Extrinsic allergic alveolitis-causing actinomycetes in indoor and farm environments
Candice Brinkmann A and İpek Kurtböke A BA University of the Sunshine Coast, GeneCology Research Centre and the Faculty of Science, Health, Education and Engineering, Maroochydore DC, Qld 4558, Australia
B Email: ikurtbok@usc.edu.au
Microbiology Australia 39(3) 149-152 https://doi.org/10.1071/MA18045
Published: 16 August 2018
Suspended airborne particles, of biological origin, can constitute bioaerosols1,2 and they can be of different origin ranging from farm environments dealing with hay, sugar cane, tobacco, mushroom and cotton to refuse disposal sites to military equipment test chambers. These bioaerosols might carry allergenic and pathogenic agents like viruses, spore forming bacteria and fungi, excreta of insects and mites, moss and fern spores, algal and plant cells; enzymes, antibiotics, endotoxins, mycotoxins and fungal glucans1. Although infections from pathogenic viruses, bacteria and fungi may occur in these work environments the commonly reported symptoms relate to allergic rhinitis and asthma, allergic alveolitis (granulomatons pneumonitis) or organic dust toxic syndrome (inhalation fever or toxic pneumonitis)1,2. This article will only provide an overview on the non-infectious lung diseases resulting from long-term exposure to the spores of thermoactinomycetes of the order Bacillales and thermophilic actinomycetes of the order Actinomycetales in indoor and farm environments.
Early reports on the above-mentioned non-infectious diseases resulting from the allergenic and/or immunotoxic properties of airborne biological agents dates to 1877 by the recognition of byssinosis (brown-lung disease) associated with cotton, hemp and flax farming3,4. The cause of this disease has later been associated with the exposure to endotoxins from Gram-negative bacteria in 19815. Another similar disease, the Farmer’s Lung Disease was linked to ‘white dust arising from mouldy hay’ in 19326 and the causative agent was only identified in 1963 as the airborne spores of actinomycetes7. Since then such diseases have been frequently reported from many different countries including Finland, Germany, Poland, Switzerland, Sweden, India, Canada, Argentina and Costa Rica due to the storage of substrates prone to infestations by spore forming and Gram-positive bacteria (e.g. farm environments, mills and compost facilities)8–13. These diseases can be more prevalent in tropical and sub-tropical environments where thermophilic actinomycetes and fungi thrive due to elevated temperatures and humidity. Farmer’s lung is also highly prevalent in regions with high rainfall, such as Doubs, France, during the haymaking season14.
In this article causative bacterial genera or species associated with the above-listed diseases and belonging to the order Actinomycetales (http://www.bacterio.net/actinomycetales.html) will be termed as thermophilic actinomycetes, whereas the ones belonging to the family of Thermoactinomycetaceae of the order Bacillales (http://www.bacterio.net/thermoactinomycetaceae.html) will be termed as thermoactinomycetes covering the 21 genera currently listed under this family, including Thermoactinomyces, Laceyella, Seinonella and Thermoflavimicrobium.
Genera of the thermophilic actinomycetes implicated in hypersensitivity pneumonitis or extrinsic allergic alveolitis (EAA)15 include Faenia, Streptomyces, Thermomonospora, Saccharopolyspora, Saccharomonospora and Nocardiopsis. Thermophilic members of these actinomycete genera are capable of growth at elevated temperatures due to diploconic acid containing spores that are responsible for their survival at elevated temperatures. Whereas, genera belonging to thermoactinomycetes produce highly heat-resistant endospores2 and such spores can easily be disseminated through bioaerosols1,2,13,16, Thermoactinomyces species can survive in organic materials, such as soil, for up to 100 years as well as at 100°C for 30–40 minutes. Improper storage of large bales of organic substrates (in airtight buildings) when temperatures reach 50–60°C with a moisture content of >30% can result in the growth and proliferation of endospore forming thermoactinomycetes13.
Thermoactinomyces species were reported to infest home humidifiers and buildings where humid conditions may be present16. Thermoactinomyces vulgaris and Thermoactinomyces intermedius were isolated from hot water and heating systems of homes and Thermoactinomyces vulgaris from the household air13,16,17. Thermophilic actinomycetes are also reported to occur in the condensate of refrigerators and air conditioners17.
The above-mentioned non-infectious lung diseases associated with different occupational environments are termed accordingly: farmer’s lung disease, mushroom worker’s lung disease and bagassosis (sugarcane mills/farms) and their occurrence may differ in work places but present with similar symptoms that include cough, fever, malaise, weight loss, chills and shortness of breath9,18,19. Eye and nose irritation may also be experienced20. Susceptible workers that are exposed to the organic dust clouds from disrupted overheated materials, due to storage rooms with inadequate ventilation, may be at risk of acquiring the acute, subacute or chronic form of extrinsic allergic alveolitis21,22. The different forms of the disease (acute, subacute or chronic) depend on the exposure concentrations of these bacteria and length of the exposure time22,23. Workers may inhale anywhere between 500 000 to 750 000 spores per minute during a concentrated exposure and this may ultimately lead to pulmonary inflammation15. Extrinsic allergic alveolitis is a T-lymphocyte dependent granulomatous inflammatory reaction of the alveoli of the lungs23. Although a clear dose-response relationship for extrinsic allergic alveolitis and concentration of thermoactinomycetes and thermophilic actinomycetes is yet to be determined symptoms increase in a dose-dependent way24.
Hypersensitivity pneumonitis has also been described in highly sensitised animals25. When animal feed, made up of hay and grain, becomes damp in environments with elevated temperatures (e.g. in a storage unit that may not be completely isolated from rain or other water entry), thermoactinomycete spore concentrations increase thus putting the animals at risk of inhaling antigens of Thermoactinomyces species25. Cattle have been known to develop hypersensitivity pneumonitis during the winter period when they are confined to their stables. Growth of Thermoactinomyces species occurs in their feed and other organic material resulting in inhalation of high concentrations of spores25,26. Hypersensitivity pneumonitis may also develop when cattle are moved from a dry stable to lush pastures at the end of summer, where these bacteria may be present26. When cattle eat feed containing thermoactinomycetes they excrete viable spores that are spread on fields and within soil that can remain there for many years until suitable germination conditions arise27.
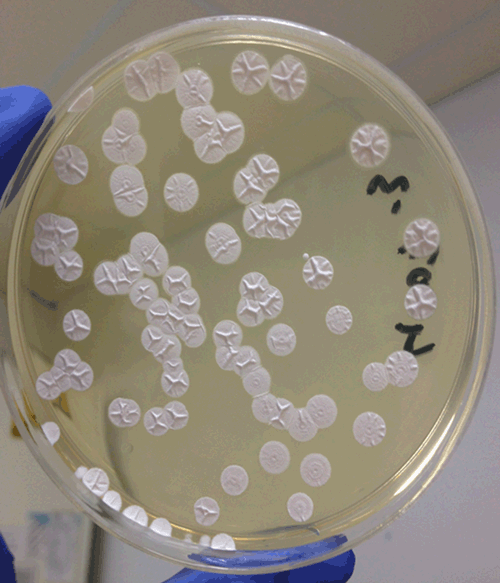
So far, the limited research that has been done in Australia has mixed results. A report by McNeill27 around Queensland sugar mills concluded that the concentration of bacteria and spores were just below the range to cause respiratory disease compared to other agricultural environments (such as sugar cane and sawmills) in other countries28. However, a pilot study, carried out in September to October 1989 around Queensland sugar mills, concluded that workers in these environments presented with symptoms related to certain occupational lung disease29. In a more recent study Brinkmann et al.30 investigated selected overheated substrates commercially available for public use in sub-tropical Queensland in Australia and detected the presence of thermoactinomycetes (Figure 1). Subsequent molecular identification of the isolates confirmed their close relationship to previously reported allergenic Thermoactinomyces vulgaris and Laceyella sacchari. The isolates were also found to display adhesion ability and cytotoxicity to human lung cells (Calu-3 (ATCC®) HTB-55™). Figure 2 illustrates the growth of the isolate USC-4002, found to be closely related to Laceyella sacchari, on an agar plate following the adhesion assay indicating a high degree of adherence as non-adherent cells would have washed away during the completion of the assay. These findings might encourage further in-depth studies and continuous screening of EAA causing thermoactinomycetes and thermophilic actinomycetes in tropical and sub-tropical regions to prevent occurrence of the Farmer’s Lung and similar diseases in Australia31. Furthermore, monitoring farm hygiene and air quality will contribute towards protection of agricultural workers and farm animals. These preventative measures are essential for the maintenance of healthy farm environments via ensuring the elimination of EAA causing bacteria.

|
|
References
[1] Lacey, J. and Dutkiewicz, J. (1994) Bioaerosols and occupational lung disease. J. Aerosol Sci. 25, 1371–1404.| Bioaerosols and occupational lung disease.Crossref | GoogleScholarGoogle Scholar |
[2] Lacey, J. and Crook, B. (1988) Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Ann. Occup. Hyg. 32, 515–533.
| Fungal and actinomycete spores as pollutants of the workplace and occupational allergens.Crossref | GoogleScholarGoogle Scholar |
[3] Proust, A.A. (1877) Affections Pulmonaires Succédant à l’Iinhalation des Poussières de Coton, Byssinosis, In : Traité D’Hygiène Publique et Privée, G. Masson, ed., Paris, 7, 171.
[4] Massoud, A. (1964) The origin of the term ‘byssinosis’. Br. J. Ind. Med. 21, 162.
[5] Rylander, R. (1981) Bacterial toxins and etiology of byssinosis. Chest 79, 34S–38S.
| Bacterial toxins and etiology of byssinosis.Crossref | GoogleScholarGoogle Scholar |
[6] Campbell, J.M. (1932) Acute symptoms following work with hay. BMJ 2, 1143–1144.
[7] Pepys, J. et al. (1963) Farmer’s lung: thermophilic actinomycetes as a source of ’farmer’s lung hay’ antigen. Lancet 2, 607–611.
| Farmer’s lung: thermophilic actinomycetes as a source of ’farmer’s lung hay’ antigen.Crossref | GoogleScholarGoogle Scholar |
[8] Gangwar, M. et al. (1989) Distribution of clinically important thermophilic actinomycetes in vegetable substrates and soil in north-western India. Antonie van Leeuwenhoek 56, 201–209.
| Distribution of clinically important thermophilic actinomycetes in vegetable substrates and soil in north-western India.Crossref | GoogleScholarGoogle Scholar |
[9] Halpin, D.M. et al. (1994) Extrinsic allergic alveolitis and asthma in a sawmill worker: case report and review of the literature. Occup. Environ. Med. 51, 160–164.
| Extrinsic allergic alveolitis and asthma in a sawmill worker: case report and review of the literature.Crossref | GoogleScholarGoogle Scholar |
[10] Paściak, M. et al. (2014) An airborne actinobacteria Nocardiopsis alba isolated from bioaerosol of a mushroom compost facility. Aerobiologia 30, 413–422.
| An airborne actinobacteria Nocardiopsis alba isolated from bioaerosol of a mushroom compost facility.Crossref | GoogleScholarGoogle Scholar |
[11] Terho, E.O. (1990) Work‐related respiratory disorders among Finnish farmers. Am. J. Ind. Med. 18, 269–272.
| Work‐related respiratory disorders among Finnish farmers.Crossref | GoogleScholarGoogle Scholar |
[12] Unaogu, I.C. et al. (1994) Occurrence of thermophilic actinomycetes in natural substrates in Nigeria. Antonie van Leeuwenhoek 65, 1–5.
| Occurrence of thermophilic actinomycetes in natural substrates in Nigeria.Crossref | GoogleScholarGoogle Scholar |
[13] Lacey, J. (1988) Actinomycetes as biodeteriogens and pollutants of the environment. In: Actinomycetes in Biotechnology, M. Goodfellow et al., eds. Academic Press, London, pp. 359-432.
[14] Depierre, A. et al. (1988) Epidemiological study of farmer’s lung in five districts of the French Doubs province. Thorax 43, 429–435.
| Epidemiological study of farmer’s lung in five districts of the French Doubs province.Crossref | GoogleScholarGoogle Scholar |
[15] Kurup, V.P. (1984) Thermophilic actinomycetes: their role in hypersensitivity pneumonitis. In: Biological, Biochemical and Biomedical Aspects of Actinomycetes, Ortiz-Ortiz et al., eds: Academic Press, Orlando, pp. 145–159.
[16] Lechevalier, M.P. (1981) Ecological associations involving actinomycetes. Zentralbl. Bakteriol. 11, 159–166.
[17] Heo, K.J. et al. (2017) Effects of human activities on concentrations of culturable bioaerosols in indoor air environments. J. Aerosol Sci. 104, 58–65.
| Effects of human activities on concentrations of culturable bioaerosols in indoor air environments.Crossref | GoogleScholarGoogle Scholar |
[18] Phoolchund, H.N. (1991) Aspects of occupational health in the sugar cane industry. Occup. Med. 41, 133–136.
| Aspects of occupational health in the sugar cane industry.Crossref | GoogleScholarGoogle Scholar |
[19] Dales, R.E. and Munt, P.W. (1982) Farmer’s lung disease. Can. Fam. Physician 28, 1817–1820.
[20] Gascon, M. et al. (2012) Respiratory, allergy and eye problems in bagasse-exposed sugar cane workers in Costa Rica. Occup. Environ. Med. , .
| Respiratory, allergy and eye problems in bagasse-exposed sugar cane workers in Costa Rica.Crossref | GoogleScholarGoogle Scholar |
[21] Abdulla, B. et al. (2010) Serological detection of Thermoactinomyces vulgaris antigen in farmer’s lung disease patients using ELISA method. Egypt. J. Biol. Sci 2, 59–64.
[22] Ismail, T. et al. (2006) Extrinsic allergic alveolitis. Respirology 11, 262–268.
| Extrinsic allergic alveolitis.Crossref | GoogleScholarGoogle Scholar |
[23] Evans, G. and Smith, I. (2016) Risks to respiratory health in the grain industry. Derbyshire, UK: Health and Saftey Executive; Contract No: RR1083.
[24] Tizard, I. (1982) (ed.) An Introduction to Veterinary Immunology, 2nd edn. Elsevier.
[25] Kerr, L.A. and Linnabary, R.D. (1989) A review of interstitial pneumonia in cattle. Vet. Hum. Toxicol. 31, 247–254.
[26] Cross, T. and Johnston, D.W. (1971) Thermoactinomyces vulgaris. II. Distribution in natural habitats. In: Spore Research, A.N. Barker et al. (eds). Academic Press, Inc., London, pp. 315–330.
[27] McNeil, K. (1982) Health problems from storage of bagasses. Queensland. Published by Queensland Sugar Research Institute.
[28] Dawson, M.W. et al. (1996) The medical and epidemiologic effects on workers of the levels of airborne Thermoactinomyces spp. spores present in Australian raw sugar mills. Am. Ind. Hyg. Assoc. J. 57, 1002–1012.
| The medical and epidemiologic effects on workers of the levels of airborne Thermoactinomyces spp. spores present in Australian raw sugar mills.Crossref | GoogleScholarGoogle Scholar |
[29] Biggins, D. and Abrahams, H. (1991) Health effects of bagasse in Queensland sugar mills. J. Occup. Health Saf 7, 168–169.
[30] Brinkmann, C.M. et al. (2014) Detection of Thermoactinomyces species in selected agricultural substrates from Queensland. Microb. Ecol. 67, 804–809.
| Detection of Thermoactinomyces species in selected agricultural substrates from Queensland.Crossref | GoogleScholarGoogle Scholar |
[31] Švajlenka, J. et al. (2018) Biomonitoring the indoor environment of agricultural buildings. Ann. Agric. Environ. Med. 25, 292–295.
| Biomonitoring the indoor environment of agricultural buildings.Crossref | GoogleScholarGoogle Scholar |
Biographies
Candice Brinkmann is a graduate of the University of the Sunshine Coast (USC), Bachelor of Biomedical Science (year 2011), Honours 1st Class (2013) and currently is a PhD student at USC. Her Honours project involved the screening of thermoactinomycetes in overheated agricultural substrates in sub-tropical Qld, Australia. In her PhD, under the principal supervision of Dr İpek Kurtböke (USC), she investigates the bioactive compounds deriving from marine sponge associated bacteria, with the co-supervision provided by the Australian Institute of Marine Science and the Griffith Institute of Drug Discovery.
Dr İpek Kurtböke has been working in the field of biodiscovery and has been an active member of the international actinomycete research community since 1982. She currently conducts research and teaches in the field of applied microbiology and biotechnology and is senior lecturer at the University of the Sunshine Coast (USC), Queensland. She has also been an active member of the World Federation of Culture Collections (WFCC) including serving as the Vice-President of the Federation (2010–2013) and currently is the President of the Federation (2017–2020).


