Campylobacter survival through poultry processing
Lesley DuffyFood Safety and Stability
CSIRO Animal, Food & Health Sciences
39 Kessels Road,
Coopers Plains Qld 4053,
Australia Tel: +61 7 3214 2055
Fax: +61 7 3214 2062
Email: Lesley.Duffy@csiro.au
Microbiology Australia 34(2) 67-69 https://doi.org/10.1071/MA13023
Published: 13 May 2013
Australia has recorded around 100 cases of campylobacteriosis per 100,000 population, each year, since the mid-1990’s. Campylobacter jejuni and C. coli are recognized as the main species isolated from clinical cases. Approximately 30% of cases have been linked to poultry. Through poultry processing, from slaughter to packaging, the prevalence and concentration of Campylobacter can be reduced. Published Australian data on the effect of current processing conditions are minimal. Data from other countries suggests that the stages of scalding and immersion chilling can have significant impact on the prevalence and concentration of Campylobacter. Understanding the complexities of these processing stages (physical, chemical and microbiological) and their effect on Campylobacter species may lead to improved control during processing and hence improved public health outcomes.
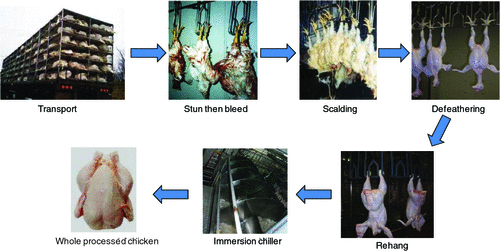
Campylobacter spp. are the leading cause of bacterial gastroenteritis in Australia and most of the western world. While most cases are sporadic in nature rather than outbreak related, poultry has been associated with 30% of all cases in Australia1. Poultry are the natural host of this organism with C. jejuni and C. coli considered the predominant species. Flocks can become contaminated from as early as 14 to 21 days of age2. Once Campylobacter enters a flock during the rearing period, it spreads rapidly such that flocks can be contaminated at high levels at slaughter, dependent on age3. Poultry are slaughtered and prepared for sale through a multistage process (Figure 1). This process can be described in stages: 1. stunning, either electrical or gas; 2. bleeding, severing of the carotid artery and jugular vein; 3. scalding, at temperatures from 53°C to 58°C for approximately two to three minutes to loosen feathers; 4. defeathering, removal of feathers; 5. evisceration, removal of the viscera; 6. washing, both inside and outside of the chicken carcase to remove gross organic contamination; 7. chilling, water immersion from 30 min to 3 h or air chilling from 60 to 80 min, to drop the temperature of the carcase and 8. packaging or further processing. Campylobacter can survive each of these processing steps and subsequent storage through to retail and food preparation for poultry to be a source of human infection. Although there is no specific processing step that will kill Campylobacter spp., good control of both scalding and chilling can significantly reduce the concentration of Campylobacter spp.4. Studies have been published in a number of countries that examine the change in prevalence and on the concentration of Campylobacter spp. at the various processing stages. A reduction in the concentration of Campylobacter spp. by 2 log10 can lead to a reduction in the number of human cases by up to 30 times5.
|
A systematic review of the prevalence of Campylobacter through poultry processing was published by Guerin4. This review of 29 separate published studies covering different stages of the process, highlights the highly variable nature of the effects of various poultry processing stages. Scalding decreased the prevalence of Campylobacter anywhere between 20 and 40% while defeathering increased the prevalence between 10 and 72% from four studies. A decrease in prevalence of between 10 and 100% was found after chilling in 6 of the 9 studies which examined this stage, while there was an increase in prevalence after chilling up to 27% in the other three studies. The process of immersion chilling has been demonstrated to lead to cross contamination events which may in part explain an increase in prevalence after chilling. A recent Australian study of four flocks found no decrease in prevalence from pre-scald to pre-chill and reductions in prevalence within two flocks after chill of 10 and 20%6. More important than prevalence alone, knowledge on the effect on the concentration of Campylobacter at each processing stage is more limited although both scalding and chilling stages are frequently reported to result in a decrease in concentration of Campylobacter4. Scalding temperature affects the extent of reduction in Campylobacter spp. concentration as does the equipment with counter-flow multi stage scalding tanks decreasing the level of contamination. In countries where chlorination of the chilling water is allowed, significant reductions can be made with improved control of chlorine and pH levels within the chilling tanks. The decrease in the concentration of Campylobacter within New Zealand processed chickens has in part been attributed to the better control of these parameters in processing7. Chlorine dissolves in water to form hypochlorous acid and hypochlorite ion8. Hypochlorous acid is the most biocidal form although the formation of these two compounds is pH dependent. The acid form is very reactive being both an oxidizing and halogenating species and therefore the level of free available chlorine in conjunction with pH and contact time will determine the effectiveness of chlorine as a disinfectant on poultry8.
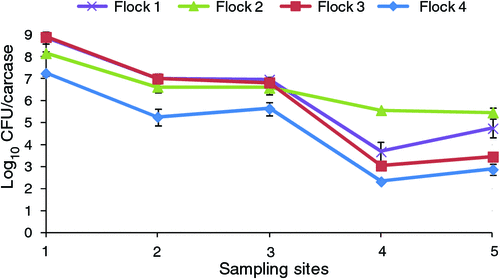
An Australian study measured the concentration of Campylobacter spp. at each stage during processing6 (Figure 2). Significant reductions were achieved after scalding and again after chilling. No significant changes in concentration were noted after evisceration or after packaging. A few studies have examined the effect of scalding temperatures and chlorine levels under laboratory conditions on the decimal reduction times (D values). A single strain of Campylobacter had D55C values in scald tank water of 0.2 min for planktonic grown cells compared with 2.2 min for cells attached to chicken skin. Sub-populations were noted that had increased D55C values of 13.9 min in water and 19.4 min attached to skin. These sub-populations may indicate a level of resistance within the Campylobacter population. When the same strain was subjected to chlorine at 50ppm, D50ppm values were recorded of 0.5 min in water compared to 73.0 min when attached to chicken skin with no sub-population detected. New Zealand Campylobacter isolates from poultry do not have unusual heat resistance and have similar heat resistance in the planktonic state as those belonging to the sub-populations mentioned above (D55C 8.5 – 17.0 min)9. No heat or chlorine resistance data are available on Australian isolates.
|
The factors that influence the effectiveness of the immersion chiller in the Australian situation where chlorine is a permissible processing aid, are numerous and complex. Examining chickens from two separate flocks, processed at the same abattoir with the same measured pH and chlorine levels in the immersion chiller does not always produce a similar decrease in the concentration of Campylobacter6. Clearly other aspects of poultry production at the chilling stage, both physical and chemical, can have a significant impact on the survival of Campylobacter. Consideration must also be given to the strain to strain variation common in Campylobacter studies and the extensive variation in the genetic makeup of this organism compared to other enteric bacteria previously noted by Park10. The genotypic variation within the Campylobacter genus may allow specific genotypes to occur or be selected for, when encountering environmental stresses11.
Understanding the changes that Campylobacter spp. undergo when subjected to typical processing temperatures and chilling (chlorine and pH) conditions in conjunction with an understanding of how these are applied within the technical aspects of poultry production, may be key to ensuring future declines in both prevalence and concentration of Campylobacter spp. on poultry products. This may lead to improved public health outcomes.
References
[1] Stafford, R.J. et al. (2008) Population-attributable risk estimates for risk factors associated with Campylobacter infection, Australia. Emerg. Infect. Dis. 14, 895–901.| Population-attributable risk estimates for risk factors associated with Campylobacter infection, Australia.Crossref | GoogleScholarGoogle Scholar | 18507899PubMed |
[2] Wagenaar, J.A. et al. (2008) Poultry colonization with Campylobacter and its control at the primary production level. In Campylobacter (Third edn) (Nachamkin, I., et al., eds), ASM Press.
[3] Berndtson, E. et al. (1996) Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int. J. Food Microbiol. 32, 35–47.
| Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FjsVKksg%3D%3D&md5=e56b94a69e0ab88dde2ea4a95f9892bcCAS | 8880326PubMed |
[4] Guerin, M.T. et al. (2010) The change in prevalence of Campylobacter on chicken carcasses during processing: a systematic review. Poult. Sci. 89, 1070–1084.
| The change in prevalence of Campylobacter on chicken carcasses during processing: a systematic review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c3kt1yisA%3D%3D&md5=08139c116a63293be7b4cd9f131a06cbCAS | 20371862PubMed |
[5] Rosenquist, H. et al. (2003) Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 83, 87–103.
| Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens.Crossref | GoogleScholarGoogle Scholar | 12672595PubMed |
[6] Duffy, L.L. et al. (2011) Survival of Campylobacter through the poultry processing chain. In 16th International Workshop on Campylobacter, Helicobacter & Related Organisms.
[7] Sears, A. et al. (2011) Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand. Emerg. Infect. Dis. 17, 1007–1015.
| Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand.Crossref | GoogleScholarGoogle Scholar | 21749761PubMed |
[8] FAO/WHO (2008) Benefits and risks of the use of chlorine-containing disinfectants in food production and food processing, report of a joint FAO/WHO expert meeting. http://www.fao.org/ag/agn/agns/files/Active%20Chlorine%20Report%20Version%20Final%20December%202009.pdf
[9] Sakkaf, A.A. and Jones, G. (2012) Thermal inactivation of Campylobacter jejuni in broth. J. Food Prot. 75, 1029–1035.
| Thermal inactivation of Campylobacter jejuni in broth.Crossref | GoogleScholarGoogle Scholar |
[10] Park, S.F. (2002) The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 74, 177–188.
| The physiology of Campylobacter species and its relevance to their role as foodborne pathogens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhslSqtLw%3D&md5=636a41c97b018c65899fd526765562f1CAS | 11981968PubMed |
[11] Wassenaar, T.M. et al. (1998) Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 64, 1816–1821.
| 1:CAS:528:DyaK1cXislWlsL4%3D&md5=cd8d187be431966d15c575629e92bb8dCAS | 9572956PubMed |
Biography
Lesley Duffy is a Research Microbiologist with CSIRO. Her research projects have included the ecology of E. coli O157 and Salmonella in red meat production systems including beef, sheep and goat; source tracking of Listeria in cooked meat and ready-to-eat food production facilities; and the survival of E. coli O157 during the manufacture of fermented meat products. Lesley’s current research project examines the ecology of Campylobacter during poultry processing and the selection and survival of specific genotypes through this process.


