The colours of durum wheat: a review
Donatella B. M. Ficco A B , Anna M. Mastrangelo A , Daniela Trono A , Grazia M. Borrelli A , Pasquale De Vita A , Clara Fares A , Romina Beleggia A , Cristiano Platani A and Roberto Papa AA Consiglio per la Ricerca e la sperimentazione in Agricoltura, Centro di Ricerca per la Cerealicoltura (CRA-CER), S.S. 673, Km 25,200, 71122 Foggia, Italy.
B Corresponding author. Email: donatellabm.ficco@entecra.it
Crop and Pasture Science 65(1) 1-15 https://doi.org/10.1071/CP13293
Submitted: 28 August 2013 Accepted: 29 October 2013 Published: 2 January 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Pigments are essential to the life of all living organisms. Animals and plants have been the subjects of basic and applied research with the aim of determining the basis of the accumulation and physiological roles of pigments. In crop species, the edible organs show large variations in colour. In durum wheat grain, which is a staple food for humans, the colour is mainly due to two natural classes of pigment: carotenoids and anthocyanins. The carotenoids provide the yellow pigmentation of the durum wheat endosperm, and consequently of the semolina, which has important implications for the marketing of end products based on durum wheat. Anthocyanins accumulate in the aleurone or pericarp of durum wheat and provide the blue, purple and red colours of the grain. Both the carotenoids and the anthocyanins are known to provide benefits for human health, in terms of decreased risks of certain diseases. Therefore, accumulation of these pigments in the grain represents an important trait in breeding programs aimed at improving the nutritional value of durum wheat grain and its end products. This review focuses on the biochemical and genetic bases of pigment accumulation in durum wheat grain, and on the breeding strategies aimed at modifying grain colour.
Additional keywords: durum wheat, pigment accumulation, pigment oxidation, pasta processing, marker-assisted selection, pigment analytical methods.
Introduction
Wheat is one of the most widely grown grain crops in the world, and durum and bread wheat represent staple foods for human nutrition, especially in the Mediterranean area. Durum wheat [Triticum turgidum (L.) subsp. turgidum (L.) convar. durum (Desf.)] is a tetraploid wheat that comprises the A and B genomes, and it is the main source of semolina for the production of pasta, couscous and burghul.
Over the last few decades, the yellow-amber colour of semolina has become an important quality trait for durum wheat end products. The yellow colour is due to the carotenoid (yellow) pigment content (YPC) in the whole kernel, and is commercially identified as the yellow index (YI) in semolina (CIE 1986). In addition to their role as an important aesthetic parameter, the carotenoids have important nutritional and health roles. Some carotenoids have provitamin A activity, which provides protection from ocular diseases (Ribaya-Mercado and Blumberg 2004), and all of the carotenoids show antioxidant capacity, which reduces the risk of chronic degenerative diseases (Abdel-Aal et al. 2007; Nishino et al. 2009).
The other class of pigments, which characterises the aleurone or pericarp of the majority of cereals including durum wheat, is the anthocyanins. The anthocyanins give rise to the so-called pigmented grains, the colour of which can range from blue to purple, and to red. The anthocyanins also have therapeutic roles for humans, against tissue inflammation, capillary fragility, cardiovascular disease, cancer, hyperglycaemia, and oxidative liver damage (Mazza 2000; Stintzing et al. 2002; Galvano et al. 2007; Ghosh and Konishi 2007; Guo et al. 2007; Abdel-Aal et al. 2008). Unlike the carotenoids, for which plant breeding is mainly in response to the needs of the pasta producers, the anthocyanins represent a new target for genetic improvement due to consumer demand for foods with greater health benefits.
The colour of the grain and the end products arises from phenotypic variations in the pigments present in the grain, which depend on genetic factors, growing conditions, and technological processes. In particular, in terms of the genetic control, the genes involved in pigment accumulation code both for enzymes involved in pigment biosynthesis and pigment degradation (e.g. enzymes with oxidase activity), and for proteins with regulatory roles (e.g. transcription factors). Information on these genes can be exploited in genetic improvement programs aimed at modifying the contents of these pigments in the processed products.
This review focuses on: (i) current knowledge of the biochemical and genetic bases of accumulation and degradation of these pigments in durum wheat grain and pasta products; (ii) breeding strategies that are aimed at modifying the accumulation of these pigments in durum wheat grain; and (iii) analytical techniques for rapid and simple screening of advanced durum wheat genotypes characterised by higher pigment contents in the grain.
Biochemical aspects of durum wheat grain colour
Carotenoid pigments: composition and distribution along the kernel
The carotenoids are a group of yellow-orange pigments that are found in many biological systems (Goodwin 1980; Krinsky 1993). The carotenoid pigments include two chemical classes: the carotenes, which are unsaturated hydrocarbons; and the xanthophylls, which are hydroxylated derivatives of the carotenes that have one or more oxygenated groups (Fig. 1). In durum wheat, the endosperm carotenoids are the main colour components, and contribute substantially to the YI of semolina. The YPC in whole kernel is highly correlated with the YI in the semolina, with correlation coefficients >0.94 reported (Fratianni et al. 2005; Abdel-Aal et al. 2007; Digesù et al. 2009).

|
The YPC reported for durum wheat is higher in the cultivated modern varieties than in the older varieties, landraces and wild populations (Digesù et al. 2009). This arises because of the more recent, intense breeding activities towards higher grain pigment concentrations. These activities have been facilitated by the high heritability of this trait, which is largely controlled by additive genetic effects and which has a strong genotypic component and low genotype × environment interaction (Elouafi et al. 2001; Clarke et al. 2006; Van Hung and Hatcher 2011). Nevertheless, despite this relatively high genetic weighting, some environmental factors can influence the final YI of semolina. Indeed, the YPC has been shown to increase in durum wheat grown under adverse environmental conditions, such as cool and wet conditions (Clarke et al. 2006), and salt and water stress (Katerji et al. 2005; Borrelli et al. 2011; Van Hung and Hatcher 2011; Fratianni et al. 2013). This might be due to an increase in the production of components of the plant defence machinery under stress conditions, which includes antioxidant molecules.
The major carotenoid in durum wheat grain is the xanthophyll lutein, which can represent 86–94% of the total carotenoids (Abdel-Aal et al. 2007; Digesù et al. 2009). For the other carotenoids, such as zeaxanthin, esterified lutein, Z-isomers of lutein and zeaxanthin, and the carotenes (e.g. α-carotene, β-carotene, β-cryptoxanthin), these are present in very low amounts that range from 3% to 5% (Panfili et al. 2004; Fratianni et al. 2005; Abdel-Aal et al. 2007; Digesù et al. 2009).
With regard to carotenoid distribution throughout the durum wheat kernel, it has been reported that the endosperm has the highest lutein and total carotenoid contents (Hentschel et al. 2002; Abdel-Aal et al. 2007; Borrelli et al. 2008). During milling, lutein is highly preserved, but there might be some loss of β-carotene, although at relatively low levels (Borrelli et al. 2008).
Carotenoid biosynthesis and degradation pathways and their contribution to the yellow colour of semolina and pasta products
The degree of yellowness in durum wheat grain and its end products is affected not only by carotenoid biosynthesis in the grain (Hentschel et al. 2002; Panfili et al. 2004), but also by carotenoid degradation during processing. This degradation has been mainly ascribed to oxidative enzymes that are responsible for the discoloration and darkening processes that can occur during pasta making (Borrelli et al. 1999; Trono et al. 1999; Dexter and Marchylo 2000; Feillet et al. 2000; Hessler et al. 2002).
The first reaction in the carotenoid biosynthesis pathway is the condensation of two molecules of geranylgeranyl pyrophosphate to form phytoene, which is catalysed by phytoene synthase (PSY) (Fig. 2). From this point, a subsequent series of cascade reactions provides increases in the numbers of conjugated double bonds from three in phytoene to eleven in lycopene; this process is catalysed by phytoene desaturase and ζ-carotene desaturase (ZDS), respectively. Lycopene cyclisation then occurs at both ends of the molecule, to generate β-carotene or α-carotene, through the activity of lycopene cyclase (ε-LCY). The formation of the xanthophylls lutein and zeaxanthin arises through the carotenoid hydroxylases. Zeaxanthin can undergo reversible double epoxidation of the rings, which is mediated by zeaxanthin epoxidase, to form violaxanthin, a precursor to abscisic acid. Zeaxanthin, antheraxanthin and violaxanthin (the xanthophyll cycle pool) are involved in the dissipation of light energy in the green tissues of plants. This pathway has at least three rate-limiting steps (Fig. 2): (i) early in the pathway, for the synthesis of phytoene; (ii) for lycopene cyclisation; and (iii) for carotene hydroxylation. Since in wheat the PSY-catalysed reaction has been reported as the rate-limiting step of the biosynthesis pathway, it is feasible that this reaction has a role in the regulation of carotenoid accumulation (Lindgren et al. 2003; Cong et al. 2009).

|
For carotenoid degradation, an important family of oxidative enzymes is responsible for the loss of the yellow colour during pasta making—the lipoxygenases (LOXs) (McDonald 1979; Trono et al. 1999; Pastore et al. 2000; De Simone et al. 2010; Verlotta et al. 2010). The LOXs are a class of non-heme iron enzymes containing dioxygenase activities and catalysing the positional and specific dioxygenation of polyunsaturated fatty acids with 1,4-cis,cis pentadiene structures, to produce the corresponding hydroperoxides. The radicals produced during the intermediate states of linoleate hydroperoxidation can cause oxidation of carotenoid pigments and, consequently, a loss of the yellow colour in pasta products (Siedow 1991). In durum wheat, partial purification of this endosperm protein has led to the definition of at least two typical LOX isoforms (Hsieh and McDonald 1989; Pastore et al. 2000). A third, atypical LOX isoform has also been reported, which also shows peroxidase activity (Hsieh and McDonald 1989). The LOX reaction has been shown to be inhibited by carotenoid compounds, and in fact, the percentage of carotenoid loss during pasta processing is inversely related to the initial carotenoid content in the semolina (Trono et al. 1999). The LOX-catalysed carotenoid degradation can also be limited by α-tocopherol (Pastore et al. 2000). Unfortunately, the content of α-tocopherol in semolina is very low because the germ, where the α-tocopherol is more concentrated (Lintas 1988), is the first portion to be removed during the milling (Fares et al. 2006).
Carotenoid pigment degradation is also affected by the peroxidases (PERs), a class of enzymes that can oxidise a large number of compounds at the expense of hydrogen peroxide (Fraignier et al. 2000). Fortunately, PERs do not show activity during pasta processing, probably because of the lack of availability of hydrogen peroxide (Icard-Vernière and Feillet1999; Feillet et al. 2000).
Finally, the polyphenol oxidases (PPOs) catalyse the oxidation of several phenols that occur naturally in wheat plants and grain. The PPO activities produce quinones, thus generating brown polymers (Sullivan 1946; Mayer and Harel 1979). The brown colour generated in this way tends to mask the yellow colour when it reaches sufficient levels. Nevertheless, the localisation of the PPOs in the aleurone layers indicates that they are unlikely to have a role in the enzymatic browning of the end products derived from semolina.
Pasta processing conditions that affect the yellow pigment degradation
Milling and pasta making (i.e. mixing, kneading, extruding, drying) are the processes involved in the preparation of the durum wheat end products. Milling is based on the opening of the tempered grain (at 16.0–16.5% humidity) and the recovery of the endosperm, step-by-step, going gradually from the inner to the outer part of the grain.
The desirable characteristics of semolina, the main product of durum milling, are a yellow-amber colour, minimum bran specks, and low oxidative enzyme activities, which are responsible for the loss of yellow pigments, and which, together with the low ash content, are involved in the browning of the semolina (Taha and Sagi 1987; Feillet et al. 2000).
These oxidative enzymes are not homogeneously distributed in the kernel; rather, there are decreasing amounts in the embryo, bran and endosperm (Rani et al. 2001; Borrelli et al. 2003, 2008). It is therefore important to consider the extraction rate, as the milling products that are richer in bran fractions might also include increased enzymatic activities (Hatcher and Kruger 1993; Okot-Kotber et al. 2001). The loss of carotenoid pigments that occurs during milling was calculated by Borrelli et al. (1999) to be ~8%, based on a laboratory semolina mill with three breaking and three sizing passages. Moreover, it has been reported that high levels of ash in the semolina, together with PERs and PPOs, can lead to an increased brown hue, which reduces the semolina and pasta yellowness (Kobrehel et al. 1974; Matsuo and Dexter 1980; Taha and Sagi 1987; Borrelli et al. 1999). Therefore, there is need for a balance between the demand of the miller for higher semolina yield and the requirement to have higher YPC and lower ash and oxidative enzyme levels.
In addition to traditional milling, the debranning process has been studied recently, and this involves the removal of the peripheral layers of the grain from the outermost to the internal regions. The debranning process has been shown to favourably affect not only the yield of semolina and the technological properties of the dough (by increasing the alveographic parameters and decreasing the α-amylase activity and microbial contamination; Dexter and Wood 1996; Gys et al. 2004), but also the yellow colour (by lowering the oxidative activities and the ash content; Fares et al. 1996; Borrelli et al. 2008).
The critical point for the final colour is the pasta processing. At the beginning of the process, kneading leads to incorporation of water and oxygen into the dough, which promotes LOX-mediated oxidation of polyunsaturated fatty acids, and which accordingly starts the oxidation of the carotenoids (Delcros et al. 1998; Borrelli et al. 2003). The next step is the pasta extrusion, during which there is further stimulation of the reduction of the total carotenoids. This decrease has been more evident in pilot laboratory pasta than in industrial processes, because the industrial kneading–extrusion operates under vacuum to limit the presence of oxygen and its oxidative effects on the dough lipid fraction and to reduce the englobing of small bubbles into the dough, which is detrimental for the final pasta structure (Hidalgo et al. 2010). In this way, the semolina particles can be hydrated more rapidly and more thoroughly.
Drying is the end phase of pasta processing, and this can also influence the final cooking quality of the pasta (Cubadda et al. 2007). Pasta yellowness is the result of concurrent chemical and physical factors. The involvement of the drying process in carotenoid losses can be considered negligible, although different time and temperature processing conditions can affect the final pasta colour in other ways (de Stefanis and Sgrulletta 1990; Borrelli et al. 2003). In particular, high or ultra-high temperatures favour the Maillard reaction, which leads to the formation of brown ‘melanoidin’ pigments. At high concentrations, these pigments can cause browning of pasta products, with consequent masking of the yellow colour (Marchylo and Dexter 1989).
Throughout pasta processing, the percentage of carotenoid loss has been shown to range from 4% for pasta produced from semolina with high YI and low LOX activity, to 20% for pasta produced from semolina with low YI and high LOX activity (Borrelli et al. 1999; De Simone et al. 2010).
Anthocyanin pigment: composition and distribution along the kernel
The anthocyanins are secondary plant products of flavonoid metabolism, and they have long sparked the interest of biologists. Chemically, the anthocyanins are based on anthocyanidin (aglycone), with sugar saccharide residues bound at different hydroxylated positions on the basic structure (Fig. 3). Individual anthocyanins differ in the numbers of hydroxyl groups and sugars, and in the aliphatic aromatic acids attached to the sugars, and they are also affected by pH, temperature, solvent and presence of co-pigments (Mazza 2007). The anthocyanins contribute almost all of the blue, purple and red colours to many fruits, vegetables and flowers (Delgado-Vargas et al. 2000; Winkel-Shirley 2001).

|
Among the cereals, many studies have investigated pigmented rice (Ryu et al. 1998; Abdel-Aal et al. 2006; Sompong et al. 2011) and maize (Moreno et al. 2005; Del Pozo-Insfran et al. 2006). In contrast, information in the literature relating to pigmented durum wheat is still lacking. In bread wheat, the anthocyanins are located in the grain and in other organs such as the culm, coleoptile, anthers and glumes (Khlestkina et al. 2010). For the kernel, the anthocyanins are predominantly in the external layers (Adom et al. 2005), similar to the other antioxidant phytochemicals. The blue wheat pigments are in the aleurone layer, whereas the purple is in the pericarp layers (Zeven 1991; Abdel-Aal and Hucl 1999).
Few studies have been conducted to evaluate the effects of the environment on the expression of anthocyanin content in the wheat kernel. Abdel-Aal and Hucl (2003) evaluated the effects of different growing seasons on the anthocyanin content of the wheat grain, and showed greater effects in a blue aleurone spring wheat line than in two commercial red and purple wheat cultivars. These effects are probably related to the different localisation of the pigments inside the wheat kernels.
Purple grain has been identified for several tetraploid wheats including Triticum dicoccum, which originates from Ethiopia and which was then introgressed into hexaploid wheats, where this trait has been widely investigated (Zeven 1991; Eticha et al. 2011). The gene for blue aleurone was transferred from the tall wheatgrass Agropyron elongatum to bread wheat (Triticum aestivum L.) (Zeller et al. 1991; Morrison et al. 2004). Similarly, translocation lines of the tall wheatgrass Thinopyrum ponticum with the blue aleurone gene(s) have also been characterised (Zheng et al. 2006).
All of the published data report greater total anthocyanin content in blue wheats and in purple wheats than in red wheat (Abdel-Aal et al. 2006, 2008; Eticha et al. 2011). With respect to anthocyanin composition, Hu et al. (2007) reported that in blue-coloured bread wheats, cyanidin-3-glucoside is the main component, and that pelargonidin-3-glucoside and cyanidin-3-galactoside are also present. Abdel-Aal and Hucl (2003) and Abdel-Aal et al. (2006) reported the first component as delphinidin-3-glucoside, followed by delphinidin-3-rutinoside, with trace levels of cyanidin-3-glucoside and peonidin-3-glucoside. For the purple-coloured bread wheats, cyanidin-3-glucoside, peonidin-3-glucoside and cyanidin-3-galactoside are the main anthocyanins found, while 10 other compounds have been seen at trace levels (Abdel-Aal and Hucl 2003; Abdel-Aal et al. 2006).
The anthocyanin biosynthesis pathway shows two main parts (Fig. 4): the general phenylpropanoid pathway, and the specific steps towards flavonoid biosynthesis. In the first of these pathways, phenylalanine is converted to 4-coumaryl-CoA through different steps. The second committed step in anthocyanin biosynthesis is catalysed by chalcone synthase, which uses 3-malonyl-CoA, the main precursor of the flavonoids, and 4-coumaroyl-CoA as substrates to produce chalcone. Chalcone isomerase then catalyses the stereospecific isomerisation of the yellow-coloured chalcone to the colourless naringenin. Naringenin is converted by flavanone 3-hydroxylase into dihydroflavonols. The conversion to the coloured anthocyanins then initially requires the reduction of the dihydroflavonols to leucoanthocyanidins by dihydroflavonol-4-reductase. Further oxidation, dehydration and glycosylation of the different leucoanthocyanidins can then produce the corresponding pigments: orange-red pelargonidin, red cyanidin, and blue delphinidin. The differences in the types of glycosides and acyl groups attached are both species- and variety-dependent.

|
For wheat, it has been reported that expression of the gene for flavanone 3-hydroxylase is the pivotal point in the regulation of anthocyanin biosynthesis (Tereshchenko et al. 2013), as previously seen in other plant species (Pelletier and Shirley 1996).
Applications to the food and colorant industry
Anthocyanin-pigmented grains can be useful for the production of foods, either from the whole grain, or following the extraction of the natural colourants from the anthocyanin-rich grain fractions, as an alternative to artificial colorants. The location of the anthocyanins in the outer layers of the kernel also facilitates their extraction.
To date, among the cereals, only pigmented rice and maize have been used for the production of foods for human consumption. In particular, blue and purple maize grains are used for blue and pink tortillas, while red rice is used in the manufacture of commercial infant cereals (Hirawan et al. 2011). For pigmented wheat, use in the food industry has been more limited; in particular, purple bread wheat is crushed into large pieces that are spread over the outside of bread, whereas blue bread wheat does not appear to have any food applications at present (see Abdel-Aal et al. 2006, and references therein). Although it has been predicted that the production and addition of anthocyanins as natural food colourants will steadily increase, particularly following the current trend away from synthetic colourants (Horbowicz et al. 2008), at present there are only a few applications regarding anthocyanin pigments extracted from red rice (Ma et al. 2000; Shipp and Abdel-Aal 2010).
In this regard, investigations aimed at better defining the total and specific contents and the distribution of the anthocyanins in pigmented durum wheat accessions and varieties will be useful.
Genetic aspects of durum wheat grain colours, and breeding strategies for their improvement
The long-term history of durum breeding has been characterised by genetic progress in terms of yield, plant height reduction, and lowering of the straw yield, which has resulted in increased kernel size. This was followed only at the end of the last Century by improvements to end-use quality attributes such as gluten strength and endosperm carotenoids (Clarke et al. 2010). In Italy, until the mid-1990s, selection for YPC did not receive particular attention from breeders, as demonstrated by the results of De Vita et al. (2007) and as previously reported by Digesù et al. (2009). However, the most recent durum wheat varieties released in the last decade show significantly higher YPC than the old varieties released before the 1970s (Digesù et al. 2009). This observation suggests that grain and semolina colour has become a particular sign of quality in durum wheat, and that in the last two decades, breeders have focussed attention on high YPC during the selection of new cultivars.
The selection process has been expedited due to the integration of traditional plant breeding methods with modern molecular marker technologies, and also due to the widespread use of non-destructive colourimetric reflectance measurements according to the Commission Internationale de l’Eclairage (CIE) scale and using near-infrared reflectance (NIR) spectroscopy. Genetic analyses based on molecular markers have allowed the identification of genomic regions involved in the accumulation of pigments in wheat grain. Moreover, the finding of molecular markers that are closely linked to genes and/or quantitative trait loci (QTL) that control the colour trait opened the way for increasing the pigment content of élite cultivars using of marker-assisted selection.
On the other hand, it must be considered that improvement of final colour will be due to the important roles of both the biosynthesis and degradation pathways in the determination of colour of the grain and end products. Therefore, molecular studies have been directed towards the genes that encode the key enzymes that regulate both of these metabolic pathways.
Genetic control of carotenoid biosynthesis
Studies using linkage and association mapping have led to the demonstration that the QTLs involved in the control of YPC in wheat grain are numerous and spread across many chromosomes of the wheat genome. Not all QTLs identified have the same importance in terms of their influence on this colour trait. The major QTLs were mapped to telomeric regions of the long arm of chromosomes of the homeologous group 7, for both durum and bread wheat. The QTLs that mapped to chromosomes 7A (Mares and Campbell 2001; Patil et al. 2008; Zhang and Dubcovsky 2008; Zhang et al. 2008, 2009; Howitt et al. 2009; Blanco et al. 2011) and 7B (Kuchel et al. 2006; Pozniak et al. 2007; Zhang and Dubcovsky 2008; Zhang et al. 2008) have been shown to explain in many cases the highest percentages of observed phenotypic variability (>50%) (Parker et al. 1998; Elouafi et al. 2001). Recent findings have shown that as well as the QTLs mapped at the telomeric region of the long arms of these chromosomes, there are other QTLs that have minor effects on colour traits on both arms of chromosomes 7A (Singh et al. 2009; Blanco et al. 2011; Roncallo et al. 2012) and 7B (Zhang and Dubcovsky 2008; Blanco et al. 2011; Roncallo et al. 2012).
The inheritance of YPC has been shown to be relatively complex, as many minor QTLs have been found on all of the chromosomes of durum wheat through different approaches. Significant marker-trait associations for YPC have been detected on all of the chromosomes by linkage mapping (Parker et al. 1998; Hessler et al. 2002; Pozniak et al. 2007; Patil et al. 2008; Zhang et al. 2008, 2009; Howitt et al. 2009; Blanco et al. 2011; Roncallo et al. 2012) and by association mapping (Reimer et al. 2008).
Most studies of QTLs for YPC have been based on the evaluation of total YPC. Recently, Blanco et al. (2011) analysed a segregating population for the accumulation of individual carotenoid components. Some of the QTLs identified explained both the individual and total carotenoids. This is in agreement with significant positive correlations among the individual carotenoids, and between the individual carotenoids and the total carotenoids and the YI within the segregating population. The QTLs have been found that explain the accumulation of more than one of these compounds, as previously shown for maize (Wong et al. 2004; Chander et al. 2008), chickpea (Abbo et al. 2005) and carrot (Just et al. 2009). Since the examined carotenoid traits represent compounds that are synthesised at different steps in the same biochemical pathway in several plant species (Just et al. 2009), the clustering of several QTLs for various carotenoid traits may be due to pleiotropy. The identification of QTLs specifically controlling the accumulation of α-carotene and β-carotene (chromosomes 2A, 3B, 7A; Blanco et al. 2011) represents a valuable tool to increase the levels of provitamin A activity of durum and bread wheat grain and, therefore, the nutritional value.
Several studies have been dedicated to the identification of candidate genes involved in the control of YPC. Two main approaches have been used in this regard: identification of significant associations between particular allelic forms of a gene and the phenotypic expression of the trait, and demonstration of co-localisation between a candidate gene and a QTL that explains the YPC. One of the genes best studied is the gene that directs the metabolism towards carotenoid synthesis, which codes for PSY. The PSY are also considered as the rate-limiting enzymes for this pathway, and the PSY1 enzymes, in particular, are required for carotenoid accumulation by the endosperm (Gallagher et al. 2004; Li et al. 2008). Almost 50 different alleles have been identified at the Psy-A1 and Psy-B1 loci in different species of wheat (He et al. 2008, 2009a; Howitt et al. 2009; Singh et al. 2009; Wang et al. 2009; Crawford et al. 2011; Ravel et al. 2013) (Table 1). The genes coding for PSY were mapped to the chromosome 7 groups by Pozniak et al. (2007); in particular, the Psy-B1 locus co-segregated with a QTL on chromosome 7B, which demonstrated an association between the position of this gene and part of the phenotypic variation for endosperm colour (Pozniak et al. 2007; Roncallo et al. 2012). Similarly, Psy-A1, located on chromosome 7A, showed a co-dominant marker based on polymorphisms between two Psy-A1 haplotypes that explained 20–28% of the phenotypic variance for YPC across three environments (He et al. 2008). Positive associations were found between YPC and the Psy1-A1o allele in a collection of 93 cultivars of durum wheat and in a recombinant inbred line population (Singh et al. 2009), with the Psy-A1t allele in a panel of 30 Australian lines of bread wheat and Psy-A1p allele in the segregating population Ajana × WAWHT2074, in which the locus controls 32–36% of the observed variability for the trait (Howitt et al. 2009; Crawford et al. 2011). Seven new alleles for Psy-A1 and two new alleles for Psy-B1 were recently identified in bread wheat by Ravel et al. (2013). Moreover, the marker Psy-A1_R_49 was shown to be associated with high YPC in a core collection of 372 genotypes.
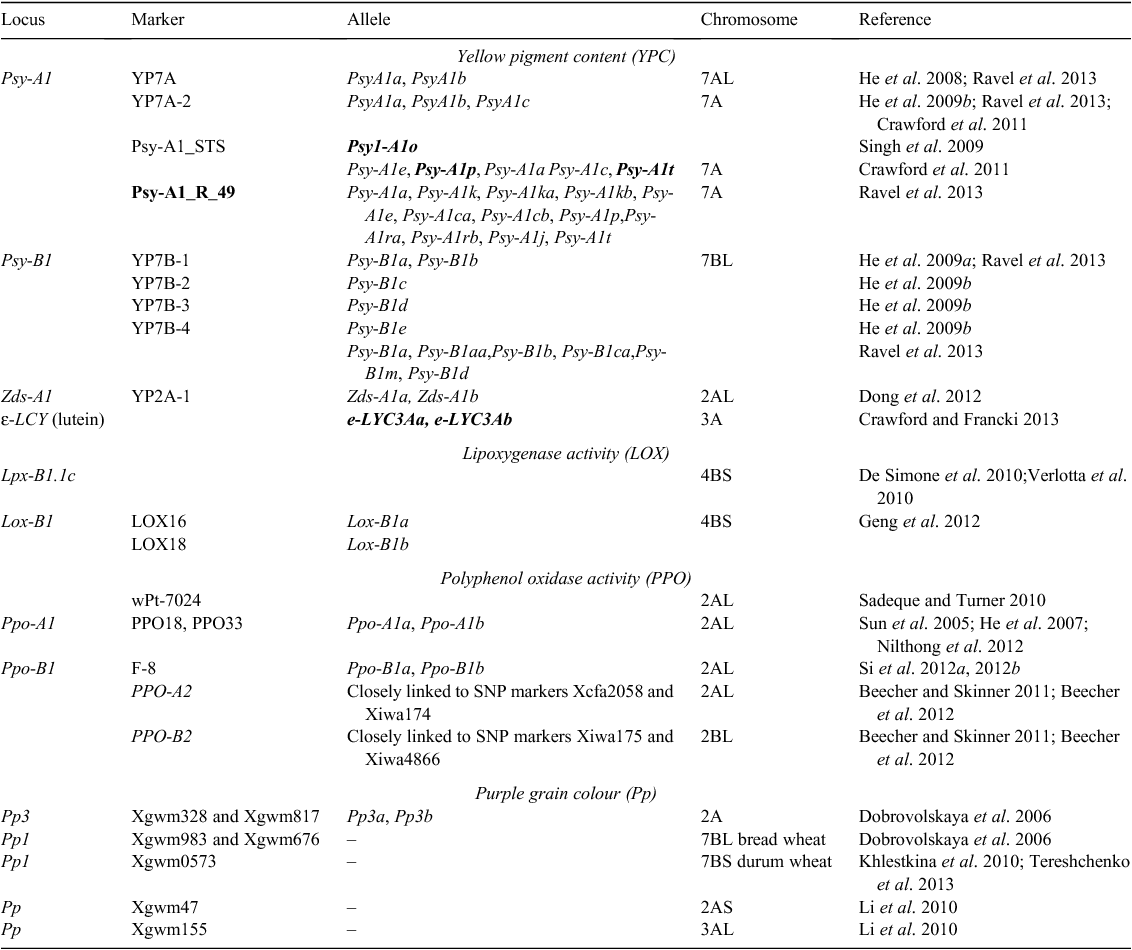
|
The Psy genes are the most studied genes in terms of their association with the YPC phenotype, although recent evidence has shown that other genes are involved in the control of this trait. One study reported that ZDS is highly associated with yellow pigment (Crawford and Francki 2013), where a co-dominant functional marker YP2A-1 showed polymorphisms of the two alleles Zds-A1a and Zds-A1b. A new QTL for yellow pigment was detected on chromosome 2A that co-segregated with the marker YP2A-1 and explained 11.3% of the phenotypic variance in a doubled haploid population. Moreover, an association between the ε-LCY gene on chromosome 3A and a QTL that explains the lutein content in seeds was described by Howitt et al. (2009) in bread wheat. More recently, a highly significant association was shown between the homologous copy of a gene on chromosome 3A (ε-LCY3A) and a QTL for b* colour in two segregating populations (Crawford and Francki 2013).
Little is known about the causal modifications that determine high YPC in associated alleles. Crawford et al. (2011) identified unique phosphorylation sites in the Psy-A1t allele. The production of alternatively spliced transcripts of Psy-A1 determines the translation of enzymatically inactive proteins (Howitt et al. 2009). Point mutations in genes coding for ε-LCY result in the substitution of a conserved amino acid in high-lutein alleles (Howitt et al. 2009; Crawford and Francki 2013).
Genetic control of carotenoid degradation during pasta processing: lipoxygenases, polyphenol oxidases and peroxidases
Lipoxygenases
The wheat genes that encode the LOX isoforms are named Lpx. The Lpx-1 and Lpx-3 genes encode the LOX-1 and LOX-2 isoforms, respectively, and they are located on chromosome 4, whereas the Lpx-2 gene that encodes the LOX-3 isoform is on chromosome 5 (Manna et al. 1998; Carrera et al. 2007; Zhang et al. 2008; Garbus et al. 2009; De Simone et al. 2010; Feng et al. 2010; Verlotta et al. 2010). Molecular studies of Lpx in developing durum wheat kernels have shown different transcript levels, with Lpx-1 transcripts being the most abundant in mature grain (De Simone et al. 2010). This suggests that the LOX-1 isoform might have a major role in oxidation of carotenoid pigments during pasta processing. This hypothesis is supported by evidence highlighting the existence of a major QTL for total LOX activity on chromosome 4BS, where three copies of the Lpx-1 gene, Lpx-B1.1, Lpx-B1.2 and Lpx-B1.3, along with three different Lpx-B1.1 alleles, Lpx-B1.1a, Lpx-B1.1b and Lpx-B1.1c, have been mapped (Nachit et al. 2001; Hessler et al. 2002; Carrera et al. 2007; Zhang et al. 2008; Verlotta et al. 2010). The Lpx-B1 genes/alleles comprise seven exons and six introns, with the exception of the Lpx-B1.1c allele, which has a large central deletion and most probably encodes a non-functional LOX-1 isoform (Verlotta et al. 2010).
Screening of a large collection of germplasm has revealed that all of the durum wheat genotypes that carry the Lpx-B1.1c allele also have very low LOX activity in the grain (Verlotta et al. 2010). Therefore, selection and fixing of this allele (Table 1) in all breeding lines will contribute to significantly reduced pigment loss during pasta processing and, consequently, to improvement of the aesthetic and nutritional qualities of the pasta products.
Recently, in hexaploid wheat, Geng et al. (2012) characterised the full-length genomic DNA sequence of a LOX gene (designated as Lox-B1) (Table 1) that is located on chromosome 4BS and shows high identity (98.6%) with the durum wheat Lpx-B1.2 gene, previously reported by Verlotta et al. (2010). Two complementary, dominant sequence-tagged site markers, LOX16 and LOX18, were developed based on the single nucleotide polymorphism of two alleles at the Lox-B1 locus, with the amplification of 489-bp and 791-bp fragments in cultivars with higher and lower LOX activities, respectively.
Polyphenol oxidases and peroxidases
A major genetic effect on PPO activity has been reported to be located on the long arm of chromosomes 2A and 2D in hexaploid wheat (Jimenez and Dubcovsky 1999; Raman et al. 2005; Sun et al. 2005; He et al. 2007), and several markers have been identified (Table 1). Sadeque and Turner (2010) reported that PPO activity in wheat is mainly controlled by a locus on the long arm of chromosome 2A that has strong genetic association with the diversity arrays technology marker wPt-7024. Recently, other paralogous PPO genes were identified on group 2, PPO-A2, PPO-B2 and PPO-D2 (Beecher and Skinner 2011). Genes PPO-A2 and PPO-D2 were shown to be expressed at high levels in developing wheat kernel. Beecher and Skinner (2011) localised the new PPO-2 gene family members to group 2 homeologous chromosomes (long arm); PPO-A2 was shown to be located 8.9 cM proximal to PPO-A1 on the long arm of chromosome 2A. Similarly, PPO-D1 and PPO-D2 are separated by 10.7 cM on the long arm of chromosome 2D, while PPO-B2 was mapped to the long arm of chromosome 2B and is the site of a novel QTL for PPO activity (Beecher et al. 2012). Due to the high similarity between bread and durum wheat genomes, the results obtained in bread wheat can be used to accelerate breeding for this trait in durum wheat. Indeed, the QTL analysis on PPO activity in durum wheat carried out by Watanabe et al. (2006) and Simeone et al. (2002) reported a major genetic effect on the long arm of chromosome 2A. For PERs, several homologous sets of loci that control the peroxidases have been identified independently in different studies (Asins and Perez de la Vega 1985; Bosch et al. 1986; Liu et al. 1990). In particular, Asins and Perez de la Vega (1985) described several seed peroxidase loci in tetraploid wheat on the short arms of homeologous groups 1, 2, and 3: Per-1 is active in coleoptile tissue; Per-2 shows some polymorphism and is most active in root tissue; Per-3 is highly variable and most active in embryo tissue; and Per-4 is carried on chromosome arms 7AS, 4AL and 7DS, and is relatively variable and most active in endosperm tissue.
Genetic control of anthocyanins
The Pp3 genes that influence the purple grain colour trait were mapped to the centromeric region of chromosomes 2A and 7BL (Pp1, at ~24 cM distal to the centromere) (Dobrovolskaya et al. 2006). The Pp1 (pericarp) gene was shown to be non-allelic to the Rc-1 (red coleoptiles) and Pc (purple culm) genes. Similar results have been found for durum wheat, except that the Pp gene maps to the short arm of chromosome 7B, instead of the long arm, as found in bread wheat (Khlestkina et al. 2010).
Using bulked segregant analysis, Li et al. (2010) detected associations of this trait with two microsatellite markers, Xgwm47and Xgwm155 (Table 1). Based on the positions of the molecular markers in bread wheat maps, the gene region indicated by the markers might correspond to that of Pp3 (Röder et al. 1998). Further studies are needed to clone and characterise the genes responsible for this trait.
In a recent study, transcriptional analysis of the five anthocyanin biosynthesis structural genes was carried out in near-isogenic lines that differed in the allelic state of loci involved in the purple colour of different organs in wheat, comprising Pp1 (Tereshchenko et al. 2013). The alleles that confer strong pigmentation promoted higher transcription levels of the structural genes, which, for Pp1 and the other genes, suggests roles as transcriptional factors in the anthocyanin biosynthesis network (Tereshchenko et al. 2013).
Analytical methods for pigment determination: classical and rapid methods
In breeding programs, there is often a need to evaluate grain quality on large numbers of plants or families, often using only small amounts of grain for each selection. In such situations, the availability of rapid, small-scale analytical methods provides opportunities to improve efficiency and complements the use of classical analytical methods. Even when molecular markers are used early in plant development to impose selection for grain-quality traits, it is still necessary to confirm the quality of the grain harvested from selected plants.
Two different approaches for durum wheat colour measurements are described below that have found widespread acceptance: one that considers the individual pigment components using sensitive techniques, and the other as a rapid method for screening purposes of total pigments.
Analytical methods for individual pigment compounds
The literature reports different solvents and extraction procedures (Konopka et al. 2006; Digesù et al. 2009) for the identification of carotenoids in grain and in various food matrices used as food additives (Breithaupt 2004), and also for the anthocyanins in grain (Abdel-Aal et al. 2006; Hosseinian et al. 2008).
The direct extraction of carotenoids with organic solvents is based on the use of water-saturated 1-butanol, ethanol, butylated hydroxytoluene, and methanol/tetrahydrofuran (Konopka et al. 2006; Digesù et al. 2009). Some of these solvents have also been used in aqueous solution for anthocyanin extraction, including ethanol, methanol and acetone (Castañeda-Ovando et al. 2009) and, as most widely used in wheat, acidified methanol (Abdel-Aal et al. 2006; Hosseinian et al. 2008).The most sensitive, although expensive, technique used for the identification and quantification of the carotenoids is based on high-performance liquid chromatography (HPLC) with a photodiode array detector system. The chromatographic separation is achieved using either normal or reverse-phase HPLC (Panfili et al. 2004; Burkhardt and Böhm 2007; Digesù et al. 2009; Van Hung and Hatcher 2011).
Analysis with HPLC was used for the quantitative determination of the main Z/E carotenoid stereoisomers, while for unequivocal structural determination of all of the main stereoisomers, HPLC-atmospheric pressure chemical ionisation mass spectrometry (HPLC-APCI-MS) and HPLC-nuclear magnetic resonance (HPLC-NMR) coupling have been used (van Breemen et al. 1996; Byrdwell 2001; Putzbach et al. 2005). Such HPLC techniques provide selective methods for the identification and quantification of novel genetic sources aimed at increasing β-carotene levels. These techniques are also useful to distinguish the two provitamin A components, α-carotene and β-cryptoxanthin, attributable to a single provitamin A structure (Wong et al. 2004; Blanco et al. 2011).
Quantification of the anthocyanins has been achieved using HPLC with anthocyanin reference standards. However, some peaks have remained undefined due to unavailability of all standards. These non-identified peaks were shown to be anthocyanins using mass spectrometry.
Indeed recently, techniques such as HPLC coupled mass spectrometry (HPLC-MS), APCI-MS and electrospray ionisation mass spectrometry (ESI-MS) have become powerful tools for anthocyanin identification in different matrices (Castañeda-Ovando et al. 2009), including cereals (Escribano-Bailón et al. 2004; Abdel-Aal et al. 2006; Hu et al. 2007; Hosseinian et al. 2008; Hirawan et al. 2011; Žilić et al. 2012). As a complement to the more widely used methods described, NMR remains a powerful method for the structural definition of the anthocyanins, as reported in a study by Aoki et al. (2002) on glycosylated derivatives in the seed of purple corn.
Rapid analytical methods for total pigment concentration
Several analytical procedures have been developed to evaluate the carotenoids and anthocyanins in durum wheat. Early methods that were designed to quantify total colour and are today still in use are based on light reflectance and spectrophotometric measures.
Methods to evaluate the yellow pigment as a colourimetric index have commonly used semolina colour, and are based on light reflectance measurements. Reflectance measurements are obtained using a Minolta CR-300 Chroma Meter (Konica Minolta Pty Ltd, Macquarie Park, NSW) equipped with a pulsed xenon arc lamp, for absolute measurements of the L* (lightness), a* (red-green chromaticity), and b* (yellow-blue chromaticity) (CIE 1986) coordinates in the Munsell colour system, using D65 lightning. The b value represents the variation in the yellow intensity.
In the same way, for the anthocyanins, the Hunter Laboratory colourimeter has been used to evaluate the L*, a* and b* colour values, as reported in a study of anthocyanin colour development in spring wheat by Knievel et al. (2009).
The yellow pigments of durum wheat flour, semolina and pasta are analysed according to the International Association for Cereal Science and Technology (ICC) Standard Method 152 (ICC 1990), or the AACC International Official Method 14-50.01 (AACC International 2013). Both are based on the extraction of durum wheat total pigments from samples (100 or 8 g, respectively) using water-saturated 1-butanol, followed by spectrophotometric evaluation of the optical density of the clear filtrate at an absorbance of 435.8 nm, and they are reported in terms of the β-carotene content. These official methods have been revised and adapted to breeding programs in which the limiting factor is the low amount of material.
In this context, Fu et al. (2013) proposed a rapid, micro-scale pigment extraction procedure for semolina that reduces the sample size to 200 mg and the volume of water-saturated 1-butanol to 1 mL. This method quantifies the pigment loss in the first step of processing due to LOX degradation, although the limiting step is the availability of durum kernels to make the semolina.
Beleggia et al. (2010) set up a micro-method on a single wheat kernel for the determination of YPC in durum wheat. This method is based on micro-sample amounts (10–100 mg, corresponding to a single wheat kernel) with micro-extraction volumes of water-saturated 1-butanol (250–500 µL) and a short extraction time (15 min, with an ultrasonic bath). The results obtained are not significantly different from those obtained with the Official AACC International Method 14-50.01 (AACC International 2013). Moreover, Beleggia et al. (2011) also applied the same micro-method to determine the difference in YPC of single kernels taken from different positions along a single ear of durum wheat.
As with the carotenoids, at present spectroscopy is the main technique used for the anthocyanins. In particular, this method is based on spectrophotometric determination at two pH levels (i.e. 4.5, 1.0) of 500-mg samples, with the reading at the maximum absorption wavelength of 520–540 nm (Abdel-Aal and Hucl 1999; Escribano-Bailón et al. 2004; Abdel-Aal et al. 2006; Hosseinianet al. 2008).
The most used methodology is based on the NIR application, as a rapid, non-destructive method with no environmental hazards; NIR does not include organic solvent extractions and is cost-effective for large-scale programs (Osborne et al. 1982; Shenk et al. 1990; Zandomeneghi et al. 2000). McCaig et al. (1992) used NIR/visible spectroscopy to estimate yellow durum pigment, where they showed a high association with a reference method based on solvent extraction (r2 = 0.94).
Recently, Sissons et al. (2006) used NIR spectra to assess the quality of Australian breeding lines of durum wheat grain, to predict kernel, flour and dough qualitative parameters such as test weight, 1000-kernel weight, grain hardness, semolina yield, semolina yellow colour, semolina browning and cooked pasta firmness. Dowell (2000) also used NIR spectroscopy to determine the vitreousness of durum wheat kernels and to predict many of their other parameters, including their b* colour value, with accuracies that are suitable for screening (Dowell et al. 2006).
Although some aspects of NIR spectroscopy are indeed advantageous for all the reasons mentioned above, this remains a comparative technique that relies on multivariate calibration of sample spectra and accurate reference analysis. It needs to take into account a sample set that covers most of the variability reported in the literature for colour of durum wheat grain. Moreover, it requires ~4 g of each sample to be packed into a black aluminium cup with a quartz window, which is not always available (Brenna and Berardo 2004). However, if properly calibrated, NIR spectroscopy can be exploited not only to measure the total quantity of pigments, but also to quantify the individual components. Indeed, over the past 25 years, the number of applications of NIR spectroscopy has grown rapidly, as demonstrated by the number of dedicated journals and large international conferences; therefore, it has the potential to be exploited as a rapid analytical method for many qualitative characters. The prediction of total anthocyanin concentrations with the NIR technique has been used for wine quality assessment (Urbano-Cuadrado et al. 2004; Janik et al. 2007), although it has not yet been applied in cereal quantification. Therefore, this technique could also be developed in durum wheat for prediction curves for beneficial health parameters.
Conclusions
The information in the literature indicates the great advances that have been made in our understanding of the biochemical and molecular basis of durum wheat colours. This has enabled the setting up of successful breeding programs. Added to this, upgraded analytical methods will help in the manipulation of the grain and the end product colour.
The combination of a high colour concentration with the presence of specific, highly nutritional coloured compounds should contribute further to the development of functional food for improved nutritional and health qualities.
The knowledge that has been obtained on the biochemistry and genetics of pigment accumulation in durum wheat grains will open the way for further studies aimed at the identification of the genes and proteins that act on the biochemical pathways, from the structural and regulatory points of view. The cloning of the genes within the major QTLs that control the accumulation of the pigments will further increase the speed and effectiveness of breeding programs, in which the selection can be based on genotype rather than phenotype.
Moreover, understanding the mechanisms by which these pigments exert their beneficial effects for humans, in terms of intestinal absorption in vivo and optimum dosage, should accelerate ongoing improvements to wheat programs and investment.
Acknowledgments
This study was supported by the Italian Ministry of Agriculture (MiPAAF), with the special grant RGV/Trattato FAO (Risorse Genetiche Vegetali), and by the Italian Ministry of Economic Development with the special grant PAQ (Pasta e Nuovi Prodotti Alimentari ad Alta Qualità da Cereali Italiani). We are grateful to Dr Christopher Berrie for scientific English language editorial assistance.
References
AACC International (2013) AACC International Official Method 14-50.01. In ‘AACC International Approved Methods Analysis’. 11th edn (Method 14-50 in the 10th edn, 2000) (AACC (formerly the American Association of Cereal Chemists): St. Paul, MN) Available at: http://methods.aaccnet.org/toc.aspxAbbo S, Molina C, Jungmann R, Grusak MA, Berkovitch Z, Reifen R, Kahl G, Winter P, Reifen R (2005) Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.). Theoretical and Applied Genetics 111, 185–195.
| Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtlOgur4%3D&md5=89230afcf232e1298f9d29c6ef12e474CAS | 15918010PubMed |
Abdel-Aal ESM, Hucl P (1999) A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chemistry 76, 350–354.
| A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjsFCqu7Y%3D&md5=1303671edd1bef46db166bc1732753b8CAS |
Abdel-Aal ESM, Hucl P (2003) Composition and stability of anthocyanins in blue grained wheat. Journal of Agricultural and Food Chemistry 51, 2174–2180.
| Composition and stability of anthocyanins in blue grained wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhvF2lt74%3D&md5=520cd43557c28c5bbce4fc012526cecfCAS |
Abdel-Aal ESM, Young JC, Rabalski I (2006) Anthocyanin composition in black, blue, pink, purple, and red cereal grains. Journal of Agricultural and Food Chemistry 54, 4696–4704.
| Anthocyanin composition in black, blue, pink, purple, and red cereal grains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltV2ktrs%3D&md5=5f84c54c80687c363ca189517ed17f6aCAS |
Abdel-Aal EMS, Young JC, Rabalski I, Hucl P, Fregeau-Reid J (2007) Identification and quantification of seed carotenoids in selected wheat species. Journal of Agricultural and Food Chemistry 55, 787–794.
| Identification and quantification of seed carotenoids in selected wheat species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlsF2m&md5=a365e77d7002d5e5590bc8873b89c3e8CAS |
Abdel-Aal ESM, Abou-Arab AA, Gamel TH, Hucl P, Young JC, Rabalski I (2008) Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. Journal of Agricultural and Food Chemistry 56, 11171–11177.
| Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlOiur3I&md5=2d22f88c643dd01606f6ad05333876d8CAS |
Adom KK, Sorrells ME, Liu RH (2005) Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. Journal of Agricultural and Food Chemistry 53, 2297–2306.
| Phytochemicals and antioxidant activity of milled fractions of different wheat varieties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsF2nurg%3D&md5=a3d625b3304fac67d1f8f65baa99f73dCAS | 15769171PubMed |
Aoki H, Kuze N, Kato Y (2002) Anthocyanins isolated from purple corn (Zea mays L.). Foods and Food Ingredients Journal of Japan 199, 41–45.
Asins MJ, Perez de la Vega M (1985) The inheritance of tetraploid wheat seed peroxidases. Theoretical and Applied Genetics 71, 61–67.
| The inheritance of tetraploid wheat seed peroxidases.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c7ot12ltw%3D%3D&md5=28035da8ffd5460751a71c039cc0d620CAS | 24247340PubMed |
Beecher B, Skinner D (2011) Molecular cloning and expression analysis of multiple polyphenol oxidase genes in developing wheat (Triticum aestivum) kernels. Journal of Cereal Science 53, 371–378.
| Molecular cloning and expression analysis of multiple polyphenol oxidase genes in developing wheat (Triticum aestivum) kernels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnt1Kku7w%3D&md5=1426bab08e9c004f451a5846ab932b98CAS |
Beecher BS, Carter AH, See DR (2012) Genetic mapping of new seed-expressed polyphenol oxidase genes in wheat (Triticum aestivum L.). Theoretical and Applied Genetics 124, 1463–1473.
| Genetic mapping of new seed-expressed polyphenol oxidase genes in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtlGitb4%3D&md5=caedb7f2fdc57468ca751a6233489ab3CAS | 22311372PubMed |
Beleggia R, Platani C, Nigro F, Cattivelli L (2010) A micromethod for the determination of yellow pigment content in durum wheat. Journal of Cereal Science 52, 106–110.
| A micromethod for the determination of yellow pigment content in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslGjtbg%3D&md5=ae8f2da7269fa94eaf54770afd1c999cCAS |
Beleggia R, Platani C, Nigro F, Papa R (2011) Yellow pigment determination for single kernels of durum wheat (Triticum durum Desf.). Cereal Chemistry 88, 504–508.
| Yellow pigment determination for single kernels of durum wheat (Triticum durum Desf.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlyitbrL&md5=f231936508eb825646237f0aecba137dCAS |
Blanco A, Colasuonno P, Gadaleta A, Mangini G, Schiavulli A, Simeone R, Digesù AM, De Vita P, Mastrangelo AM, Cattivelli L (2011) Quantitative trait loci for yellow pigment concentration and individual carotenoid compounds in durum wheat. Journal of Cereal Science 54, 255–264.
| Quantitative trait loci for yellow pigment concentration and individual carotenoid compounds in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFahsrvE&md5=47e9829dc159abd8f6a9953654849424CAS |
Borrelli GM, Troccoli A, Di Fonzo N, Fares C (1999) Durum wheat lipoxygenase activity and other quality parameters that affect pasta colour. Cereal Chemistry 76, 335–340.
| Durum wheat lipoxygenase activity and other quality parameters that affect pasta colour.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjsFCqu7g%3D&md5=f275ea24e2cf21e839098c593b683490CAS |
Borrelli GM, De Leonardis AM, Fares C, Platani C, Di Fonzo N (2003) Effects of modified processing conditions on oxidative properties of semolina dough and pasta. Cereal Chemistry 80, 225–231.
| Effects of modified processing conditions on oxidative properties of semolina dough and pasta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitlOrtLc%3D&md5=8b4da22d5f8a22449e1b6519206db4fcCAS |
Borrelli GM, De Leonardis AM, Platani C, Troccoli A (2008) Distribution along durum wheat kernel of the components involved in semolina colour. Journal of Cereal Science 48, 494–502.
| Distribution along durum wheat kernel of the components involved in semolina colour.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVarsLjE&md5=1b0c7554bf193b13723873f20a3f9333CAS |
Borrelli GM, Ficco DBM, Giuzio L, Pompa M, Cattivelli L, Flagella Z (2011) Durum wheat salt tolerance in relation to physiological, yield and quality characters. Cereal Research Communications 39, 525–534.
| Durum wheat salt tolerance in relation to physiological, yield and quality characters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmsFKjtg%3D%3D&md5=7e725328aab464cd5f69460d3069e7ecCAS |
Bosch A, Figueiras Ana M, Gonzales-Jean MT, Benito C (1986) Leaf peroxidases – abiochemical marker for the group 2 chromosomes in the Triticianae. Genetical Research 47, 103–107.
| Leaf peroxidases – abiochemical marker for the group 2 chromosomes in the Triticianae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XksFyjtL8%3D&md5=5f99c749af1b9d3d15552054989c9fc8CAS |
Breithaupt DE (2004) Simultaneous HPLC determination of carotenoids used as food colouring additives: applicability of accelerated solvent extraction. Food Chemistry 86, 449–456.
| Simultaneous HPLC determination of carotenoids used as food colouring additives: applicability of accelerated solvent extraction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXitlajsrs%3D&md5=39ea2a232890724df1e348c9c5b24343CAS |
Brenna OV, Berardo N (2004) Application of near-infrared reflectance spectroscopy (NIRS) to the evaluation of carotenoids content in maize. Journal of Agricultural and Food Chemistry 52, 5577–5582.
| Application of near-infrared reflectance spectroscopy (NIRS) to the evaluation of carotenoids content in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmt1Gntb0%3D&md5=eafbf5b0aeeca6f177ebe5df2ea10a9bCAS | 15373395PubMed |
Burkhardt S, Böhm V (2007) Development of a new method for the complete extraction of carotenoids from cereals with special reference to durum wheat (Triticum durum Desf.). Journal of Agricultural and Food Chemistry 55, 8295–8301.
| Development of a new method for the complete extraction of carotenoids from cereals with special reference to durum wheat (Triticum durum Desf.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVCqtLzP&md5=289c2f36cbc0729aca3ef7ba8b493276CAS | 17874841PubMed |
Byrdwell WC (2001) Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids 36, 327–346.
| Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjsl2gs7w%3D&md5=409bee3f7712f0e7fa1bb0da3e5eb130CAS | 11383683PubMed |
Carrera A, Echenique V, Zhang W, Helguera M, Manthey F, Schrager A, Picca A, Cervigni G, Dubcovsky J (2007) A deletion at the Lpx-B1 locus is associated with low lipoxygenase activity and improved pasta colour in durum wheat, Triticum turgidum ssp. durum. Journal of Cereal Science 45, 67–77.
| A deletion at the Lpx-B1 locus is associated with low lipoxygenase activity and improved pasta colour in durum wheat, Triticum turgidum ssp. durum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht12rtbnJ&md5=6138a895fd4751c7501152181e6758bbCAS |
Castañeda-Ovando A, Pacheco-Hernández Md L, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chemistry 113, 859–871.
| Chemical studies of anthocyanins: a review.Crossref | GoogleScholarGoogle Scholar |
Chander S, Guo YQ, Yang XH, Zhang J, Lu XQ, Yan JB, Song TM, Rocheford TR, Li JS (2008) Using molecular markers to identify two major loci controlling carotenoid contents in maize grain. Theoretical and Applied Genetics 116, 223–233.
| Using molecular markers to identify two major loci controlling carotenoid contents in maize grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVGhurrP&md5=920f176e84893bf1adc0eb2f0f4f50b2CAS | 17960357PubMed |
CIE (1986) ‘Publication 15.2. Colorimetry.’ 2nd edn (CIE (Commission Internationale de l’Eclairage) Central Bureau: Vienna)
Clarke FR, Clarke JM, McCaig TN, Knox RE, DePauw RM (2006) Inheritance of yellow pigment concentration in seven durum wheat crosses. Canadian Journal of Plant Science 86, 133–141.
| Inheritance of yellow pigment concentration in seven durum wheat crosses.Crossref | GoogleScholarGoogle Scholar |
Clarke JM, Clarke FR, Pozniak CJ (2010) Forty-six years of genetic improvement in Canadian durum wheat cultivars. Canadian Journal of Plant Science 90, 791–801.
| Forty-six years of genetic improvement in Canadian durum wheat cultivars.Crossref | GoogleScholarGoogle Scholar |
Cong L, Wang C, Chen L, Liu H, Yang G, He G (2009) Expression of phytoene synthase1 and carotene desaturase crtI genes result in an increase in the total carotenoids content in transgenic elite wheat (Triticum aestivum L.). Journal of Agricultural and Food Chemistry 57, 8652–8660.
| Expression of phytoene synthase1 and carotene desaturase crtI genes result in an increase in the total carotenoids content in transgenic elite wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVWrsr7M&md5=a53b71c245343dc7dc6ea528b2eff53bCAS | 19694433PubMed |
Crawford AC, Francki MG (2013) Lycopene-ε-cyclase (ε-LCY3A) is functionally associated with quantitative trait loci for flour b* colour on chromosome 3A in wheat (Triticum aestivum L.). Molecular Breeding 31, 737–741.
| Lycopene-ε-cyclase (ε-LCY3A) is functionally associated with quantitative trait loci for flour b* colour on chromosome 3A in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjs1yhtbo%3D&md5=fde8c36739eb26bdbe8ed7a5ae9b7577CAS |
Crawford AC, Stefanova K, Lambe W, McLean R, Wilson R, Barclay I, Francki MG (2011) Functional relationships of phytoene synthase 1 alleles on chromosome 7A controlling flour colour variation in selected Australian wheat genotypes. Theoretical and Applied Genetics 123, 95–108.
| Functional relationships of phytoene synthase 1 alleles on chromosome 7A controlling flour colour variation in selected Australian wheat genotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtFeisL4%3D&md5=b809cf1d531f729dfc13c205f628eda8CAS | 21442411PubMed |
Cubadda RE, Carcea M, Marconi E, Trivisonno MC (2007) Influence of gluten proteins and drying temperature on the cooking quality of durum wheat pasta. Cereal Chemistry 84, 48–55.
| Influence of gluten proteins and drying temperature on the cooking quality of durum wheat pasta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsFGrtrg%3D&md5=d91ca51f38367c96d9ab61d19a83f6fcCAS |
De Simone V, Menzo V, De Leonardis AM, Ficco DBM, Trono D, Cattivelli L, De Vita P (2010) Different mechanisms control lipoxygenase activity in durum wheat kernels. Journal of Cereal Science 52, 121–128.
| Different mechanisms control lipoxygenase activity in durum wheat kernels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFCrtbrL&md5=78440a2ef18055a5381094f2f55a1f67CAS |
de Stefanis E, Sgrulletta D (1990) Effect of high temperature drying on technological properties of pasta. Journal of Cereal Science 12, 97–104.
| Effect of high temperature drying on technological properties of pasta.Crossref | GoogleScholarGoogle Scholar |
De Vita P, Li Destri Nicosia O, Nigro F, Platani C, Riefolo C, Di Fonzo N, Cattivelli L (2007) Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century. European Journal of Agronomy 26, 39–53.
| Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century.Crossref | GoogleScholarGoogle Scholar |
Del Pozo-Insfran D, Brenes CH, Serna Saldivar SO, Talcott ST (2006) Polyphenolic and antioxidant content of white and blue corn (Zea mays L.) products. Food Research International 39, 696–703.
| Polyphenolic and antioxidant content of white and blue corn (Zea mays L.) products.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjs1Gru74%3D&md5=ee86945f3e3745cf36fb90a3fbeea93bCAS |
Delcros JF, Rakotozafy L, Boussard A, Davidou S, Porte C, Potus J, Nicolas J (1998) Effect of mixing conditions on the behavior of lipoxygenase, peroxidase, and catalase in wheat flour doughs. Cereal Chemistry 75, 85–93.
| Effect of mixing conditions on the behavior of lipoxygenase, peroxidase, and catalase in wheat flour doughs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXotVegug%3D%3D&md5=af5775c1e0334f33878dd0c989d13d35CAS |
Delgado-Vargas F, Jiménez AR, Paredes-López O (2000) Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing and stability. Critical Reviews in Food Science and Nutrition 40, 173–289.
| Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing and stability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktlahsbk%3D&md5=f2b1613c6b81f5da83aad044ed946350CAS | 10850526PubMed |
Dexter JE, Marchylo BA (2000) Recent trends in durum wheat milling and pasta processing: impact on durum wheat quality requirements. In ‘Proceedings International Workshop on Durum Wheat, Semolina and Pasta Quality: Recent Achievements and New Trends’. Montpellier, France. (Ed. P Feillet) pp. 77–101. (International Association of Cereal Science and Technology (ICC—Europe): Vienna)
Dexter JE, Wood PJ (1996) Recent application of debranning of wheat before milling. Trends in Food Science & Technology 7, 35–41.
| Recent application of debranning of wheat before milling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhsVWqtb8%3D&md5=27bde848e6798c9a9984253eb76fccbdCAS |
Digesù AM, Platani C, Cattivelli L, Mangini G, Blanco A (2009) Genetic variability in yellow pigment components in cultivated and wild tetraploidwheats. Journal of Cereal Science 50, 210–218.
| Genetic variability in yellow pigment components in cultivated and wild tetraploidwheats.Crossref | GoogleScholarGoogle Scholar |
Dobrovolskaya O, Arbuzova VS, Lohwasser U, Röder MS, Börner A (2006) Microsatellite mapping of complementary genes for purple grain colour in bread wheat (Triticum aestivum L.). Euphytica 150, 355–364.
| Microsatellite mapping of complementary genes for purple grain colour in bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVSitLbI&md5=5672b456f0cac631b2270be9ee0ca9abCAS |
Dong CH, Ma ZY, Xia XC, Zhang LP, He ZH (2012) Allelic variation at the TaZds-A1 locus on wheat chromosome 2A and development of a functional marker in common wheat. Journal of Integrative Agriculture 11, 1067–1074.
| Allelic variation at the TaZds-A1 locus on wheat chromosome 2A and development of a functional marker in common wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtV2itr7L&md5=be794efa9b355385594a66cee1c3a558CAS |
Dowell FE (2000) Differentiating vitreous and nonvitreous durum wheat kernels by using near-infrared spectroscopy. Cereal Chemistry 77, 155–158.
| Differentiating vitreous and nonvitreous durum wheat kernels by using near-infrared spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXitFersb0%3D&md5=67851b7b4f21fff13049ca8492d2970aCAS |
Dowell FE, Maghirang EB, Xie F, Lookhart GL, Pierce RO, Seabourn BW, Bean SR, Wilson JD, Chung OK (2006) Predicting wheat quality characteristics and functionality using near-infrared spectroscopy. Cereal Chemistry 83, 529–536.
| Predicting wheat quality characteristics and functionality using near-infrared spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVWntbzN&md5=f65fdb00594333774ed296134bf80c98CAS |
Elouafi I, Nachit MM, Martin LM (2001) Identification of a microsatellite on chromosome 7B showing a strong linkage with yellow pigment in durum wheat (Triticum turgidum L. var. durum). Hereditas 135, 255–261.
| Identification of a microsatellite on chromosome 7B showing a strong linkage with yellow pigment in durum wheat (Triticum turgidum L. var. durum).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmsFaisb4%3D&md5=20d1c69d7bef98106acb20ee7331ad99CAS | 12152344PubMed |
Escribano-Bailón MT, Santos-Buelga C, Rivas-Gonzalo JC (2004) Anthocyanins in cereals. Journal of Chromatography. A 1054, 129–141.
| Anthocyanins in cereals.Crossref | GoogleScholarGoogle Scholar | 15553138PubMed |
Eticha F, Grausgruber H, Siebenhandl-Ehn S, Berghofer E (2011) Some agronomic and chemical traits of blue aleurone and purple pericarp wheat (Triticum L.). Journal of Agricultural Science and Technology B 1, 48–58.
Fares C, Troccoli A, Di Fonzo N (1996) Use of friction debranning to evaluate ash distribution in Italian durum wheat cultivars. Cereal Chemistry 73, 232–234.
Fares C, Platani C, Dattoli MA, Menga V, Borrelli GM (2006) Tocols in tetraploid wheat: their location in the kernel and evolution during milling and pasta processing. Tecnica Molitoria 11, 1170–1176.
Feillet P, Autran JC, Icard-Verniere C (2000) Pasta brownness: an assessment. Journal of Cereal Science 32, 215–233.
| Pasta brownness: an assessment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXisVCisQ%3D%3D&md5=fa419394a44a008f447827e6182ad946CAS |
Feng B, Dong Z, Xu Z, An Z, Qin H, Wu N, Wang D, Wang T (2010) Molecular analysis of lipoxygenase (LOX) genes in common wheat and phylogenetic investigation of LOX proteins from model and crop plants. Journal of Cereal Science 52, 387–394.
| Molecular analysis of lipoxygenase (LOX) genes in common wheat and phylogenetic investigation of LOX proteins from model and crop plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsV2gt7%2FM&md5=daa2a0a6315300c9a6e9a5601a950ac6CAS |
Fraignier MP, Michaux-Ferrière N, Kobrehel K (2000) Distribution of peroxidases in durum wheat (Triticum durum). Cereal Chemistry 77, 11–17.
| Distribution of peroxidases in durum wheat (Triticum durum).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmt1eltQ%3D%3D&md5=a4d656200801b2b60db3d38e98a997bbCAS |
Fratianni A, Irano M, Panfili G, Acquistucci R (2005) Estimation of colour of durum wheat comparison of WSB, HPLC, and reflectance colourimeter measurements. Journal of Agricultural and Food Chemistry 53, 2373–2378.
| Estimation of colour of durum wheat comparison of WSB, HPLC, and reflectance colourimeter measurements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsl2qsrk%3D&md5=c4b5acd46420d15112eb050f994752bdCAS | 15796565PubMed |
Fratianni A, Giuzio L, Di Criscio T, Flagella Z, Panfili G (2013) Response of carotenoids and tocols of durum wheat in relation to water stress and sulfur fertilization. Journal of Agricultural and Food Chemistry 61, 2583–2590.
| Response of carotenoids and tocols of durum wheat in relation to water stress and sulfur fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXivFChurg%3D&md5=2efb4d266d4bbb1e7d88b08bf22f02d3CAS | 23425658PubMed |
Fu BX, Schlichting L, Pozniak CJ, Singh AK (2013) Pigment loss from semolina to dough: rapid measurement and relationship with pasta colour. Journal of Cereal Science 57, 560–566.
| Pigment loss from semolina to dough: rapid measurement and relationship with pasta colour.Crossref | GoogleScholarGoogle Scholar |
Gallagher CE, Matthews PD, Li F, Wurtel ET (2004) Gene duplication in the carotenoid biosynthetic pathway preceded evolution of the grasses. The Plant Cell 135, 1776–1783.
Galvano F, Fauci LL, Vitaglione P, Fogliano V, Vanella L, Felgines C (2007) Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Annali dell’Istituto Superiore di Sanita 43, 382–393.
Garbus I, Carrera AD, Dubcovsky J, Echenique V (2009) Physical mapping of durum wheat lipoxygenase. Journal of Cereal Science 50, 67–73.
| Physical mapping of durum wheat lipoxygenase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVaks7Y%3D&md5=36091c22080c03becb0f1f5903d1b2f0CAS |
Geng HW, He ZH, Zhang LP, Qu YY, Xia XC (2012) Development of functional markers for a lipoxygenase gene TaLox-B1 on chromosome 4BS in common wheat. Crop Science 52, 568–576.
Ghosh D, Konishi T (2007) Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pacific Journal of Clinical Nutrition 16, 200–208.
Goodwin TW (1980) ‘The biochemistry of the carotenoids. Vol. 1: Plants.’ (Chapman and Hall)
Guo H, Ling W, Wang Q (2007) Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods for Human Nutrition 62, 1–6.
| Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitFKnu7g%3D&md5=7b83d02809952a1c92b5308fb206f588CAS | 17187297PubMed |
Gys W, Gebruers K, Sørensen JF, Courtin CM, Delcour JA (2004) Debranning of wheat prior to milling reduces xylanase but not xylanase inhibitor activities in wholemeal and flour. Journal of Cereal Science 32, 215–233.
Hatcher DW, Kruger JE (1993) Distribution of polyphenol oxidase in the flour millstreams of Canadian bread wheat classes milled to three extraction rates. Cereal Chemistry 70, 51–55.
He XY, He ZH, Zhang LP, Sun DJ, Morris CF, Fuerst EP, Xia XC (2007) Allelic variation of polyphenol oxidase (PPO) genes located on chromosomes 2A and 2D and development of functional markers for the PPO genes in common wheat. Theoretical and Applied Genetics 115, 47–58.
| Allelic variation of polyphenol oxidase (PPO) genes located on chromosomes 2A and 2D and development of functional markers for the PPO genes in common wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1Sgsrg%3D&md5=e0f51a008aa06f519affef732e4ca906CAS | 17426955PubMed |
He HY, Zhang YL, He ZH, Wu YP, Xiao YG, Ma CX, Xia XC (2008) Characterization of phytoene synthase 1 gene (Psy1) located on bread wheat chromosome 7A and development of a functional marker. Theoretical and Applied Genetics 116, 213–221.
| Characterization of phytoene synthase 1 gene (Psy1) located on bread wheat chromosome 7A and development of a functional marker.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVGhurrE&md5=1b6e04b5892e7efbafe34f14f12e6815CAS |
He X, Wang J, Ammar K, Javier Peña R, Xia X, He Z (2009a) Allelic variants at the Psy-A1and Psy-B1 loci in durum wheat and their associations with grain yellowness. Crop Science 49, 2058–2064.
| Allelic variants at the Psy-A1and Psy-B1 loci in durum wheat and their associations with grain yellowness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFWjsr3J&md5=75d4f1a40f97d40cc33e25a06b5cfd9cCAS |
He XY, He ZH, Ma W, Appels R, Xia XC (2009b) Allelic variants of phytoene synthase 1 (Psy1) genes in Chinese and CIMMYT wheat cultivars and development of functional markers for flour colour. Molecular Breeding 23, 553–563.
| Allelic variants of phytoene synthase 1 (Psy1) genes in Chinese and CIMMYT wheat cultivars and development of functional markers for flour colour.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvFOmtr4%3D&md5=8a35a7afc687de455f2d424aa62395b1CAS |
Hentschel V, Kranl K, Hollmann J, Lindhauer MG, Bhom V, Bitsch R (2002) Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain. Journal of Agricultural and Food Chemistry 50, 6663–6668.
| Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsFWmurs%3D&md5=2e66e8a41de99bbae666d64982b322f9CAS | 12405758PubMed |
Hessler TG, Thomson MJ, Benscher D, Nachit MM, Sorrells ME (2002) Association of a lipoxygenase locus, Lpx-B1, with variation in lipoxygenase activity in durum wheat seeds. Crop Science 42, 1695–1700.
| Association of a lipoxygenase locus, Lpx-B1, with variation in lipoxygenase activity in durum wheat seeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsFOhtrk%3D&md5=fb158e347b07ae7f0907fec3e52b02c9CAS |
Hidalgo A, Brandolini A, Pompei C (2010) Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours. Food Chemistry 121, 746–751.
| Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXivVOrt70%3D&md5=b56ab29deb692ddc80fe7682563ca46aCAS |
Hirawan R, Diehl-Jones W, Beta T (2011) Comparative evaluation of the antioxidant potential of infant cereals produced from purple wheat and red rice grains and LC-MS analysis of their anthocyanins. Journal of Agricultural and Food Chemistry 59, 12330–12341.
| Comparative evaluation of the antioxidant potential of infant cereals produced from purple wheat and red rice grains and LC-MS analysis of their anthocyanins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlyhtLzM&md5=2e38ce1af3f231e55e851efa22a4bfb3CAS | 22035073PubMed |
Horbowicz M, Kosson R, Grzesiuk A, Debski H (2008) Anthocyanins of fruits and vegetables—their occurrence, analysis and role in human nutrition. Vegetable Crops Research Bulletin 68, 5–22.
| Anthocyanins of fruits and vegetables—their occurrence, analysis and role in human nutrition.Crossref | GoogleScholarGoogle Scholar |
Hosseinian FS, Li W, Beta T (2008) Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chemistry 109, 916–924.
| Measurement of anthocyanins and other phytochemicals in purple wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkt1Oht7k%3D&md5=b03e678f55b0d5c2147a646c17f7ffe4CAS |
Howitt CA, Cavanagh CR, Bowerman AF, Cazzonelli C, Rampling L, Mimica JL, Pogson BJ (2009) Alternative splicing, activation of cryptic exons and amino-acid substitutions in carotenoid biosynthetic genes are associated with lutein accumulation in wheat endosperm. Functional & Integrative Genomics 9, 363–376.
| Alternative splicing, activation of cryptic exons and amino-acid substitutions in carotenoid biosynthetic genes are associated with lutein accumulation in wheat endosperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXns1Chur4%3D&md5=9f12c2823e93b3e8df2631c9f38750e1CAS |
Hsieh CC, McDonald CE (1989) Isolation of lipoxygenase isoenzymes from flour of durum wheat endosperm. Cereal Chemistry 61, 249–253.
Hu C, Cai YZ, Li W, Corke H, Kitts DD (2007) Anthocyanin characterization and bioactivity assessment of a dark blue grained wheat (Triticum aestivum L. cv. Hedong Wumai) extract. Food Chemistry 104, 955–961.
| Anthocyanin characterization and bioactivity assessment of a dark blue grained wheat (Triticum aestivum L. cv. Hedong Wumai) extract.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlsFaksbs%3D&md5=a4bd595d5e2e5e6a25d3950d4d791727CAS |
Icard-Vernière C, Feillet P (1999) Effects of mixing conditions on pasta dough development and biochemical changes. Cereal Chemistry 76, 558–565.
| Effects of mixing conditions on pasta dough development and biochemical changes.Crossref | GoogleScholarGoogle Scholar |
ICC (1990) ICC Method 152. In ‘Standard methods of the International Association for Cereal Science and Technology’. (ICC, Verlag Moritz Schäfer: Detmold, Germany)
Janik LJ, Cozzolino D, Dambergs R, Cynkar W, Gishen M (2007) The prediction of total anthocyanin concentration in red-grape homogenates using visible-near-infrared spectroscopy and artificial neural networks. Analytica Chimica Acta 594, 107–118.
Jimenez M, Dubcovsky J (1999) Chromosome location of genes affecting polyphenol oxidase activity in common and durum wheat seeds. Plant Breeding 118, 395–398.
| Chromosome location of genes affecting polyphenol oxidase activity in common and durum wheat seeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsVCmtQ%3D%3D&md5=0eabd3e04d6ad9daf0ecf23d324598a4CAS |
Just BJ, Santos CAF, Yandell BS, Simon PW (2009) Major QTL for carrot colour are positionally associated with carotenoid biosynthetic genes and interact epistatically in a domesticated × wild carrot cross. Theoretical and Applied Genetics 119, 1155–1169.
| Major QTL for carrot colour are positionally associated with carotenoid biosynthetic genes and interact epistatically in a domesticated × wild carrot cross.Crossref | GoogleScholarGoogle Scholar | 19657616PubMed |
Katerji N, van Hoorn JW, Fares C, Hamdy A, Mastrorilli M, Oweis T (2005) Salinity effect on grain quality of two durum wheat varieties differing in salt tolerance. Agricultural Water Management 75, 85–91.
| Salinity effect on grain quality of two durum wheat varieties differing in salt tolerance.Crossref | GoogleScholarGoogle Scholar |
Khlestkina EK, Röder MS, Börner A (2010) Mapping genes controlling anthocyanin pigmentation on the glume and pericarp in tetraploid wheat (Triticum durum L.). Euphytica 171, 65–69.
| Mapping genes controlling anthocyanin pigmentation on the glume and pericarp in tetraploid wheat (Triticum durum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFantL7L&md5=32bbe95ffb3ee830a0a503dd32b31ae5CAS |
Knievel DC, Abdel-Aal ESM, Rabalski I, Nakamura T, Hucl P (2009) Grain colour development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.). Journal of Cereal Science 50, 113–120.
| Grain colour development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVaksrY%3D&md5=92aa600aa7f59420486f8a9f21318efcCAS |
Kobrehel K, Laignelet B, Feillet P (1974) Study of some factors of macaroni brownness. Cereal Chemistry 51, 675–683.
Konopka I, Czaplicki S, Rotkiewicz D (2006) Differences in content and composition of free lipids and carotenoids in four of spring and winter wheat cultivated in Poland. Food Chemistry 95, 290–300.
| Differences in content and composition of free lipids and carotenoids in four of spring and winter wheat cultivated in Poland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVamsLvK&md5=c2f3d786fbe5615138d0ba78d7229cb2CAS |
Krinsky NI (1993) Actions of carotenoids in biological systems. Annual Review of Nutrition 13, 561–587.
| Actions of carotenoids in biological systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXms12mtLw%3D&md5=d1a42decf231ee4c341238da597322d5CAS | 8369159PubMed |
Kuchel H, Langridge P, Mosionek L, Williams K, Jefferies SP (2006) The genetic control of milling yield, dough rheology and baking quality of wheat. Theoretical and Applied Genetics 112, 1487–1495.
| The genetic control of milling yield, dough rheology and baking quality of wheat.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283ntVansA%3D%3D&md5=e437032931ede1d2c1e13e057220487fCAS | 16550398PubMed |
Li F, Vallabhaneni R, Yu J, Rochefort T, Wurtzel ET (2008) The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiology 147, 1334–1346.
| The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXoslyisb4%3D&md5=3a112d1c2da8dcfcea059a08033c549eCAS | 18508954PubMed |
Li XP, Lan SQ, Zhang YL, Liu YP (2010) Identification of molecular markers linked to the genes for purple grain colour in wheat (Triticum aestivum). Genetic Resources and Crop Evolution 57, 1007–1012.
| Identification of molecular markers linked to the genes for purple grain colour in wheat (Triticum aestivum).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVSmsb3F&md5=a38970a9eca5bcab76cbee6447cb162dCAS |
Lindgren LO, Stalberg KG, Hoglund AS (2003) Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in delayed germination and increased levels of carotenoids, chlorophyll, and abscisic acid. Plant Physiology 132, 779–785.
| Seed-specific overexpression of an endogenous Arabidopsis phytoene synthase gene results in delayed germination and increased levels of carotenoids, chlorophyll, and abscisic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkslert70%3D&md5=674f1c218965def41a7fc804e8b0bfffCAS | 12805607PubMed |
Lintas C (1988) Durum wheat vitamins and minerals. In ‘Durum wheat: chemistry and technology’. (Eds G Fabriani, C Lintas) pp. 149–159. (American Association of Cereal Chemists: St. Paul, MN)
Liu CS, Chao S, Gale MD (1990) The genetical control of tissue-specific peroxidase, Per-1, Per-2, Per-3, Per-4, and Per-5 in wheat. Theoretical and Applied Genetics 79, 305–313.
| The genetical control of tissue-specific peroxidase, Per-1, Per-2, Per-3, Per-4, and Per-5 in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXkvFCqsrw%3D&md5=74ae4329e55174a7507c67acf5071220CAS |
Ma J, Li Y, Li J, Hua Y, Ju D, Zhang D, Cooper R, Chang M (2000) Constituents of red yeast rice, a traditional Chinese food and medicine. Journal of Agricultural and Food Chemistry 48, 5220–5225.
| Constituents of red yeast rice, a traditional Chinese food and medicine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnt1SlsLo%3D&md5=669abcc5195575754e011e4af70b2d2dCAS | 11087463PubMed |
Manna F, Borrelli GM, Massardo DM, Wolf K, Alifano P, Del Giudice L, Di Fonzo N (1998) Differential expression of lipoxygenase genes among durum wheat cultivars. Cereal Research Communications 26, 23–30.
Marchylo BA, Dexter JE (1989) Pasta production. In ‘Cereals processing technology’. (Ed. G Owens) pp. 109–130.
Mares DJ, Campbell AW (2001) Mapping components of flour and noodle colour in Australian wheat. Australian Journal of Agricultural Research 52, 1297–1309.
| Mapping components of flour and noodle colour in Australian wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltlOmtw%3D%3D&md5=5035ee4c2ba9e3f2164364ec4b1a3226CAS |
Matsuo RR, Dexter JE (1980) Relationship between some durum wheat physical characteristics and semolina milling properties. Canadian Journal of Plant Science 60, 49–53.
| Relationship between some durum wheat physical characteristics and semolina milling properties.Crossref | GoogleScholarGoogle Scholar |
Mayer AM, Harel E (1979) Polyphenol oxidases in plants. Phytochemistry 18, 193–215.
| Polyphenol oxidases in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXkvVOhsrk%3D&md5=fc7c995c7b7017cb90a880c931aa63f5CAS |
Mazza G (2000) Health aspects of natural colours. In ‘Natural food colourants—science and technology’. (Eds GJ Lauro, FJ Francis) pp. 289–314. (Marcel Dekker: New York)
Mazza G (2007) Anthocyanins and heart health. Annali dell’Istituto Superiore di Sanita 43, 369–374.
McCaig TN, McLeod JG, Clarke JM, DePauw RM (1992) Measurement of durum pigment with an NIR instrument operating in the visible range. Cereal Chemistry 69, 671–672.
McDonald CE (1979) Lipoxygenase and lutein bleaching activity of durum wheat semolina. Cereal Chemistry 56, 84–89.
Moreno YS, Sanchez GS, Hernandez DR, Lobato NR (2005) Characterization of anthocyanin extracts from maize kernels. Journal of Chromatographic Science 43, 483–487.
| Characterization of anthocyanin extracts from maize kernels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGitrbN&md5=145296c4a50c60d42b06d7829a5800a1CAS | 16212795PubMed |
Morrison LA, Metzger RJ, Lukaszewski AJ (2004) Origin of the blue-aleurone gene in sebesta blue wheat genetic stocks and a protocol for its use in apomixes screening. Crop Science 44, 2063–2067.
| Origin of the blue-aleurone gene in sebesta blue wheat genetic stocks and a protocol for its use in apomixes screening.Crossref | GoogleScholarGoogle Scholar |
Nachit MM, Elouafi I, Pagnotta MA, El Saleh A, Iacono E, Labhilili M, Asbati A, Azrak M, Hazzam H, Benscher D, Khairallah M, Ribaut JM, Tanzarella OA, Porceddu E, Sorrells ME (2001) Molecular linkage map for an intraspecific recombinant inbred population of durum wheat (Triticum turgidum L. var. durum). Theoretical and Applied Genetics 102, 177–186.
| Molecular linkage map for an intraspecific recombinant inbred population of durum wheat (Triticum turgidum L. var. durum).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXisVynsrk%3D&md5=e2279ca80ae0ed2bccf3ded8851c3786CAS |
Nilthong S, Graybosch RA, Baenziger PS (2012) Inheritance of grain polyphenol oxidase (PPO) activity in multiple wheat (Triticum aestivum L.) genetic backgrounds. Theoretical and Applied Genetics 125, 1705–1715.
| Inheritance of grain polyphenol oxidase (PPO) activity in multiple wheat (Triticum aestivum L.) genetic backgrounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1GqsL7E&md5=90d1b77dcc9e58d76e751d1c1c795340CAS | 22864385PubMed |
Nishino H, Murakoshi M, Tokuda H, Yoshiko S (2009) Cancer prevention by carotenoids. Archives of Biochemistry and Biophysics 483, 165–168.
| Cancer prevention by carotenoids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsFWqsL8%3D&md5=92b62e26721deb21965d4745010934aaCAS | 18848517PubMed |
Okot-Kotber M, Liavoga A, Yong KJ, Bagorogoza K (2001) Activity and inhibition of polyphenol oxidase in extracts of bran and other milling fractions from a variety of wheat cultivars. Cereal Chemistry 78, 514–520.
| Activity and inhibition of polyphenol oxidase in extracts of bran and other milling fractions from a variety of wheat cultivars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmvV2hu7s%3D&md5=6b59e7d58a03981ebb48d548c6e195beCAS |
Osborne BG, Douglas S, Fearn T (1982) The application of near infrared reflectance analysis to rapid flour testing. Journal of Food Technology 17, 355–363.
| The application of near infrared reflectance analysis to rapid flour testing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XkslSrurY%3D&md5=a29c1710952066503bc4c96e5aa93798CAS |
Panfili G, Fratianni A, Irano M (2004) Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. Journal of Agricultural and Food Chemistry 52, 6373–6377.
| Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnslartLY%3D&md5=318b45ea8bf553f081a02fd84592f213CAS | 15478994PubMed |
Parker GD, Chalmers KJ, Rathjen AJ, Langridge P (1998) Mapping loci associated with our colour in wheat. Theoretical and Applied Genetics 97, 238–245.
| Mapping loci associated with our colour in wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlslaisrs%3D&md5=c2f994dd5bc93514eaa9691aea35655cCAS |
Pastore D, Trono D, Padalino L, Simone S, Valenti D, Di Fonzo N, Passerella S (2000) Inhibition by α-tocopherol and L-ascorbate of linoleate hydroperoxidation and β-carotene bleaching activities in durum wheat semolina. Journal of Cereal Science 31, 41–54.
| Inhibition by α-tocopherol and L-ascorbate of linoleate hydroperoxidation and β-carotene bleaching activities in durum wheat semolina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjt1emsg%3D%3D&md5=816f24b7ea019b9c32c56c337f7beddbCAS |
Patil RM, Oak MD, Tamhankar SA, Sourdille P, Rao VS (2008) Mapping and validation of a major QTL for yellow pigment content on 7AL in durum wheat (Triticum turgidum L. ssp. durum). Molecular Breeding 21, 485–496.
| Mapping and validation of a major QTL for yellow pigment content on 7AL in durum wheat (Triticum turgidum L. ssp. durum).Crossref | GoogleScholarGoogle Scholar |
Pelletier MK, Shirley BW (1996) Analysis of flavanone 3-hydroxylase in Arabidopsis seedlings. Coordinate regulation with chalcone synthase and chalcone isomerase. Plant Physiology 111, 339–345.
| Analysis of flavanone 3-hydroxylase in Arabidopsis seedlings. Coordinate regulation with chalcone synthase and chalcone isomerase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XivFeku70%3D&md5=fcd82b43b314a240936896ea2f5932b3CAS | 8685272PubMed |
Pozniak CJ, Knox RE, Clarke FR, Clarke JM (2007) Identification of QTL and association of a phytoene synthase gene with endosperm colour in durum wheat. Theoretical and Applied Genetics 114, 525–537.
| Identification of QTL and association of a phytoene synthase gene with endosperm colour in durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXosFKrsw%3D%3D&md5=461265f61aaa7f1cd336c1dd33f58e9dCAS | 17131106PubMed |
Putzbach K, Krucker M, Albert K, Grisak MA, Tang G, Dolnikowski GG (2005) Structure determination of partially deuterated carotenoids from intrinsically labeled vegetables by HPLC-MS and 1H-NMR. Journal of Agricultural and Food Chemistry 53, 671–677.
| Structure determination of partially deuterated carotenoids from intrinsically labeled vegetables by HPLC-MS and 1H-NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltlSh&md5=ac0b492ef7fb898ca1e91c00d2853905CAS | 15686418PubMed |
Raman R, Raman H, Johnstone K, Lisle C, Smith A, Martin P, Allen H (2005) Genetic and in-silico comparative mapping of the polyphenol oxidase gene in bread wheat (Triticum aestivum L.). Functional & Integrative Genomics 5, 185–200.
| Genetic and in-silico comparative mapping of the polyphenol oxidase gene in bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVajsLnN&md5=7e53e79d0842464a7bbf42af0f390247CAS |
Rani KU, Prasada Rao UJS, Leelavathi K, Haridas Rao P (2001) Distribution of enzymes in wheat flour mill streams. Journal of Cereal Science 34, 233–242.
| Distribution of enzymes in wheat flour mill streams.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXpt12rsbg%3D&md5=5516e442a30ab564c8b1fff12b03286cCAS |
Ravel C, Dardevet M, Leenhardt F, Bordes J, Joseph JL, Perretant MR, Exbrayat F, Poncet C, Balfourier F, Chanliaud E, Charmet G (2013) Improving the yellow pigment content of bread wheat flour by selecting the three homoeologous copies of Psy1. Molecular Breeding 31, 87–99.
| Improving the yellow pigment content of bread wheat flour by selecting the three homoeologous copies of Psy1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvVWrsA%3D%3D&md5=06056c1e1d0a9b1496a7c710496c1b80CAS |
Reimer S, Pozniak CJ, Clarke FR, Clarke JM, Somers DJ, Knox RE, Singh AK (2008) Association mapping of yellow pigment in an elite collection of durum wheat cultivars and breeding lines. Genome 51, 1016–1025.
| Association mapping of yellow pigment in an elite collection of durum wheat cultivars and breeding lines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFSmtbvJ&md5=7b1d78309dd15bda2694c7e90ee59cadCAS | 19088814PubMed |
Ribaya-Mercado JD, Blumberg JB (2004) Lutein and zeaxanthin and their potential roles in disease prevention. Journal of the American College of Nutrition 23, 567S–587S.
| Lutein and zeaxanthin and their potential roles in disease prevention.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht12jt7o%3D&md5=399fe29ee83b23e047ab7c150412e239CAS | 15640510PubMed |
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149, 2007–2023.
Roncallo PF, Cervigni GL, Jensen C, Miranda R, Carrera AD, Helguera M, Echenique V (2012) QTL analysis of main and epistatic effects for flour colour traits in durum wheat. Euphytica 185, 77–92.
| QTL analysis of main and epistatic effects for flour colour traits in durum wheat.Crossref | GoogleScholarGoogle Scholar |
Ryu SN, Park SZ, Ho CT (1998) High performance liquid chromatographic determination of anthocyanin pigments in some varieties of black rice. Journal of Food and Drug Analysis 6, 729–736.
Sadeque A, Turner MA (2010) QTL mapping of polyphenol oxidase (PPO) activity and yellow alkaline noodle (YAN) color components in an Australian hexaploid wheat population. Thai Journal of Agricultural Science 43, 109–118.
Shenk JS, Workman JJ, Westerhaus MO (1990) ‘Handbook of near infrared analysis.’ (Marcel Dekker: New York)
Shipp J, Abdel-Aal E-SM (2010) Food applications and physiological effects of anthocyanins as functional food ingredients. The Open Food Science Journal 4, 7–22.
| Food applications and physiological effects of anthocyanins as functional food ingredients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktValtrg%3D&md5=fd9070538c6775cf2468e0cdab3c00d9CAS |
Si H, Zhou Z, Wang X, Ma C (2012a) A novel molecular marker for the polyphenol oxidase gene located on chromosome 2B in common wheat. Molecular Breeding 30, 1371–1378.
| A novel molecular marker for the polyphenol oxidase gene located on chromosome 2B in common wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVCgurjI&md5=0e28ff7ec2d2e4a5e2738533678a9ca4CAS |
Si H, Ma C, Wang X, He X (2012b) Variability of polyphenol oxidase (PPO) alleles located on chromosomes 2A and 2D can change the wheat kernel PPO activity. Australian Journal of Crop Science 6, 444–449.
Siedow JN (1991) Plant lipoxygenase: structure and function. Annual Review of Plant Physiology and Plant Molecular Biology 42, 145–188.
| Plant lipoxygenase: structure and function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXltFSmsrs%3D&md5=9f5894c9808bd250f375b9d548f19506CAS |
Simeone R, Pasqualone A, Clodoveo ML, Blanco A (2002) Genetic mapping of polyphenol oxidase in teraploid wheat. Cellular & Molecular Biology Letters 7, 763–769.
Singh A, Reimer S, Pozniak CJ, Clarke FR, Clarke JM, Knox RE, Singh AK (2009) Allelic variation at Psy1-A1 and association with yellow pigment in durum wheat grain. Theoretical and Applied Genetics 118, 1539–1548.
| Allelic variation at Psy1-A1 and association with yellow pigment in durum wheat grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXls1OltrY%3D&md5=69fb3e743e6598cc68486882a45a3b25CAS | 19319504PubMed |
Sissons M, Osborne B, Sissons S (2006) Application of near infrared reflectance spectroscopy to a durum wheat breeding programme. Journal of Near Infrared Spectroscopy 14, 17–25.
| Application of near infrared reflectance spectroscopy to a durum wheat breeding programme.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xkslamtbw%3D&md5=9cbfe9e0f70262c441eec85731ec7313CAS |
Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer GE (2011) Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chemistry 124, 132–140.
| Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpsFWmu74%3D&md5=7f5f205f388e980740fc143a2ab55ac7CAS |
Stintzing FC, Stintzing AS, Carle R, Frei B, Wrolstad RE (2002) Colour and antioxidant properties of cyanidin-based anthocyanin pigments. Journal of Agricultural and Food Chemistry 50, 6172–6181.
| Colour and antioxidant properties of cyanidin-based anthocyanin pigments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmslGnt7o%3D&md5=a3ffff6a77e8787f76daf2f1186ff94aCAS | 12358498PubMed |
Sullivan B (1946) Oxidizing enzyme systems of wheat and flour. In ‘Enzymes and their role in wheat technology’. (Ed. JA Anderson) p. 215. (Interscience Publishers: New York)
Sun DJ, He ZH, Xia XC, Zhang LP, Morris CF, Appels R, Ma WJ, Wang H (2005) A novel STS marker for polyphenol oxidase activity in bread wheat. Molecular Breeding 16, 209–218.
| A novel STS marker for polyphenol oxidase activity in bread wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1GlsLvL&md5=1c86664678cb62b7c601a7ba7abdd7dcCAS |
Taha SA, Sagi F (1987) Relationships between chemical composition of durum wheat semolina and macaroni quality. II. Ash, carotenoid pigments, and oxidative enzymes. Cereals Research Message 15, 123–129.
Tereshchenko OY, Arbuzova VS, Khlestkina EK (2013) Allelic state of the genes conferring purple pigmentation in different wheat organs predetermines transcriptional activity of the anthocyanin biosynthesis structural genes. Journal of Cereal Science 57, 10–13.
| Allelic state of the genes conferring purple pigmentation in different wheat organs predetermines transcriptional activity of the anthocyanin biosynthesis structural genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsF2nu77J&md5=d92b3b259efd3e4d5e4772cd04b74c5eCAS |
Trono D, Pastore D, Di Fonzo N (1999) Carotenoid dependent inhibition of durum wheat lipoxygenase. Journal of Cereal Science 29, 99–102.
| Carotenoid dependent inhibition of durum wheat lipoxygenase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhtlSjsbo%3D&md5=4176e6e04bf3d0c5d3a26405bc736568CAS |
Urbano-Cuadrado M, De Castro MDL, Pérez-Juan PM, García-Olmo J, Gómez-Nieto MA (2004) Near infrared reflectance spectroscopy and multivariate analysis in enology determination or screening of fifteen parameters in different types of wines. Analytica Chimica Acta 527, 81–88.
van Breemen RB, Huang CR, Tan Y, Sander LC, Schilling AB (1996) Liquid chromatography/mass spectrometry of carotenoids using atmospheric pressure chemical ionization. Journal of Mass Spectrometry 31, 975–981.
| Liquid chromatography/mass spectrometry of carotenoids using atmospheric pressure chemical ionization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvFCgu78%3D&md5=4107498e4e9461803bc6e5ca4fe04a29CAS |
Van Hung P, Hatcher DW (2011) Ultra-performance liquid chromatography (UPLC) quantification of carotenoids in durum wheat: influence of genotype and environment in relation to the colour of yellow alkaline noodles (YAN). Food Chemistry 125, 1510–1516.
| Ultra-performance liquid chromatography (UPLC) quantification of carotenoids in durum wheat: influence of genotype and environment in relation to the colour of yellow alkaline noodles (YAN).Crossref | GoogleScholarGoogle Scholar |
Verlotta A, De Simone V, Mastrangelo AM, Cattivelli L, Papa R, Trono D (2010) Insight into durum wheat Lpx-B1: a small gene family coding for the lipoxygenase responsible for carotenoid bleaching in mature grains. BMC Plant Biology 10, 263
| Insight into durum wheat Lpx-B1: a small gene family coding for the lipoxygenase responsible for carotenoid bleaching in mature grains.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFagtrjE&md5=ef37863e905cec2c9134c1365c6979bdCAS | 21110856PubMed |
Wang JW, He XY, He ZH, Xia XC (2009) Cloning and phylogenetic analysis of Psy1 genes in bread wheat and related species. Hereditas 146, 208–256.
| Cloning and phylogenetic analysis of Psy1 genes in bread wheat and related species.Crossref | GoogleScholarGoogle Scholar |
Watanabe N, Masum Akond ASMG, Nachit MM (2006) Genetic mapping of the gene affecting polyphenol oxidase activity in tetraploid durum wheat. Journal of Applied Genetics 47, 201–205.
| Genetic mapping of the gene affecting polyphenol oxidase activity in tetraploid durum wheat.Crossref | GoogleScholarGoogle Scholar | 16967558PubMed |
Winkel-Shirley B (2001) Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology 126, 485–493.
| Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXks1Gnu74%3D&md5=163d15206a2084b87fd2d3b7b4ce7033CAS | 11402179PubMed |
Wong JC, Lambert RJ, Wurtzel ET, Rocheford TR (2004) QTL and candidate genes phytoene synthase and zeta-carotene desaturase associated with the accumulation of carotenoids in maize. Theoretical and Applied Genetics 108, 349–359.
| QTL and candidate genes phytoene synthase and zeta-carotene desaturase associated with the accumulation of carotenoids in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjtFCntw%3D%3D&md5=aeedf824e232394ec12b93a3493de084CAS | 14523521PubMed |
Zandomeneghi M, Festa C, Carbonaro L, Galleschi L, Lenzi A, Calducci L (2000) Front surface absorbance spectra of wheat flour: determination of carotenoids. Journal of Agricultural and Food Chemistry 48, 2216–2221.
| Front surface absorbance spectra of wheat flour: determination of carotenoids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtlGruro%3D&md5=11f542800fa8897cad9eac79530ba3cdCAS | 10888525PubMed |
Zeller FJ, Cermeno MC, Miller TE (1991) Cytogenetical analysis on the distribution and origin of the alien chromosome pair conferring blue aleurone colour in several European bread wheat (Triticum aestivum L.) strains. Theoretical and Applied Genetics 81, 551–558.
| Cytogenetical analysis on the distribution and origin of the alien chromosome pair conferring blue aleurone colour in several European bread wheat (Triticum aestivum L.) strains.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c7lvVKlsQ%3D%3D&md5=81be62362a35f74578fbf1eb05a632deCAS | 24221323PubMed |
Zeven AC (1991) Wheats with purple and blue grains: a review. Euphytica 56, 243–258.
| Wheats with purple and blue grains: a review.Crossref | GoogleScholarGoogle Scholar |
Zhang W, Dubcovsky J (2008) Association between allelic variation at the phytoene synthase 1 gene and yellow pigment content in the wheat grain. Theoretical and Applied Genetics 116, 635–645.
| Association between allelic variation at the phytoene synthase 1 gene and yellow pigment content in the wheat grain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFCqtbs%3D&md5=d0fb2d2633d204d1f6f82187f14df25cCAS | 18193186PubMed |
Zhang W, Chao S, Manthey F, Chicaiza O, Brevis JC, Echenique W, Dubcovsky J (2008) QTL analysis of pasta quality using a composite microsatellite and SNP map of durum wheat. Theoretical and Applied Genetics 117, 1361–1377.
| QTL analysis of pasta quality using a composite microsatellite and SNP map of durum wheat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlSgsLnI&md5=d7fe23c666daafc37876752dd375d892CAS | 18781292PubMed |
Zhang YL, Wu YP, Xiao YG, He ZH, Zhang Y, Yan J, Zhang Y, Xia XC, Ma CX (2009) QTL mapping for flour colour components, yellow pigment content and polyphenol oxidase activity in bread wheat (Triticum aestivum L.). Euphytica 165, 435–444.
| QTL mapping for flour colour components, yellow pigment content and polyphenol oxidase activity in bread wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFCmsLjJ&md5=7ac834fdd48f2ea33a80d22be66b53d8CAS |
Zheng Q, Li B, Zhang X, Mu S, Zhou H, Li Z (2006) Molecular cytogenetic characterization of wheat Thinopyrum ponticum translocations bearing blue-grained gene(s) induced by r-ray. Euphytica 152, 51–60.
| Molecular cytogenetic characterization of wheat Thinopyrum ponticum translocations bearing blue-grained gene(s) induced by r-ray.Crossref | GoogleScholarGoogle Scholar |
Žilić S, Serpen A, Akillioğlu G, Gökmen V, Vančetović J (2012) Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of coloured maize (Zea mays L.) kernels. Journal of Agricultural and Food Chemistry 60, 1224–1231.
| Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of coloured maize (Zea mays L.) kernels.Crossref | GoogleScholarGoogle Scholar | 22248075PubMed |


