment concentration in seven durum wheat crosses.Crossref | GoogleScholarGoogle Scholar |
Clarke FR, Clarke JM, Pozniak CJ, Knox RE (2009) Inheritance of test weight and kernel weight in eight durum wheat crosses. Canadian Journal of Plant Science 89, 1047–1057.
| Inheritance of test weight and kernel weight in eight durum wheat crosses.Crossref | GoogleScholarGoogle Scholar |
De Vita P, Li Destri Nicosia O, Nigro F, Platani C, Riefolo C, Di Fonzo N, Cattivelli L (2007) Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century. European Journal of Agronomy 26, 39–53.
| Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Di Fonzo N, Ravaglia S, DeAmbrogio E, Blanco A, Troccoli A (2005) Durum wheat improvement in Italy. In ‘Durum wheat breeding: current approaches and future strategies. Vol. 2’. (Eds C Royo, MM Nachit, N Di Fonzo, JL Araus, WH Pfeiffer, GA Slafer) pp. 825–881. (Food Products Press: New York)
Digesù AM, Platani C, Cattivelli L, Mangini G, Blanco A (2009) Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. Journal of Cereal Science 50, 210–218.
| Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats.Crossref | GoogleScholarGoogle Scholar |
Dotlačil L, Hermuth J, Stenho Z, Dvořáček V, Bradová J, Leišová L (2010) How can wheat landraces contribute to present breeding? Czech Journal of Genetics and Plant Breeding 46, S70–S74.
Finlay KW, Wilkinson GN (1963) The analysis of adaptation in a plant breeding programme. Australian Journal of Agricultural Research 14, 742–754.
| The analysis of adaptation in a plant breeding programme.Crossref | GoogleScholarGoogle Scholar |
Genstat (2010) ‘Genstat, 13th edn.’ (VSN International: Hemel Hempstead, UK)
Gregová E, Medvecká E, Jómová K, Šliková S (2012) Characterization of durum wheat (Triticum durum desf.) quality from gliadin and glutenin protein composition. Journal of Microbiology Biotechnology and Food Sciences 1, 610–615.
Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. Journal of Experimental Botany 53, 773–787.
| Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivFSntLw%3D&md5=a0545d4cc05884e4c04501f485eefa69CAS | 11912221PubMed |
Liu CY, Rathjen AJ (1996) Association of high and low molecular weight glutenin subunits with dough strength in durum wheats [Triticum turgidum ssp. turgidum L. conv. durum (Desf.)] in southern Australia. Australian Journal of Experimental Agriculture 36, 451–458.
| Association of high and low molecular weight glutenin subunits with dough strength in durum wheats [Triticum turgidum ssp. turgidum L. conv. durum (Desf.)] in southern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XkvFWjsL8%3D&md5=905fb6774bb4a5c5ebddd36b513b512bCAS |
Maliani C (1979) Nazareno Strampelli, a forerunner in green revolution. Genetica Agraria 33, 1–4.
Martínez MC, Ruiz M, Carrillo JM (2005) Effects of different prolamin alleles on durum wheat quality properties. Journal of Cereal Science 41, 123–131.
| Effects of different prolamin alleles on durum wheat quality properties.Crossref | GoogleScholarGoogle Scholar |
Martos V, Royo C, Rharrabti Y, Garcia del Moral LF (2005) Using AFLPs to determine phylogenetic relationships and genetic erosion in durum wheat cultivars released in Italy and Spain throughout the 20th century. Field Crops Research 91, 107–116.
| Using AFLPs to determine phylogenetic relationships and genetic erosion in durum wheat cultivars released in Italy and Spain throughout the 20th century.Crossref | GoogleScholarGoogle Scholar |
Martre P, Porter JR, Jamieson PD, Triboï E (2003) Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat. Plant Physiology 133, 1959–1967.
| Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhvFKm&md5=8e0177d3349bacf15b58e9cca91d6b69CAS | 14630962PubMed |
Matz SA, Larsen RA (1954) Evaluating semolina colour with photoelectric reflectometers. Cereal Chemistry 31, 73–86.
Moragues M, Zarco-Hernández J, Moralejo MA, Royo C (2006) Genetic diversity of glutenin protein subunits composition in durum wheat landraces [Triticum turgidum ssp. turgidum convar. durum (Desf.) MacKey] from the Mediterranean basin. Genetic Resources and Crop Evolution 53, 993–1002.
| Genetic diversity of glutenin protein subunits composition in durum wheat landraces [Triticum turgidum ssp. turgidum convar. durum (Desf.) MacKey] from the Mediterranean basin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotlSnsr4%3D&md5=f21e40ebd1ec0c2390a2ca4b3227195aCAS |
Motzo R, Fois S, Giunta F (2004) Relationship between grain yield and quality of durum wheats from different eras of breeding. Euphytica 140, 147–154.
| Relationship between grain yield and quality of durum wheats from different eras of breeding.Crossref | GoogleScholarGoogle Scholar |
MPAAF (2011) Statistiche agronomiche di superficie, resa e produzione (Agronomical statistics of surface, yield and production). Bulletin 30 June 2011. Ministero delle Politiche Agricole Alimentari e Forestali, Rome. Available at: www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/3892
Nazco R, Villegas D, Ammar K, Peña RJ, Moragues M, Royo C (2012) Can Mediterranean durum wheat landraces contribute to improved grain quality attributes in modern cultivars? Euphytica 185, 1–17.
| Can Mediterranean durum wheat landraces contribute to improved grain quality attributes in modern cultivars?Crossref | GoogleScholarGoogle Scholar |
Nazco R, Peña RJ, Ammar K, Villegas D, Crossa J, Moragues M, Royo C (2013) Variability in glutenin subunit composition of Mediterranean durum wheat germplasm and its relationship with gluten strength. Journal of Agricultural Science, Cambridge
| Variability in glutenin subunit composition of Mediterranean durum wheat germplasm and its relationship with gluten strength.Crossref | GoogleScholarGoogle Scholar | In press
Nieto-Taladriz MT, Ruiz M, Martínez MC, Vázquez JF, Carrillo JM (1997) Variation and classification of B low-molecular-weight glutenin subunit alleles in durum wheat. Theoretical and Applied Genetics 95, 1155–1160.
| Variation and classification of B low-molecular-weight glutenin subunit alleles in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhsFajsA%3D%3D&md5=1296fff3f1d7918d5cdbefe5c7ac03d6CAS |
Pagnotta MA, Blanco A, Gadaleta A, Fares C (2005) Functional determinants of grain quality. In ‘Durum wheat breeding: current approaches and future strategies. Vol. 1’. (Eds C Royo, MM Nachit, N Di Fonzo, JL Araus, WH Pfeiffer, GA Slafer) pp. 483–527. (Food Products Press: New York)
Payne PI, Lawrence GJ (1983) Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1, and Glu-D1 which code for high-molecular-weight subunits of glutenin in hexaploid wheat. Cereal Research Communications 11, 29–35.
Payne PI, Jackson EA, Holt LM (1984) The association between γ-gliadin 45 and gluten strength in durum wheat varieties: a direct causal effect on the result of genetic linkage? Journal of Cereal Science 2, 73–81.
| The association between γ-gliadin 45 and gluten strength in durum wheat varieties: a direct causal effect on the result of genetic linkage?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXksFWrt7c%3D&md5=61d37fecda6473d04f30650c78f6fec7CAS |
Pecetti L, Annicchiarico P (1998) Agronomic value and plant type of Italian durum wheat cultivars from different eras of breeding. Euphytica 99, 9–15.
| Agronomic value and plant type of Italian durum wheat cultivars from different eras of breeding.Crossref | GoogleScholarGoogle Scholar |
Peña RJ (2000) Durum wheat for pasta and bread-making. Comparison of methods used in breeding to determine gluten quality-related parameters. In ‘Durum wheat improvement in the Mediterranean region: New challenges. Zaragoza (Spain)’. (Eds C Royo, MM Nachit, N Di Fonzo, JL Araus). Options Méditerranéennes: Série A. Séminaires Méditerranéens No. 40, pp. 423–430. (CIHEAM: Paris)
Peña RJ, Amaya A, Rajaram S, Mujeeb-Kazi A (1990) Variation in quality characteristics associated with some spring 1B/1R translocation wheats. Journal of Cereal Science 12, 105–112.
| Variation in quality characteristics associated with some spring 1B/1R translocation wheats.Crossref | GoogleScholarGoogle Scholar |
Peña RJ, Trethowan R, Pfeiffer WH, van Ginkel M (2002) Quality (end-use) improvement in wheat: Compositional, genetic, and environmental factors. In ‘Quality improvement in field crops’. (Eds AS Basra, LS Randhawa) pp. 1–37. (The Haworth Press Inc.: London)
Peña RJ, Gonzalez-Santoyo H, Cervantes F (2004) Relationship between Glu-D1/Glu-B3 allelic combinations and breadmaking quality-related parameters commonly used in wheat breeding. In ‘Proceedings of the 8th Gluten Workshop’. (Eds S Masci, D Lafiandra) pp. 156–157. (Cambridge Royal Society of Chemistry: Cambridge, UK)
Pogna NE, Autran JC, Mellini F, Lafiandra D, Feillet P (1990) Chromosome 1B-encoded gliadins and glutenin subunits in durum wheat: genetics and relationship to gluten strength. Journal of Cereal Science 11, 15–34.
| Chromosome 1B-encoded gliadins and glutenin subunits in durum wheat: genetics and relationship to gluten strength.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhtlKis78%3D&md5=001af8f0c2ae81fe790cb045fc91749cCAS |
Rharrabti Y, Villegas D, García del Moral LF, Aparicio N, Elhani S, Royo C (2001) Environmental and genetic determination of protein content and grain yield in durum wheat under Mediterranean conditions. Plant Breeding 120, 381–388.
| Environmental and genetic determination of protein content and grain yield in durum wheat under Mediterranean conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlvFKhtg%3D%3D&md5=eb22025c99333f8bcbb29de09ddc12aaCAS |
Rharrabti Y, Royo C, Villegas D, Aparicio N, García del Moral LF (2003) Durum wheat quality in Mediterranean environments I. Quality expression under different zones, latitudes and water regimes across Spain. Field Crops Research 80, 123–131.
| Durum wheat quality in Mediterranean environments I. Quality expression under different zones, latitudes and water regimes across Spain.Crossref | GoogleScholarGoogle Scholar |
Royo C (2005) Durum wheat improvement in Spain. In ‘Durum wheat breeding: Current approaches and future strategies. Vol. 2’. (Eds C Royo, MM Nachit, N Di Fonzo, JL Araus, WH Pfeiffer, GA Slafer) pp. 883–906. (Food Products Press: New York)
Royo C, Briceño-Félix G (2011) Wheat breeding in Spain. In ‘The world wheat book: A history of wheat breeding’. 2nd edn (Eds AP Bonjean, WJ Angus, Mv Ginkel) pp. 121–154. (Lavoisier Publishing Inc.: Paris)
Royo C, Álvaro F, Martos V, Ramdani A, Isidro J, Villegas D, García del Moral LF (2007) Genetic changes in durum wheat yield components and associated traits in Italian and Spanish varieties during the 20th century. Euphytica 155, 259–270.
| Genetic changes in durum wheat yield components and associated traits in Italian and Spanish varieties during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Royo C, Martos V, Ramdani A, Villegas D, Rharrabti Y, García del Moral LF (2008) Changes in yield and carbon isotope discrimination of Italian and Spanish durum wheat during the 20th century. Agronomy Journal 100, 352–360.
| Changes in yield and carbon isotope discrimination of Italian and Spanish durum wheat during the 20th century.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvFyku7w%3D&md5=24b43bb98e4a07e0cafcb02127a0b338CAS |
Royo C, Elias EM, Manthey FA (2009) Durum wheat breeding. In ‘Cereals—Handbook of plant breeding’. (Ed. MJ Carena) pp. 199–226. (Springer: Berlin)
Royo C, Maccaferri M, Álvaro F, Moragues M, Sanguineti MC, Tuberosa R, Maalouf F, García del Moral LF, Demontis A, Rhouma S, Nachit M, Nserallah N, Villegas D (2010) Understanding the relationships between genetic and phenotypic structures of a collection of elite durum wheat accessions. Field Crops Research 119, 91–105.
| Understanding the relationships between genetic and phenotypic structures of a collection of elite durum wheat accessions.Crossref | GoogleScholarGoogle Scholar |
Ruiz M, Carrillo JM (1995) Relationships between different prolamin proteins and some quality properties in durum wheat. Plant Breeding 114, 40–44.
| Relationships between different prolamin proteins and some quality properties in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmsVKgtb8%3D&md5=bf46a4dbb5146b9d8a5b648f7d828cefCAS |
Ruiz M, Vázquez JF, Carrillo JM (2005) Genetic bases of grain quality. In ‘Durum wheat breeding: Current approaches and future strategies. Vol. 1’. (Eds C Royo, MM Nachit, N Di Fonzo, JL Araus, WH Pfeiffer, GA Slafer) pp. 349–375. (Food Products Press: New York)
SAS Institute Inc (2009a) ‘SAS/STAT Version 9.2.’ (SAS Institute: Cary, NC)
SAS Institute Inc (2009b) ‘SAS JMP Version 8.0.1.’ (SAS Institute: Cary, NC)
Singh NK, Sheperd KW, Cornish GB (1991) A simplified SDS-PAGE procedure for separating LMW subunits of glutenin. Journal of Cereal Science 14, 203–208.
| A simplified SDS-PAGE procedure for separating LMW subunits of glutenin.Crossref | GoogleScholarGoogle Scholar |
Sissons M (2008) Role of durum wheat composition on the quality of pasta and bread. Food 2, 75–90.
Sissons MJ, Ames NP, Hare RA, Clarke JM (2005) Relationship between glutenin subunit composition and gluten strength measurements in durum wheat. Journal of the Science of Food and Agriculture 85, 2445–2452.
| Relationship between glutenin subunit composition and gluten strength measurements in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFKjsbjK&md5=f0f97f8e84f9d65e611f472574d6c41aCAS |
Soil Survey Staff (1999) ‘Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys.’ 2nd edn. Natural Resources Conservation Service. U.S. Department of Agriculture Handbook 436. (USDA)
Taghouti M, Gaboun F, Nsarellah N, Rhrib R, El-Haila M, Kamar M, Abbad-Andaloussi F, Udupa SM (2010) Genotype × environment interaction for quality traits in durum wheat cultivars adapted to different environments. African Journal of Biotechnology 9, 3054–3062.
Vallega V (1988) High molecular weight glutenin subunit composition of 115 cultivars of Triticum turgidum var. durum from various origins. Genetica Agraria 42, 235–240.
Walsh DE, Gilles KA, Shuey WC (1969) Color determination of spaghetti by the tristimulus method. Journal of Cereal Chemistry 46, 7–13.
Weegels PL, Hamer RJ, Schofield JD (1996) Functional properties of wheat glutenin. Journal of Cereal Science 23, 1–18.
| Functional properties of wheat glutenin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhsFKls74%3D&md5=772252182483474f6ec2f01787f77eaaCAS |