Foodborne viruses: a focus on challenges associated with detection methods
Gail E GreeningEnvironmental and Food Virology, Food Group,
Institute of Environmental Science and Research
Kenepuru Science Centre
PO Box 50-348
Porirua, New Zealand
Tel: +64 4 9140 700/+61 7 3410 8794
Email: Gail.greening@esr.cri.nz
Microbiology Australia 34(2) 63-66 https://doi.org/10.1071/MA13022
Published: 13 May 2013
Human enteric viruses are now recognised as one of the commonest causes of foodborne disease with norovirus and hepatitis A virus (HAV) the main viruses implicated in foodborne outbreaks. Norovirus is the main cause of acute nonbacterial gastroenteritis worldwide. Foods at risk of virus contamination are bivalve shellfish, fresh produce, manually prepared ready to eat (RTE) foods and bakery products. Analysis of foods for virus presence is challenging for many reasons. Complex food matrices present processing problems for efficient recovery and detection of viruses, current molecular methods do not allow for determination of virus infectivity and low virus copy number in foods means that exquisitely sensitive methods and multiple controls are required for virus detection. There are still no international standard methods for viral analysis of foods. However significant progress towards a standard method for detection of norovirus and HAV in foodstuffs has been made by a European Committee for Standardisation (CEN) technical working group. This method is due for publication in 2013 as a two-part joint ISO/CEN Technical Specification. In later years it will be replaced by a fully validated standard.
Foodborne viral disease has emerged as a major public health problem in recent years. The globalisation of our food supply means that fresh produce may be sourced from countries where quality control and hygiene practices do not meet international standards. Food can become contaminated at either at the pre-harvest or post-harvest stage. Bivalve shellfish such as mussels, oysters, clams and fresh produce, including lettuce, salad greens, herbs and berry fruits, are most at risk of preharvest contamination. Food may be irrigated or washed in water containing human faecal material, or handled by field-workers with poor hygiene practices. Filter feeding bivalve shellfish grown in sewage-contaminated waters accumulate and concentrate enteric viruses and other microbial contaminants as they feed. Bacteria are eliminated from shellfish within a few days, but viruses are known to persist and retain their infectivity for several weeks or months in the shellfish gut. Although molecular methods do not determine whether these viruses are infectious, their presence and low infectious dose (<100 particles may cause infection) indicate there may be a risk of infection if shellfish are consumed either raw or lightly cooked.
Enteric viruses such as norovirus and HAV are transmitted by the faecal – oral route, excreted in human faeces and hence pass into the sewage systems. They are present in sewage effluent and discharges, septic tank leachates and also drinking, growing, irrigation and bathing waters contaminated with sewage or faecal material. These viruses are very resistant to environmental stressors and may persist in the environment for long periods.
Postharvest contamination results from unsafe foodhandling practices. Foods most at risk are uncooked or lightly cooked foods, salads, cold meats, bakery products and other RTE foods subject to extensive handling during preparation (Figure 1). Foods implicated in recent norovirus and HAV foodborne outbreaks in Australia and New Zealand include blueberries1, semi-dried tomatoes2,3, shellfish4–6 and foodhandling7,8. In some of these outbreaks, norovirus or HAV were identified in both the implicated food and associated cases. Additional genotyping of virus strains may aid in establishing the source of outbreaks.
|
Analysis for pathogenic viruses is very different to bacterial analysis. Neither enrichment culture techniques nor in vitro culture can be used because human norovirus and wild-type HAV are not able to be grown in cell culture yet. Therefore real-time quantitative (RT-) PCR is generally used for virus detection but this technique does not determine infectivity. Detection of viruses in foods is challenging due to the following issues:
-
Low virus copy number in foods
-
Complex food matrices are difficult to analyse and may be inhibitory to PCR
-
Efficiency of virus recovery is generally poor and can be as low as 1–10%
-
Many controls are required to monitor each stage of the detection process
-
Infectivity data are not provided by current molecular methods and culture is not an option for norovirus and wild-type HAV
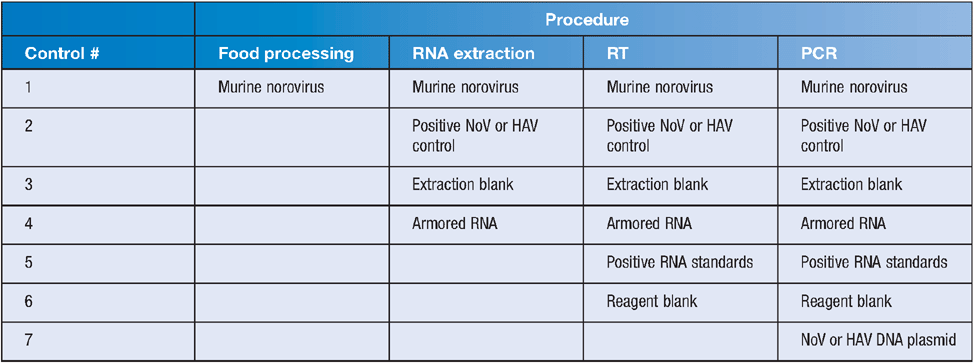
The internationally required agreements for PCR controls for food testing are described in ISO 22174 : 2005. Many controls are required to check each stage of the viral analysis process (Table 1). These include a process control added at the start of the viral extraction procedure to provide information on virus recovery efficiency and, in the RT-PCR assay, an internal amplification control (IAC) for RT-PCR inhibition, a positive RT-PCR control, viral RNA standards, negative RT-PCR controls and an extraction blank. The IAC and process controls monitor for cross contamination, false positives, assay sensitivity and reproducibility between runs. Virus quantitation is often required for shellfish analysis. This can be achieved by establishing the limit of detection and the limit of quantitation for each assay, then using plasmid copy standard curves to determine sample quantities. The target DNA sequence is cloned into a plasmid to produce a range of standard copy numbers which are included in each run. As these DNA transcripts do not provide quality control for the RT step, RNA standards and the IAC are included to monitor RT efficiency and detect inhibition. Analysis of foods for viruses is costly because of the number of replicate samples tested, the number of controls and standards required per sample and the controls required for each PCR run.
|
There are currently no international standard methods for virus detection in foods and shellfish. However, in 2004 the European Committee for Standardization (CEN) established a technical advisory group (CEN/TC275/WG6/TAG4) comprising 30 members from 13 countries to develop a standard method for detection of norovirus and HAV in foodstuffs - namely bivalve shellfish, salad crops, soft fruits, bottled water and hard surfaces9. This method is to be published in 2013 as Technical Specification CEN ISO/TS 15216; Microbiology of food and animal feed – Horizontal method for detection of hepatitis A virus and norovirus in food using real-time RT-PCR (TS). Methods for virus detection in RTE foods are not included in the CEN method and there are few published methods10–12. The range of RTE food matrices is extensive (e.g. pasta, potato salad, dairy and bakery products, sliced meats) and so virus recovery processes need to be adapted for each matrix.
The CEN method is currently undergoing validation and, when completed, the TS will be replaced with a fully validated CEN/ISO standard in 2018. Despite the lack of formal validated methods, a European National Reference Laboratory (NRL) ring trial for detection and quantitation of norovirus and HAV in lenticules and shellfish tissue is carried out annually with over 25 laboratories worldwide participating, including New Zealand, Australian, Asian and North American laboratories. Several laboratories use their own methods in combination with components of the CEN method. The results show that overall the CEN methods are consistently performing well. The EU plans to introduce legislation for regulatory testing once the method is complete (personal communication Dr J Lowther, CEFAS, UK).
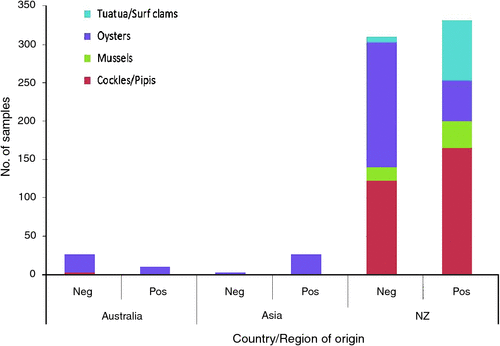
Since 2007, the ESR Environmental and Food Virology Laboratory (EFVL) has gained 100% accuracy in the European NRL trial using its ISO 17025 accredited method for detection of norovirus in shellfish13. This method, based on the CEN method, has been used for analysis of over 700 Australasian and Asian shellfish samples referred to the EFVL laboratory from water and shellfish quality monitoring programmes, outbreak investigations, product clearances, export screening and following sewage discharge events (Figure 2). Norovirus was detected in varying concentrations from low (<80 copies/g of gut tissue) to high (>10,000 copies/g) in 368/709 (52%) of shellfish analysed (Figure 3) including 48/74 (65%) shellfish associated with outbreak investigations.

|
|
The main drawback of molecular methods is their inability to provide information on virus infectivity. Recently, new approaches to differentiate between inactivated and infectious viruses have been evaluated. The principal hypothesis is that non-infectious viruses have damaged capsids and so may be more susceptible to stressors and may also be unable to bind to cellular antigens. Capsid damage may expose the RNA genome and allow it to be degraded. Researchers have evaluated pre-treatment or exposure of samples to different agents, including heat, chlorine/hypochlorite, UV, RNAse, proteinase K, oxidative damage and propidium monoazide (PMA) prior to RT-PCR14–17. Pretreatment by enzymes may damage capsids and/or viral RNA14,15. Oxidative damage to capsid is measured by biotin labeling of the damaged capsid16. PMA is a light activated intercalating chemical which can damage RNA once capsid damage has occurred 17.
In a different approach, long target (~3kb) and short target (~100bp) RT18 or PCR19 were used to distinguish between infectious and inactivated viruses based on the premise that long genome sequences could be amplified in infectious viruses, but only short sequences could be amplified in inactivated viruses where the RNA genome may be partially degraded.
All of these approaches have been carried out using culturable viruses so that the methodologies can be compared with conventional cell culture. They have shown that it may be possible to distinguish between infectious and inactivated viruses by molecular methods but further research to validate these methods is required. As some of these approaches are technically complex, they may more useful in research applications rather than for routine analysis.
Current molecular detection and quantification methods for viruses are used in many applications. These include food and water quality control and monitoring programmes, outbreak investigations, prevalence studies, and for tracing contamination sources. In conjunction with real-time RT-PCR methods, where culture methods are available for culturable HAV and surrogate viruses, virus persistence, survival and inactivation studies can be carried out to develop appropriate intervention and control strategies.
References
[1] Calder, L. et al. (2003) An outbreak of hepatitis A associated with consumption of raw blueberries. Epidemiol. Infect. 131, 745–751.| An outbreak of hepatitis A associated with consumption of raw blueberries.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3svgvFSmuw%3D%3D&md5=6f3405e9367f246847a8fe7b233fe959CAS | 12948375PubMed |
[2] Petrignani, M. et al. (2010) Update: a food-borne outbreak of hepatitis A in the Netherlands related to semi-dried tomatoes in oil. Eurosurveillance 15, January–February 2010.
[3] European Centre for Disease Control Threat Assessment (2010) Food-borne HAV outbreaks in Australia, France and the Netherlands. 26 February 2010.
[4] Simmons, G. et al. (2007) A New Zealand outbreak of norovirus gastroenteritis linked to the consumption of imported raw Korean oysters. N. Z. Med. J. 120, U2773.
| 17972982PubMed |
[5] Webby, R.J. et al. (2007) Internationally distributed frozen oyster meat causing multiple outbreaks of norovirus infection in Australia. Clin. Infect. Dis. 44, 1026–1031.
| Internationally distributed frozen oyster meat causing multiple outbreaks of norovirus infection in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s7msV2iug%3D%3D&md5=8134073eb4f871a7e2249e7b6503d45dCAS | 17366444PubMed |
[6] Conaty, S. et al. (2000) Hepatitis A in New South Wales, Australia from consumption of oysters: the first reported outbreak. Epidemiol. Infect. 124, 121–130.
| Hepatitis A in New South Wales, Australia from consumption of oysters: the first reported outbreak.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7osFaitg%3D%3D&md5=a5b0873abb64063f6292b00cdf59a84cCAS | 10722139PubMed |
[7] Thornley, C.N. et al. (2013) Multiple outbreaks of norovirus linked to an infected post symptomatic food handler. Epidemiol. Infect. , .
| Multiple outbreaks of norovirus linked to an infected post symptomatic food handler.Crossref | GoogleScholarGoogle Scholar | 23388349PubMed |
[8] McLean, M. et al. (2001) Norwalk-like virus gastroenteritis linked to a food handler. New Zealand Public Health Report 8, 65–68.
[9] Lees, D. and CEN WG6 TAG4 (2010) International standardisation of a method for detection of human pathogenic viruses in molluscan shellfish. Food Environ. Virol. 2, 146–155.
| International standardisation of a method for detection of human pathogenic viruses in molluscan shellfish.Crossref | GoogleScholarGoogle Scholar |
[10] Stals, A. et al. (2011) Evaluation of a norovirus detection methodology for ready-to-eat foods. Int. J. Food Microbiol. 145, 420–425.
| Evaluation of a norovirus detection methodology for ready-to-eat foods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1SlsL4%3D&md5=88dbbe584a73bbaad5aff1242edf3dedCAS | 21333370PubMed |
[11] Baert, L. et al. (2008) Evaluation of viral extraction methods on a broad range of ready-to-eat foods with conventional and real-time RT-PCR for Norovirus GII detection. Int. J. Food Microbiol. 123, 101–108.
| Evaluation of viral extraction methods on a broad range of ready-to-eat foods with conventional and real-time RT-PCR for Norovirus GII detection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtVChsrw%3D&md5=7e7b02b464561f7b72b7d79131294732CAS | 18258325PubMed |
[12] Rutjes, S.A. et al. (2006) Detection of noroviruses in foods: a study on virus extraction procedures in foods implicated in outbreaks of human gastroenteritis. J. Food Prot. 69, 1949–1956.
| 16924922PubMed |
[13] Greening, G.E. and Hewitt, J. (2008) Norovirus detection in shellfish using a rapid, sensitive virus recovery and real-time RT-PCR detection protocol. Food Analyt. Meth. 1, 109–118.
| Norovirus detection in shellfish using a rapid, sensitive virus recovery and real-time RT-PCR detection protocol.Crossref | GoogleScholarGoogle Scholar |
[14] Nuanualsuwan, S. and Cliver, D.O. (2002) Pretreatment to avoid positive RT-PCR results with inactivated viruses. J. Virol. Methods 104, 217–225.
| Pretreatment to avoid positive RT-PCR results with inactivated viruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkslGnsLw%3D&md5=0a0dcc62fcfbda4985bb5e1f148a9176CAS | 12088831PubMed |
[15] Topping, J.R. et al. (2009) Temperature inactivation of feline calicivirus vaccine strain FCV F-9 in comparison with human noroviruses using an RNA exposure assay and reverse transcribed quantitative real-time polymerase chain reaction – A novel method for predicting virus infectivity. J. Virol. Methods 156, 89–95.
| Temperature inactivation of feline calicivirus vaccine strain FCV F-9 in comparison with human noroviruses using an RNA exposure assay and reverse transcribed quantitative real-time polymerase chain reaction – A novel method for predicting virus infectivity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitVClur4%3D&md5=f8571563515817c2bb72922e6783b08eCAS | 19028524PubMed |
[16] Sano, D. et al. (2010) Detection of oxidative damages on viral capsid protein for evaluating structural integrity and infectivity of human norovirus. Environ. Sci. Technol. 44, 808–812.
| Detection of oxidative damages on viral capsid protein for evaluating structural integrity and infectivity of human norovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGkurfO&md5=020be03e69b509f7da2aa6094e110cb6CAS | 20000802PubMed |
[17] Parshionikar, S. et al. (2010) Use of propidium monoazide in reverse transcriptase PCR to distinguish between infectious and noninfectious enteric viruses in water samples. Appl. Environ. Microbiol. 76, 4318–4326.
| Use of propidium monoazide in reverse transcriptase PCR to distinguish between infectious and noninfectious enteric viruses in water samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptFOjtr8%3D&md5=ab2b5d68d5623d51fab66816d84fe83bCAS | 20472736PubMed |
[18] Wolf, S. et al. (2009) Long-range reverse transcription as a useful tool to assess the genomic integrity of norovirus. Food Environ. Virol. 1, 129–136.
| Long-range reverse transcription as a useful tool to assess the genomic integrity of norovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFOhtLzI&md5=5c5f8968d673677d5ad5ac2184758c8cCAS |
[19] Simonet, J. and Gantzer, C. (2006) Degradation of the Poliovirus 1 genome by chlorine dioxide. J. Appl. Microbiol. 100, 862–870.
| Degradation of the Poliovirus 1 genome by chlorine dioxide.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksFWns7o%3D&md5=76e72adce5532ce872d5a845b8b1dcd8CAS | 16553743PubMed |
Biography
Dr Gail Greening is a Science Leader/Consultant in Environmental and Food Virology for the Institute of Environmental Science and Research, New Zealand (and now based in Queensland). Her research interests include development of molecular virology methods for clinical, environmental and food applications, molecular epidemiology of noroviruses and occurrence of human enteric viruses in shellfish, foods and the environment.


