A challenging future for Carnaby’s Cockatoo (Zanda latirostris) under a changing climate
Denis A. Saunders A , Peter R. Mawson
A , Peter R. Mawson  B * , Rick Dawson B and Geoffrey Pickup C
B * , Rick Dawson B and Geoffrey Pickup C
A
B
C
Abstract
With climate change causing unprecedented and rapid changes, conservation agencies need to establish the impacts on vulnerable and threatened species to prioritise actions to minimise threats associated with those impacts.
Carnaby’s Cockatoo (Zanda latirostris) is endemic to south-western Australia and this paper provides data to underpin future conservation management actions.
We used data on the commencement of egg laying, breeding success, nestling condition, fledgling survival over their first year, and annual survival from the first year to construct a life table to examine the impacts of decreasing annual rainfall and increasing temperature in south-western Australia on the future viability of Carnaby’s Cockatoos.
Long-term survival of Carnaby’s Cockatoos will be impacted by changes in rainfall, projected to be 16% drier in winter and up to 20% drier in spring, and by an increase in the number of days with maxima ≥35°C, conditions when the birds are unable to forage.
This drying and warming is likely to lead to a further contraction in the range of Carnaby’s Cockatoo.
Conservation management needs to address revegetation of foraging and breeding areas, repairs to derelict natural hollows and their maintenance, and provision of artificial hollows. Management should concentrate on areas with the best prospects for species survival and recovery including in the areas identified in this paper based on life table analysis and mapped across regions.
Keywords: Carnaby’s Cockatoos, fledgling survival, heatwaves, impacts of changes in annual rainfall, life history analyses, longevity, nestling condition and rainfall, nestling condition and temperature.
Introduction
The world is going through an unprecedented, rapid period of climate change with challenging impacts on biota (IPCC 2023). While impacts vary between regions, the changes pose major concerns for policy makers addressing detrimental impacts. South-western Australia, with its Mediterranean climate of hot, dry summers and cool, wet winters is no exception. It is already going through rapid change, in line with predictions from the Government of Western Australia (2022) that winter will be 16% drier and up to 20% drier in spring. These changes are manifesting themselves locally. For example, annual rainfall in Albany, on the south coast of Western Australia (see Fig. 1 for locations named in text) decreased by 16.8% between 1960–1969 and 2014–2023, and mean annual minimum temperature increased by 0.7°C (Hopkins et al. 2024). Impacts of climate change on regional biota will vary, with both losers and winners. Conservation agencies will need to assess potential impacts, particularly on vulnerable and threatened species, and prioritise management to mitigate the risks to those species.
Map of south-western Australia showing extent of remnant native vegetation (green), extent of the wheatbelt (cereal cropping/sheep grazing), the Coomallo Creek study area (yellow circle), locations mentioned in the text (red circles), locations of weather stations (black triangles), and Perth city and coastal towns (black squares).
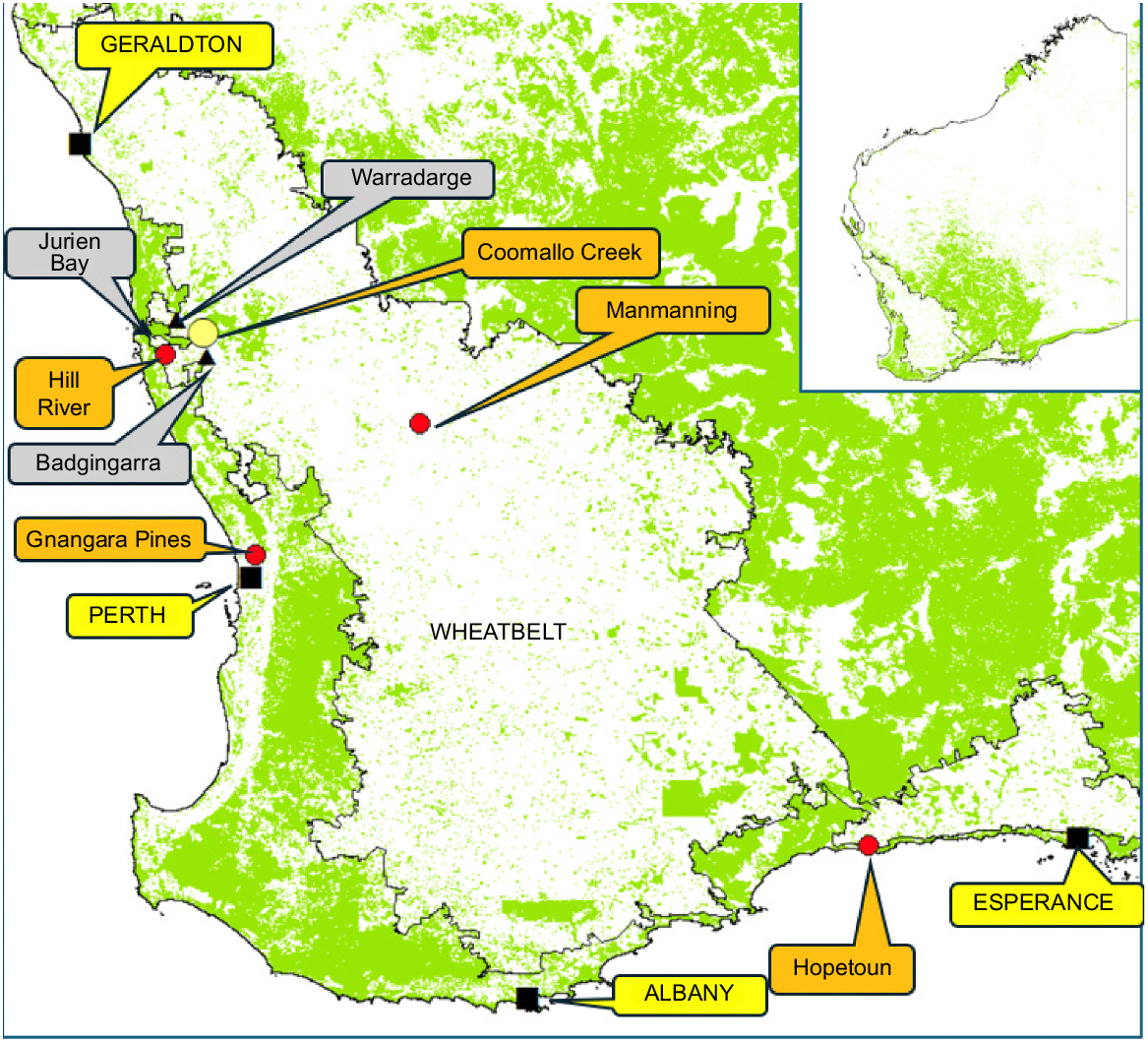
Carnaby’s Cockatoo (Zanda latirostris Carnaby, 1948) is one such species. It is endemic to south-western Australia (Saunders and Pickup 2023). It is a large (660 g), black bird, with a distinctive white sub-terminal tail band. Sexes are the same size. They reach sexual maturity aged 3 or 4 years, form pair bonds that last the life of one of the partners, and breed in the late austral winter through to early summer in large hollows in mature eucalypt trees. One to two eggs are laid, on average 8 days apart, from early June to late December with the timing of the commencement of egg laying each season strongly influenced by rainfall in the first half of autumn. Both eggs usually hatch after 29 days, with the second nestling usually dying before fledging in 97% of breeding attempts; occasionally both nestlings fledge. Once they have fledged their nestling(s), the family moves away from the breeding area and spends the non-breeding season (February–May) in flocks that mainly forage on native vegetation, particularly seeds of the Proteaceae (Banksia, Grevillea, and Hakea), and exotic vegetation, including pines (Pinus spp.), almonds (Prunus amygdalus), and macadamias (Macadamia spp.) (information from Saunders 1974, 1980, 1982; Saunders and Ingram 1998; Saunders et al. 2013, 2018, 2024a, 2024b; Saunders and Dawson 2018).
Since European settlement, the species has lost much of its breeding and foraging habitat due to government-sponsored extensive clearing of native vegetation for agricultural development, urbanisation, infrastructure development, and mining, particularly for bauxite and mineral sands (Saunders et al. 1985, 2024a; Saunders 1990; Department of Environment and Conservation 2012; Fig. 1). It is now classified as endangered by the Western Australian and Australian governments, as well as internationally (Saunders et al. 2021). Climate change now poses a major, and additional, threat to the survival of the species (Saunders et al. 2011a).
One breeding population of Carnaby’s Cockatoo at Coomallo Creek (Fig. 1) in the northern wheatbelt of south-western Australia has been studied in detail from 1969 to 2023, with data available for 34 years. This population provides a record of timing of egg laying, breeding success, fledgling survival, adult survival, and fidelity to breeding area and nest site. Using these data, we constructed a life table analysis with rates of fecundity and mortality based on the impacts of variations in rainfall and temperature on the commencement of egg laying, breeding success, nestling condition, and fledgling survival. Among other variables, life table analysis yields information on the lifetime reproduction potential of an average female, and the intrinsic rate of population increase that may be used to predict the size of the population in the future. Together, these variables can identify whether a population is increasing, stable or in decline.
We use the results of this analysis, together with information on regional variations in climate change, to provide guidance for conservation management of Carnaby’s Cockatoo and to discuss the implications of climate change for the future viability of the species.
The Coomallo Creek dataset is of importance as it is the longest study of its kind carried out in Australia so far (Lunney et al. 2018).
Materials and methods
Coomallo Creek breeding population – survey methods
Carnaby’s Cockatoos at Coomallo Creek (Fig. 1) breed in a 9-km long, narrow belt of wandoo (Eucalyptus wandoo) woodland on four private cereal and sheep producing properties, surrounded by cleared agricultural land and uncleared native Kwongkan heath/shrublands. Between 1969 and 1996, the area was visited at least twice (September and November) during each of 22 years. There was a hiatus from 1997 to 2008. Visits resumed in 2009 continuing to 2023. Since 2009, the area has been visited at least three times each year in September, November, and January (Saunders and Ingram 1998; Saunders and Dawson 2018).
During each visit, every hollow known to be used by the cockatoos was inspected and contents noted. If nestlings were present, and at least 3 weeks old, they were measured and leg banded. In addition, searches were conducted throughout the area to find any hollows not previously recorded. These hollows were added to the list of breeding hollows and inspected subsequently as long as they remained useable (Saunders et al. 2020).
Any nestling old enough to be handled was removed from the hollow, the length of its folded left wing measured (mm), its body mass (g) recorded, its sex noted, and an Australian Bird and Bat Banding Scheme (ABBBS) uniquely numbered metal leg band fitted. Between 1971 and 1976, large nestlings and any adults trapped in their hollow were fitted with a stainless steel patagial tag on each wing. Unfortunately, cockatoos fitted with patagial tags were heavily predated by wedge-tailed eagles (Aquila audax Latham, 1801). Tagged adults had an annual survival rate of 0.59 compared with 1.00 for those leg banded only (Saunders 1988) making the method unsuitable for calculating survival. Accordingly, the use of tags was discontinued (Saunders 1982, 1988) and from 1977, only uniquely numbered ABBBS leg bands were used to identify individuals. Between 1978 and 1990, 20 breeding females were banded with a unique combination of two-colour bands in addition to their numbered metal band.
Female and male nestlings are the same size. They were sexed on the size and colour of their cheek patch (Saunders et al. 2014a). They were aged using the relationship between the length of the nestling’s folded left wing and their age (Saunders et al. 2015). Hatching dates were calculated, working back from nestling age, and then establishing laying dates using an incubation period of 29 days (Saunders 1982).
From 1969 to 1996, attempts were made to identify wing tagged/leg banded individuals by telescope, but it was extremely difficult to identify individuals if they were only carrying a numbered leg band. Carnaby’s Cockatoos have short tarsi, leg bands are narrow, and could only be read reliably by catching the cockatoo. Females with a unique colour band combination could be identified without recourse to trapping. More recently, digital cameras and telephoto lenses allowed numbered leg bands of free-flying cockatoos to be read reliably (Saunders et al. 2011b). From 2009, every female found in a breeding hollow was photographed on leaving its nest hollow to establish her identity, and flocks encountered within and outside Coomallo Creek were scanned for banded individuals. Observations of banded individuals were also reported by members of the public who sent photographs to the authors (Dawson and Saunders 2014; Saunders et al. 2024a).
Life table analysis to assess the viability of the Coomallo Creek population
Life table analysis was used to explore the possible effects on Carnaby’s Cockatoo of projected decreases in rainfall under climate change (Government of Western Australia 2022). A sensitivity analysis was also conducted to understand how changes in annual fledgling production/breeding pair, fledgling survival rate over their first year, and annual survival rate from Year 2, affect population dynamics.
While life table analysis calculations based on climate data alone provide estimates of potential changes in populations over time, they do not allow for other factors such as loss of foraging and breeding sites associated with clearing of native vegetation for agriculture, mining, infrastructure development, and urban development. Nor do they factor in the potential impact of other biotic factors such as avian diseases (Le Souëf et al. 2024) and the loss of nesting sites over time due to senescence of old growth trees in remnant vegetation patches (Saunders et al. 2003, 2014b).
Establishing a relationship between climate change and the viability of the Coomallo Creek population used data on population productivity and survival rates that have been calculated from the survey data. Resightings/recoveries of leg banded birds were used to calculate annual survival rates of breeding adults and fledglings. Survival rates, together with average annual productivity/breeding pair, were used to construct a life table following the methods of Donovan and Welden (2022) and Shoemaker (undated). The life table estimated female lifetime reproduction potential, average age of females in various cohorts, life expectancy of females, the intrinsic rate of increase of the population, and the number of surviving females at age 30 years.
The parameters derived from band re-sightings/recoveries are minimum values as females may have not returned to their natal area to breed but have survived elsewhere, undetected. This is likely, as a female fledgling from Coomallo Creek was recorded breeding 69 km away (Saunders et al. 2024a).
A nestling condition index was calculated allowing comparison of nestling condition with periods of climate stress such as heatwaves. Calculated for every nestling from 1969 to 2023, the index was derived by subtracting the expected mass of a nestling at its estimated age from its actual mass and dividing the result by the measure of variation applicable at that age from the relationship with nestling mass, as presented in Saunders et al. (2019). An overall nestling condition index for each year was calculated as the mean condition index of all that year’s successful fledglings.
Data from fledglings produced during the period 1969–1976 were not used in the life table analysis as the search effort required to identify individually marked birds was much greater and less effective, compared with birds from 2009 on. Also, the number of cockatoos surviving was too small for statistical analysis.
Climate data – rainfall analyses
The Australian Bureau of Meteorology provides online climate data and analyses. Data on daily, monthly, and annual rainfall are available from Badgingarra Research Station #009037, located 30 km south-east of Coomallo Creek (Fig. 1), the closest inland weather station to Coomallo Creek that provides data from 1962 until the present (http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=136&p_display_type=dailyDataFile&p_startYear=&p_c=&p_stn_num=9037). Rainfall records from Badgingarra were occasionally incomplete and required infill with data from Warradarge Station #008278 (30 km north of Coomallo Creek) (Fig. 1). (http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=136&p_display_type=dailyDataFile&p_startYear=&p_c=&p_stn_num=8278). The Badgingarra rainfall data were used to establish relationships with each of the biological parameters used in the life table analysis. Prior to establishing a relationship, several rainfall time periods were investigated, from 3 months to 2 years with the best predictor of the biological parameter being selected for further analysis.
Analysing data on commencement of egg laying (1969–2011), Saunders et al. (2013) showed there was a significant negative linear correlation between the commencement of egg laying and rainfall in the first half of autumn of that year. In view of this relationship, we examined rainfall as a key variable on annual breeding success (percentage of breeding attempts that successfully fledged nestlings) (1970–2023) and survival rates of immature cockatoos for the first year after fledging (2009–2020). Survival was only calculated until 2020 as fledglings hatched after that date had not yet reached sexual maturity and were unlikely to have returned to their natal breeding area.
In analysing the relationship between rainfall and breeding success, nestling condition, and fledgling survival for their first year, we used rainfall data from October to December the year before breeding along with the following January to September; i.e. 12 months rainfall that we refer to hereafter as annual offset rainfall. This provides a result more in line with the breeding season of Carnaby’s Cockatoo. We also found it useful to include the offset rainfall total from the previous year in several of the rainfall analyses where food availability is a critical variable affecting the biological parameters described above. There is a lag effect on seed productivity in that most native proteaceous vegetation that makes up much of Carnaby’s Cockatoo diet flowers in Year 1, but seed only becomes available in Year 2, and may remain available to cockatoos for even longer in several species that demonstrate serotiny.
Temperature analysis
We examined the number of days during the breeding season where daytime maxima were equal to or above 35°C, and the number of heatwaves experienced, where a heatwave is defined as three or more consecutive days with daytime maxima equal to or above 35°C. This is a critical threshold as Saunders (1982) showed that Carnaby’s Cockatoos cease to forage at temperatures exceeding 35°C.
Australian Bureau of Meteorology data on daily maxima are available from Badgingarra Research Station (#009037). Where Badgingarra records were incomplete, we filled in the missing data with records from Jurien Bay (#009031) on the coast, 50 km west of the Coomallo Creek (Fig. 1). We initially limited our analysis of temperature to the period September–December inclusive. This period encompassed the bulk of the breeding season for Carnaby’s Cockatoos at Coomallo Creek, but subsequently added the following January to include late hatching fledglings and older birds that were free-flying, but still being fed by parents and not yet ready to move from the breeding area. Our initial analysis of temperatures ≥35°C used data for all 54 years of our study. We were particularly interested in the number of heatwaves that were recorded during the breeding season since these are periods of stress for nestlings. More detailed heatwave analysis examined changes over time and the relationship with the nestling condition index and used data for the 37 years where we had nestling condition index data between 1969 and 2023. We also examined the relationship between rainfall offset and temperature metrics, finding correlations in both cases.
Spatial analysis of rainfall data
In predicting the impact of the decreasing rainfall in south-western Australia on Carnaby’s Cockatoo, we investigated whether insights from the Coomallo Creek breeding population might be applied more widely. This work relied on long-term gridded rainfall data from the Bureau of Meteorology’s rainfall monitoring stations (Evans et al. 2020).
Data are available on the Bureau’s website for a variety of time intervals from monthly, seasonal, and annual, and use a 0.05-degree (~30.8 km2) grid. In our study of climate impact, we used 1966–1995 as the baseline and 2011–2022 to represent the period of recent climate change.
While the gridded data are an excellent resource, they show the limitations usually experienced when gridding an irregularly-spaced point dataset, so there are some artefacts, especially when interpolating across long distances. Despite these problems, we consider the data to reflect real trends and to be reliable enough to calculate regional variations in life table parameters.
Time series analysis of rainfall indicates first order serial autocorrelation; i.e. 1 year’s annual rainfall is correlated with the previous year, usually with a correlation coefficient of about 0.3. Using both each year’s, and the previous year’s rainfall as independent variables in a regression analysis to represent a rainfall effect on food supply that occurs over 2 years (as with proteaceous species) raises problems of multi-collinearity because the two independent rainfall variables are correlated. This problem has been addressed by applying principal components analysis to the two rainfall variables to produce two unrelated variables: PC1 and PC2. The first is a single composite of the two rainfall variables and is correlated with several of the breeding biology variables. It produces substantially higher R2-values than either of the individual rainfall variables when correlated with both nestling condition and fledgling survival up to Year 1. PC2 is a largely random variable and is unrelated to any of the breeding biology variables. Regression analysis can then be carried out with a single independent rainfall variable.
Statistical analysis used standard linear regression to identify relationships between the climate and biological variables using the NCSS (2024) statistical software (https://www.ncss.com/) and Microsoft Excel. Principal component analysis and t-tests used the same packages.
Ethical approval
Field work and animal handling were conducted under appropriate ethics approvals (held by CSIRO staff for the period 1969–1996, and Western Australian Department of Biodiversity, Conservation and Attractions Animal Ethics Committee project approval numbers 2011/30, 2014/23, 2017/21, and 2020/10C for 2009–2023), and bird banding approvals for the same periods (Australian Bird and Bat Banding Authority #418 held by DAS and #1862 held by PRM).
Results
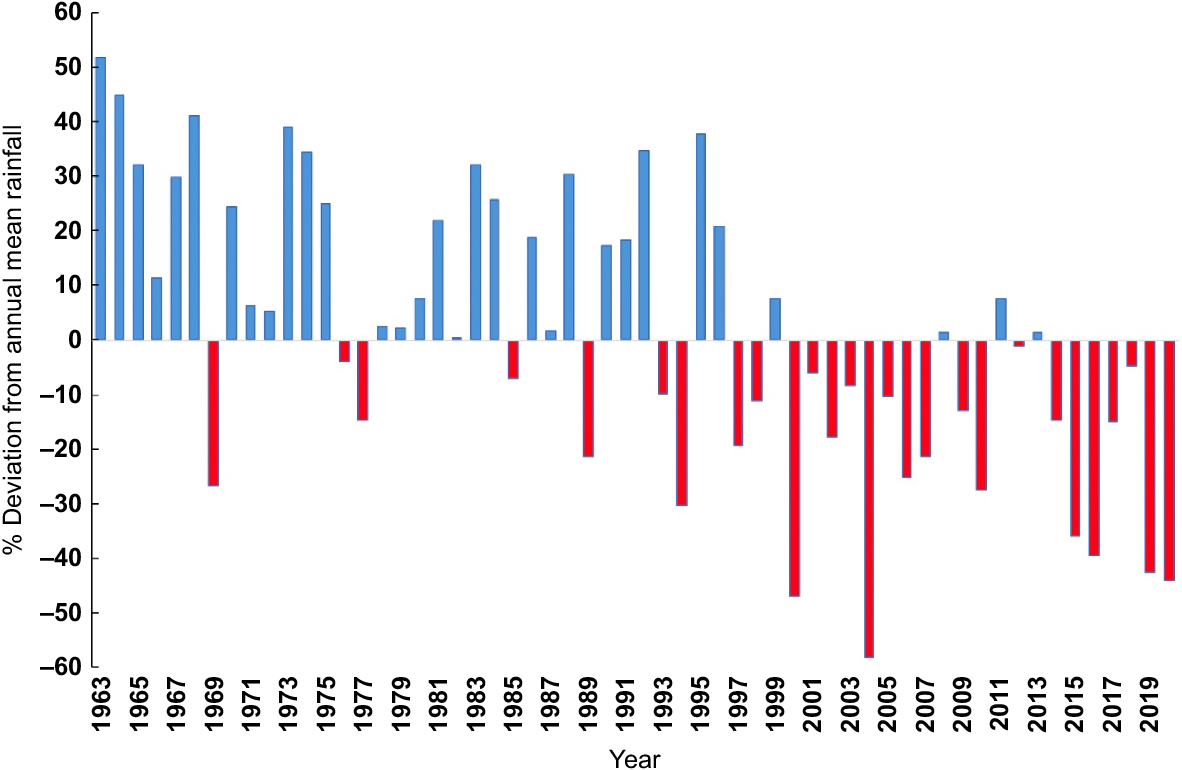
Change in rainfall 1963–2020
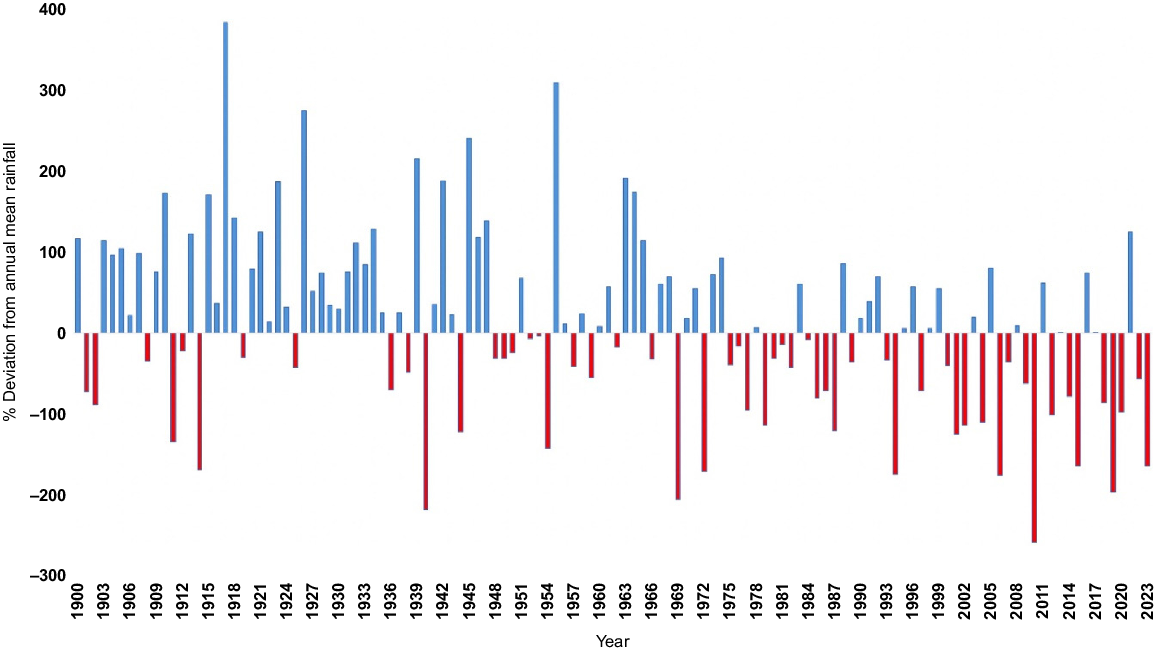
Rainfall records for Badgingarra the period 1963–2020 reveal that the annual rainfall for 1963–1992 was below the long term (1963–2020) mean for only 5 out of 30 years (Fig. 2). The reduction in rainfall beginning in the mid-1990s suggests increasing severity, but with some particularly dry years. The period 2009–2020 was mainly dry, with the exceptions of 2011 and 2013, when annual rainfall was close to the long-term average. The drying pattern at Badgingarra is part of the same pattern shown by the rainfall anomaly for south-western Australia from 1990 to 2020 (Fig. 3), although there are some local variations (see below).
Incidence of hot days (≥35°C) during the breeding season
Using data for years when the breeding sites were monitored, the incidence of hot (≥35°C) days increased by 43.9% from the period 1969–1996 for the September to December period, when there was an average of 11.4 ± 0.90 (s.e.) days with day time maxima ≥35°C, to the period 2003–2023 when there was an average of 16.4 ± 1.8 days (Fig. 4; Student’s t-test: t = 3.62, P < 0.0001). The number, average duration, and maximum length of heat waves have also increased since the mid-1990s. For example, using data for those years where breeding area monitoring was carried out shows that the average longest heatwave recorded each year during the period 1969–1996 was 3.48 days, significantly shorter than 5.56 days for the period 2003–2023 (Student’s unpaired t-test t = 3.18, d.f. = 35, P = 0.003). However, there was no significant correlation between heatwave data and nestling condition index, so it was not used in subsequent investigations.
Change in the number of days for all years with a maximum temperature ≥35°C recorded during Carnaby’s Cockatoo (Zanda latirostris) breeding season (September–December–January) at Coomallo Creek, south-western Australia during 1969–2023.
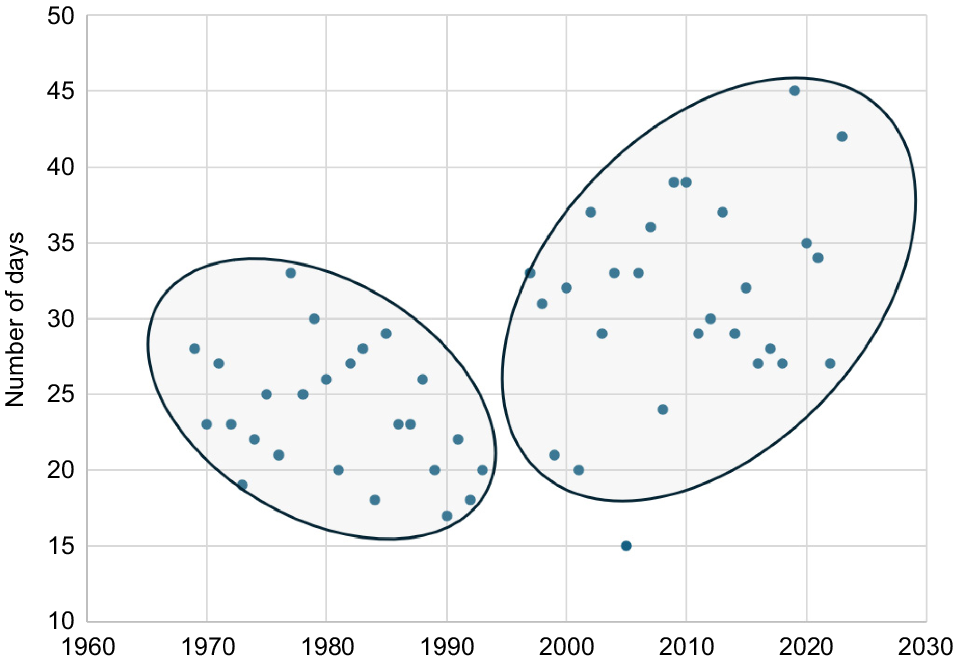
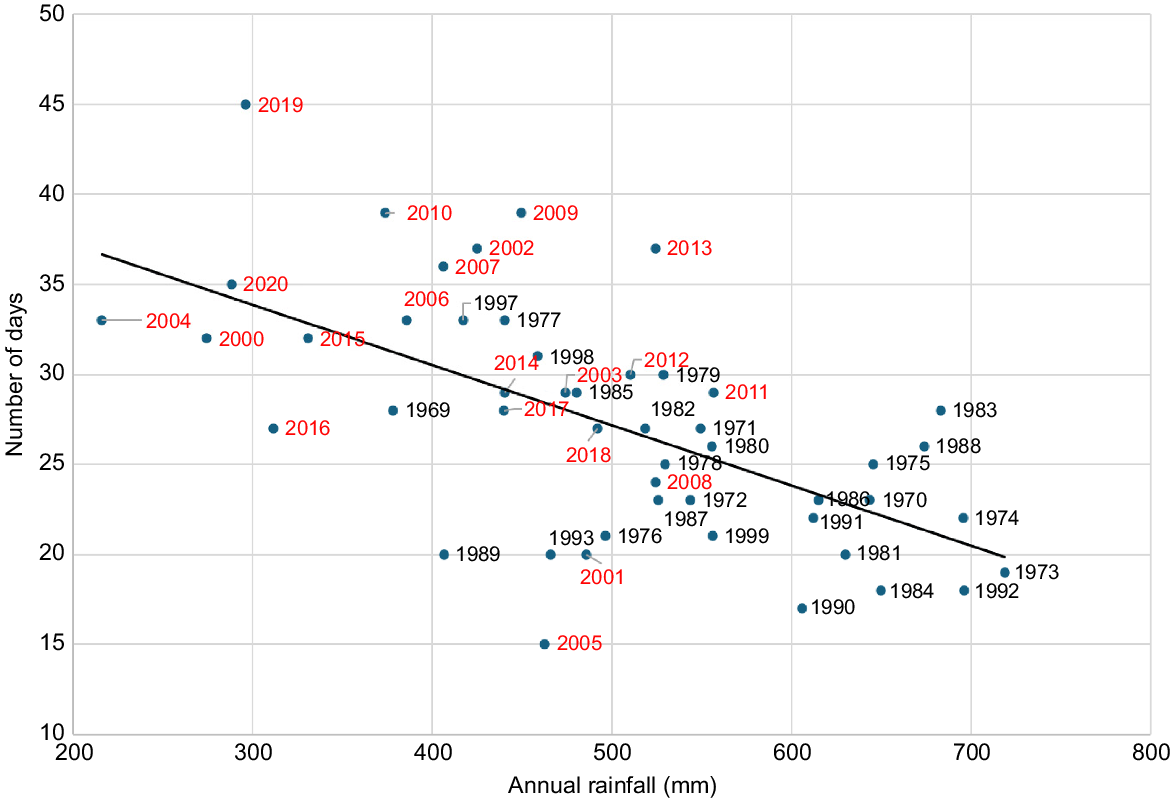
Using the full climate dataset from 1963 to 2023, there is a weak downward trend in the number of hot days up to the mid-1990s then a step change to a gradually increasing number (Fig. 4), indicating similar behaviour to the rainfall series (Fig. 2). Clearly, they are part of the same climatic process. There is also a significant negative relationship with the offset rainfall (Fig. 5), emphasising how closely increasing temperature and decreasing rainfall are linked. This makes it difficult, and probably unnecessary, to separate temperature and rainfall when investigating effects on the breeding biology parameters as they are essentially measuring the same underlying climatic process. Where this is the case, we used what is likely to be the most relevant climate variable on biological grounds rather than its surrogate.
Relationship between offset (October–September) rainfall (mm) and the number of days in Carnaby’s Cockatoo breeding season (September–December–January) with maxima ≥35°C for the period 1969–2023 (y = −0.033x + 43.874, R2 = 0377, P = 0.0005). Red date labels indicate the period when climate change was becoming apparent.
Fidelity to natal area
Of the 59 fledgling females (2009–2020) known to have survived their first year, 56 (94.9%) were recorded as adults at Coomallo Creek. With a known sex ratio of adults of 1:1 (see below), it is reasonable to assume that if males demonstrated the same breeding site fidelity to their natal site, they would show a similar rate of return. However, 19 (19/59 = 32.2%) males that fledged from Coomallo Creek were recorded in the area as adults; one-third of the return rate of female fledglings.
Commencement of egg laying and autumn rainfall
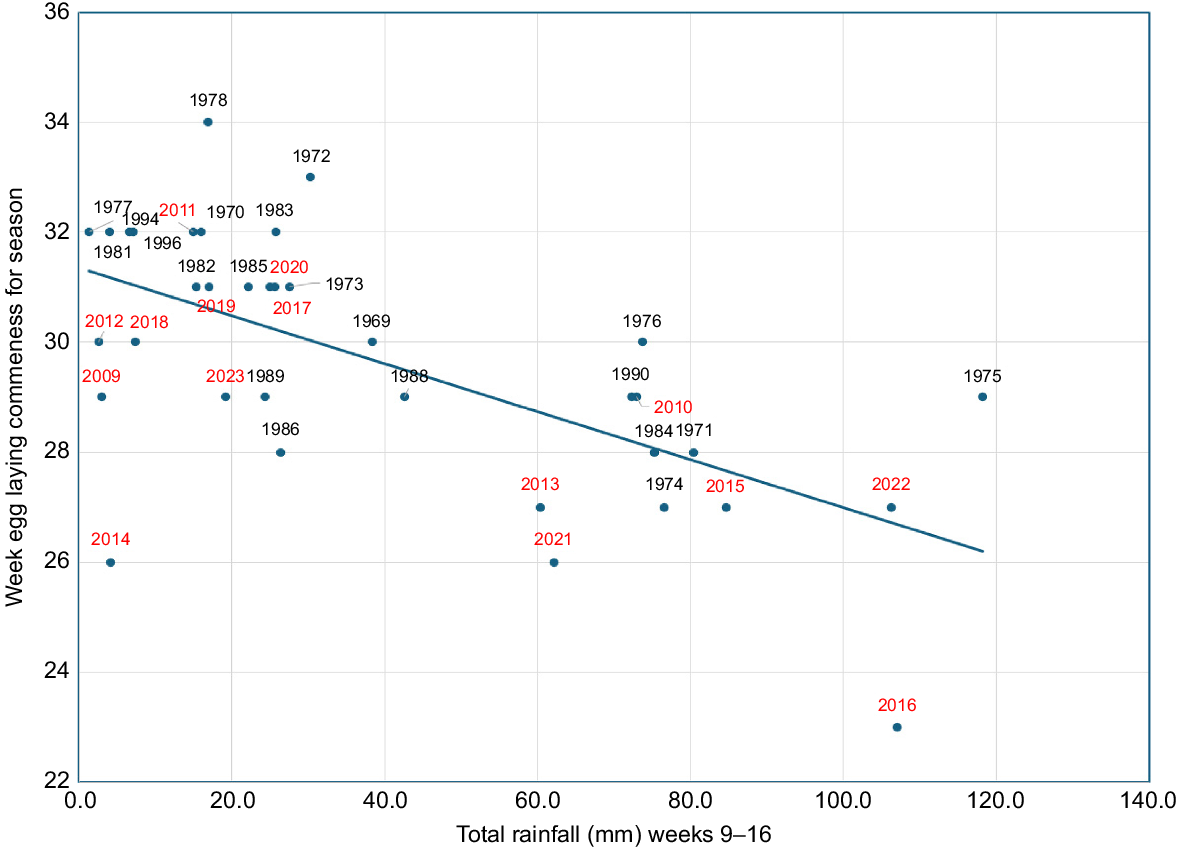
There is a significant linear correlation between commencement of egg laying and rainfall in the first half of autumn (Fig. 6) with egg laying starting later in years with lower autumn rainfall. A 100-mm decrease in rainfall in the first half of autumn results in the commencement of egg laying occurring 4.4 weeks later. This pattern is likely to persist as the decline in rainfall continues. The post-2009 results are scattered throughout the same range as the earlier values, suggesting that rainfall influence on the timing of egg laying has remained constant from 1969 to 2023.
Relationship between the week egg-laying commences at Coomallo Creek for Carnaby’s Cockatoos (Zanda latirostris) and total rainfall in weeks 9–16 of the same year for the period 1969–2023 (y = −0.044x + 31.351; R2 = 0.404; P < 0.0001).
Timing of egg laying is unlikely to be strongly affected by heat waves since most occur late in the breeding season; accordingly, heatwave effects were not considered in the analysis.
Nestling condition, temperature, and rainfall
We expected nestling condition, as measured by the condition index, to be related to both rainfall (as a driver of food availability) and the number of days ≥35°C (as a variable affecting foraging by parents), so both were examined.
Nestling condition index was related to both autumn rainfall in the year of breeding and total rainfall in the previous year (Fig. 7). The R2-value (0.189) is substantially higher than that resulting from a comparison of nestling condition index with the offset annual rainfall alone (R2 = 0.110), the July–October rainfall (R2 = 0.037), the March–June rainfall (R2 = 0.113), and the November–February rainfall (R2 = 0.021). Thus, autumn rainfall is a better predictor of nestling condition. However, PC1 that combines both offset annual rainfall of the current and previous year gave the best prediction from rainfall alone. This may reflect the reduced foraging range that parents use to feed their nestling(s), hence the importance of having 2 years’ vegetation growth.
Relationship between Carnaby’s Cockatoo (Zanda latirostris) nestling condition index and rainfall as described by the first principal component PC1 for the period 1969–2020 (y = 0.096x − 0.129; R2 = 0.189; P = 0.019).
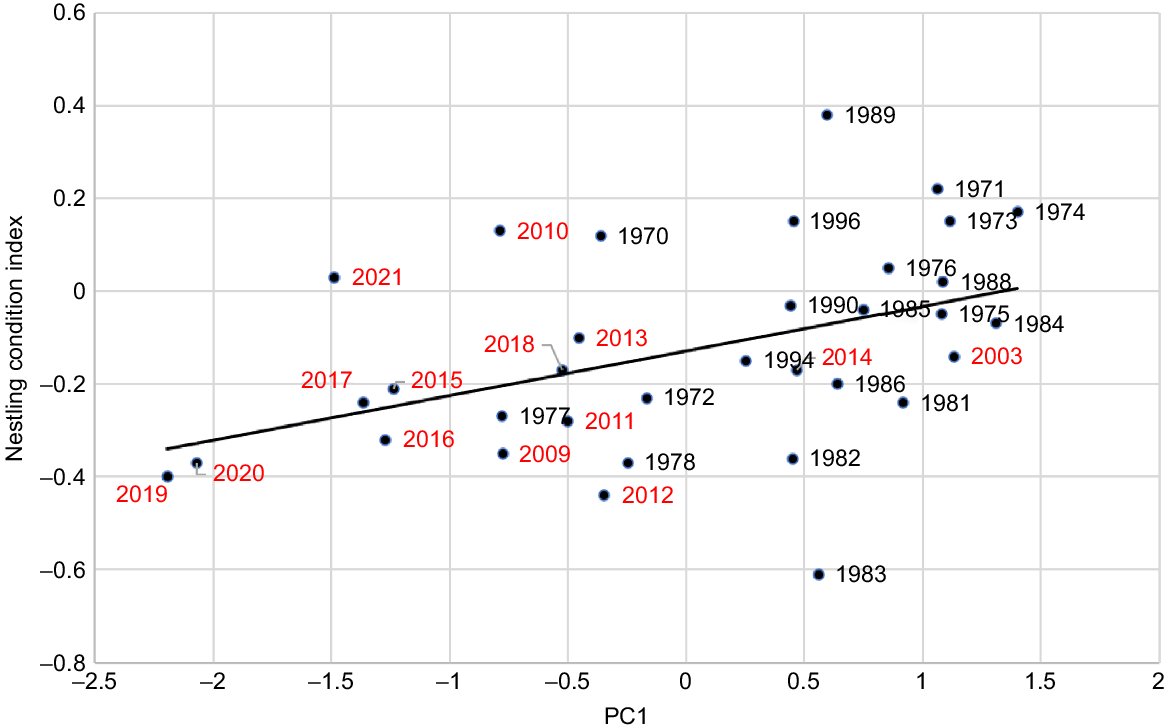
There are two major climate-related outliers in the data (Fig. 7): 1983 and 1989. Both are related to climate events that are not captured in a simple correlation between the nestling condition index and the smoothing that occurs when using annual or seasonal rainfall. Both reflect extreme events in years preceding the year when the condition index was calculated. In 1983, one of Australia’s most severe droughts (Bureau of Meteorology 2024) led to a reduction in available food and egg laying commenced later (Week 32) and breeding success was lower (78.3%). The 1989 outlier followed one of the wettest periods on record with the previous three consecutive years exceeding the long-term average. Egg laying commenced earlier (Week 29) and breeding success was higher (85.7%).
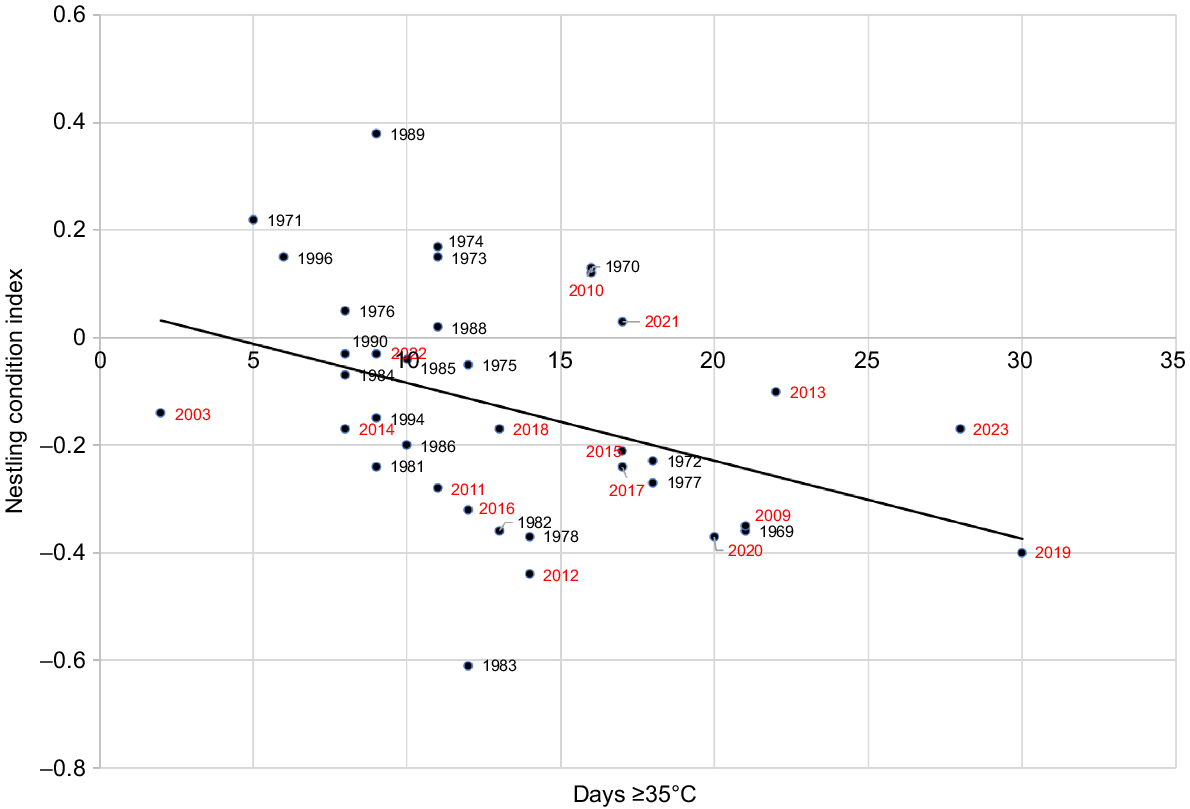
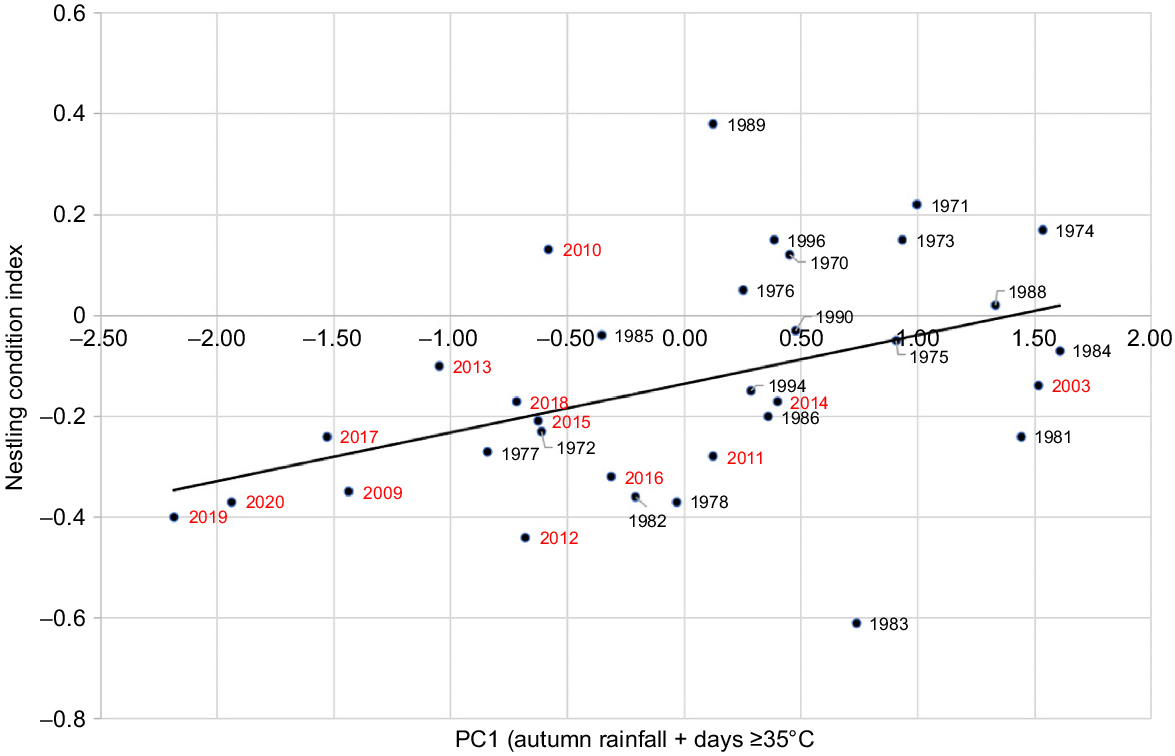
Comparing nestling condition index with the number of hot days ≥35°C was expected to produce a closer or similar correlation than autumn rainfall and PC1 as it is a variable directly affecting food supply to nestlings. However, it results in a marginally lower R2-value of 0.166 (Fig. 8). Combining autumn rainfall with the number of days ≥35°C in a principal component analysis gives the best result (R2-value of 0.202, Fig. 9). Overall, the analysis shows that reasonable predictions of nestling condition may be obtained from either rainfall or temperature, with only a marginal improvement from combining both.
Relationship between Carnaby’s Cockatoo (Zanda latirostris) nestling condition index and the number of days during the breeding season (September–December) with maxima ≥35°C, during the period 1969–2023 (y = 0.015x + 0.061, R2 = 0.166, P = 0.006).
Relationship between Carnaby’s Cockatoo (Zanda latirostris) nestling condition index, autumn rainfall and the number of days during the breeding season (September–December) with maxima ≥35°C, during the period 1969–2023 expressed as the first principal component of the combined dataset (y = 0.09x – 0.1361, R2 = 0.202, P = 0.0001).
Average annual fledgling production per pair
Between 1971 and 1976, the study area was visited most weeks during the breeding season. With patagial tagged cockatoos it was possible to obtain an estimate of number of breeding attempts. A total of 184 breeding attempts were made by tagged females and 14 (7.6%) of these were re-lays after the first attempts failed. Most females that re-laid only made one attempt, but one female, tag LT/band 210-00261, made three attempts, the last one being successful. The extent of re-laying later in the study could not be determined with site visits only being conducted in September and November.
Annual production/breeding pair for the period 1970–2023 was calculated by taking the number of breeding attempts each season, adjusting for the fact that on average 7.6% of attempts were made after a failure, and accounting for the number of attempts that season where pairs successfully fledged two young. The average annual fledgling production per breeding pair was 0.678 ± 0.023 (s.e.) [range 0.456–0.953; n (years) = 34].
Annual breeding success and annual rainfall
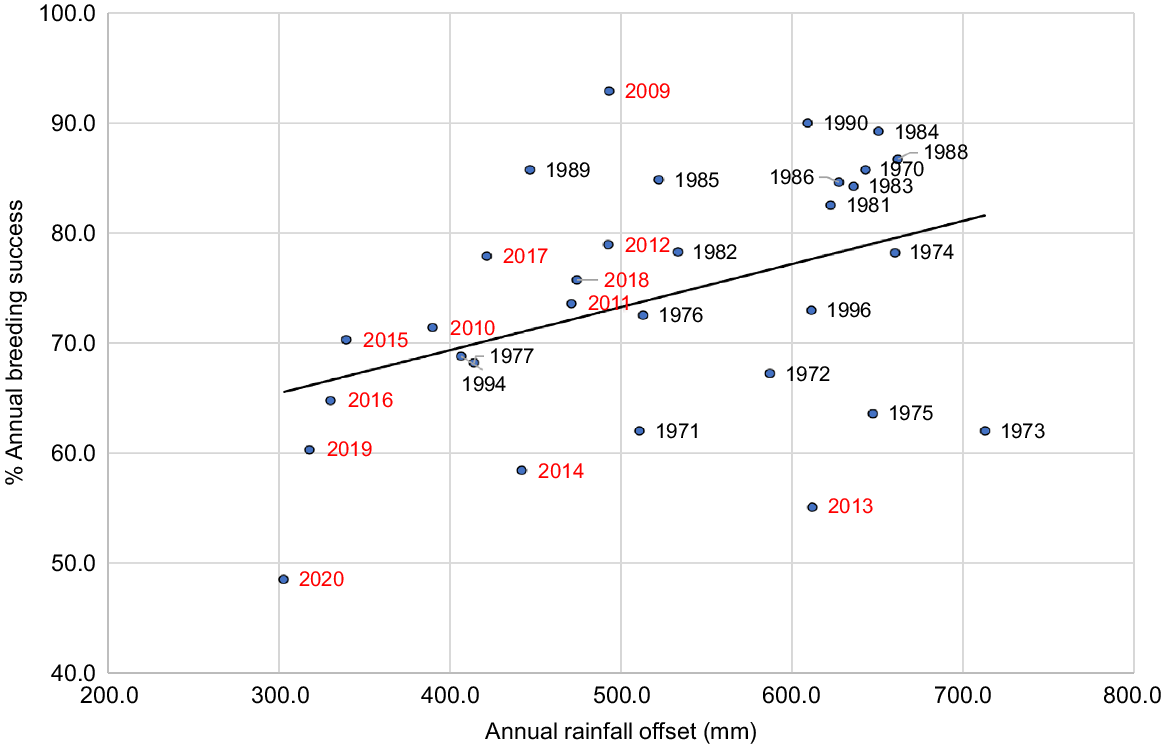
Over the period 1979 to 2020, there was a significant positive linear correlation between percentage annual breeding success and annual offset rainfall (Fig. 10), with an increase of 3.9% in breeding success for every additional 100 mm in annual offset rainfall. Including the rainfall of any 1 year and previous year in the analysis in the form of PC1 produces a lower R2-value (0.063 compared with 0.167), so the influence of rainfall on breeding success is confined to the year of breeding.
Fledgling survival and annual rainfall the first year after fledging
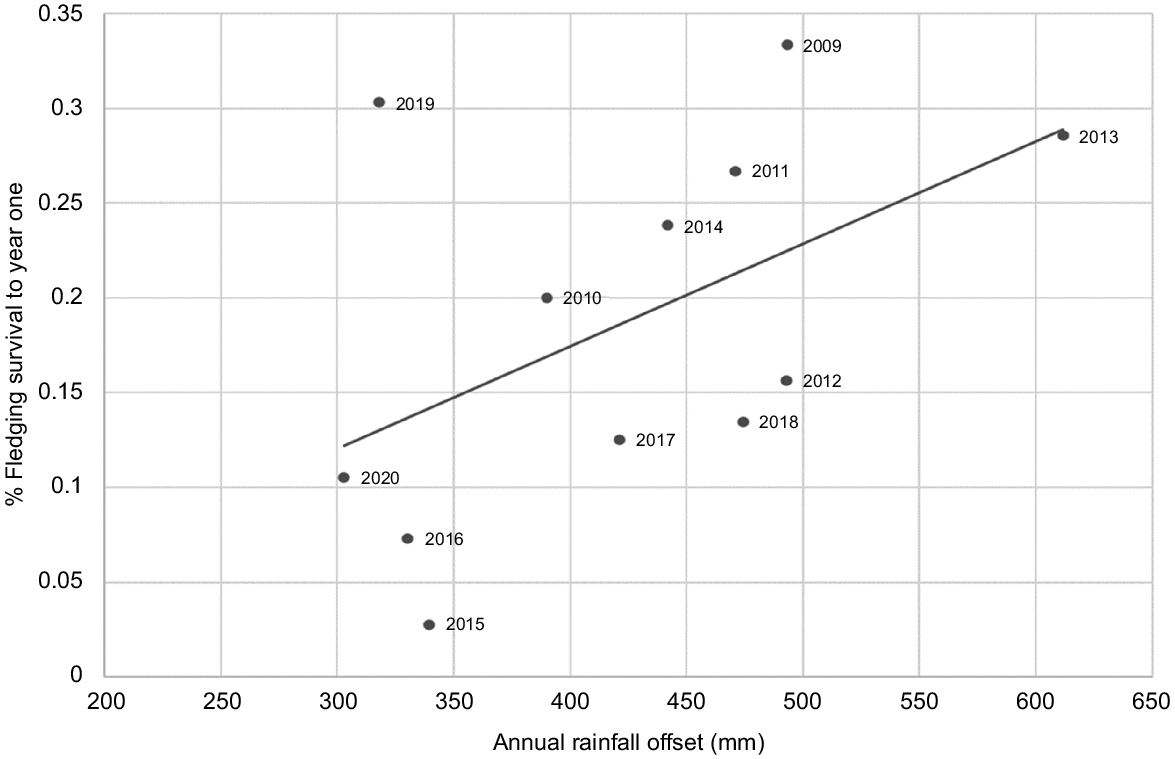
There is a positive linear trend between fledgling survival in their first year and annual offset rainfall (Fig. 11). An increase of 100 mm in annual offset rainfall results in an increased survival rate of between 5% and 6%. Adding a second year of offset rainfall by replacing the independent variable with PC1 increases the R2-value from 0.253 to 0.299, suggesting that the increase in food supply from having an extra year of vegetation growth from a year of previous higher rainfall provides a small advantage.
Annual survival rates for Carnaby’s Cockatoos
Between 1969 and 2023, 1251 nestlings were sexed, measured, and leg banded. Fledglings from Coomallo Creek have a sex ratio of 1:1 (637 females: 614 males). As females demonstrate greater natal site fidelity than males, only data from female fledglings were used for calculating survival. A random population sample of 130 Carnaby’s Cockatoos collected in Gnangara Pine Plantation (Fig. 1) over 4 months in 1970 revealed: 41 adult males; 41 adult females; 23 immature males; and 25 immature females (D. A. Saunders, unpubl. data). As the sex ratios of both adults and immature birds were 1:1, it is reasonable to assume males have the same survival rates as females throughout their lives.
Between 1977 and 1990, 86 banded female nestlings fledged at Coomallo Creek. Ten fledglings were known to have survived their first year; a mean survival rate of 0.116 ± 0.048 (s.e.) [range 0.059– 0.167; n (years) = 6]. Between 2009 and 2020, 367 female banded fledglings were produced. Fifty-nine were known to have survived their first year; a mean survival rate of 0.195 ± 0.028 (0.028–0.333; n = 12).
Of the nine fledgling females (1977–1990) that survived their first year, mean annual survival over the following 17 years was 0.918 ± 0.029 (0.667–1.00). Of the 59 fledgling females (2009–2020) that survived their first year, mean annual survival over the following 9 years was 0.874 ± 0.025 (0.667–0.906). Of the 20 females that were colour-banded as adults (1974–1989), mean annual survival over the 24 years following banding was 0.914 ± 0.021 (0.667–1.000).
Viability of the Coomallo Creek population
Using the life table analysis, an estimate of the lifetime reproduction potential based on the average number of surviving fledglings produced by a female over her life while allowing for female mortality over time was produced. The analysis used birth rates (average annual fledgling production/breeding female) and mortality rates (derived from survival of fledglings over their first year and survival of birds from their second year and older) to calculate the number of birds in each cohort of the population that survive to time (x). The life table calculated the following variables:
R0 = lifetime reproduction potential; a value of 1.0 indicates a stable population, <1.0 indicates decline, and >1.0 indicates increase;
LE = life expectancy; i.e. how much longer a female of a given age can be expected to live beyond its present age;
G = average age of the females in all cohorts; and
r = intrinsic rate of increase that may be used to predict the size of the population in the future. A value of 0 indicates a stable population, a negative value indicates one in decline and a positive value indicates one that is increasing.
We ran a life table analysis using a set of input parameters covering the range of values encountered during our investigation of the variables described above. There were three values for annual fledgling production/breeding pair after the birds begin breeding at Year 4 (minimum 0.456, average 0.678, maximum 0.953), three survival rates (0.028, 0.195, 0.333) for the first year after fledging, and two long-term survival rates after Year 1 (0.874, 0.914). Seven of the 18 combinations of parameters of life history attributes indicate that the Coomallo Creek population is either stable or capable of increasing (Table 1). Four of the six combinations involving the highest first year survival (0.333) with annual fledgling production/breeding pair of 0.678 or 0.953, and annual long-term survival of 0.874 or 0.914 result in a stable or increasing population. Two of the six combinations involving the average first year survival (0.195) with annual fledgling production/breeding pair of 0.678 or 0.953, and annual long-term survival of 0.914 result in a stable or potentially increasing population. All other combinations indicate a declining population. These results suggest that to be stable, the population should have a fledgling survival rate over their first year of around 20%, with an annual fledgling production per breeding pair of at least 67%, and a long-term survival rate of at least 90%. These six combinations have a predicted survival to age 30 years of between 6 and 24 females out of 1000 female fledglings; the initial number used in the model (Table 1).
| Survival rate Year 0–1 | Annual fledgling productivity per pair | Annual survival rate from Year 2 | R0 | G | LE | r | Survivors at Year 30 | |
|---|---|---|---|---|---|---|---|---|
| 0.028 | 0.456 | 0.914 | 0.103 | 12.016 | 0.803 | −0.189 | 2 | |
| 0.028 | 0.456 | 0.874 | 0.066 | 10.206 | 0.718 | <1 | ||
| 0.028 | 0.678 | 0.914 | 0.154 | 12.016 | 0.803 | −0.156 | 2 | |
| 0.028 | 0.678 | 0.874 | 0.098 | 10.206 | 0.718 | −0.228 | <1 | |
| 0.028 | 0.953 | 0.914 | 0.216 | 12.016 | 0.803 | −0.128 | 2 | |
| 0.028 | 0.953 | 0.874 | 0.216 | 12.016 | 0.803 | −0.128 | 2 | |
| 0.195 | 0.456 | 0.914 | 0.720 | 12.016 | 2.607 | −0.027 | 14 | |
| 0.195 | 0.456 | 0.874 | 0.459 | 10.206 | 2.018 | −0.076 | 3 | |
| 0.195 | 0.678 | 0.914 | 1.070 | 12.016 | 2.607 | 0.006 | 14 | |
| 0.195 | 0.678 | 0.874 | 0.682 | 10.206 | 2.018 | −0.037 | 3 | |
| 0.195 | 0.953 | 0.914 | 1.504 | 12.016 | 2.607 | 0.034 | 14 | |
| 0.195 | 0.953 | 0.874 | 0.959 | 10.206 | 2.018 | −0.004 | 3 | |
| 0.333 | 0.456 | 0.914 | 1.229 | 12.016 | 4.098 | 0.017 | 24 | |
| 0.333 | 0.456 | 0.874 | 0.783 | 10.206 | 3.093 | −0.024 | 6 | |
| 0.333 | 0.678 | 0.914 | 1.828 | 12.016 | 4.098 | 0.05 | 24 | |
| 0.333 | 0.678 | 0.874 | 1.165 | 10.206 | 3.093 | 0.015 | 6 | |
| 0.333 | 0.953 | 0.914 | 2.569 | 12.016 | 4.098 | 0.079 | 24 | |
| 0.333 | 0.953 | 0.874 | 1.637 | 10.206 | 3.093 | 0.048 | 6 |
R0, the lifetime reproduction potential; G, the average age of the females in all cohorts; LE, life expectancy (i.e. how much longer an individual of a given age can be expected to live beyond its present age); r, the intrinsic rate of increase that may be used to predict the size of the population in the future. R0 = 1.00 and r = 0 indicate the population is stable over time (values in bold). The analysis was run for 30 years after fledging, commencing with 1000 fledglings. Survivors at Year 30 are rounded to lower whole number.
Discussion
Likely impacts of changes in future rainfall on the viability of Carnaby’s Cockatoo
Three values for annual fledgling production per breeding pair were used in our sensitivity analysis. Annual fledgling production per breeding pair is a component of annual breeding success that is influenced by annual offset rainfall (Fig. 10). Three survival rates from fledging to Year 1 were applied in the analysis and there is a trend of increasing survival with increasing annual offset rainfall (Fig. 10). Our values for fledgling survival over the first year, and annual survival from Year 1 differ from those used by Williams et al. (2017) in their analysis of the impact of potential clearing of pine plantations and Kwongkan heath around the Perth metropolitan area on Carnaby’s Cockatoo. Their value for fledgling survival (0.264) to Year 1 is higher than our mean, but within the range used in our sensitivity analysis. Their value of annual survival from Year 1 (0.927) is higher than our value of 0.914, but was not derived from a detailed analysis of data from 1970 to 2023, as ours was.
The results of the sensitivity analysis (Table 1) suggest that under sustained low annual rainfall, with associated low breeding success and fledgling survival over their first year, the population would decline over time. Under higher rainfall with associated higher fledgling production per breeding pair, the population would be stable or increasing. Under average long-term rainfall, perhaps reflecting the period before the obvious impact of climate change on rainfall at the turn of the 21st century, the R0-value was close to 1.0, implying a stable population. The analysis suggests that some females survive up to age 30 years, and we assume they may still be breeding, as shown by records of females over 21 years old breeding (Saunders et al. 2024b).
There are uncertainties in using a life table analysis using our dataset. The two obvious ones are annual survival rates and the average age when females cease breeding. Data in Table 1 assumes that all female fledglings that survive their first year have been resighted/recovered. Assuming that some females have survived and moved elsewhere, have not returned to their natal area to breed, or have not been recorded elsewhere (Saunders et al. 2024a), we calculated R0-values for an additional 5%, 10%, 15%, and 20% of female fledglings that survived their first year, but not recorded subsequently (Table 2). R0-values increase with the increasing number of missing cockatoos, and all remain greater than 1.0 as might be expected since they effectively increase the number of survivors and the associated values of breeding success. However, the resultant variation is within the range in Table 1, so the increase in uncertainty is not great. Given that females demonstrate high natal site fidelity, we consider that R0-value associated with the 5% figure, or the assumption that all survivors have been identified, are the most likely ones and that the 5% value indicates a slightly increasing population.
| % of female fledglings that survived their first year, but were not resighted | 5 | 10 | 15 | 20 | |
|---|---|---|---|---|---|
| R0 | 1.124 | 1.177 | 1.232 | 1.284 |
Assumes annual survival rate of 0.914 from Year 2, annual fledgling productivity/pair of 0.678, and a survival rate for the first year of 0.195. The analysis was run for 30 years.
The average age when females cease breeding and thus, the number of years to include in the life table will also affect the value of R0 because it will affect the number of fledglings produced in total. The results of calculations in Table 1 are based on 30 years, which may be somewhat arbitrary, but within the range of likely values (Saunders et al. 2024b). However, we have also experimented with the impact of changing the age when females cease breeding by using the same parameters as the average condition, but varying the number of breeding years (Table 3). This suggests that the population is stable when females cease breeding from ages 20–25 years. However, life spans of at least 20 years and up to 35 years in wild birds are known (Saunders et al. 2024b), so the values we are using for life span are reasonable.
| Number of years females breed | 15 | 20 | 25 | 30 | |
|---|---|---|---|---|---|
| R0 | 0.696 | 0.919 | 1.011 | 1.070 |
The analysis was run for 30 years.
Effects of changes in temperature
There was a 43.9% increase in the number of days at Coomallo Creek with maxima ≥35°C from 1969–1996 to 2003–2023. There was a significant negative relationship between the number of days with maxima ≥35°C and annual average nestling condition index during the study (Fig. 8). The impact of increasing number of days with maxima ≥35°C is being exacerbated by the significant negative relationship between rainfall offset and the number of hot days (Fig. 5), meaning that in the years when plant productivity is reduced due to low rainfall, nestlings are at risk of both reduced food supplies (at least those derived from annual seeding species) and reduced provisioning from their parents due to an increased number of days with maxima ≥35°C.
Saunders et al. (2011a) reported on the impact of two extreme weather events on Carnaby’s Cockatoo. The first was the effect of 1 day of extreme heat (maximum 48.0°C following a maximum of 26.4°C the previous day) at Hopetoun (Fig. 1). A total of 208 cockatoos were found dead over the next 2 days. The region has a low human population density, so many more cockatoo deaths may have gone unrecorded. The second was a hail storm in the Perth metropolitan area, with the largest hail stones (up to 60 mm diameter) recorded falling in the central business district. Fifty-seven cockatoos were known to be killed outright and 24 so badly injured that they were taken into rehabilitation. Under a changing climate such extreme events may become more frequent and intense (https://www.swclimatechange.com.au/cb_pages/extreme_weather_events.php, accessed 29 July 2024), with detrimental effects on Carnaby’s Cockatoos. When the results reported here on the effect of rising temperature, the interaction between reduced rainfall and increased temperature are considered along with Saunders et al.’s (2011a) report on the impact of two extreme weather events on Carnaby’s Cockatoo, climatic conditions for Carnaby’s Cockatoos will become more challenging.
Conservation implications
We divided the Badgingarra rainfall data into two periods: (1) 1963–1996, representing pre- or early climate change impact on rainfall; and (2) 1997–2020, when drying became increasingly apparent (Fig. 2). Values of R0 and r for the first period (1993–1996) are 1.045 and 0.004, respectively, indicating a stable or slightly increasing population. While this may be the case using the survival and breeding success rates, it may be unrepresentative, given the effect of continuing clearing native vegetation on breeding and foraging habitat throughout the range of the species. Values of R0 and r for the later period (1997–2020) are 0.705 and −0.030, respectively, clearly representative of a declining population, other things being equal.
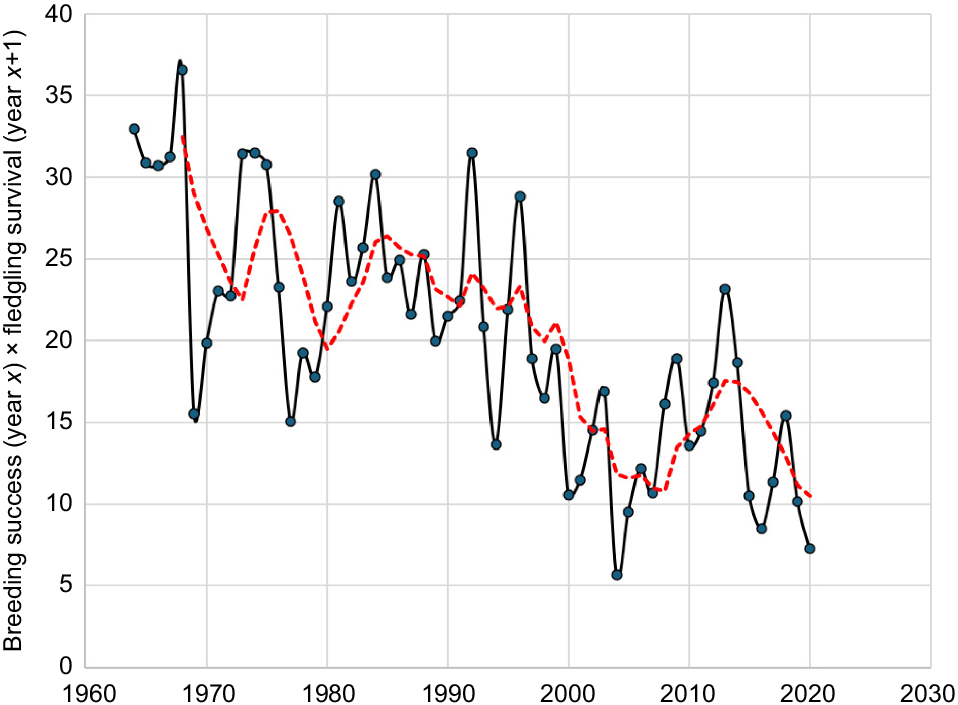
A further limitation of the life table analysis is that it uses breeding success and survival rates derived from an average rainfall value for the period of interest. Clearly, the annual rates vary through time. At this stage, we do not have enough long-term adult mortality data to attempt a life table or population viability analysis using time-variant rainfall. However, we can calculate the potential population recruitment for each year from the period 1963–2020 annual rainfall using breeding success and fledgling survival after the first year (Fig. 12). While there is year-to-year variability, data suggest that the observed reduction in rainfall is having an adverse effect on population recruitment, and if it continues, could lead to a population decline.
Potential Carnaby’s Cockatoo (Zanda latirostris) population recruitment for the period 1963–2020. Black line is annual data, red line shows a 5-year moving average. Recruitment is calculated from the Badgingarra Research Station rainfall data and the regression equations relating breeding success and fledgling survival after Year 1, presented in the captions of Fig. 10 and Fig. 11.
As availability of suitable natural nest hollows was limiting the breeding population at Coomallo Creek (Saunders et al. 2020, Saunders et al. 2023), total fledgling productivity was greatly aided by the provision of 82 artificial nest hollows from 2011 onwards, along with renovations to more than 100 derelict natural nest hollows (Fig. 13). The recent loss of natural hollows at Coomallo is mostly the product of bushfires and senescence and decay of old growth trees, rather than deliberate clearing. Without this intervention, the number of breeding females at Coomallo Creek would likely have been limited to older or aging females resulting in overall declining production. We observed that many breeding females after 2022 were younger birds, in some cases 3-year-old cockatoos (Dawson et al. 2013). With the benefit of these extra nest sites, the population was not limited by availability of nest sites, otherwise the observed decline in Fig. 12 might have been more precipitous.
(a) Repairs to derelict natural hollow. This hollow was unusable as the side of the floor was exposed. Before the breeding season of 2024 it was repaired with tin covering the formerly open sides. Carnaby’s Cockatoos nested in it immediately after it was repaired. (b) Artificial hollow attached to a wandoo with leafy canopy providing shade (photographs Rick Dawson).

Regional impact of climate change
The long-term mean annual rainfall (Fig. 14, upper map) and the rainfall anomaly in south-western Australia that has developed since 2011 are on the lower map of Fig. 14. The anomaly map shows the largest reduction in rainfall has occurred on the western coastal plain with the reduction in rainfall decreasing inland. In contrast, the south coastal region experienced only small reductions in total rainfall.
Mean annual rainfall for the period 1966–1995 (upper map) and rainfall anomaly for the period 2011–2020 minus mean annual rainfall 1996–1995 (lower map). The scales indicate data in mm including the contours on both maps. The highlighted and labelled contours identify the lower rainfall limits that separate breeding sites from the lower rainfall areas to the east. The large green circle identifies Coomallo Creek. Yellow circles identify Carnaby’s Cockatoo (Zanda latirostris) breeding sites currently monitored (Saunders et al. 2024a). White circles identify breeding sites identified in the Department of Biodiversity, Conservation and Attractions’ Carnaby’s Cockatoo database. These maps, Fig. 1 and Fig. 15 cover the Western Australian wheatbelt and adjacent coastal regions.
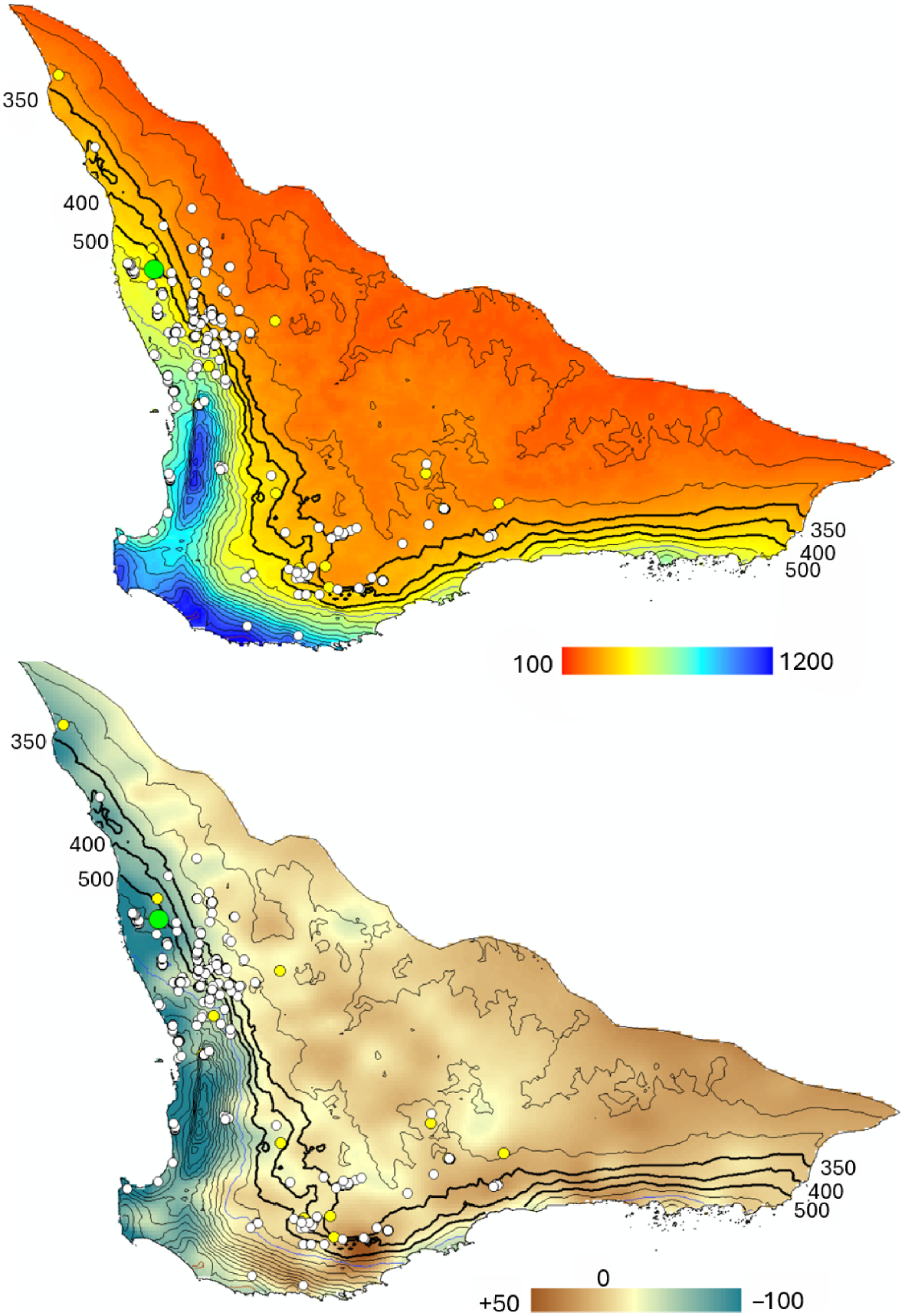
Known breeding site locations are in Fig. 14. Most of the sites that are monitored are located where the long-term rainfall exceeded 350 mm, although a few are located within the 300–350 mm rainfall zone. Life table analysis with a long-term survival rate of 0.874 indicates that rainfall of less than 550 mm yields an R0-value of less than 1.0, suggesting long-term population decline. A long-term survival rate of 0.914 produces an R0-value of about 1.0 for a long-term mean annual rainfall of 400 mm, and a stable or potentially increasing population for rainfalls above that.
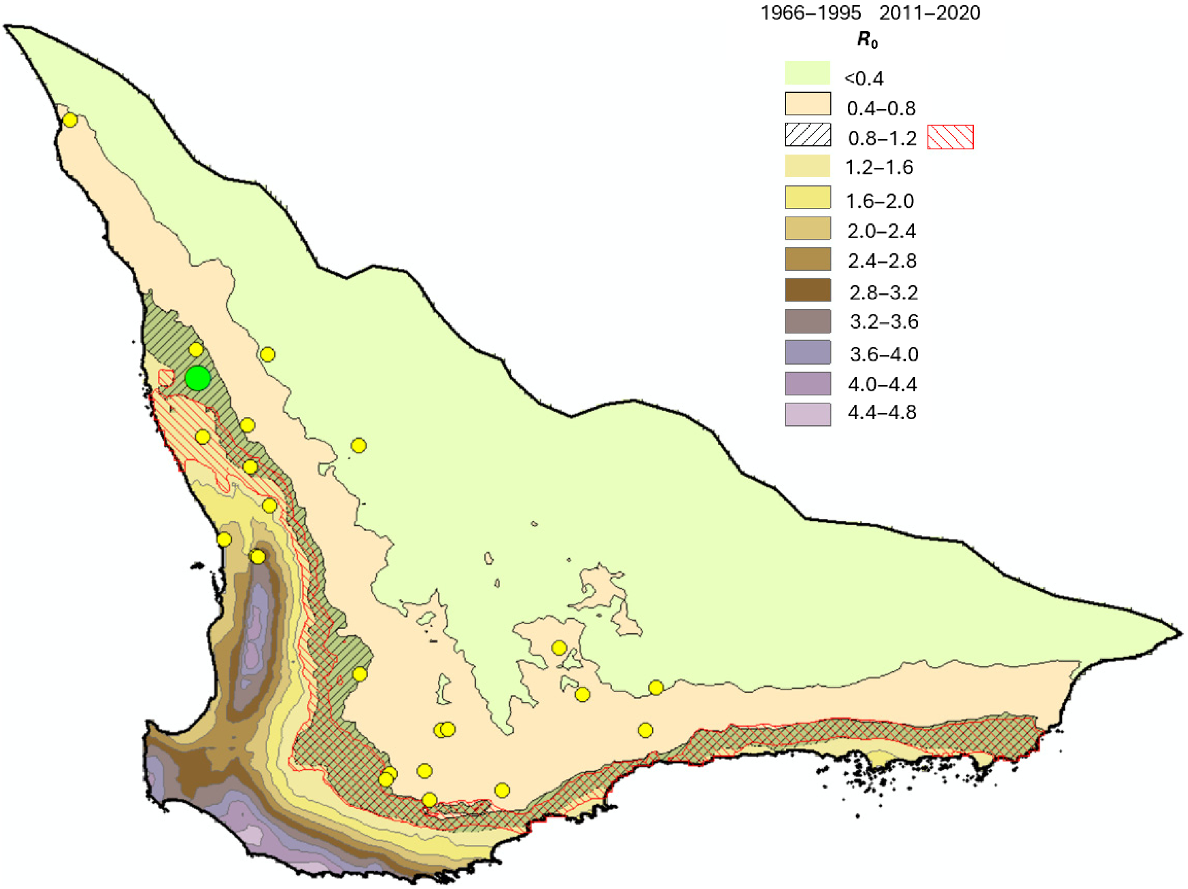
The relationship between R0 and rainfall is slightly non-linear, but still makes it possible to produce gridded maps of R0 for different life table parameter values (Fig. 15) for a baseline rainfall period from 1966 to 1995 and a long-term survival rate of 0.914. The area with rightwards sloping diagonal lines is identified specifically since this represents a possible error range (0.8–1.2) around a value of R0 = 1.0 with the range selected given the level of accuracy of the various regression equations used to construct the life table. The area with red leftward sloping diagonal lines produces an equivalent R0 gridded map for the period 2011–2020 representing the period with a clear rainfall decrease and shows how far the 0.8–1.2 R0 region moves into the previously, but still higher rainfall zone to the west. The greatest shift into the higher rainfall zone is in the northwest of the species’ breeding range; Coomallo Creek is in this zone. Changes in the R0 zone location in the southeast coastal region are minimal.
R0 (lifetime reproduction potential) values for the periods 1966–1995 and 2011–2020 for a Carnaby’s Cockatoo (Zanda latirostris) long-term survival rate 0.914. The diagonal lines identify the 0.8–1.2 values for each period. The large green circle identifies Coomallo Creek. Yellow circles identify breeding sites currently monitored or that have been monitored in the past (Saunders et al. 2024a).
Conclusion
We offer the following guidance for managing Carnaby’s Cockatoo populations in future. There are few opportunities to influence both Year 1 and the long-term survival rates of Carnaby’s Cockatoos. The principal opportunities are vegetation management and addressing the threat posed by collisions with vehicles at sites where cockatoos are drawn to feed on roadside vegetation, spilt grain, or pooled water sources along major transport routes (Saunders et al. 2011a; Coyle 2021). Addressing both will be challenging, however they would have significant effects on the survival of the species across its range.
Preservation of breeding and foraging habitat in the wheatbelt is critical because even under long-term average rainfall, life table analysis points to future population decline. Saunders et al. (1985, 2003, 2014b) demonstrated nett loss of hollow-bearing trees in the Western Australian wheatbelt with little to no regeneration. Given the enormous amount of habitat lost to date for agriculture, mining, infrastructure development, and urban development, no further loss of breeding or foraging habitat should be permitted. In planning for regeneration, planning must be done with a time-frame of centuries, not decades. Trees must be large and old enough to develop hollows suitable for Carnaby’s Cockatoos and consideration should also be given to ensure seed provenance of tree species used for revegetation will survive under a drier and hotter climate.
Breeding success rates can also be enhanced by provision of artificial nesting sites (Saunders et al. 2020) and repair of derelict natural hollows (Saunders et al. 2023). However, artificial hollows must be attached to trees with leafy canopies, providing adequate shade to the hollow (Fig. 13b) to offset the small increase in temperatures experienced inside artificial nest hollows (Saunders et al. 2020). Saunders et al. (2020) showed that artificial hollows without shade may be ‘death traps’ in extreme hot weather. The greatest conservation gain for Carnaby’s Cockatoos would be to concentrate revegetation in locations where R0-values are currently close to 1.0, since this is the zone where negative values are more easily moved into the positive.
It remains to be seen whether life table analysis based solely on rainfall and rainfall changes proves useful for conservation planning under a changing climate, but it should help when developing regional strategies. It is likely that our results, particularly those relating to population reproduction and mortality are of wider application since they come from a situation where climate change and associated biological stress are well advanced. They should provide parallels with those species where food supply is limited by reducing rainfall and increasing temperature, where foraging distance is limited during the breeding season, and where annual reproductive output is low. The low mortality and longevity of adult cockatoos does provide a buffer, but the climate effect on other species may be more severe.
Dedication
This paper is dedicated to Emeritus Professor Paul Ehrlich, pioneer population biologist and conservation biologist, who was a major factor in DAS continuing this study of Carnaby’s Cockatoos for over 50 years.
Conflicts of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
Denis A. Saunders: conceptualisation, methodology, formal analysis, investigation, writing – original draft, writing – review and editing. Peter R. Mawson: conceptualisation, methodology, formal analysis, investigation, writing – original draft, writing – review and editing. Rick Dawson: conceptualisation, methodology, investigation, writing – review and editing. Geoffrey Pickup: conceptualisation, methodology, formal analysis, spatial and climate change analysis, writing – original draft, writing – review and editing.
Acknowledgements
We are grateful to John Ingram for technical support 1972–1996; the Hayes, McAlpine, Paish, and Raffan families for supporting this research on their properties; the Raffan family for providing accommodation during the field work; Brooke Richards for technical support in 2020–2023; Dr Manda Page and Dr Juanita Renwick for administrative support; Claire Sands for provision of the Department of Biodiversity, Conservation and Attractions’ Carnaby’s Cockatoo database; Professor Lesley Hughes and Professor Stuart Marsden for critical comment on a pre-submission draft of our manuscript; and Emeritus Professor Mike Calver, Dr Hugh Finn, and an anonymous reviewer for critical comments on a post-submission draft of the manuscript.
References
Bureau of Meteorology (2024) Previous droughts. Available at http://www.bom.gov.au/climate/drought/knowledge-centre/previous-droughts.shtml [Accessed 5 August 2024]
Dawson R, Saunders DA (2014) Individually marked wild Carnaby’s Cockatoos: a challenge and opportunity for keen photographers. Western Wildlife 18, 4-5.
| Google Scholar |
Dawson R, Saunders DA, Lipianin E, Fossey M (2013) Young-age breeding by a female Carnaby’s cockatoo. Western Australian Naturalist 29, 63-65.
| Google Scholar |
Government of Western Australia (2022) Western Australian climate projections summary. Available at https://www.wa.gov.au/system/files/2022-01/Western_Australian_Climate_Projections_Summary.pdf.
Hopkins AJM, Smith GT, Saunders DA (2024) Introduction to the special issue of the natural history of Two Peoples Bay Nature Reserve, Western Australia. Pacific Conservation Biology 30, PC24023.
| Crossref | Google Scholar |
IPCC (2023) Summary for Policymakers. In ‘Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change’. (Eds Core Writing Team, H Lee, J Romero) pp. 1–34. (IPCC: Geneva, Switzerland) 10.59327/IPCC/AR6-9789291691647.001
Le Souëf AT, Bruce M, Barbosa A, Shephard JM, Mawson PR, Dawson R, Saunders DA, Warren KS (2024) Health parameters for wild Carnaby’s cockatoo (Zanda latirostris) nestlings in Western Australia: results of a long-term study. Conservation Physiology 12(1), coae005.
| Crossref | Google Scholar |
Lunney D, Dickman CR, Predavec M (2018) The critical value of long-term field studies and datasets: An editorial perspective. Australian Zoologist 39(4), 559-567.
| Crossref | Google Scholar |
NCSS (2024) Statistical Software. Data Analysis & Graphics. NCSS, LLC, Kaysville, Utah, USA. Available at ncss.com/software/ncss
Saunders DA (1974) Subspeciation in the White-tailed Black Cockatoo, Calyptorhynchus baudinii, in Western Australia. Australian Wildlife Research 1, 55-69.
| Crossref | Google Scholar |
Saunders DA (1980) Food and movements of the short-billed form of the White-tailed Black Cockatoo. Australian Wildlife Research 7, 257-269.
| Crossref | Google Scholar |
Saunders DA (1982) The breeding behaviour and biology of the short-billed form of the White-tailed Black Cockatoo Calyptorhynchus funereus. Ibis 124, 422-455.
| Crossref | Google Scholar |
Saunders DA (1988) Patagial tags: do the benefits for the research worker outweigh the risks to the animal? Australian Wildlife Research 15, 565-569.
| Crossref | Google Scholar |
Saunders DA (1990) Problems of survival in an extensively cultivated landscape: the case of Carnaby’s cockatoo Calyptorhynchus funereus latirostris. Biological Conservation 54, 277-290.
| Crossref | Google Scholar |
Saunders DA, Dawson R (2018) Cumulative learnings and conservation implications of a long-term study of the endangered Carnaby’s cockatoo Calyptorhynchus latirostris. Australian Zoologist 39, 591-609.
| Crossref | Google Scholar |
Saunders DA, Ingram JA (1998) Twenty-eight years of monitoring a breeding population of Carnaby’s cockatoo. Pacific Conservation Biology 4, 261-270.
| Crossref | Google Scholar |
Saunders DA, Pickup G (2023) A review of the taxonomy and distribution of Australia’s endemic Calyptorhynchinae black cockatoos. Australian Zoologist 43, 145-191.
| Crossref | Google Scholar |
Saunders DA, Rowley I, Smith GT (1985) The effects of clearing for agriculture on the distribution of cockatoos in the Southwest of Western Australia. In ‘Birds of eucalypt forests and woodlands: ecology, conservation, management’. (Eds A Keast, HF Recher, H Ford, D Saunders) pp. 309–321. (Surrey Beatty & Sons: Chipping Norton, NSW, Australia)
Saunders DA, Smith GT, Ingram JA, Forrester RI (2003) Changes in a remnant of salmon gum Eucalyptus salmonophloia and York gum E. loxophleba woodland, 1978 to 1997. Implications for woodland conservation in the wheat-sheep regions of Australia. Biological Conservation 110, 245-256.
| Crossref | Google Scholar |
Saunders DA, Mawson P, Dawson R (2011a) The impact of two extreme weather events and other causes of death on Carnaby’s Black Cockatoo: a promise of things to come for a threatened species? Pacific Conservation Biology 17, 141-148.
| Crossref | Google Scholar |
Saunders DA, Dawson R, Mawson P (2011b) Photographic identification of bands confirms age of breeding Carnaby’s Black Cockatoo Calyptorhynchus latirostris. Corella 35, 52-54.
| Google Scholar |
Saunders DA, Wintle BA, Mawson PR, Dawson R (2013) Egg-laying and rainfall synchrony in an endangered bird species: implications for conservation in a changing climate. Biological Conservation 161, 1-9.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R (2014a) One fledgling or two in the endangered Carnaby’s Cockatoo (Calyptorhynchus latirostris): a strategy for survival or legacy from a bygone era? Conservation Physiology 2, cou001.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R (2014b) Use of tree hollows by Carnaby’s Cockatoo and the fate of large hollow-bearing trees at Coomallo Creek, Western Australia 1969–2013. Biological Conservation 177, 185-193.
| Crossref | Google Scholar |
Saunders DA, Dawson R, Nicholls N (2015) Aging nestling Carnaby’s cockatoo, Calyptorhynchus latirostris, and estimating the timing and length of the breeding season. Nature Conservation 12, 27-42.
| Crossref | Google Scholar |
Saunders DA, White NE, Dawson R, Mawson PR (2018) Breeding site fidelity, and breeding pair infidelity in the endangered Carnaby’s cockatoo Calyptorhynchus latirostris. Nature Conservation 27, 59-74.
| Crossref | Google Scholar |
Saunders DA, Dawson R, Mawson PR, Nicholls AO (2019) Factors affecting nestling condition and timing of egg-laying in the endangered Carnaby’s cockatoo Calyptorhynchus latirostris. Pacific Conservation Biology 26, 22-34.
| Crossref | Google Scholar |
Saunders DA, Dawson R, Mawson PR, Cunningham RB (2020) Artificial hollows provide an effective short-term solution to the loss of natural nesting hollows for Carnaby’s cockatoo Calyptorhynchus latirostris. Biological Conservation 245, 108556.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R, Johnstone RE, Kirkby T, Warren K, Shepherd J, Rycken SJE, Stock WD, Williams MR, Yates CJ, Peck A, Barrett GW, Stokes V, Craig M, Burbidge AH, Bamford M, Garnett ST (2021) Carnaby’s Black-Cockatoo Zanda latirostris. In ‘Action Plan for Australian Birds 2020’. (Eds ST Garnett, GB Baker) pp. 402–407. (CSIRO Publishing: Melbourne)
Saunders D, Dawson R, Mawson PR (2023) Artificial nesting hollows for the conservation of Carnaby’s Cockatoo Calyptorhynchus latirostris: definitely not a case of erect and forget. Pacific Conservation Biology 29, 119-129.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R, Beswick H, Pickup G, Usher K (2024a) Movements of adult and fledgling Carnaby’s cockatoos (Zanda latirostris Carnaby, 1948) from eleven breeding areas throughout their range. Pacific Conservation Biology 30, PC24042.
| Crossref | Google Scholar |
Saunders DA, Mawson PR, Dawson R (2024b) Longevity in Carnaby’s Cockatoo (Zanda latirostris) Carnaby, 1948. Pacific Conservation Biology 30, PC24066.
| Crossref | Google Scholar |
Shoemaker K (undated) Lab 1 Excel demo. Life Tables. NRES 470/670. Available at https://www.youtube.com/watch?v=xv1CMsoKd0s [accessed 27 June 2024]
Williams MR, Yates CJ, Saunders DA, Dawson R, Barrett GW (2017) Combined demographic and resource models quantify the effects of potential land-use change on the endangered Carnaby’s cockatoo (Calyptorhynchus latirostris). Biological Conservation 210, 8-15.
| Crossref | Google Scholar |