Catch, bycatch, and mitigation options for endangered sharks in data poor fisheries: a case study on pelagic thresher sharks (Alopias pelagicus) in the eastern Indian Ocean
Muhammad Ichsan A B * , Shoimatul Ula C , Hollie Booth D ,
A B * , Shoimatul Ula C , Hollie Booth D , A
B
C
D
E
F
Abstract
Endangered pelagic thresher sharks (Alopias pelagicus) are facing high fishing pressure in many countries. As the world’s largest shark fishing nation, with particularly high catch rates of thresher sharks, the impact of Indonesian fisheries has become a global priority for thresher shark conservation. Therefore, baseline data is needed to inform management and implement international agreements.
This research provides data and management recommendations for data-poor targeted shark fishing in Western Indonesia, where thresher sharks are regularly caught.
Daily landings data was recorded from June 2019 to December 2020 in Southwest Aceh and analysed to explore catch patterns, risk factors for thresher sharks, and potential mitigation options.
Twenty-five wooden vessels of <30 gross tonnage target sharks in the Indian Ocean. We recorded a total of 109 pelagic thresher individuals during the study period, with a female-skewed sex ratio (1 female:0.39 male). Almost all pelagic threshers were caught by the bottom longline.
Based on this data, we offer some potential management measures to reduce fisheries impacts on pelagic thresher sharks by applying a simple mitigation hierarchy framework.
These results provide fisheries management recommendations, including effort limits and gear modifications.
Keywords: Aceh, conservation, elasmobranchs, endangered species, fisheries management, Indian Ocean, Indonesia, pelagic thresher shark.
Introduction
In recent decades, the scale of global fisheries has put high pressure on marine species both in industrial and small-scale fisheries. In developing countries, where the majority of fishers are categorised as small-scale fisheries, these fisheries contribute around half of global fish supplies (Food and Agriculture Organization 2020; Sari et al. 2021). Due to its scale, the impact of small-scale fisheries on shark and ray populations is considered to be significant either as a target or as a secondary catch (Davidson and Dulvy 2017; Tiralongo et al. 2018; Di Lorenzo et al. 2022).
Sharks, rays, and their cartilaginous relatives (Class Chondrichthyes) are among the world’s most threatened marine species groups, with an estimated one-third of all species threatened with extinction (Dulvy et al. 2021). Within this group, thresher sharks (Family Alopiidae) are of particular conservation priority (Dulvy et al. 2008; Aalbers et al. 2010; Oliver et al. 2013). Thresher sharks comprise three species within the family Alopiidae: common thresher shark (Alopias vulpinus), bigeye thresher shark (Alopias supercilious) and pelagic thresher shark (Alopias pelagicus), which are all epi- and mesopelagic sharks occupying tropical and temperate seas (Compagno 1984).
Thresher sharks are circumglobal and highly migratory species, which means they are susceptible to exploitation by coastal and high-seas fisheries. In particular, they are commonly found throughout the Indian Ocean, including in the Exclusive Economic Zone (EEZ) of Indonesia, the world’s largest shark fishing nation (Dent and Clarke 2015). In this region, the range of thresher sharks overlaps with many under-reported and unregulated gillnet and longline fisheries (Rigby et al. 2019; Ichsan et al. 2020). Thresher sharks are one of Indonesia’s most frequently caught groups of shark species, with the highest catches recorded in 2002–2004, with around 50,000–60,000 tonnes annually (Fahmi and Dharmadi 2013). Hotspots of targeted shark fishing occur throughout Indonesia, such as in Western Aceh, Northern Java, West Nusa Tenggara, East Nusa Tenggara and North Maluku (Booth et al. 2018).
The biological and ecological characteristics of pelagic thresher sharks (A. pelagicus) are poorly understood; in particular, there are few studies on age, growth, and reproductive characteristics, limiting science-based fisheries management for this vulnerable species. The pelagic thresher attains sizes of up to 365 cm total length (TL) and reaches sexual maturity at the age of 7–8 years for males and 8–9.2 years for females or a TL of 250–280 cm for both sexes (Compagno 1984; Liu et al. 1999). Furthermore, they have small litter sizes, usually giving birth to two pups per year (Otake and Mizue 1981; Compagno 1984; Liu et al. 1999). As a result, the intrinsic rate of population increase is very low, estimated at 2–4% (Smith et al. 1998; Dulvy et al. 2008). These life-history traits make the pelagic thresher particularly vulnerable to over-exploitation, with the species recently classified as endangered according to the IUCN Red List of threatened species (Rigby et al. 2019). However, additional detailed information regarding population structure, exploitation rates, and areas that support important biological and ecological processes in Indonesia and the Indian Ocean is needed to improve management.
In 2012, the Indonesian government incorporated Indian Ocean Tuna Commission (IOTC) Resolution 10/12 into a Ministerial Regulation, which regulates thresher shark catches on the high seas (Kementerian Kelautan dan Perikanan Republik Indonesia 2012; The Indian Ocean Tuna Commission (IOTC) 2012). Indonesia is also a party to the Convention on International Trade of Endangered Species (CITES), which lists thresher sharks (family Alopiidae) in Appendix II. Thus, the government must ensure that any international trade in thresher sharks is sustainable. This requirement led to the regulation of international trade; however, within this regulatory framework, thresher sharks can still be caught, retained, traded, and utilised within the country, and thresher sharks remain one of the most commonly caught shark species in Indonesia (Fahmi and Dharmadi 2013; Kementerian Kelautan dan Perikanan Republik Indonesia 2018). Most management measures have focused on international trade (e.g. CITES) and high-seas fisheries (e.g. IOTC), but small-scale shark fisheries remain challenging and neglected (Booth et al. 2020). As a result, there is a gap in practical fisheries management measures for target and bycatch fish species, which is urgently needed to halt population declines and move pelagic thresher sharks on a pathway to recovery.
This research aims to fill this data and management gap by (1) documenting pelagic thresher shark catches in data-poor targeted shark fisheries in Southwest Aceh and operational fishing characteristics that are associated with these catches; (2) providing information on the biology of pelagic thresher sharks in the eastern Indian Ocean, and (3) using this baseline information to outline some practical management recommendations for reducing thresher shark fishing mortality in fisheries in the Indian Ocean, based on a mitigation hierarchy framework (Booth et al. 2019; Gupta et al. 2020).
Materials and methods
Study area
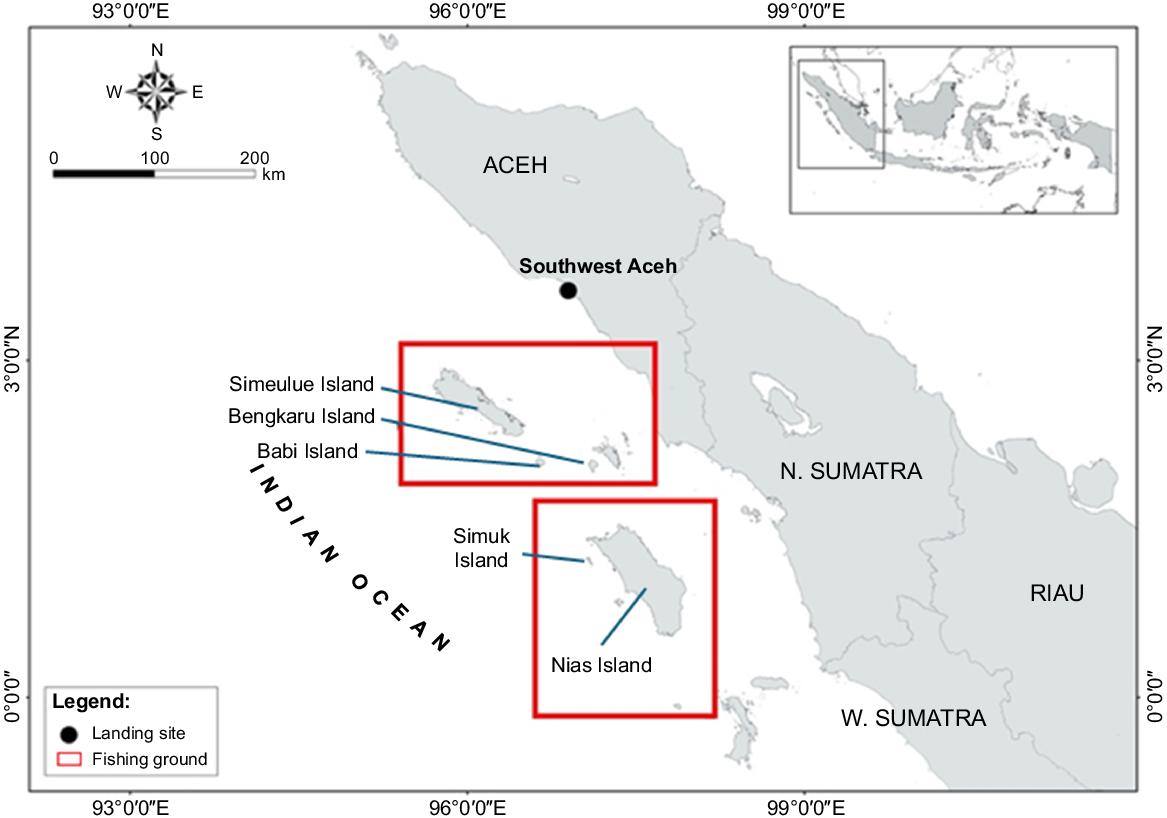
Southwest Aceh is a district in Aceh Province located at 3°34′24″ to 4°05′37″N and 96°34′57″ to 97°09′19″E. The Aceh province has one of the largest shark fisheries in Indonesia (Simeon et al. 2020). Like many shark fisheries hotspots in Indonesia, shark fisheries in Aceh started as early as the 1970s when the global fin market was emerging (Pacoureau et al. 2021). The Southwest Aceh district has one of the largest shark fisheries in Aceh, with more than 25 shark-specialised longline vessels operating throughout the year based at two sites in Southwest Aceh: Ujong Serangga fishing port and Lhok Pawoh community landing site (Booth et al. 2018; Simeon et al. 2020) (Fig. 1).
Data collection
We monitored fishing activities and landings 6 days a week from June 2019 to December 2020. The operational fishing characteristics associated with the catch were recorded by interviewing each boat owner, captain, and crew member and recording the vessel’s name, gear type, and fishing ground. For shark landing information, we recorded the length, sex, and stage of maturity for all pelagic thresher shark landing sites (Nugraha et al. 2020). Sex was determined by the presence of claspers in males or the absence of claspers in females. The degree of calcification and length of claspers determined maturity levels for males. Males were divided into three reproductive stages, immature: short and noncalcified clasper, subadult: long and semicalcified claspers and mature: fully calcified claspers. For females, due to rapid processing of landed sharks, it was not possible to conduct internal examinations of reproduction organs and we only recorded the presence of embryos opportunistically in some processed specimens. Prior to biological and fisheries monitoring we conducted interviews with key actors in the fishery using snowball sampling with shark fishers (n = 17), traders (n = 13) and local leaders (n = 10). During interviews we collected general information about shark fisheries, particularly for thresher sharks including fishing method (vessel type, fishing ground, fishing gear) and socio-economic aspects (price, product types and market destination).
Analysis
We summarised the characteristics of shark fisheries, including the fishing gear used, the fishing grounds and seasonality, and their catch utilisation. In addition, the Catch Per Unit Effort (CPUE) estimate for pelagic thresher sharks was based on the total catch per soaking hour and trip with soak time estimated by fishers.
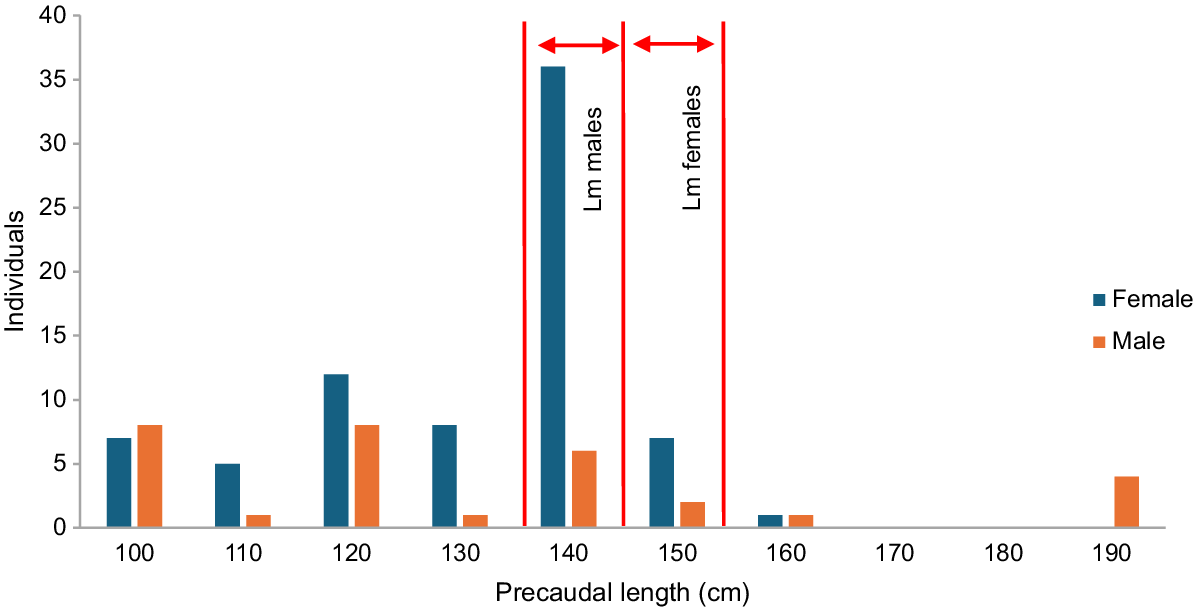
We analysed size distribution for both sexes and plotted size–frequency distributions. We tested for a skewed sex ratio using a chi-squared test with a null hypothesis of a 1:1 sex ratio (Zar 2010). Estimation of length at first maturity (Lm) was compared to a previous study on pelagic threshers landed in Taiwanese waters, which states that Lm is 140–145 cm precaudal length, PL (8 years) for males and 145–150 cm PL (8 years) for females (Liu et al. 1999).
Finally, we contextualised the catch and biological data alongside data on fishing effort to examine potential management options for reducing the mortality of pelagic thresher sharks based on a mitigation hierarchy (MH) framework (Booth et al. 2020). The MH framework is a precautionary approach for reducing the risks to sharks in fisheries, which, if fully applied, can halt and reverse declines in fishery-affected populations. The MH structures management options into four steps: (1) avoid interactions as far as possible (e.g. through fishery closures); (2) minimise capture as far as possible (e.g. through gear modifications) if the interactions cannot be avoided; (3) remediate capture that cannot be avoided or minimised (e.g. through live release protocols); (4) compensate for residual mortality (e.g. through population restoration). We use these four steps to structure a range of management options for pelagic thresher sharks and offer a qualitative assessment of their potential effectiveness and feasibility (Business and Biodiversity Offsets Programme 2012; Booth et al. 2020).
Ethics statement
This work was conducted under a Memorandum of Understanding (MoU) and Technical Cooperation Agreement (TCA) between the Wildlife Conservation Society (WCS) and the Ministry of Environment and Forestry (MoEF), Ministry of Marine Affairs and Fisheries (MMAF) and the Marine and Fisheries Agency (MFA) of Aceh Province and supported by ZSL EDGE of Existence Fellowship Programme and Fondation Segré. Due to this MoU and TCA, no specific research permit was required. We collected data by measuring sharks that were already caught, dead, and landed by fishers in Southwest Aceh, with no incentives, compensation, or specific requests for killing sharks for this study. WCS participates in the Conservation Initiative on Human Rights and the rules and guidelines of our Internal Review Board ensures that any research protects the rights of human subjects. We did not apply for an institutional review board permit for this study because our study design focused on collecting fish and fisheries data as opposed to personal socio-economic data. The focus group discussions and interviews were conducted to obtain early scoping information about fishing practices. We used a protocol developed by Ministry Marine Affairs and Fisheries (MMAF) for data collection.
Results
Fisheries characteristics
Of all vessels that landed sharks and rays, we recorded 31 fishing trips from 17 vessels that landed pelagic threshers. Of these 17 vessels that landed pelagic thresher sharks, 12 are shark vessels using bottom longlines. The fishing gear comprised the mainline, branch lines, and 150– 250 hooks. This fishing gear is operated by lowering baited hooks horizontally along the mainline, which is left submerged at about 30 metres deep for several hours. The main target of fishing are requiem shark species such as silky and spot-tail shark (Carcharhinus falciformis and Carcharhinus sorrah), however, thresher sharks are sometimes caught since these species share the same habitat. Fisher used skipjack (Katsuwonus pelamis) or pilchard (Sardinella sp.) as bait. Fishers typically spend 20–25 days at sea with a crew of three to six people. The fishing season is divided into low (January–May) and high season (June–November). According to fishers, the sea condition is calmer in high season, and more boats depart with shorter fishing days, deploying their gear twice a day but with shorter soaking hours.
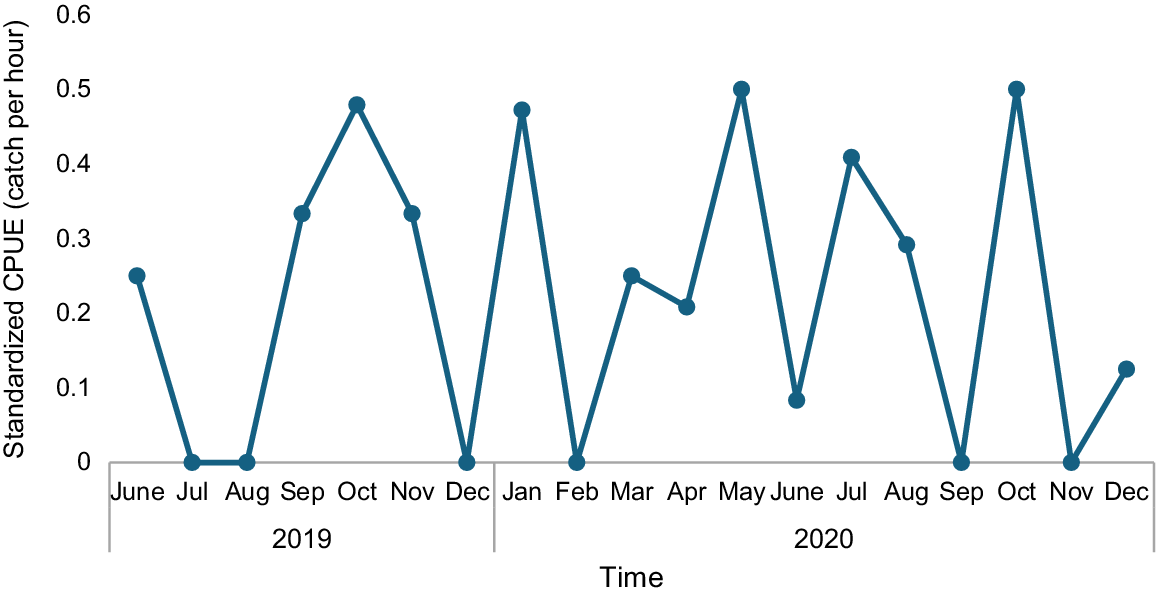
During the study, 109 pelagic thresher sharks were recorded as caught and landed. Of all pelagic threshers landed, 94% were caught using bottom longline, with the rest by surface longline, gillnet, handline, and purse seine as bycatch in the reef and pelagic fisheries. The average catch rate for pelagic thresher catches using longline was 0.22 (s.d. ±0.19) individuals per soaking hour or about 3–4 individuals per trip. Monthly CPUE fluctuated widely throughout the year, with no seasonal trend (Fig. 2). Most pelagic thresher sharks (94%) are caught around small islands in Western Sumatra in two different provinces: Simuk Island and Nias Island in North Sumatra Province and Bengkaru and Babi Island in Aceh Province (Fig. 1). In this fishing ground, the bottom longline is the only fishing gear used to catch pelagic thresher sharks and not used anywhere else.
According to interviews shark meat is made into shark curry and served as a local delicacy, whereas they sent fins to big cities such as Medan and Banda Aceh. The fins are priced higher per kilogram than other fisheries’ products. However, for thresher sharks, the fin price is relatively low (IDR70,000 or around USD5 per kg) compared to the fins of main target species (IDR400,000 or around USD26 per kg), whereas the value of meat (between IDR10,000 and 28,000 or between USD0.7 and 2 per kg) and other body parts is similar to with other shark species. Other than fins and meat, utilised body parts include cartilage, skin, and liver oil.
Biological information
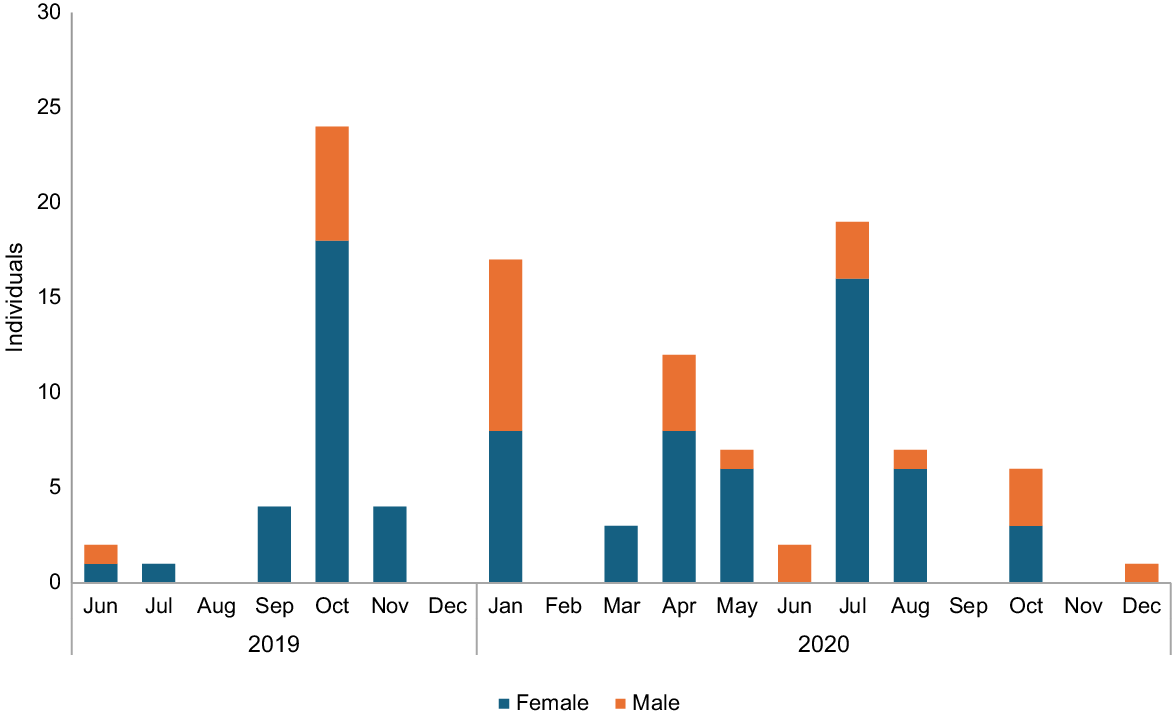
Of the 109 pelagic thresher sharks we recorded, 31 were males, and 78 were females, with a statistically significant skew in the sex ratio of 1:0.39 females:males (X2 = 3.84, P < 0.05). The results show no distinctive trends in the average monthly landing (Fig. 3).
Average monthly landing of pelagic thresher sharks (individual per month). Sex ratio of 1:0.39 for females:males (X2 = 3.84, P < 0.05).
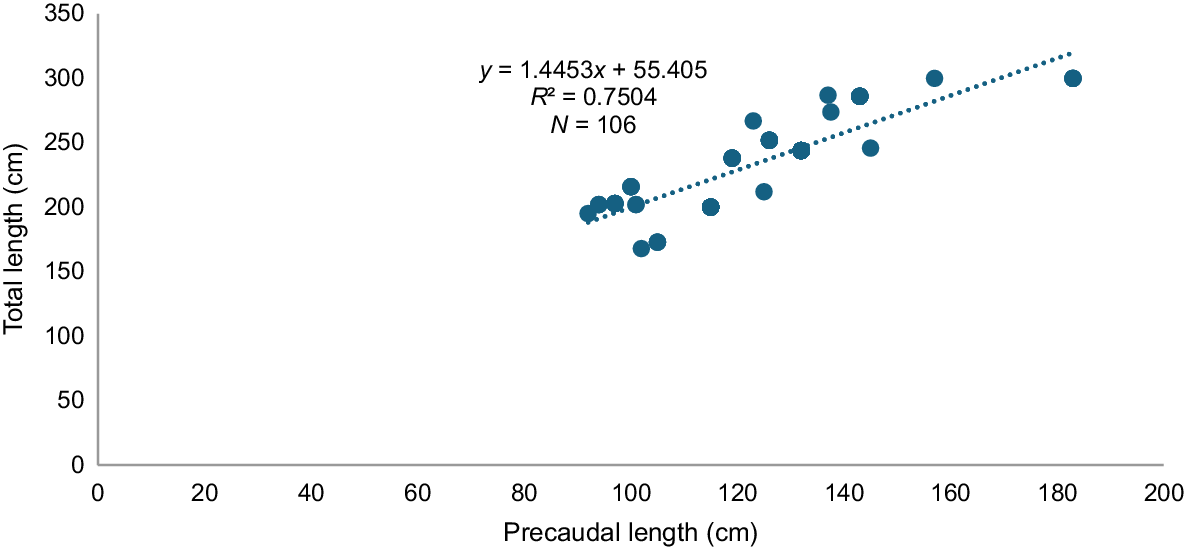
We present the correlation between total length (TL) and precaudal length (PL), which shows a linear trend (Fig. 4). PL is a length from the tip of the snout to the base of the tail. We are using PL as the primary measure of body length due to its higher accuracy for thresher sharks, as their tails are regularly damaged or removed. Sizes ranged from 63–183 cm PL. Male PL varied from 97–183 cm, with a mode of 132 cm, and female PL ranged from 63–152 cm, with a mode of 132 cm. Most individuals are immature, with very few individuals of mature length of either sexes represented in the landings (Fig. 5).
Size distribution of pelagic thresher shark. Length at first maturity (Lm) is 140–145 cm PL for males and 145–150 cm PL for females (Liu et al. 1999).
Management options
Data on the operational fishing characteristics that lead to higher catches of thresher sharks can be used to inform management options via an MH framework (Table 1). Following the mitigation hierarchy, a number of options are possible. The most effective option to prevent fishing mortality could be a ban on specialised shark bottom longlines, as they contribute 94% of the reported pelagic thresher shark catch. Another approach is establishing one or more no-take zones around small islands in Western Sumatra, as these fishing grounds contribute 94% of the reported total pelagic thresher shark catch, however, the efficacy of spatial closures would need to be carefully examined to understand how movement and migration, and the population structure may affect their contribution to reducing thresher shark catches (Chin et al. 2023).
| Mitigation hierarchy step | Management measure | Technical effectiveness | |
|---|---|---|---|
| Avoid | Complete closure/ban of specialised shark bottom longline | Could reduce pelagic thresher catches | |
| Establish no-take zone(s) in/around small islands in the western part of Sumatra Island | Could reduce pelagic thresher catches | ||
| Minimise | Effort limits (e.g. trip length or hook number, hook size, bait) | Could minimise the total catch of pelagic threshers | |
| Catch quota or trip limits | Could keep a total catch of pelagic threshers within a specified limit | ||
| Temporal closure | Limiting fishing to one fishing season or any particular period in a year could reduce the catch of pelagic threshers and other targeted species | ||
| Remediate | Retention bans with postcapture handling and live-release training | Up to 87.5% of released thresher sharks may survive, depending on soak time and other variables based on the study on bigeye thresher sharks in the Pacific (Hutchinson and Bigelow 2019) | |
| Compensate | Payments in-kind (e.g. fishers contribute to critical habitat conservation) | Required further exploration and a case-specific approach (Booth et al. 2022, 2023) |
Temporal closure scenarios may reduce total pelagic thresher catches. In 2020, 46% of pelagic threshers were caught in high season. Depending on needs, fisheries managers could choose to limit fisheries activities within a certain time frame (e.g. in high season, low season, or any particular period of the year) to reduce fishing effort, however, the potential effect of displacing this fishing effort to other areas or species would need to be carefully considered and planned in close consultation with fishers to avoid unintended consequences and adverse outcomes (Jaiteh et al. 2016). Other options include effort limits and/or setting catch limits for certain species.
In addition, live release protocols could be used for the remaining residual catch with an estimated 87.5% survival rate based on previous studies of bigeye thresher shark (Alopias supercilious) postcapture survival in Pacific tuna longline fisheries (Hutchinson and Bigelow 2019). Finally, compensatory mechanisms, such as fishers contributing toward critical habitat conservation, could be explored (Booth et al. 2022, 2023). This mechanism can be in form of Payment for Ecosystem Service (PES) where fisheries managers pay fishers to release specific target species.
Discussion
Thresher sharks are commonly caught in targeted and bycatch fisheries in Indonesia (Fahmi and Dharmadi 2015). In addition, artisanal semicommercial fisheries may represent a significant source of fishing mortality for endangered sharks, yet are often unmonitored and unregulated (Lam and Sadovy de Mitcheson 2011; Tiralongo et al. 2018). This phenomenon drives management challenges due to ethical dilemmas regarding conservation impacts on coastal communities (Muttaqin et al. 2018; Booth et al. 2019), with the need for management approaches that can mitigate threats to priority species while maintaining the livelihoods and wellbeing of fishers and their families. Our study helps to fill this gap, first by providing information on pelagic thresher shark catches in Aceh, a priority species for conservation and a priority region for shark fisheries management in Indonesia; and secondly by exploring potential management options using a mitigation hierarchy approach.
Biological implications
Subadult individuals of both sexes dominated the size composition of documented pelagic thresher sharks. This composition could indicate population structuring between sizes or age classes, which is evident in many shark and ray populations (Jacoby et al. 2012; Mourier et al. 2013; Araujo et al. 2017; Meyers et al. 2017) and fishing activity might be causing selective mortality on the juveniles in the population.
In terms of fisheries management, the selective harvest of juveniles may actually present a lower risk scenario as for some sharks, restricting mortality to juveniles while protecting breeding adults can actually be a foundational approach to improve sustainability (Prince et al. 2008). However, this approach requires information about the movement and mortality of the adults as well as population modeling to inform catch limits which are also a suggested minimisation measure (Smart et al. 2020) (Table 1). In the future, we also suggest exploring how gear modifications, such as using different hook types or sizes, influence the length of the thresher shark catches. A study in the northern Gulf of Mexico shows that hook type has significantly influenced the catchability and size of Atlantic sharpnose shark (Rhizoprionodon terraenovae) and blacknose shark (Carcharhinus acronotus) (Hannan et al. 2013), and gear modification could be used to reduce catches of pelagic threshers.
Differences in sizes between the sexes were insignificant, though males recorded had a larger maximum PL than females (190 and 163 cm). In contrast, a previous study in Taiwanese waters found females with a larger maximum PL than males (188 cm and 170 cm PL, respectively) (Liu et al. 1999). Nevertheless, the individuals recorded in this study were mostly juveniles and the maximum sizes of males and females in this population, and comparisons on size differences between the sexes, are presently unknown. For comparison, other studies on the pelagic thresher shark in Indonesia found numerous individuals >300 cm TL (Drew et al. 2015). It is possible that fisheries in those locations in Central Java, Bali and Lombok were taking adults from a different portion of this population or a completely different population, however, it is also possible that maximum sizes of thresher sharks have declined over time, which could potentially indicate overfishing. This difference in historical versus present size structures in the pelagic thresher population in Indonesia warrants further investigation.
Lastly, the significant catch of endangered pelagic thresher sharks in this fishery indicates that further catch monitoring is required. At present, catch rates and fishing locations are only estimated as these are based on fisher interviews, with fishers estimating gear soak times and recounting fishing locations. Further quantification of fishing effort using electronic monitoring, onboard observers, and/or low-cost GPS trackers (Lara-Lopez et al. 2012; Hold et al. 2015; Navarrete Forero et al. 2017; Bartholomew et al. 2018) would provide more detailed information on fishing effort. Over the long term, standardised catch-per-unit-effort data would provide baseline data and contemporary information to understand population trends and thus indicate the performance of any introduced management measures.
The female-skewed sex ratio suggests sexual segregation in this thresher shark population. Sexual segregation commonly occurs in elasmobranch populations, where studies on different shark species, such as spurdog (Squalus acanthias) and shortfin mako (Isurus oxyrinchus), also showed similar results (Jacoby et al. 2012; Hannan et al. 2013). The selective fishing of juvenile females as evident in this study will likely affect population dynamics and, given the K-selected life history of this species (Drew et al. 2015), the survival of females should be prioritised to enhance fisheries sustainability. Population viability analyses that integrates existing biological and fisheries data would help mangers understand how this population may respond to different types of fishing pressure and could provide the bases for choosing potential management measures to minimise impacts (Table 1) as has been performed for other shark fisheries and populations (Smart et al. 2017, 2020).
Management options
Our results indicate that the fishing method targeting sharks in Southwest Aceh is highly specialised, with similarities to other small-scale semicommercial shark fisheries in Indonesia, such as Tanjung Luar in Lombok, West Nusa Tenggara (Yulianto et al. 2018; Glaus et al. 2019). Based on the data available, we could adopt a range of potential management measures to reduce thresher shark catches in Aceh, with limitations on specialised shark bottom longlines and spatial closures being the most technically effective. However, these measures require further investigation. The effects of gear modification on catches and resulting population dynamics need to be explored to understand how different modifications may enhance sustainability. Other than that, some strict measures such as banning the most effective gear will be unrealistic in species-specific fisheries, because such measures would most likely be rejected by the fishing community despite their high potential conservation effectiveness.
Likewise, the contribution of a marine protected area (MPA) to protecting a shark population depends on numerous factors. Given that the pelagic thresher shark is a strongly K-selected species (Drew et al. 2015) and that the individuals in this area are juveniles, an MPA in this area may be less effective than an MPA that protects adult females (Chin et al. 2023), which highlights the need to better understand the wider movements and population structure of the species in Indonesia.
However, these results are based only on technical considerations. Besides technical measures, the socio-economic aspects of shark fisheries and compliance management must also be considered, especially in small-scale shark fisheries (Booth et al. 2019; Ichsan et al. 2021). For example, poverty alleviation, alternative livelihood options and incentives for shark conservation are needed to reduce dependence on threatened sharks whilst maintaining or improving wellbeing (Weeratunge et al. 2014; Bennett et al. 2019). Furthermore, introducing management measures that do not account for livelihoods and social and economic factors can lead to broad noncompliance and even cause other unwanted conservation outcomes (Jaiteh et al. 2017).
There is limited information regarding the economic value of specific species and their relative benefits to fisher households. Yet, to implement these measures in Southwest Aceh, understanding the potential socio-economic costs of different management measures to fishers and their relative feasibility will be essential (Booth et al. 2019). Thus, we need more information to determine the cost-effectiveness of management measures and the needs and preferences of fishers to support effective implementation (Muttaqin et al. 2018; Yulianto et al. 2018; Booth et al. 2020; Seidu et al. 2022). This information could also help to identify where novel interventions such as incentives and payments for ecosystem services could be introduced (Bladon et al. 2016; Leduc and Hussey 2019; Wosnick et al. 2020; Booth et al. 2021). Further data on which operational fishing characteristics create the highest risks to thresher sharks, with robust statistical analysis, and considering the results of population viability analyses (PVA) will also help clarify which measures might be the most effective at reducing mortality (Smart et al. 2017).
A recent study on the potential effectiveness of Payment for Ecosystem Services (PES) in artisanal shark fisheries focuses on two CITES-listed and critically endangered taxa, hammerhead sharks (Family: Sphyrnidae) and wedgefish (Family: Rhinidae) in two locations in Indonesia (Booth et al. 2023). This study suggests that PES could save >20,000 hammerheads and wedgefish annually for an estimated cost of USD71,000–236,000. Based on this study, the factors that can influence the effectiveness of PES include incentive design, individual socio-demographic characteristics, and broader contextual and market-related factors. Further, this study suggests that all stakeholders, including government, funders, scientists, and private sector, strengthen partnerships with local communities and create an environment for testing and developing PES projects (Booth et al. 2023). These studies also demonstrate the importance of gaining in-depth knowledge of the social and economic factors that influence fisher behaviours, and a measured, iterative approach in designing and testing PES schemes to maximise positive behaviours and avoid potential adverse outcomes.
More broadly, there is also a need to appropriately manage other fisheries operating in Aceh and the Indian Ocean. Ensuring the survival of highly migratory and threatened shark species in Indonesia needs robust bycatch mitigation in both industrial and small-scale fisheries that interact with these species. Measures for industrial fishing will require a different approach than managing targeted and small-scale fisheries. For example, as a member of IOTC, Indonesia must comply with Resolution 12/09 on the conservation of thresher sharks (family Alopiidae) caught in association with fisheries in the IOTC area of competence, which prohibits fishing vessels flying the flag of IOTC Members and Cooperating Non-Contracting Parties (CPCs) from retaining, transshipping, landing, storing, selling or offering for sale any part or whole carcass of thresher sharks of all the species of the family Alopiidae (The Indian Ocean Tuna Commission (IOTC) 2012). Further, the contracting parties also need to release thresher shark catches and records by observers. However, implementation of this resolution may be constrained by limited monitoring and compliance. Other than that, IOTC only requires a minimum ‘5% observer coverage of the number of operations/sets for each gear type by the fleet of each CPC’ (The Indian Ocean Tuna Commission (IOTC) 2022). More significant investments in observer programs and landings monitoring could help improve compliance with IOTC resolutions, reduce the mortality of thresher sharks in commercial fisheries, and protect marine resources for the livelihoods and wellbeing of coastal communities.
Overall, the data and management options we have presented can be part of a holistic management strategy for conserving the charismatic, evolutionarily distinct, and globally endangered thresher shark in the Indian Ocean. Therefore, this information on data-poor targeted shark fisheries in the Indian Ocean from two sites in Aceh province, Indonesia, could help to inform local and national management for endangered and CITES-listed sharks, such as pelagic thresher sharks (Ichsan et al. 2021; Seidu et al. 2022).
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This research was funded by Fondation Segré via ZSL EDGE of Existence Fellowship.
Acknowledgements
Authors thanks the Government of Aceh Province and all the fishers and people who work at Ujong Serangga and Lhok Pawoh landing site for the guidance, partnership and support in this study. The authors also thank the Fondation Segré, ZSL EDGE of Existence fellowship and Wildlife Conservation Society – Indonesia Programme for financial and technical support during this study.
References
Aalbers SA, Bernal D, Sepulveda CA (2010) The functional role of the caudal fin in the feeding ecology of the common thresher shark Alopias vulpinus. Journal of Fish Biology 76, 1863-1868.
| Crossref | Google Scholar |
Araujo G, Snow S, So CL, Labaja J, Murray R, Colucci A, Ponzo A (2017) Population structure, residency patterns and movements of whale sharks in Southern Leyte, Philippines: results from dedicated photo-ID and citizen science. Aquatic Conservation: Marine and Freshwater Ecosystems 27, 237-252.
| Crossref | Google Scholar |
Bartholomew DC, Mangel JC, Alfaro-Shigueto J, Pingo S, Jimenez A, Godley BJ (2018) Remote electronic monitoring as a potential alternative to on-board observers in small-scale fisheries. Biological Conservation 219, 35-45.
| Crossref | Google Scholar |
Bennett NJ, Cisneros-Montemayor AM, Blythe J, Silver JJ, Singh G, Andrews N, Calò A, Christie P, Di Franco A, Finkbeiner EM, et al. (2019) Towards a sustainable and equitable blue economy. Nature Sustainability 2, 991-993.
| Crossref | Google Scholar |
Bladon AJ, Short KM, Mohammed EY, Milner-Gulland EJ (2016) Payments for ecosystem services in developing world fisheries. Fish and Fisheries 17, 839-859.
| Crossref | Google Scholar |
Booth H, Squires D, Milner-Gulland EJ (2019) The neglected complexities of shark fisheries, and priorities for holistic risk-based management. Ocean & Coastal Management 182, 104994.
| Crossref | Google Scholar |
Booth H, Squires D, Milner-Gulland EJ (2020) The mitigation hierarchy for sharks: a risk-based framework for reconciling trade-offs between shark conservation and fisheries objectives. Fish and Fisheries 21, 269-289.
| Crossref | Google Scholar |
Booth H, Arlidge WNS, Squires D, Milner-Gulland EJ (2021) Bycatch levies could reconcile trade-offs between blue growth and biodiversity conservation. Nature Ecology & Evolution 5, 715-725.
| Crossref | Google Scholar | PubMed |
Booth H, Mourato S, Milner-Gulland EJ (2022) Investigating acceptance of marine tourism levies, to cover the opportunity costs of conservation for coastal communities. Ecological Economics 201, 107578.
| Crossref | Google Scholar |
Booth H, Ramdlan MS, Hafizh A, Wongsopatty K, Mourato S, Pienkowski T, Adrinato L, Milner-Gulland EJ (2023) Designing locally-appropriate conservation incentives for small-scale fishers. Biological Conservation 277, 109821.
| Crossref | Google Scholar |
Chin A, Molloy FJ, Cameron D, Day JC, Cramp J, Gerhardt KL, Heupel MR, Read M, Simpfendorfer CA (2023) Conceptual frameworks and key questions for assessing the contribution of marine protected areas to shark and ray conservation. Conservation Biology 37, e13917.
| Crossref | Google Scholar |
Davidson LNK, Dulvy NK (2017) Global marine protected areas to prevent extinctions. Nature Ecology & Evolution 1, 40.
| Crossref | Google Scholar | PubMed |
Di Lorenzo M, Calò A, Di Franco A, Milisenda G, Aglieri G, Cattano C, Milazzo M, Guidetti P (2022) Small-scale fisheries catch more threatened elasmobranchs inside partially protected areas than in unprotected areas. Nature Communications 13, 4381.
| Crossref | Google Scholar | PubMed |
Drew M, White WT, Dharmadi, Harry AV, Huveneers C (2015) Age, growth and maturity of the pelagic thresher Alopias pelagicus and the scalloped hammerhead Sphyrna lewini. Journal of Fish Biology 86, 333-354.
| Crossref | Google Scholar | PubMed |
Dulvy NK, Baum JK, Clarke S, Compagno LJV, Cortés E, Domingo A, Fordham S, Fowler S, Francis MP, Gibson C, et al. (2008) You can swim but you can’t hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conservation: Marine and Freshwater Ecosystems 18, 459-482.
| Crossref | Google Scholar |
Dulvy NK, Pacoureau N, Rigby CL, Pollom RA, Jabado RW, Ebert DA, Finucci B, Pollock CM, Cheok J, Derrick DH, et al. (2021) Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Current Biology 31, 4773-4787.e8.
| Crossref | Google Scholar |
Fahmi, Dharmadi (2015) Pelagic shark fisheries of Indonesia’s Eastern Indian Ocean Fisheries Management Region. African Journal of Marine Science 37, 259-265.
| Crossref | Google Scholar |
Food and Agriculture Organization (2020) ‘The State of World Fisheries and Aquaculture 2020.’ (Food and Agriculture Organization) 10.4060/ca9229en
Glaus KBJ, Adrian-Kalchhauser I, Piovano S, Appleyard SA, Brunnschweiler JM, Rico C (2019) Fishing for profit or food? Socio-economic drivers and fishers’ attitudes towards sharks in Fiji. Marine Policy 100, 249-257.
| Crossref | Google Scholar |
Gupta T, Booth H, Arlidge W, Rao C, Manoharakrishnan M, Namboothri N, Shanker K, Milner-Gulland EJ (2020) Mitigation of elasmobranch bycatch in trawlers: a case study in indian fisheries. Frontiers in Marine Science 7, 571.
| Crossref | Google Scholar |
Hannan KM, Fogg AQ, Driggers WB, III, Hoffmayer ER, Walter Ingram G, Jr., Grace MA (2013) Size selectivity and catch rates of two small coastal shark species caught on circle and J hooks in the northern Gulf of Mexico. Fisheries Research 147, 145-149.
| Crossref | Google Scholar |
Hold N, Murray LG, Pantin JR, Haig JA, Hinz H, Kaiser MJ (2015) Video capture of crustacean fisheries data as an alternative to on-board observers. ICES Journal of Marine Science 72, 1811-1821.
| Crossref | Google Scholar |
Hutchinson M, Bigelow K (2019) Quantifying post release mortality rates of sharks incidentally captured in Pacific tuna longline fisheries and identifying handling practices to improve survivorship. Pacific Islands Fisheries Science Center WP-19-003, 26. (NOAA) 10.25923/2SXY-S659
Ichsan M, Ula S, Simeon B, Muttaqin E, Booth H (2020) Thresher sharks (Alopiidae) catch in the pelagic fisheries of Western Indonesia. IOP Conference Series: Earth and Environmental Science 420, 012013.
| Crossref | Google Scholar |
Ichsan M, Ula S, Herman (2021) Silky Shark (Carcharhinus falciformis) fisheries in Southwest Aceh. In ‘Prosiding Simposium Hiu dan Pari Indonesia-3 “Penguatan Kolaborasi dan Sinergi dalam Pengelolaan Hiu dan Pari”. Simposium Hiu dan Pari Indonesia Ke 3’. p. 492. (Pusat Riset Perikanan - KKP: Jakarta, Indonesia)
Jacoby DMP, Croft DP, Sims DW (2012) Social behaviour in sharks and rays: analysis, patterns and implications for conservation: Shark social behaviour. Fish and Fisheries 13, 399-417.
| Crossref | Google Scholar |
Jaiteh VF, Lindfield SJ, Mangubhai S, Warren C, Fitzpatrick B, Loneragan NR (2016) Higher abundance of marine predators and changes in fishers’ behavior following spatial protection within the world’s biggest shark fishery. Frontiers in Marine Science 3, 43.
| Crossref | Google Scholar |
Jaiteh VF, Loneragan NR, Warren C (2017) The end of shark finning? Impacts of declining catches and fin demand on coastal community livelihoods. Marine policy 82, 224-233.
| Crossref | Google Scholar |
Kementerian Kelautan dan Perikanan Republik Indonesia (2018) 61/PERMEN-KP/2018 Peraturan Menteri Kelautan dan Perikanan tentang Pemanfaatan Jenis Ikan Yang Dilindungi Dan/Atau Jenis Ikan Yang Tercantum Dalam Appendiks Convention On International Trade In Endangered Species Of Wild Fauna And Flora. Kementerian Kelautan dan Perikanan Republik Indonesia.
Lam VYY, Sadovy de Mitcheson Y (2011) The sharks of South East Asia – unknown, unmonitored and unmanaged: Sharks of South East Asia. Fish and Fisheries 12, 51-74.
| Crossref | Google Scholar |
Leduc AOHC, Hussey NE (2019) Pay to protect at-risk bycatch species. Science 364, 342.
| Crossref | Google Scholar |
Liu K-M, Chen C-T, Liao T-H, Joung S-J (1999) Age, growth, and reproduction of the Pelagic Thresher Shark, Alopias pelagicus in the Northwestern Pacific. Copeia 1999, 68-74.
| Crossref | Google Scholar |
Meyers EKM, Tuya F, Barker J, Jiménez Alvarado D, Castro-Hernández JJ, Haroun R, Rödder D (2017) Population structure, distribution and habitat use of the Critically Endangered Angelshark, Squatina squatina, in the Canary Islands. Aquatic Conservation: Marine and Freshwater Ecosystems 27, 1133-1144.
| Crossref | Google Scholar |
Mourier J, Mills SC, Planes S (2013) Population structure, spatial distribution and life-history traits of blacktip reef sharks Carcharhinus melanopterus. Journal of Fish Biology 82, 979-993.
| Crossref | Google Scholar | PubMed |
Navarrete Forero G, Miñarro S, Mildenberger TK, Breckwoldt A, Sudirman, Reuter H (2017) Participatory boat tracking reveals spatial fishing patterns in an indonesian artisanal fishery. Frontiers in Marine Science 4, 409.
| Crossref | Google Scholar |
Oliver SP, Turner JR, Gann K, Silvosa M, D’Urban Jackson T (2013) Thresher sharks use tail-slaps as a hunting strategy. PLoS ONE 8, e67380.
| Crossref | Google Scholar |
Otake T, Mizue K (1981) Direct evidence for oophagy in Thresher Shark Alopias pelagicus. Japanese Journal of Ichthyology 28, 171-172.
| Crossref | Google Scholar |
Pacoureau N, Rigby CL, Kyne PM, Sherley RB, Winker H, Carlson JK, Fordham SV, Barreto R, Fernando D, Francis MP, et al. (2021) Half a century of global decline in oceanic sharks and rays. Nature 589, 567-571.
| Crossref | Google Scholar | PubMed |
Prince JD, Loneragan NR, Okey TA (2008) Contraction of the banana prawn (Penaeus merguiensis) fishery of Albatross Bay in the Gulf of Carpentaria, Australia. Marine and Freshwater Research 59, 383-390.
| Crossref | Google Scholar |
Rigby CL, Barreto R, Carlson J, Fernando D, Fordham S, Francis MP, Herman K, Jabado RW, Liu KM, Marshall A, et al. (2019) Alopias pelagicus, Pelagic Thresher. The IUCN Red List of Threatened Species 2019: e.T161597A68607857. Available at https://www.iucnredlist.org/species/161597/68607857
Sari I, Ichsan M, White A, Raup SA, Wisudo SH (2021) Monitoring small-scale fisheries catches in Indonesia through a fishing logbook system: challenges and strategies. Marine Policy 134, 104770.
| Crossref | Google Scholar |
Seidu I, Brobbey LK, Danquah E, Oppong SK, van Beuningen D, Seidu M, Dulvy NK (2022) Fishing for survival: Importance of shark fisheries for the livelihoods of coastal communities in Western Ghana. Fisheries Research 246, 106157.
| Crossref | Google Scholar |
Simeon BM, Fajri I, Ula S, Muttaqin E, Ichsan M, Dharmadi, Damora A, Sarong A (2020) Laporan Teknis Pemantauan Hasil Tangkapan: Hiu dan Pari di Provinsi Aceh. Wildlife Conservation Society. Available at https://data-ikan.org/hiu_aceh/dokumen.php
Smart JJ, Chin A, Tobin AJ, White WT, Kumasi B, Simpfendorfer CA (2017) Stochastic demographic analyses of the silvertip shark (Carcharhinus albimarginatus) and the common blacktip shark (Carcharhinus limbatus) from the Indo-Pacific. Fisheries Research 191, 95-107.
| Crossref | Google Scholar |
Smart JJ, White WT, Baje L, Chin A, D’Alberto BM, Grant MI, Mukherji S, Simpfendorfer CA (2020) Can multi-species shark longline fisheries be managed sustainably using size limits? Theoretically, yes. Realistically, no. Journal of Applied Ecology 57, 1847-1860.
| Crossref | Google Scholar |
Smith SE, Au DW, Show C (1998) Intrinsic rebound potentials of 26 species of Pacific sharks. Marine and Freshwater Research 49, 663-678.
| Crossref | Google Scholar |
The Indian Ocean Tuna Commission (IOTC) (2012) Resolution 12/09 On The Conservation of Thresher Sharks (Family Alopiidae) Caught in Association With Fisheries in The IOTC Area of Competence. Available at https://www.iotc.org/cmm/resolution-1209-conservation-thresher-sharks-family-alopiidae-caught-association-fisheries-iotc
The Indian Ocean Tuna Commission (IOTC) (2022) Resolution 22/04 on a Regional Observer Scheme. Available at https://www.fao.org/faolex/results/details/en/c/LEX-FAOC212810
Tiralongo F, Messina G, Lombardo BM (2018) Discards of elasmobranchs in a trammel net fishery targeting cuttlefish, Sepia officinalis Linnaeus, 1758, along the coast of Sicily (central Mediterranean Sea). Regional Studies in Marine Science 20, 60-63.
| Crossref | Google Scholar |
Weeratunge N, Béné C, Siriwardane R, Charles A, Johnson D, Allison EH, Nayak PK, Badjeck M-C (2014) Small-scale fisheries through the wellbeing lens. Fish and Fisheries 15, 255-279.
| Crossref | Google Scholar |
Wosnick N, Da Costa De Lima Wosiak C, Machado Filho OC (2020) Pay to conserve: what we have achieved in 10 years of compensatory releases of threatened with extinction guitarfishes. Animal Conservation 24, 537-539.
| Crossref | Google Scholar |
Yulianto I, Booth H, Ningtias P, Kartawijaya T, Santos J, Sarmintohadi, Kleinertz S, Campbell SJ, Palm HW, Hammer C (2018) Practical measures for sustainable shark fisheries: Lessons learned from an Indonesian targeted shark fishery. PLoS ONE 13, e0206437.
| Crossref | Google Scholar |