Antimicrobial resistance in production animals
Darren J. Trott A * , Andrea McWhorter B , Kylie Hewson C , Rebecca Abraham D and Sam Abraham DA
B
C
D

Prof. Darren Trott is a veterinarian and microbiologist with research interests including zoonotic, companion and production animal bacterial diseases, focusing on molecular epidemiology, microbial pathogenesis, microbial ecology, antibiotic resistance and development and repurposing of new antimicrobials. He is the inaugural Director of the Australian Centre of Antimicrobial Resistance Ecology. He teaches veterinary microbiology, antimicrobial chemotherapy and antimicrobial stewardship to veterinary science students and co-ordinates the DVM-1 Clinical Research Project. |

Andrea McWhorter is a microbiologist specialising in poultry health and zoonotic foodborne pathogens. Her research also extends to understanding the transmission of foodborne pathogens from farms to the food supply chain, leading to human disease. Andrea collaborates closely with the egg and chicken meat industries to address the challenges of improving bird health and mitigating zoonotic bacteria in food. |

Kylie Hewson has worked for over 15 years in the Australian agriculture sector as a scientist, research manager, policy manager and advocate for ensuring quality science is not only produced, but also made accessible and able to be used in setting policy and achieving meaningful impact. Kylie has represented industry on several high-level cross-sectoral and government committees, with extensive experience as Chair, across issues such as biosecurity, food safety, animal health and antimicrobial stewardship. |

Rebecca Abraham is an early career researcher with experience in bacterial AMR surveillance and drug discovery for microorganisms including fungi, bacteria, viruses and parasites. She is involved in the development and use of automated high-throughput screening assays to identify novel antimicrobial compounds and to detect bacterial AMR. |

Prof. Sam Abraham is Professor of Microbiology and Director, Centre for Biosecurity and One Health, Harry Butler Institute, Murdoch University. He leads the Antimicrobial Resistance and Infectious Disease Laboratory. His research encompasses AMR, robotics, genomics, One Health and veterinary microbiology. |
Abstract
There is growing recognition of the significant role food production animals play in the dissemination of antimicrobial-resistant bacteria. This issue is particularly relevant in the realm of food production, where the administration of antibiotics of importance to human health to animals can foster the emergence of resistant bacterial strains that may be transmitted to humans through the food supply chain. Further, resistance can develop to antibiotics of importance for animal health which can create health and productivity issues. Cross-sectional surveys of Australian food animal production systems have revealed that very low levels of antimicrobial resistance (AMR) are present in key bacterial species. Expansion of AMR surveillance in food production systems is, however, needed to improve the detection sensitivity of emerging resistances of importance to human and animal health. Scalable high throughput technologies, such as the robotic antimicrobial susceptibility platform (RASP), represents a step-fold change in the capacity to accurately detect AMR. Enhanced surveillance along with improved biosecurity, industry engagement and antimicrobial stewardship will contribute to maintaining low levels of AMR in Australia’s food production systems.
Keywords: animal production, antimicrobial resistance, antimicrobial susceptibility testing, critically important antimicrobials, food-producing animals, livestock, robotic processing, surveillance.
Background
The history of agricultural usage of antimicrobials began in the mid-1930s with the use of synthetic sulfonamides to treat mastitis in dairy cattle.1 Since, then, antibiotics have been used in agriculture to prevent and treat disease, increase feed conversion efficiency, average weight gain and contribute to food preservation. Little more than 30 years later, a multidrug-resistant Salmonella Typhimurium phage type 29 caused a spike in infection in calves in the United Kingdom2 that was also linked with many human cases of salmonellosis. Multidrug-resistant bacterial species subsequently quickly became more globally widespread following the adoption of intensive farming practices which often included the imprudent addition of antibiotics to animal feed without veterinary prescription.
Regulation of antimicrobials
Australia has long recognised the importance of regulating the production and usage of antimicrobial therapeutic drugs.3 High level committees comprised of representatives from the Australian Government and its agencies in human medicine and agriculture, as well as human and veterinary health professionals informing regulation and providing national governance and leadership on antimicrobials in Australia have existed since the 1960s.3,4 Australia’s first National AMR [Antimicrobial Resistance] Strategy was released in 2015,5 and, in March 2020, the updated Australia’s National Antimicrobial Resistance Strategy – 2020 and Beyond6 was released alongside a One Health antimicrobial resistance action plan7 that takes a One Health approach to mitigating AMR across humans, animals, food, agriculture and the environment. This strategy has been expanded to include the responsible usage of antifungals, antiparasitics and antivirals. The ‘2020 Strategy’ outlines seven key objectives including governance, AMR surveillance, AMR research, infection prevention and control, communication and engagement, antimicrobial stewardship, as well as global partnerships. Australia’s Animal Sector Antimicrobial Resistance Action Plan 2023–20288 was developed as the plan for the animal sector to progress relevant activities under the ‘2020 Strategy’ and is inclusive of multiple animal sectors including both terrestrial and aquatic food and fibre producing animals, companion animals, zoological collections, laboratory animals and wildlife treated with antimicrobials.
Antimicrobial stewardship is one of the seven key objectives of the ‘2020 Strategy’ and represents the coordinated efforts to promote optimal antimicrobial usage that includes optimal drug selection, dose and duration of treatment that leads to the best clinical outcome while minimising the potential for resistance. World-wide, Australia has one of the most conservative approaches surrounding the regulation and usage of antimicrobials in food-producing animals. Most antimicrobials have been classified as Schedule 4 medicines since the 1970s and require a prescription from a licensed veterinarian.9,10 Fluoroquinolones have never been registered for use in food-producing animals in Australia and label directives limit the administration of extended-spectrum cephalosporins to only individual animals with respiratory disease though there is some reported off-label use.11,12 In food-producing animals, polymyxins are only present in two registered preparations with infrequent use as topical agents for ear and ocular conditions.
AMR surveillance in food production animals
Surveillance programs focused on AMR are vital within veterinary and food production sectors to detect emerging threats to human and animal health. AMR surveillance in food production animals in Australia is ad hoc with various industry-led studies in the red-meat industries, and government co-investment in studies in poultry, pigs as well as commercial barramundi and salmon. AMR in E. coli and Salmonella isolated from Australian food-producing animals substantially differs from what has been observed in other countries. Gram-negative bacteria isolated from Australian livestock have no or extremely low levels of resistance to a wide range of critically important antimicrobials.13–15 Recent AMR surveys in poultry have also revealed low levels of resistance in key indicator bacterial species, including E. coli and enterococci, as well as the foodborne pathogen Salmonella spp.16–18 To date, there have been no reports of carbapenem or colistin resistance in E. coli and Salmonella from Australian livestock and resistance to fluoroquinolones and extended-spectrum cephalosporins has been observed at only very low frequencies. Low levels of AMR are believed to be in part due to Australia’s unique geography, quarantine restrictions and governance regarding the use of critically important antimicrobials in food-producing animals. Effective ongoing surveillance, however, requires continuous repeated evaluation (rather than one-off surveys) and expansion to include pathogens of animal health significance so that AMR of human and animal importance can be effectively monitored and reported. Importantly, this strategy would provide the data needed to identify emerging AMR trends and contribute to public health and animal health related decision making.19
Antimicrobial usage practices in humans represent one of the most significant risks to the emergence of AMR in Australia. Given the global nature of the world’s economies and the extent of human travel, the contribution of reverse-zoonotic transfer of antimicrobial-resistant bacterial species (human to animals) cannot be discounted. For example, workers at an Australian piggery with close animal contact exhibited a higher risk for carriage of methicillin-resistant Staphylococcus aureus.20 Despite the absence of fluoroquinolone usage in Australian food animal species, fluoroquinolone-resistant Campylobacter spp. were detected in broiler faeces collected from major poultry processing plants.21 Comparative genomic analysis revealed that these Campylobacter isolates clustered with international isolates previously detected in both humans and animals overseas.21
Movement of wild animals through natural migration and urban encroachment may also contribute to the emergence of AMR in Australia. Recent genomic analysis of fluoroquinolone and extended-spectrum cephalosporin-resistant E. coli and Salmonella isolates detected from Australian livestock has shown that these strains are very dissimilar to those previously observed in clinically ill humans in Australia but have previously been observed in humans and wild birds overseas.22,23 These results suggest that the few fluoroquinolone- and extended-spectrum cephalosporin-resistant E. coli and Salmonella isolated from Australian food-producing animals are unlikely to have evolved locally but rather have potentially been introduced, by human carriers or wild birds.
Robotic platform technologies in AMR surveillance
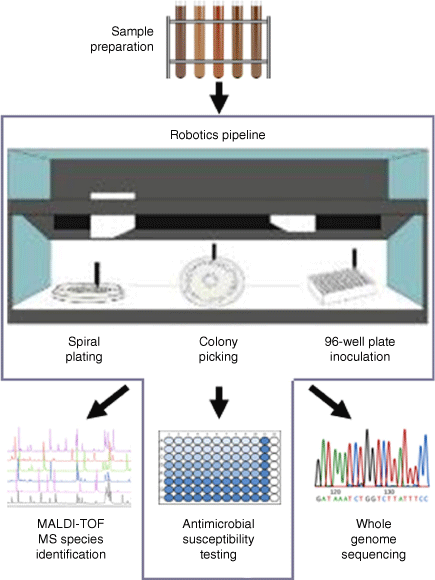
Combining automation and genomics is the future of AMR surveillance. Current AMR surveillance strategies utilise the broth microdilution minimal inhibitory concentration (MIC) method,24 which is labour intensive and costly. These limitations restrict the number of bacterial isolates that can be tested, ultimately decreasing the sensitivity of detection of emerging resistances, including to critically important antimicrobials. A high-throughput robotic antimicrobial susceptibility platform (RASP) has recently been developed representing a significant advancement in AMR surveillance. Combining laboratory automation from Tecan, spiral plating and colony picking with SciRobotics, MALDI-TOF from Bruker, and Illumina sequencing into a single high-throughput solution, it is possible to rapidly screen thousands of samples and study AMR in a large number of colonies, while adhering to internationally recognised standards (Fig. 1).25 Comparison of MIC results between RASP and a human technician revealed a high degree of agreement.25 With funding from the Australian Government Department of Health, Food Standards Australia New Zealand has recently used RASP to conduct a survey of AMR in retail food products.
Industry engagement and communication
Industry engagement in the control and prevention of AMR is critical to reduce the public health and animal health impacts of antimicrobial use in all areas of intensive food production systems. Business management, veterinarians, farm managers and workers must be committed to implementing high standards of antimicrobial stewardship and biosecurity, and refining antimicrobial use to maintain bacterial susceptibility among pathogens in both intensive and extensive animal production systems. This approach will have a profoundly beneficial effect as healthy animals result in improved productivity and lower production costs (and, ultimately, lower costs to the consumer).
Conclusion
AMR in animals and food is a complex and multifaceted issue. Efforts to address this issue should focus on strengthening existing conservative regulations targeted at reducing unnecessary use of antimicrobials in humans and animals, improving biosecurity practices, implementing high standards of antimicrobial stewardship, raising awareness, education, and conducting research and surveillance. By taking a comprehensive and coordinated One Health approach to combating AMR, stakeholders can help to protect human and animal health, preserving the efficacy antimicrobials for future use.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Darren Trott and Andrea McWhorter are guest editors for this issue of Microbiology Australia but did not at any stage have editor-level access to this manuscript or those that they authored while they were in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Microbiology Australia encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
Development of the RASP described in this paper was supported by funding from the Australian Government Department of Agriculture, Water and the Environment as part of its Rural R&D for Profit programme.
References
1 Kirchhelle C (2018) Pharming animals: a global history of antibiotics in food production (1935–2017). Palgrave Commun 4, 96.
| Crossref | Google Scholar |
2 Anderson ES (1968) Drug resistance in Salmonella Typhimurium and its implications. Br Med J 3, 333-339.
| Crossref | Google Scholar | PubMed |
3 McEwan J (2007) A history of therapeutic goods regulation in Australia. Therapeutic Goods Administration, Canberra, ACT, Australia. https://www.tga.gov.au/sites/default/files/history-tg-regulation.pdf
5 Department of Health and Aged Care and Department of Agriculture, Fisheries and Forestry (2015) National AMR Strategy. Commonwealth of Australia. https://www.amr.gov.au/australias-response/national-amr-strategy
6 Department of Health and Aged Care and Department of Agriculture, Fisheries and Forestry (2020) Australia’s National Antimicrobial Resistance Strategy – 2020 and beyond. Commonwealth of Australia. https://www.amr.gov.au/resources/australias-national-antimicrobial-resistance-strategy-2020-and-beyond
7 Department of Health and Aged Care and Department of Agriculture, Fisheries and Forestry (2021) One Health Master Action Plan for Australia’s National Antimicrobial Resistance Strategy – 2020 and beyond. Commonwealth of Australia. https://www.amr.gov.au/resources/one-health-master-action-plan-australias-national-antimicrobial-resistance-strategy-2020-and-beyond
8 Department of Agriculture, Fisheries and Forestry (2023) Australia’s Animal Sector Antimicrobial Resistance: Action Plan 2023 to 2028. September 2023. DAFF, Canberra, ACT, Australia. https://www.agriculture.gov.au/sites/default/files/documents/australias-animal-sector-amr-action-plan-2023-2028.pdf
11 Mukerji S et al. (2017) Development and transmission of antimicrobial resistance among Gram-negative bacteria in animals and their public health impact. Essays Biochem 61, 23-35.
| Crossref | Google Scholar | PubMed |
12 Cheng AC et al. (2012) Control of fluoroquinolone resistance through successful regulation, Australia. Emerg Infect Dis 18, 1453-1460.
| Crossref | Google Scholar | PubMed |
13 Abraham S et al. (2014) Salmonella enterica isolated from infections in Australian livestock remain susceptible to critical antimicrobials. Int J Antimicrob Agents 43, 126-130.
| Crossref | Google Scholar | PubMed |
14 Pande VV et al. (2015) Antimicrobial resistance of non-typhoidal Salmonella isolates from egg layer flocks and egg shells. Int J Food Microbiol 203, 23-26.
| Crossref | Google Scholar | PubMed |
15 Barlow RS et al. (2015) Prevalence and antimicrobial resistance of Salmonella and Escherichia coli from Australian cattle populations at slaughter. J Food Prot 78, 912-920.
| Crossref | Google Scholar | PubMed |
16 Abraham S et al. (2019) Escherichia coli and Salmonella spp. isolated from Australian meat chickens remain susceptible to critically important antimicrobial agents. PLoS ONE 14, e0224281.
| Crossref | Google Scholar | PubMed |
17 O’Dea M et al. (2019) Genomic, antimicrobial resistance, and public health insights into Enterococcus spp. from Australian chickens. J Clin Microbiol 57,.
| Crossref | Google Scholar | PubMed |
18 Sodagari HR et al. (2019) Non-typhoidal Salmonella contamination in egg shells and contents from retail in Western Australia: Serovar diversity, multilocus sequence types, and phenotypic and genomic characterizations of antimicrobial resistance. Int J Food Microbiol 308, 108305.
| Crossref | Google Scholar | PubMed |
19 Shaban RZ et al. (2014) Surveillance and reporting of antimicrobial resistance and antibiotic usage in animals and agriculture in Australia. Report to the Department ofAgriculture. Griffith University and University of Adelaide, Australia. https://www.agriculture.gov.au/agriculture-land/animal/health/amr/surveillance-antimicrobial-resistance#daff-page-main
20 Sahibzada S et al. (2018) Emergence of highly prevalent CA-MRSA ST93 as an occupational risk in people working on a pig farm in Australia. PLoS ONE 13, e0195510.
| Crossref | Google Scholar | PubMed |
21 Abraham S et al. (2020) Emergence of fluoroquinolone-resistant Campylobacter jejuni and Campylobacter coli among Australian chickens in the absence of fluoroquinolone use. Appl Environ Microbiol 86, e02765-19.
| Crossref | Google Scholar | PubMed |
22 Abraham S et al. (2015) First detection of extended-spectrum cephalosporin- and fluoroquinolone-resistant Escherichia coli in Australian food-producing animals. J Glob Antimicrob Resist 3, 273-277.
| Crossref | Google Scholar | PubMed |
23 Abraham S et al. (2018) Dissemination and persistence of cephalosporin- and cephalosporin-resistance encoding IncI1-bla CTXM-1 plasmid among Escherichia coli in pigs. ISME J 12, 2352-2362.
| Crossref | Google Scholar | PubMed |
24 World Health Organization (2015) WHA68.7: Global Action Plan onAntimicrobial Resistance. In Sixty-Eighth World Health Assembly, 26 May 2015, Geneva, Switzerland. WHO. https://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_R7-en.pdf
25 Truswell A et al. (2021) Robotic antimicrobial susceptibility platform (RASP): a next-generation approach to One Health surveillance of antimicrobial resistance. J Antimicrob Chemother 76(7), 1800-1807.
| Crossref | Google Scholar | PubMed |
 Prof. Darren Trott is a veterinarian and microbiologist with research interests including zoonotic, companion and production animal bacterial diseases, focusing on molecular epidemiology, microbial pathogenesis, microbial ecology, antibiotic resistance and development and repurposing of new antimicrobials. He is the inaugural Director of the Australian Centre of Antimicrobial Resistance Ecology. He teaches veterinary microbiology, antimicrobial chemotherapy and antimicrobial stewardship to veterinary science students and co-ordinates the DVM-1 Clinical Research Project. |
 Andrea McWhorter is a microbiologist specialising in poultry health and zoonotic foodborne pathogens. Her research also extends to understanding the transmission of foodborne pathogens from farms to the food supply chain, leading to human disease. Andrea collaborates closely with the egg and chicken meat industries to address the challenges of improving bird health and mitigating zoonotic bacteria in food. |
 Kylie Hewson has worked for over 15 years in the Australian agriculture sector as a scientist, research manager, policy manager and advocate for ensuring quality science is not only produced, but also made accessible and able to be used in setting policy and achieving meaningful impact. Kylie has represented industry on several high-level cross-sectoral and government committees, with extensive experience as Chair, across issues such as biosecurity, food safety, animal health and antimicrobial stewardship. |
 Rebecca Abraham is an early career researcher with experience in bacterial AMR surveillance and drug discovery for microorganisms including fungi, bacteria, viruses and parasites. She is involved in the development and use of automated high-throughput screening assays to identify novel antimicrobial compounds and to detect bacterial AMR. |
 Prof. Sam Abraham is Professor of Microbiology and Director, Centre for Biosecurity and One Health, Harry Butler Institute, Murdoch University. He leads the Antimicrobial Resistance and Infectious Disease Laboratory. His research encompasses AMR, robotics, genomics, One Health and veterinary microbiology. |