Mixed community biofilms and microbially influenced corrosion
Enrico Marsili A E , Staffan Kjelleberg A B C and Scott A Rice A C DA Singapore Centre for Environmental Life Sciences Engineering, Nanyang Technological University, Singapore
B School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW, Australia
C School of Biological Sciences, College of Science, Nanyang Technological University, Singapore
D ithree Institute, The University of Technology Sydney, Sydney, NSW, Australia
E Email: enrico.marsili1@gmail.com
Microbiology Australia 39(3) 152-157 https://doi.org/10.1071/MA18046
Published: 10 August 2018
Metals are used in most marine infrastructures for energy extraction and production. Metal corrosion is a serious concern, due to the environmental, safety, and replacement costs associated with it. Microbially influenced corrosion (MIC) contributes to the overall corrosion process, through several chemical, electrochemical and biochemical mechanisms, particularly in the presence of microbial biofilms. In this short article, we discuss briefly recent advances in MIC research, comparing corrosion in single species and mixed species biofilms, and outline possible strategies for biofilm and corrosion control.
The direct loss from corrosion in 1994 to the US industry was approximately 4% of the Gross National Product (GNP), with the highest cost in the power industry. A recent study estimates that the cost of corrosion in China is about 3.3% of the GNP, with the transportation and electronics industries bearing the highest costs. Older reports from Australia estimate a cost around 1.5% of the GNP, highlighting how corrosion control measures will contribute to national economy1–3.
While most materials are affected by corrosion and weathering processes, it is a concern for metals due to their ubiquitous applications and their importance in advanced technology infrastructures such as oil rigs and processing plants. The corrosion of metals in marine environments is much faster than in freshwater and particularly problematic due to the possible release of crude and processed oil in a sensitive environment, which might affect marine ecosystems.
Corrosion of metals in seawater depends primarily on the charge imbalance in the electrical double layer at the metal/water interface; the presence of impurities that start pitting corrosion; and the co-occurrence of different metals, which create sites for galvanic corrosion. However, it is well established that microorganisms also play an important role in the corrosion process, known as microbially influenced corrosion (MIC). Mechanisms invoked to explain MIC include the formation of differential aeration cells caused by oxygen respiration; production of corrosive agents such as sulfide by sulfate-reducing bacteria (SRB) and organic and/or inorganic acids; metal-deposition; hydrogen embrittlement; the metal-binding effect of extracellular polymeric substance (EPS), and inactivation of corrosion inhibitors, with no specific mechanism playing a major role3. Most MIC mechanisms hypothesise that SRB play the major role in MIC, yet corrosion clearly also occurs in sulfate-free, anaerobic and even aerobic environments, although at lower rates. This highlights that many other microorganisms might contribute to MIC in mixed microbial communities.
Most recent strategies for corrosion inhibition draw from current experience in medical biofilm removal and focus on dispersal/inhibition, rather than lethal treatments. This opens new avenues for sustainable corrosion control in the shipping and oil and gas industries. In the following, we will outline a few aspects of MIC in biofilms and seawater, and the related research prospects.
Single species biofilms
Research on simple systems, such as monospecies biofilms, has revealed the existence of multiple MIC mechanisms. In fact, biofilms can either protect the metal surface from corrosion or enhance the corrosion rate, depending on the species considered. For example, Pseudomonas aeruginosa was shown to accelerate the corrosion of different grades of duplex steel and nickel-free stainless steel4. The same species promoted corrosion of nickel-copper coatings yet inhibited corrosion of nickel-zinc coatings5. Biofilms formed by Vibrio neocaledonicus appeared to inhibit corrosion of carbon steel in artificial seawater6, while Chlorella vulgaris accelerated stainless steel corrosion in seawater7. The dual roles of biofilms in MIC were recently reviewed8, and it was suggested that these variable effects might depend on biofilm matrix overproduction in monospecies biofilm or removal of oxygen through aerobic respiration.
Using these monospecies systems, some of the MIC mechanisms that have been demonstrated or hypothesised include adsorption or chelation of metals by proteins and the formation of an anaerobic/aerobic interface, which results in surface deterioration and depletion of the passivation layer, respectively. Work on electroactive bacteria, such as Geobacter sp.9 and Shewanella sp.10 has shown that direct electron transfer from the metal to the cells in the biofilm can enhance corrosion rates. The extracted EPS from iron-oxidising bacteria can either inhibit or enhance corrosion effects, depending on its concentration, age, and the presence of active enzymes11. Further, some of these differences in whether an organism is protective or corrosive could also be related to differences in medium composition and environmental conditions, which strongly affect the physiological activity of the microorganisms involved. SRB biofilms show enhanced corrosion under starvation conditions and this has been linked to their use of elemental iron as energy source in the absence of an organic energy source12. Similar results were observed for P. aeruginosa biofilms on carbon steel13, where the starved sessile cells switched to elemental iron as an electron donor. Further, deaeration methods change the outcome of biocorrosion for stainless and mild steel as well as titanium14. It is also possible that the source and quality of the steel plays a role in these differences, due to the presence of impurities, which initiate the corrosion process. Overall, these and other studies on monospecies biofilms suggests that MIC is common in microorganisms and one should look beyond SRB to explain MIC in sulfate-depleted environments. They also highlight the multiplicity of MIC mechanisms and the need for standardisation of approaches to readily compare results across different studies14.
Mixed biofilms
While research on monospecies biofilms has revealed much about the roles and mechanisms of microorganisms with respect to corrosion, there are relatively few habitats that are comprised of a single bacterial species. Indeed, most habitats would be comprised of hundreds or thousands of species of microorganisms and this would certainly be true for steel structures in marine habitats. However, studies of multispecies biofilms are complex, with higher levels of variation (thus requiring increased replication). The challenges here are that most studies of complex biofilms have investigated natural communities, which are highly variable with season and environmental conditions (e.g. nutrient concentration, temperature, etc). In contrast, fewer studies on defined communities that enable more detailed mechanistic studies have been reported.
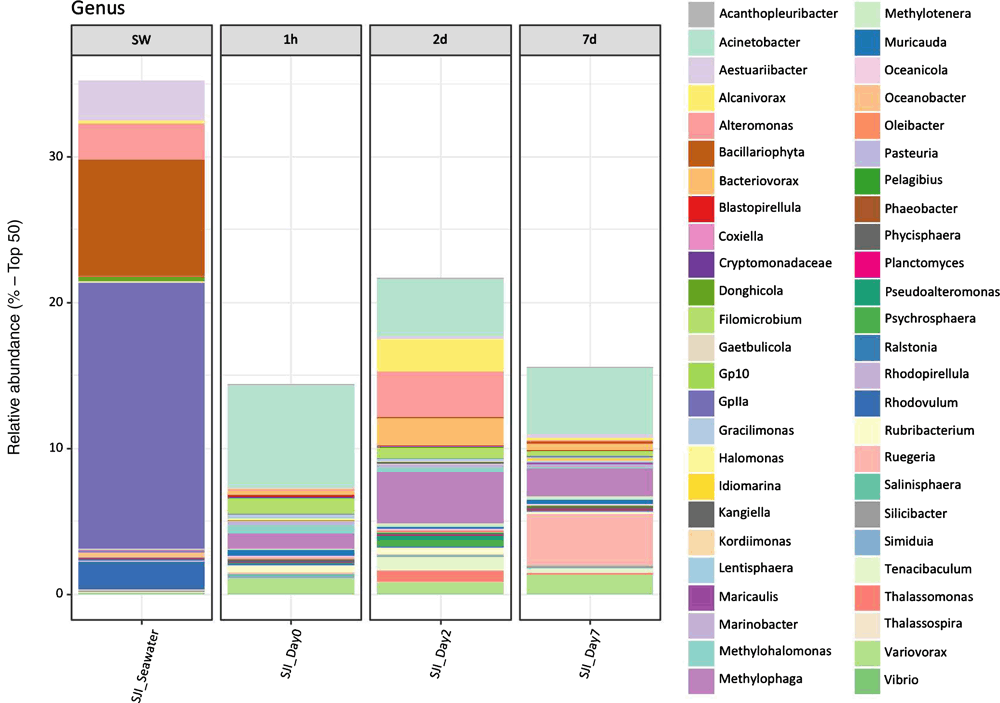
In contrast to monospecies systems, mixed microbial communities change with time, and this should be accounted for when studying MIC. For example, the succession of microorganisms during colonisation and the community diversity observed on concrete materials indicate that the community decreases in diversity as MIC proceeds and the pH decreases. A similar study on mild steel in the marine environment showed that iron-oxidising bacteria are early colonisers and other bacteria join the community later, which can accelerate the MIC process15. Our work16 showed a marked difference between mixed microbial communities from seawater before and after biofilm formation on stainless steel coupons (Figure 1), which is similar to what observed in oral biofilm development, where there is a reproducible pattern of colonisation. The different succession patterns observed in concrete and steel suggest that the formation of a mixed community associated with MIC is a complex phenomenon, and it may be that the early colonisers are also habitat-modifying organisms that produce the niches appropriate for the corroding organisms. For example, the formation of a biofilm under aerobic conditions can nonetheless result in anaerobic pockets where the anaerobes thrive. Similarly, the activity of community members can change nutrient availability, alter nitrogen and sulfate species as well as provide a spatially structured environment for microorganisms to interact through the exchange of metabolites. It is our opinion, supported by some experimental evidence, that such community systems can be used in the laboratory to incorporate this high level of diversity to study how microorganisms function at the system level to cause corrosion.
|
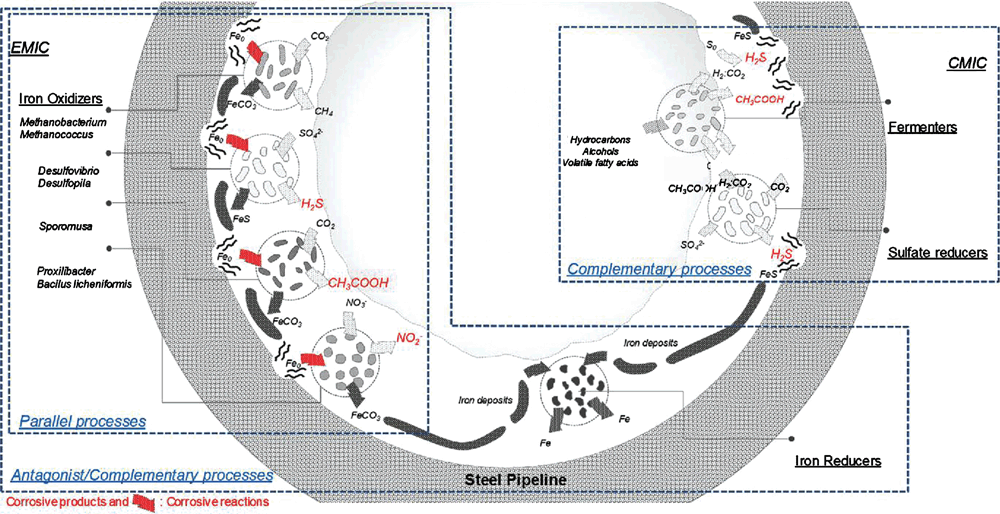
Research on the microstructure and microbial ecology of mixed microbial communities and biofilms has helped to increase our understanding of MIC and its underlying mechanisms. Recent studies on the biofilm matrix17 have shown how the matrix can concentrate corrosive metabolites at the interface, thus accelerating local corrosion rates. Examples include sulfide accumulation in SRB18 and the formation of SRB biofilms on inorganic sulfides deposited on steel19. The matrix can also facilitate the concentration of quorum-sensing effectors, and thus accelerate cell–cell communication-based gene expression responses20. Finally, biofilm electroactivity has provided further theoretical background to explain MIC in terms of direct electron transfer between the metal surface and biofilms, particularly under starvation conditions21. The type of metal used can also select for different communities, where we have observed strikingly different communities on low-grade stainless steel and superduplex stainless steel, most likely due to the presence of metals that are toxic to microorganisms (unpublished data). The complex microbial ecology of marine biofilms elicits multiple corrosion routes, which are affected by iron oxidisers, iron reducers, methanobacteria, fermenters and other specialised microorganisms (Figure 2)22.
|
Control of biofilms associated with MIC
One of the highly sought-after goals of MIC studies is to develop effective control measures. These can either be in the form of ways to keep the corroding microorganisms in check or to develop advanced surveillance methods that can readily give operators better information on in situ corrosion rates. For example, biocide enhancers such as D-tyrosine and D-methionine increase activity of tetrakis hydroxymethyl phosphonium sulfate (THPS) and can help to delay biofilm formation23, thus maintaining an intact passivation layer, which reduces the corrosion rate. Further, hydrogen peroxide inhibits microbial colonisation but did not increase abiotic corrosion, and the addition of MgO2 slows down SRB growth, thus inhibiting corrosion on carbon steel24. Virulent phage have been also proposed to target microorganisms associated to MIC in mixed microbial communities, although there are few demonstrations of this in practice.
Microbial composition is not the only MIC determinant. The biofilm microstructure seems also important, as patchy biofilms do not protect from corrosion in saline media, while a uniform, homogenous biofilm layer does appear to protect25. The presence of inorganic deposits can protect bacteria from the external environment and thus decrease the activity of biocides in real seawater26.
Sub-lethal biofilm treatments
While conventional treatments for MIC site sanitation use highly toxic agents, such as glutaraldehyde and benzalkonium chloride, MIC control strategies typically use sub-lethal concentrations of chemical agents that disperse biofilm and minimise the extent of corrosion. This approach was first developed for biomedical equipment and only recently extended to MIC. However, no commercial product is available yet. For example, 100 ppm D-methionine decreased corrosion of monospecies Desulfovibrio biofilms by 50% without any effect on the planktonic cells, indicating the mitigation is due to biofilm dispersal only27. Similar effects were observed for mixed species biofilm consortia from an oilfield12. Additionally, a mixture of amino acids has been shown to partially inhibit MIC in mixed biofilms23, presumably by altering community metabolism such that the corrosive metabolites were not produced or did not accumulate. MIC can be enhanced by the presence of microbially produced mediators, such as flavins, which enhance electron uptake from the metal surface28. Thus, removal of these mediators might help in mitigating MIC. Other MIC control strategies use surfactants29 and mixed-type inhibitors carbazole derivatives to inhibit SRB biofilm formation30. Ideally, biofilm inhibitors should be applied at the metal surface, and engineered to allow slow release over long periods of time. Alternatively, the inhibitors could be triggered for release in response to the presence of corrosion-related organisms, which could be achieved by metabolic, physiological or physical conditions (e.g. low pH) specific for corroding organisms. Thus, there are a range of mild biofilm control approaches that could be used to delay or reduce corrosion rates, although they remain to be demonstrated in situ. Some of the challenges around such approaches are related to the cost (due to the large volume/surface to be treated) and method of application. For example, a coating would be most likely to localise activity where it is needed, but is subject to loss of function due to damage to the coating and depletion of active compounds over time31.
Summary and Conclusions
There is a considerable appreciation for the roles of microorganisms in mediating corrosion processes in conjunction with abiotic mechanisms. However, further studies on how microbial biofilm communities form on, and contribute to, the corrosion of metals and other materials, are needed. The challenges partly lie in the complexity of the problem, with multiple organisms working together, each influencing their neighbours as well as the surface in question. Then there is the challenge of understanding the microbe-surface nexus which is further complicated by batch variations of materials and compositional differences. However, the introduction of reproducible community systems, coupled with solid interdisciplinary collaboration and advanced analysis approaches may help to further elucidate how microorganisms manage to modify their environment in such a dramatic fashion.
References
[1] El-Sherik, A.M. (2017) Trends in Oil and Gas Corrosion Research and Technologies. Woodhead Publishing.[2] Hou, B. et al. (2017) The cost of corrosion in China. Npj Mater. Degrad. 1, 4.
| The cost of corrosion in China.Crossref | GoogleScholarGoogle Scholar |
[3] Javaherdashti, R. (2008) Microbiologically Influenced Corrosion: An Engineering Insight. Springer-Verlag.
[4] Li, H.B. et al. (2017) Microbiologically influenced corrosion behavior of S32654 super austenitic stainless steel in the presence of marine Pseudomonas aeruginosa biofilm. J. Mater. Sci. Technol. 33, 1596–1603.
| Microbiologically influenced corrosion behavior of S32654 super austenitic stainless steel in the presence of marine Pseudomonas aeruginosa biofilm.Crossref | GoogleScholarGoogle Scholar |
[5] San, N.O. et al. (2014) Microbially influenced corrosion and inhibition of nickel-zinc and nickel-copper coatings by Pseudomonas aeruginosa. Corros. Sci. 79, 177–183.
| Microbially influenced corrosion and inhibition of nickel-zinc and nickel-copper coatings by Pseudomonas aeruginosa.Crossref | GoogleScholarGoogle Scholar |
[6] Moradi, M. et al. (2015) Introducing a novel bacterium, Vibrio neocaledonicus sp., with the highest corrosion inhibition efficiency. Electrochem. Commun. 51, 64–68.
| Introducing a novel bacterium, Vibrio neocaledonicus sp., with the highest corrosion inhibition efficiency.Crossref | GoogleScholarGoogle Scholar |
[7] Liu, H.W. et al. (2018) Microbiologically influenced corrosion of 316L stainless steel in the presence of Chlorella vulgaris. Int. Biodet. Biodeg. 129, 209–216.
| Microbiologically influenced corrosion of 316L stainless steel in the presence of Chlorella vulgaris.Crossref | GoogleScholarGoogle Scholar |
[8] Kip, N. et al. (2015) The dual role of microbes in corrosion. ISME J. 9, 542–551.
| The dual role of microbes in corrosion.Crossref | GoogleScholarGoogle Scholar |
[9] Mehanna, M. et al. (2009) Effect of Geobacter sulfurreducens on the microbial corrosion of mild steel, ferritic and austenitic stainless steels. Corros. Sci. 51, 2596–2604.
| Effect of Geobacter sulfurreducens on the microbial corrosion of mild steel, ferritic and austenitic stainless steels.Crossref | GoogleScholarGoogle Scholar |
[10] Miller, R.B. et al. (2018) Uniform and pitting corrosion of carbon steel by Shewanella oneidensis MR-1 under nitrate-reducing conditions. Appl. Environ. Microbiol. 84, e00790-18.
| Uniform and pitting corrosion of carbon steel by Shewanella oneidensis MR-1 under nitrate-reducing conditions.Crossref | GoogleScholarGoogle Scholar |
[11] Liu, H.W. et al. (2017) The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria. Corros. Sci. 114, 102–111.
| The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria.Crossref | GoogleScholarGoogle Scholar |
[12] Liu, H.W. et al. (2018) Corrosion of X80 pipeline steel under sulfate-reducing bacterium biofilms in simulated CO2-saturated oilfield produced water with carbon source starvation. Corros. Sci. 136, 47–59.
| Corrosion of X80 pipeline steel under sulfate-reducing bacterium biofilms in simulated CO2-saturated oilfield produced water with carbon source starvation.Crossref | GoogleScholarGoogle Scholar |
[13] Jia, R. et al. (2017) Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation. Corros. Sci. 127, 1–9.
| Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation.Crossref | GoogleScholarGoogle Scholar |
[14] Javed, M.A. et al. (2017) Effect of sulphate-reducing bacteria on the microbiologically influenced corrosion of ten different metals using constant test conditions. Int. Biodet. Biodeg. 125, 73–85.
| Effect of sulphate-reducing bacteria on the microbiologically influenced corrosion of ten different metals using constant test conditions.Crossref | GoogleScholarGoogle Scholar |
[15] McBeth, J.M. and Emerson, D. (2016) In situ microbial community succession on mild steel in estuarine and marine environments: exploring the role of iron-oxidizing bacteria. Front. Microbiol. 7, 767.
| In situ microbial community succession on mild steel in estuarine and marine environments: exploring the role of iron-oxidizing bacteria.Crossref | GoogleScholarGoogle Scholar |
[16] Jogdeo, P. et al. (2017) Onset of microbial influenced corrosion (MIC) in stainless steel exposed to mixed species biofilms from equatorial seawater. J. Electrochem. Soc. 164, C532–C538.
| Onset of microbial influenced corrosion (MIC) in stainless steel exposed to mixed species biofilms from equatorial seawater.Crossref | GoogleScholarGoogle Scholar |
[17] Flemming, H.C. et al. (2016) Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575.
| Biofilms: an emergent form of bacterial life.Crossref | GoogleScholarGoogle Scholar |
[18] Li, Y. et al. () Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: a review. J. Mater. Sci. Technol. , .
[19] Dec, W. et al. (2017) Characterization of Desulfovibrio desulfuricans biofilm on high-alloyed stainless steel: XPS and electrochemical studies. Mater. Chem. Phys. 195, 28–39.
| Characterization of Desulfovibrio desulfuricans biofilm on high-alloyed stainless steel: XPS and electrochemical studies.Crossref | GoogleScholarGoogle Scholar |
[20] McDougald, D. et al. (2012) Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 10, 39–50.
| Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal.Crossref | GoogleScholarGoogle Scholar |
[21] Xu, D. and Gu, T. (2011) Bioenergetics explains when and why more severe MIC pitting by SRB can occur. NACE Corrosion Conference.
[22] Vigneron, A. et al. (2018) Damage to offshore production facilities by corrosive microbial biofilms. Appl. Microbiol. Biotechnol. 102, 2525–2533.
| Damage to offshore production facilities by corrosive microbial biofilms.Crossref | GoogleScholarGoogle Scholar |
[23] Jia, R. et al. (2017) Electrochemical testing of biocide enhancement by a mixture of D-amino acids for the prevention of a corrosive biofilm consortium on carbon steel. Ind. Eng. Chem. Res. 56, 7640–7649.
| Electrochemical testing of biocide enhancement by a mixture of D-amino acids for the prevention of a corrosive biofilm consortium on carbon steel.Crossref | GoogleScholarGoogle Scholar |
[24] Chang, Y.J. et al. (2015) A study of microbial population dynamics associated with corrosion rates influenced by corrosion control materials. Int. Biodet. Biodeg. 102, 330–338.
| A study of microbial population dynamics associated with corrosion rates influenced by corrosion control materials.Crossref | GoogleScholarGoogle Scholar |
[25] Batmanghelich, F. et al. (2017) Influence of multispecies biofilms of Pseudomonas aeruginosa and Desulfovibrio vulgaris on the corrosion of cast iron. Corros. Sci. 121, 94–104.
| Influence of multispecies biofilms of Pseudomonas aeruginosa and Desulfovibrio vulgaris on the corrosion of cast iron.Crossref | GoogleScholarGoogle Scholar |
[26] Skovhus, T.L. et al. (2017) Management and control of microbiologically influenced corrosion (MIC) in the oil and gas industry-Overview and a North Sea case study. J. Biotechnol. 256, 31–45.
| Management and control of microbiologically influenced corrosion (MIC) in the oil and gas industry-Overview and a North Sea case study.Crossref | GoogleScholarGoogle Scholar |
[27] Xu, D. et al. (2014) D-Methionine as a biofilm dispersal signaling molecule enhanced tetrakis hydroxymethyl phosphonium sulfate mitigation of Desulfovibrio vulgaris biofilm and biocorrosion pitting. Mat. Corr. Werkst. Korr. 65, 837–845.
| D-Methionine as a biofilm dispersal signaling molecule enhanced tetrakis hydroxymethyl phosphonium sulfate mitigation of Desulfovibrio vulgaris biofilm and biocorrosion pitting.Crossref | GoogleScholarGoogle Scholar |
[28] Zhang, P.Y. et al. (2015) Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 101, 14–21.
| Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm.Crossref | GoogleScholarGoogle Scholar |
[29] Labena, A. et al. (2015) The biocidal effect of a novel synthesized gemini surfactant on environmental sulfidogenic bacteria: Planktonic cells and biofilms. Mat. Sci. Engin. C-Mat. Biol. Appl. 47, 367–375.
[30] Nwankwo, H.U. et al. (2017) Experimental, quantum chemical and molecular dynamic simulations studies on the corrosion inhibition of mild steel by some carbazole derivatives. Sci. Rep. 7, 2436.
[31] Guo, J. et al. (2018) Polymers for combating biocorrosion. Front. Mater. 5, 10.
| Polymers for combating biocorrosion.Crossref | GoogleScholarGoogle Scholar |
Biographies
Dr Enrico Marsili received his PhD in 2005 from University of Rome, Italy. Following a 2-year postdoc at University of Minnesota and 4 years as Lecturer in Ireland, he joined the Singapore Centre for Environmental Life Sciences Engineering, Singapore, as Principal Scientist. He contributes to research in microbial corrosion, biosensor development and microbial ecology of electroactive biofilms.
Staffan Kjelleberg is centre director of the Singapore Centre for Environmental Life Sciences Engineering (SCELSE) at Nanyang Technological University and National University of Singapore. SCELSE conducts fundamental and translational research of the behaviour of microbial biofilm communities across natural and urban ecosystems and habitats, including bacterial biofilm biology, chemically mediated interactions used by bacteria and higher organisms, and harnessing/controlling biofilms for engineering and public health applications. Prior to establishing SCELSE, Prof Kjelleberg was co-founder/co-director of the Centre for Marine Bio-Innovation at University of New South Wales, Australia.
Scott A Rice completed his PhD in 1996 from the University of Tennessee, USA, after which time he took up a post-doctoral position at The Centre for Marine Bio-Innovation at UNSW. More recently, he has taken a position at The Singapore Centre for Environmental Life Sciences Engineering and the School of Biological Sciences at Nanyang Technological University. His interests are in microbial communities, with a focus on their function and formation.


