Coupling anaerobic bacteria and microbial fuel cells as whole-cell environmental biosensors
Lara T Bereza-Malcolm A and Ashley E Franks A BA Applied and Environmental Microbiology
Department of Physiology, Anatomy and Microbiology
School of Life Sciences
College of Science, Health and Engineering
La Trobe University
Melbourne, Vic. 3086, Australia
B Tel: +61 3 9479 2206, Email: a.franks@latrobe.edu.au
Microbiology Australia 36(3) 129-132 https://doi.org/10.1071/MA15045
Published: 14 August 2015
Microorganisms have evolved to respond to environmental factors allowing adaption to changing conditions and minimisation of potential harm. Microbes have the ability to sense a wide range of biotic and abiotic factors including nutrient levels, analytes, temperature, contaminants, community quorum, and metabolic activity. Due to this ability, the use of whole-cell microbes as biosensors is attractive as it can provide real-time in situ information on biologically relevant factors through qualitative and quantitative outputs. Interestingly, many of the environments where these biosensors will be of most of use lack oxygen; and as such the use of anaerobic microorganisms to sense environmental factors with easy to use outputs is essential. Furthermore, sensing of contaminants can be linked with bioremediation of known contaminated environments, allowing a flexible, multiplexed device.
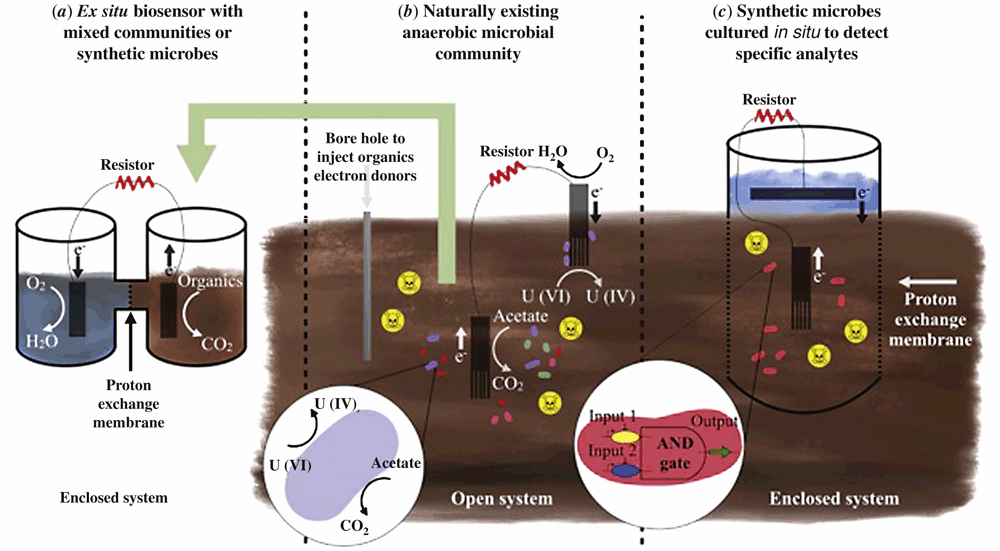
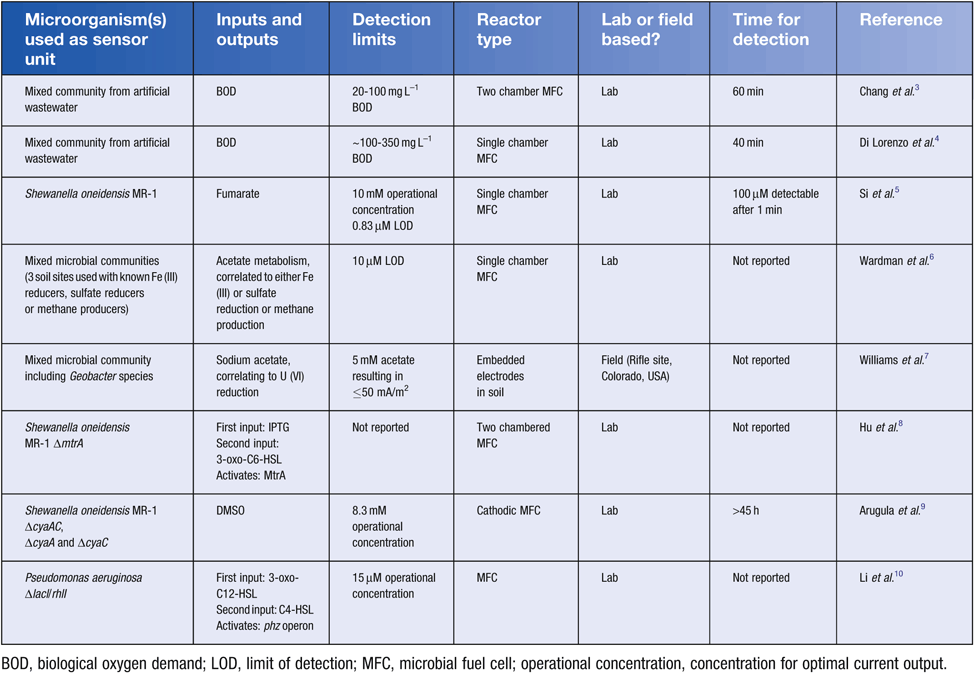
Traditionally, a whole-cell microbial biosensor is described as an analytic device consisting of microorganisms that produce a measurable output in response to a particular stimulus1. In the current literature, microbial biosensors are developed in laboratory based E. coli strains, consisting of a cloned gene pathway incorporating regulatory and reporter genes1, e.g. gfp expression under regulation by an arsenic sensitive regulatory protein2. Microbial fuel cell (MFC) technology however, is now being incorporated with both naturally existing and synthetic microorganisms to produce novel biosensing devices, for detection of environmentally relevant compounds (Table 1). Initially MFCs were developed for energy production via the oxidation of organic matter, utilising anaerobic bacteria such as Geobacter, Shewanella, Desulfuromonas, Rhodopseudomonas, and Desulfovibrio11. Species from these genera are essential in microbial community driven oxidation-reduction reactions, necessary for biochemical cycling, and transformation of elements, such as carbon (C), nitrogen (N), sulfur (S) and Iron (Fe), through the biosphere12. MFC designs typically consist of electrodes that act as electron acceptors and donors, allowing redox reactions to occur at a distance while creating an electrical current (Figure 1). In the environment, MFCs take advantage of the microorganisms associated with biogeochemical cycles that are able to utilise electrodes as electron acceptors or donors. The electrode-associated microbes are able to extracellularly transfer electrons directly from their central metabolism to an electrode surface through a network of shuttles, cytochromes, and electrical conductive nanowires11. Since the electrons come directly from central metabolism, the current produced is a direct measurement of metabolic activity of the electrode associated community11. However, such processes can be limited by factors, such as O2 availability, pH levels, available substrates and electron donor availability12,13. Nevertheless, there has been interest in utilising naturally existing anaerobic microbial communities as biosensors in the environment where a correlation can be determined between available substrates and current production14.
|
Electrically integrated microbial biosensor
Organic matter content in an environmental system is normally evaluated in terms of the biochemical oxygen demand (BOD). BOD is a measurement of the required dissolved oxygen to completely degrade the organic matter15. MFCs allow the use of the electrode for anaerobic respiration to gain similar information about organic oxidation rates in real-time. A single-chamber MFC containing a mixed anaerobic community has been used as a biosensor for the quantification of glucose. The sensor response was linear for concentration of glucose between 0.025 g L−1 up to 25 g L−1. Unfortunately, such sensors are difficult to implement outside of laboratory conditions due to issues with the reliability of the biosensors, as a new anaerobic community is required for each sample tested15.
Investigation into developing a cost-effective MFC biosensor has resulted in a Subsurface Microbial Activity in Real Time (SMART) system, which allows constant monitoring of anaerobic microbial activity in soils6. Three sediment samples were tested with known anaerobic communities responsible for either Fe(III) reduction, sulfate reduction, or methane production. Electrical current production was correlated with the degradation of tracer acetate [uniformly 14C labelled] with Fe (III), sulfate, or methane acting as a terminal electron acceptor6. These lab-based MFC biosensors thus allow the ex situ monitoring of organic compound degradation from added sediment samples (Figure 1a). When placed in situ, these devices will primarily be useful for monitoring relative changes in microbial activity in response to environmental perturbations. These microbial systems are sensitive to environmental variations, such as temperature and seasonal changes, and may not be ideal to be implemented as in situ standalone devices. However, investigation of current production in these anode-resistor-cathode systems provides insight into microbial activity in sediments, and may allow monitoring of the microbial activity of communities responsible for the transformations of important organic compounds in anoxic environments.
Bioremediation of contaminants
Direct or mediated electron transfer between microbes and an electrode may allow degradation or transformation of pollutants in bioremediation processes16. Compounds such as metals are unable to be degraded, but can have their solubility reduced. This is essential in preventing their spread and contamination16,17. For example, Geobacter sulfurreducens is capable of dissimilatory metal reduction, a process where energy is conserved through oxidizing organic or inorganic electron donors while reducing a metal or metalloid18. Geobacter species capable of dissimilatory metal reduction have been applied in a practical on-site in situ MFC biosensor, using acetate, an intermediate compound produced during metabolic processes7, to drive reduction of soluble U(VI) to less-soluble U(IV). Anodes were installed down-gradient of the site of bioremediation and a cathode was embedded on the soil surface (Figure 1b). This allowed a correlation to be determined between the injections of acetate at the site of contamination, with a significant increase in current7. This spike in activity also allowed monitoring of changing subsurface water flow through the use of microbial activity measurements, in a grid pattern well set up. Thus this multiplexed sensor allows for monitoring of uranium reduction, while acting as an on-site bioreactor, removing the need for continuous sampling and off-site testing7. The use of a cathode alone can provide the redox potential required for promoting microbial activity, while simplifying the system. In this case, the cathode acts as an electron donor and provides metabolic energy to the subsoil microbial population. These devices can be solar powered, left in remote locations indefinitely, and not require any further input. It is important to note that in subsoil systems undergoing bioremediation, a good understanding of electron donor or acceptor limitations, metabolic activity and community function are essential for efficient remediation.
Synthetically derived electric biosensors
Currently, anaerobic biosensors rely on naturally existing microbial communities to degrade organics, however there has been investigation into incorporating synthetically derived biosensing pathways into MFC devices. Such systems have utilised Boolean logic gate ideas, which are based on modular computer based decision circuits, such as AND, NOT and OR gates10,19. For example, for an AND gate, two inputs must be present for a target gene to be transcribed. A synthetic biosensor has been produced that allows incorporation of a range of known regulator genes, and is based on a quorum sensing system. In a demonstration system, IPTG and quorum sensing modules were created through the use of lacl and luxR8. In the presence of both IPTG and 3-oxo-C6-HSL inducers, an output module would be activated. To integrate this reporter system to an electrode, MtrA was utilised as an output in a S. oneidensis ΔmtrA mutant strain. This mutant is unable to produce an electrical current due to disruption of the Mtr extracellular electron transfer (EET) system8. Thus, to return this pathway back to functionality, both IPTG and 3-oxo-C6-HSL need to be present to activate the logic gate and MtrA production. This synthetic biosensor demonstrates the potential to clone alternate input/output systems, in particular regulator elements with a high specificity for dangerous contaminants in place of Lacl and LuxR, with the detection as an electrical output8. However, problems can arise through mutations to the sensors through random mutation events, or selection via the contaminants present, which may affect functionality. Further research is also necessary to incorporate safety mechanisms into the synthetic microbes to deter fears of accidental release of genetically modified microorganisms. However with further research synthetic microbes may effectively be utilised in ex situ (Figure 1a) or in situ (Figure 1c) MFC biosensing systems.
Future directions and conclusions
Anaerobic bacteria may provide value for biosensing technologies to monitor anoxic zones6,20. Their ability to naturally cycle contaminants, along with survivability in contaminated soils and waterways is allowing incorporation into both biosensing devices and bioreactors. Many anaerobic biosensors developed are generally reliant on MFC type designs because the ability to utilise an external electron acceptor provides an easy-to-monitor output, in the form of current. Furthermore, these designs have already been linked to degradation of organics, or immobilisation of metals. However, such outputs are subject to environmental fluctuations and as thus may not always be a reliable detection method. Further investigation by monitoring the metabolism of microbes associated with anodes21 is also still necessary. Currently, there is promise in incorporating synthetic microbes into the anode compartment to produce a biosensing device for a range of contaminants. Hence, whole-cell microbial biosensors based in anaerobic microbes may provide a cost effective means of detection and bioremediation, allowing long-term monitoring that may be deployed in a variety of environments.
References
[1] Bereza-Malcolm, L.T. et al. (2015) Environmental sensing of heavy metals through whole cell microbial biosensors: a synthetic biology approach. ACS Synth. Biol. 4, 535–546.| 1:CAS:528:DC%2BC2cXhslahsLfO&md5=26225debd2297f9300e3809471fc4823CAS | 25299321PubMed |
[2] Liao, V.H.C. and Ou, K.L. (2005) Development and testing of a green fluorescent protein‐based bacterial biosensor for measuring bioavailable arsenic in contaminated groundwater samples. Environ. Toxicol. Chem. 24, 1624–1631.
| Development and testing of a green fluorescent protein‐based bacterial biosensor for measuring bioavailable arsenic in contaminated groundwater samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlslWku70%3D&md5=7d2c3bd31658f9daa69d9df3b393080bCAS |
[3] Chang, I.S. et al. (2004) Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens. Bioelectron. 19, 607–613.
| Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpvVWrs7w%3D&md5=87b304f540074c37842ebcd1d7b11e10CAS | 14683644PubMed |
[4] Di Lorenzo, M. et al. (2009) A single-chamber microbial fuel cell as a biosensor for wastewaters. Water Res. 43, 3145–3154.
| A single-chamber microbial fuel cell as a biosensor for wastewaters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnslGrurk%3D&md5=9eb1f6df40446dbdbf737a88d9b57aedCAS | 19482326PubMed |
[5] Si, R.W. et al. (2015) A whole-cell electrochemical biosensing system based on bacterial inward electron flow for fumarate quantification. Biosens. Bioelectron. 68, 34–40.
| A whole-cell electrochemical biosensing system based on bacterial inward electron flow for fumarate quantification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXlvFyhtg%3D%3D&md5=333ee9c2bed6e86fc52ad88c49a85bbaCAS | 25558872PubMed |
[6] Wardman, C. et al. (2014) Real-time monitoring of subsurface microbial metabolism with graphite electrodes. Front. Microbiol. 5, 621.
| Real-time monitoring of subsurface microbial metabolism with graphite electrodes.Crossref | GoogleScholarGoogle Scholar | 25484879PubMed |
[7] Williams, K.H. et al. (2010) Electrodic voltages accompanying stimulated bioremediation of a uranium-contaminated aquifer. J. Geophys. Res. 115, G00G05.
[8] Hu, Y. et al. (2015) Programming the quorum sensing-based AND gate in Shewanella oneidensis for logic gated-microbial fuel cells. Chem. Commun. 51, 4184–4187.
| Programming the quorum sensing-based AND gate in Shewanella oneidensis for logic gated-microbial fuel cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvFCmsbw%3D&md5=177dcf354d343cfade714d1a7c657bf6CAS |
[9] Arugula, M.A. et al. (2012) Molecular AND logic gate based on bacterial anaerobic respiration. Chem. Commun. 48, 10174–10176.
| Molecular AND logic gate based on bacterial anaerobic respiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtl2hur7M&md5=b9273c8e807c8b6e79bdd05ba056e7fdCAS |
[10] Li, Z. et al. (2011) Bacteria-based AND logic gate: a decision-making and self-powered biosensor. Chem. Commun. 47, 3060–3062.
| Bacteria-based AND logic gate: a decision-making and self-powered biosensor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1eqsrg%3D&md5=2b28fe818aff661072434b8f069c0ea9CAS |
[11] Semenec, L. and Franks, A.E. (2014) The microbiology of microbial electrolysis cells. Microbiol. Aust. 35, 201–206.
| The microbiology of microbial electrolysis cells.Crossref | GoogleScholarGoogle Scholar |
[12] Paerl, H.W. and Pinckney, J.K. (1996) A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb. Ecol. 31, 225–247.
| A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling.Crossref | GoogleScholarGoogle Scholar | 8661534PubMed |
[13] Rivett, M.O. et al. (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res. 42, 4215–4232.
| Nitrate attenuation in groundwater: a review of biogeochemical controlling processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOjsb3M&md5=861def5d94ed5cf76c75a776bdce3404CAS | 18721996PubMed |
[14] Bond, D.R. and Lovley, D.R. (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69, 1548–1555.
| Electricity production by Geobacter sulfurreducens attached to electrodes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitlCls7k%3D&md5=67fdf4951bf111674a34e2ccb1f28bbcCAS | 12620842PubMed |
[15] Kumlanghan, A. et al. (2007) Microbial fuel cell-based biosensor for fast analysis of biodegradable organic matter. Biosens. Bioelectron. 22, 2939–2944.
| Microbial fuel cell-based biosensor for fast analysis of biodegradable organic matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXlsFKmsrw%3D&md5=feaab25e5f1647cb862b0196cd3ae57fCAS | 17223031PubMed |
[16] Lovley, D.R. (2003) Cleaning up with genomics: applying molecular biology to bioremediation. Nat. Rev. Microbiol. 1, 35–44.
| Cleaning up with genomics: applying molecular biology to bioremediation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptFWlu7w%3D&md5=1caab38bf2eabc199062585f093f810eCAS | 15040178PubMed |
[17] Anderson, R.T. et al. (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69, 5884–5891.
| Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotlGqurg%3D&md5=8bb8a4826d78f3194b2a31af4e88e951CAS | 14532040PubMed |
[18] Caccavo, F. et al. (1994) Geobacter sulfurreducens sp. nov., a hydrogen-and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60, 3752–3759.
| 1:CAS:528:DyaK2cXmt1KgsL4%3D&md5=2f2dd74fbf525cfcb692fc160823fb97CAS | 7527204PubMed |
[19] Wang, B. et al. (2013) A modular cell-based biosensor using engineered genetic logic circuits to detect and integrate multiple environmental signals. Biosens. Bioelectron. 40, 368–376.
| A modular cell-based biosensor using engineered genetic logic circuits to detect and integrate multiple environmental signals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlKjsr3E&md5=bce333a09080ccbe35e19f6dbad1f0bfCAS | 22981411PubMed |
[20] Coates, J.D. et al. (1997) Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl. Environ. Microbiol. 63, 3589–3593.
| 1:CAS:528:DyaK2sXmtVKhtbo%3D&md5=fa7ee5efbbd2a8cfe83e90653f3dec98CAS | 9341091PubMed |
[21] Aracic, S. et al. (2014) Investigating microbial activities of electrode-associated microorganisms in real-time. Front. Microbiol. 5, 663.
| 25506343PubMed |
Biographies
Lara Bereza-Malcolm is a PhD candidate at Dr Ashley Franks laboratory in the College of Science, Health and Engineering, at La Trobe University. Her research focus is on the development of novel whole-cell microbial biosensors for the detection of heavy metals in the environment.
Ashley E Franks is an Associate Professor in the Department of Physiology, Anatomy and Microbiology at La Trobe University and head of the Applied and Environmental Microbiology research Laboratory. He conducted his doctorate research as part of the Centre of Marine Biofouling and Bioinnovation at the University of New South Wales by investigating antifungal compounds produced by marine bacteria in biofilms. During his PhD he spent 4 months at the University of Exeter in the UK on an Adrian Lee fellowship to develop dual bacterial/yeast biofilm systems. On graduating he moved to the Biomerit Research Centre in Cork, Ireland to work on bacterial plant interactions at as a Government of Ireland Fellow in Science Technology and Engineering. This research looked at how to use bacteria to help plant growth. He then took a position as a Senior Scientist and Research Professor within the Geobacter Project at the University of Massachusetts Amherst in the USA where he worked on microbes that make electricity. His lab currently has research interests in ecology, electric microbiology and microbiome research.