Is nitrite from nitrification the only cause of microbiologically induced chloramine decay?
KC Bal Krishna A , Maneesha P Ginige B and Arumugam Sathasivan A CA School of Computing Engineering and Mathematics, Western Sydney University, Locked Bag 1797, Penrith, NSW 2750, Australia
B CSIRO Land and Water, Floreat, WA 6014, Australia
C Email: S.Sathasivan@westernsydney.edu.au
Microbiology Australia 39(3) 145-148 https://doi.org/10.1071/MA18044
Published: 13 August 2018
Nitrite, produced by ammonia oxidizing bacteria (AOB), was traditionally thought to be the only cause of microbiologically mediated decay of chloramine. The development and application of microbial decay factor method and bacterial community studies, for the first time have revealed many other factors such as soluble microbial products (SMPs) and bacteria other than AOB mediating the decay of chloramine.
In all states of Australia, chloramine (NH2Cl is formed using chlorine and ammonia – Cl2+NH3→NH2Cl+HCl) has been used as a secondary disinfectant in drinking water distribution systems to comply with drinking water quality guidelines. About seven million people in Australia drink chloraminated water. Chloramine offers several advantages over chlorine: chloramine continues to remain active in long water-supply systems even in warmer climates; it inhibits the growth of pathogens such as Naegleria fowleri1; and it produces fewer carcinogenic disinfection by-products2 in water distribution systems.
Although somewhat resilient towards decay (i.e. to Cl– and free NH3), as the chloraminated water travels along the distribution system, the disinfection residual gradually starts to decrease due to self-decay and chemical interactions of chloramine with water constituents (such as organic carbon, bromide, iron) that are prevalent in the distribution system3. The continuous release of free NH3 in smaller concentrations trigger growth of ammonia oxidising bacteria (AOB) in chloraminated distribution systems and AOB oxidise ammonia to nitrite that accelerates the decay of chloramine. With a focus on controlling accelerated decay of chloramine, much of the research was focused traditionally towards AOB communities in chloraminated systems. The AOB Nitrosomonas oligotropha and Nitrosospira were found ubiquitous in chloraminated systems4,5. In addition to AOB, heterotrophic bacteria (measured by the heterotrophic plate count method) were observed dominating specifically after/or closer to the onset of nitrification and in some instances, an increase of abundance has even been observed prior to the onset of nitrification4,6. The pH and temperature can impact AOB activity as well as chemical instability of chloramine3,6. The utility operators find it difficult to predict at which combination of chlorine, ammonia and temperature, nitrification starts to take place in a distribution system. Hence, they operate their reservoirs with frequent rechlorination to control free NH3 to the lowest possible level. However, even small operational interruptions lead to nitrification with an accelerated loss of chloramine in distribution systems.
Questioning the traditional belief that nitrite produced by AOB is the only cause of microbial chloramine decay and with the idea that chloramine decay should be measured to control residuals, Sathasivan et al.7 developed a microbial decay factor method to distinguish chemical and microbial mediated components of the decay. The method used filtration (0.2 µm) or inhibition (100 µg-silver L–1) to eliminate the microbial activity. According to the results of many full-scale water supply systems, chloramine decays rapidly despite nitrite concentrations being insignificant in bulk water. Unware of the overall microbial diversity along a chloraminated system, past research concluded nitrifiers were responsible for such decay. On comprehensively analysing bulk water samples of chloraminated distribution systems, Sathasivan et al.8 for the first-time reported the prevalence of many phases that have varying influence on the decay of chloramine. The first phase identified refers to chemical decay that is often observed in treated water and is at the front end of the system. The second is the mildly nitrified phase recognisable by the presence of a low nitrite concentration (<0.010 mg-N L–1) and a mild decay of chloramine (<0.0101 h–1). This phase is distinctly apparent prior to the onset of nitrification. The third and final phase is called severe nitrification, where an accelerated decay of chloramine (>0.0101 h–1) is noted. The prevalence of these three distinct phases suggests several different mechanisms of decay and there could be different microbes that are responsible and involved in each of these phases. Understanding and quantifying the decay mechanisms in each of these phases could lead to a better control of chloramine decay.
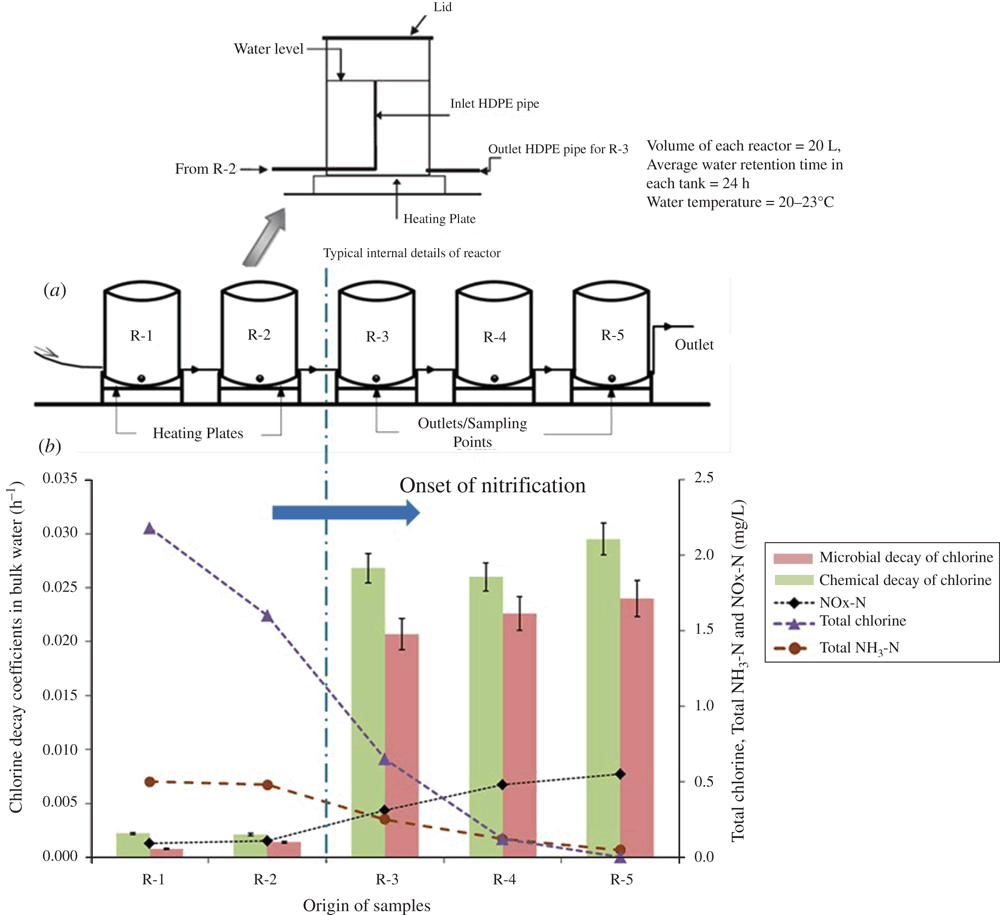
A lab-scale chloraminated distribution system9 was operated to capture the aforementioned three phases with the aim to facilitate a deeper understanding of the microbial decay mechanisms taking place in chloraminated distribution systems. The lab-scale system successfully simulated all three phases8 that are encountered in full-scale chloraminated systems. The chloramine residuals in the first two reactors (R-1 and R-2) were high with mild nitrification (nitrite < 0.010 mg-N L–1) and a rapid decrease of the residual was observed in the subsequent reactors (R-3 to R-5) with an onset of severe nitrification in R-3 (Figure 1). The bulk water pH dropped from 8.1 (in R-1) to 7.7 (in R-5) along the reactors.
The accelerated decay of chloramine with soluble microbial products (SMPs)
When the microbial decay factor method was applied to infer the chemical decay of the nitrified bulk water in each of the reactors in the lab-scale system (Figure 1a), the chemical decay rates derived simply could not be explained by the increase of bulk water nitrite concentrations and the decrease of pH alone10. The reason for the observed higher chemical decay rates were unclear and were hypothesised to be a result of novel dissolved organic carbon (DOC) constituent and/or SMPs released by microorganisms. A similar behaviour was also noted with bulk water in a full-scale chloraminated distribution system11. Further experiments with DOC free chloraminated water in the lab-scale reactor system revealed that accelerated chemical decay was primarily a result of SMPs12. SMPs are largely amines, carbohydrates, nucleic acids, proteins, and, polysaccharides. When bulk water was subjected to protein denaturing conditions (such as heat treatment, pH treatment, microwave irradiation, silver addition), it negatively impacted the chemical decay rate and according to this observation, the SMPs that accelerate chloramine decay were hypothesised to be protein-like in nature12.
In our studies10,12, the SMPs accelerating chloramine decay was evident only after the onset of nitrification. The reactors R-1 and R-2 (Figure 1a), which are upstream of R-3 (where the onset of nitrification occurs (Figure 1b)), also foster bacterial growth and these bacteria may also have released SMPs into the bulk water. However, due to a low abundance of bacteria, the bulk water SMPs concentration in these two reactors may not have reached the minimum specific concentration required to facilitate a noticeable decay of chloramine. Similarly, within the nitrified waters, there were microbes other than nitrifiers present9 and these microbes may also have contributed towards production of these SMPs13. While SMPs may accelerate chloramine decay, only a more comprehensive understanding of SMPs could enable development of engineering solutions to mitigate chloramine decay in chloraminated drinking water distribution systems.
Chloramine decay by microbes other than nitrifiers
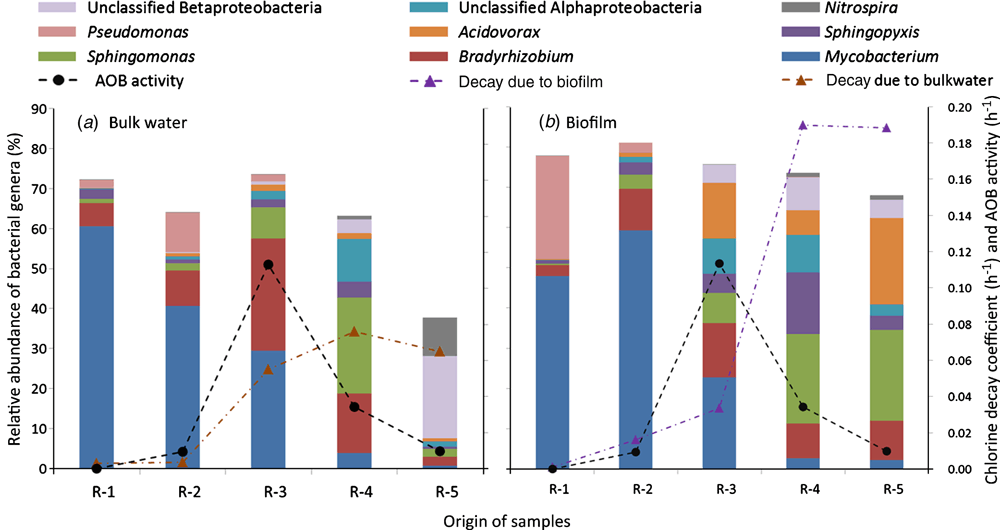
Both full and lab-scale studies have clearly demonstrated the prevalence of microbial decay of chloramine before the onset of nitrification in chloraminated distribution systems8,9,13. Well-known nitrifiers were rarely detected in both bulk water and biofilm (inlet HDPE pipe shown in Figure 1a) prior to the onset of nitrification, and bacterial genera such as Mycobacterium and Pseudomonas were found dominating in R-1 and R-2 of our lab-scale chloramination system (Figure 2). High abundances of Mycobacterium (41–61% in bulk water and 48–59% in biofilm) and Pseudomonas (2–10% in bulk water and 3–26% in biofilm) were observed before the onset of nitrification (Figure 2). Mycobacterium and Pseudomonas are both known to resist chlorine and chloramine and have also been observed in full-scale distribution systems14. While these bacterial genera are known to resist chloramine, the mechanism behind resistance and their role, if at all any, in the initial decay of chloramine remains unknown.
|
The AOB activity measured as NOx-N (nitrite-N + nitrate-N) production rate (detailed in Bal Krishna et al.9) peaks in R-3, and then rapidly declines in R-4 and in R-5 (Figure 2). Even with a decrease of AOB activity in R-4 and R-5, an increase of chloramine decay was observed (Figure 2), which could be a result of non-AOB bacterial activities. For instance, when Herath et al.15 operated the lab-scale reactor with chloraminated water containing a high DOC concentration of 10–12 mg L–1, mild nitrification prevailed (nitrite <0.012 mg-N L–1) but chloramine decay rates remained similar to when AOB activity prevailed in the lab-scale system. This observation provides evidence towards the possible involvement of non-AOB in the decay of chloramine.
The abundance of the AOB genus Nitrosomonas in reactors R-3 to R-5 ranged between 1.4–2.9% in bulk water and 0.9–1.4% in the biofilm (Figure 2). Accordingly, our lab-scale system was dominated by heterotrophic bacteria, both before and after the onset of nitrification (Figure 2). A decrease of AOB activity coincided with an increased abundance of other bacterial genera such as Sphingomonas, and Acidovorax (in the biofilm). These bacteria are metabolically diverse and have been demonstrated capable of decomposing halogenated compounds such as chlorinated biphenyls, halogenated diphenyl ethers, haloacetic acids, etc.16. The diverse metabolic capacity of these bacteria raises questions on whether they are able to directly or indirectly utilise chloramine.
In summary, accelerated chloramine loss after the onset of nitrification was noted due to SMPs that are protein-like in nature. There was no positive correlation between AOB activity and the chloramine decay rate (both in bulk water and biofilm) in the lab-scale system and this suggests a possible involvement of other bacteria in the decay of chloramine.
References
[1] Trolio, R. et al. (2008) Operational management of Naegleria spp. in drinking water supplies in Western Australia. Water Sci. Technol. Water Supply 8, 207–215.| Operational management of Naegleria spp. in drinking water supplies in Western Australia.Crossref | GoogleScholarGoogle Scholar |
[2] Brodtmann, N. et al. (1979) The use of chloramines for reduction of trihalomethanes and disinfection of drinking water. J. Am. Water Works Assoc. 71, 40–42.
| The use of chloramines for reduction of trihalomethanes and disinfection of drinking water.Crossref | GoogleScholarGoogle Scholar |
[3] Vikesland, P.J. et al. (2000) Reaction pathways involved in the reduction of monochloramine by ferrous iron. Environ. Sci. Technol. 34, 83–90.
| Reaction pathways involved in the reduction of monochloramine by ferrous iron.Crossref | GoogleScholarGoogle Scholar |
[4] Regan, J.M. et al. (2002) Ammonia- and nitrite-oxidizing bacterial communities in a pilot-scale chloraminated drinking water distribution system. Appl. Environ. Microbiol. 68, 73–81.
| Ammonia- and nitrite-oxidizing bacterial communities in a pilot-scale chloraminated drinking water distribution system.Crossref | GoogleScholarGoogle Scholar |
[5] Regan, J.M. et al. (2003) Diversity of nitrifying bacteria in full-scale chloraminated distribution systems. Water Res. 37, 197–205.
| Diversity of nitrifying bacteria in full-scale chloraminated distribution systems.Crossref | GoogleScholarGoogle Scholar |
[6] Wilczak, A. et al. (1996) Occurrence of nitrification in chloraminated distribution systems. J. Am. Water Works Assoc. 88, 74–85.
| Occurrence of nitrification in chloraminated distribution systems.Crossref | GoogleScholarGoogle Scholar |
[7] Sathasivan, A. et al. (2005) Simple method for quantifying microbiologically assisted chloramine decay in drinking water. Environ. Sci. Technol. 39, 5407–5413.
| Simple method for quantifying microbiologically assisted chloramine decay in drinking water.Crossref | GoogleScholarGoogle Scholar |
[8] Sathasivan, A. et al. (2008) Onset of severe nitrification in mildly nitrifying chloraminated bulk waters and its relation to biostability. Water Res. 42, 3623–3632.
| Onset of severe nitrification in mildly nitrifying chloraminated bulk waters and its relation to biostability.Crossref | GoogleScholarGoogle Scholar |
[9] Bal Krishna, K.C. et al. (2013) Microbial community changes with decaying chloramine residuals in a lab-scale system. Water Res. 47, 4666–4679.
| Microbial community changes with decaying chloramine residuals in a lab-scale system.Crossref | GoogleScholarGoogle Scholar |
[10] Bal Krishna, K.C. et al. (2010) Does an unknown mechanism accelerate chemical chloramine decay in nitrifying waters? J. Am. Water Works Assoc. 102, 96–104.
[11] Bal Krishna, K.C. et al. (2013) Wider presence of accelerated chemical chloramine decay in severely nitrifying conditions. Water Sci. Technol. Water Supply 13, 1090–1098.
| Wider presence of accelerated chemical chloramine decay in severely nitrifying conditions.Crossref | GoogleScholarGoogle Scholar |
[12] Bal Krishna, K.C. et al. (2012) Evidence of soluble microbial products accelerating chloramine decay in nitrifying bulk water samples. Water Res. 46, 3977–3988.
| Evidence of soluble microbial products accelerating chloramine decay in nitrifying bulk water samples.Crossref | GoogleScholarGoogle Scholar |
[13] Sathasivan, A. et al. (2011) Role of nitrification in accelerating chloramine decay through application of Microbial Decay Factor (Fm) method. Water Quality Technology Conference and Exposition 2011; Phoenix, AZ; 13 November 2011.
[14] Taylor, R.H. et al. (2000) Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 66, 1702–1705.
| Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium.Crossref | GoogleScholarGoogle Scholar |
[15] Herath, B.S. et al. (2015) Can microbes significantly accelerate chloramine decay without severe nitrification? Int. Biodeterior. Biodegradation 102, 231–236.
| Can microbes significantly accelerate chloramine decay without severe nitrification?Crossref | GoogleScholarGoogle Scholar |
[16] Hoefel, D. et al. (2009) Biodegradation of geosmin by a novel Gram-negative bacterium; isolation, phylogenetic characterisation and degradation rate determination. Water Res. 43, 2927–2935.
| Biodegradation of geosmin by a novel Gram-negative bacterium; isolation, phylogenetic characterisation and degradation rate determination.Crossref | GoogleScholarGoogle Scholar |
[17] Ginige, M. P. et al. (2017) Effectiveness of devices to monitor biofouling and metals deposition on plumbing materials exposed to a full-scale drinking water distribution system. PloS One 12, e0169140.
| Effectiveness of devices to monitor biofouling and metals deposition on plumbing materials exposed to a full-scale drinking water distribution system.Crossref | GoogleScholarGoogle Scholar |
Biographies
KC Bal Krishna is working as a postdoctoral research fellow at Western Sydney University, Australia. His research interests are understanding the role of microbes in degrading disinfectants residuals in water/recycled water distribution systems, investigating health-related microbes in water distribution systems, nutrients removal and recovery from wastewaters, polycyclic aromatic hydrocarbon removal from stormwater.
Maneesha Ginige is a senior research scientist at CSIRO Land and Water. His research interests are resource recovery from municipal wastewater, municipal and industrial wastewater treatment, membrane fouling, bioelectrochemical systems and anaerobic digestion.
Arumugam Sathasivan is a Professor of Water Quality Engineering in Western Sydney University. His research interests are chemical and microbial disinfectant (chlorine and chloramine) decay mechanisms, bacterial regrowth, biological water treatment, modelling of disinfectant decay and disinfection by-products formation, and sewer corrosion.