Contribution of the community pharmacist workforce to primary care through the lens of medicines classification: comparison of Aotearoa New Zealand and Australia
Chloë Campbell 1 * , Caroline Morris 1 , Bruce Sunderland 2 , Lynn McBain 1 , Petra Czarniak 2
1 * , Caroline Morris 1 , Bruce Sunderland 2 , Lynn McBain 1 , Petra Czarniak 2
1
2
Abstract
Optimal use of the workforce in primary care is critical due to increasing complexity and demand resulting from multi-morbidity in ageing populations. Improving public access to medicines by making them available via a pharmacist without prescription can support self-care while ensuring oversight by a health professional.
The aim of this paper was to identify and explore key differences between New Zealand and Australia in medicines classified nationally for pharmacist-only non-prescription supply.
Medicines legally classified to allow sale by a pharmacist without a prescription were identified and compared between the two countries as of 1 February 2024. Based on consensus among the research team, notable differences were subjected to qualitative consideration about how medicines classification may be used to extend the role of pharmacists in primary care.
Overall, New Zealand has a less restrictive approach to classification than Australia providing New Zealanders increased access to medicines via a pharmacist in two key therapeutic areas: sexual and reproductive health and infection. Oral contraceptives, sildenafil, antibiotics for urinary tract infection and two COVID-19 antivirals were classified for supply without prescription via pharmacists in New Zealand but not nationally in Australia, although some alternative legislative mechanisms are emerging at state level.
Medicines classification has an ongoing role in enabling pharmacist contribution to primary care. Medicines classification needs to be considered alongside commissioning of services and other policy to facilitate integration of community pharmacy-provided care within the wider primary care environment. Digital tools supporting information sharing, collaboration and communication are key.
Keywords: Australia, community pharmacy, medicines access, New Zealand, pharmacist, pharmacy services, primary care, regulation, workforce.
| WHAT GAP THIS FILLS |
| What is already known: Primary care faces significant challenges with multi-morbidity in ageing populations causing increased health care demand and complexity. Optimal use of the whole primary care workforce is key in addressing these challenges. |
| What this study adds: This analysis provides a comparison between New Zealand and Australia regarding medicines that can be accessed via a community pharmacy through their classification as pharmacist-only medicines. It considers potential elements for a successful model of care including commissioning and tools for communication and collaboration with the wider health care team. |
Introduction
Pharmacists contribute to primary care in multiple ways, with the foundation of their contributions being to facilitate safe access to medicines and optimise outcomes from their use.1 Primary care globally faces significant challenges with multi-morbidity in ageing populations causing increased health care demand and complexity.2 Policy documents in both Australia and Aotearoa New Zealand (NZ) articulate a need to make better use of the full scope of pharmacists’ skills and knowledge.3,4 The COVID-19 pandemic has further emphasised that maintenance of an efficient and functioning health system relies on the available workforce being used to its maximum capability.5
Australia and NZ have similar public health systems and legal frameworks that are designed to ensure safe access to medicines for the public by classifying them into different categories.6–8 Both countries have a prescription medicine category which includes some controlled drugs (known to have dependency/abuse potential) and three non-prescription medicine categories. The non-prescription categories are:
general sale (unscheduled),
pharmacy-only/pharmacy (also known as Schedule 2 in Australia), and
pharmacist-only (also known as restricted in NZ and Schedule 3 in Australia).
General sale medicines may be sold from any retail outlet. Pharmacy-only medicines may only be sold from a licenced pharmacy. Pharmacist-only medicines may only be supplied by a pharmacist from a licenced pharmacy. Pharmacist-only medicines may also require a more detailed record of the sale (all in NZ and pseudoephedrine in Australia) and must be stored in a way that members of the public are not able to self-select, for example, behind a pharmacy counter or barrier. Sometimes medicines may have multiple classifications with a maximum quantity, strength or particular indication associated with the level of availability.6,9
Both Australia and NZ have committees enshrined in legislation to advise their respective Governments on medicine classification. In Australia, the Advisory Committee on Medicines Scheduling (ACMS) makes recommendations to the Delegate of the Secretary to the Department of Health and Aged Care.6 In NZ, the Medicines Classification Committee (MCC) makes recommendations to the Minister of Health.10
The pharmacist-only classification helps to improve public access to medicines that enable self-care while ensuring there is supervision by a health professional who can identify or confirm the need for the medicine and provide tailored information and advice. However, pharmacist-only medicines are not usually funded by the health system in either country which can lead to questions about socioeconomic factors affecting access to these medicines and the potential to exacerbate health inequities.11
Several classes of medicines have moved from prescription-only to pharmacist-only over the years, increasing public access in both countries.12 Research comparing this type of reclassification between Australia and NZ covering the period up until 2013 concluded that NZ was more progressive in their use of the pharmacist-only category.8 A decade has since passed with no further evaluation of alignment in this area between the two closely related countries. Pharmacists in Australia consider it important to improve the availability of effective therapeutic options for the management of minor ailments.13 Given ongoing pressure on primary care and with significant health system structural changes progressing in NZ, including new therapeutic products legislation, it is timely to re-examine this area.14,15
The aim of this paper was to explore the key differences in medicines (excluding vaccines) currently classified for pharmacist-only non-prescription supply in the two countries in relation to the implications for optimal use of the primary care workforce. Ethical approval was not required for this analysis as data was drawn from medicines-related legislation, health policy and professional body documents in both countries, all of which are in the public domain.
Methods
Medicines (excluding vaccines) classified to allow sale by a pharmacist (only) in Australia or NZ as of 1 February 2024 were identified, providing a sampling frame to enable comparisons to be made between the two countries. An initial table was created by a NZ research pharmacist, listing medicines allowed to be supplied by pharmacists without a prescription in NZ and their associated classification statements.7 The medicines were listed alphabetically and the classification along with any specific terms of the classification (age restriction, number of units, dosage forms) were included. A column was added for Australia and populated by an Australian research pharmacist using data extracted from Schedule 3 of the Standard for the Uniform Scheduling of Medicines and Poisons (also known as The Poisons Standard).6 Once the Australian data was incorporated, the NZ classification of any additional items identified from the Australian data were added to the table.
Based on consensus among the research team that included pharmacists (two from Australia and two from New Zealand) and a general practitioner (GP), notable differences were extracted for further qualitative consideration. Extraction was based on consideration of how medicine classification and access may contribute to extending the role of pharmacists in primary care within the different jurisdictions, and the implications for policy around primary care and the optimal use of medicines. Extracted medicines were grouped by therapeutic area and where necessary for context (eg whether an application for reclassification in either of the countries had been declined and why), additional detail was sought from Therapeutic Good Administration (TGA) publications (Australia), MCC meeting minutes (NZ), professional and government websites and other policy documents.16,17
Results
The full comparative list of medicines available via a pharmacist without a prescription (pharmacist-only) in Australia and NZ is available in Supplementary File S1. While there is broad consistency overall, key differences were identified between the two countries in three therapeutic areas: respiratory, infection and sexual and reproductive health. The findings in each area are outlined briefly below with the classification details presented in Table 1.
| Medicine | NZ classification9 | Australian classification6 | |
|---|---|---|---|
| Respiratory | |||
| Salbutamol |
|
| |
| Terbutaline | |||
| Infection | |||
| Trimethoprim |
|
| |
| Nitrofurantoin |
|
| |
| Note: NZ uses a modified release nitrofurantoin 100 mg 12-hourly for 5 days whereas all Australian states are using 100 mg normal release 6-hourly for 5 days. | |||
| Cefalexin |
| ||
| Oseltamivir |
| ||
| Molnupiravir |
| ||
| Nirmatrelvir and ritonavir (Paxlovid™) |
| ||
| Sexual & reproductive health | |||
| Levonorgestrel |
| ||
| Ulipristal |
| ||
| Selected oral contraceptives |
|
| |
| COC with ≤35 micrograms of ethinylestradiol combined with levonorgestrel or norethisterone (patients aged 16–39 years) | |||
| POP with levonorgestrel, norethisterone or desogrestrel alone (patients aged 16–52 years) | |||
| Sildenafil and its structural analogues |
|
| |
KEY: S3, Schedule three pharmacist-only medicine; S4, Schedule four prescription medicine; UTI, urinary tract infection; COC, Combined oral contraceptive; POP, Progestogen only pill; MCC, Medicines Classification Committee;  , Medicines classification status allows pharmacist supply;
, Medicines classification status allows pharmacist supply;  , Prescription-only classification;
, Prescription-only classification;  , Alternative arrangement at state level allowing pharmacist supply;
, Alternative arrangement at state level allowing pharmacist supply; 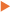 , State level trial/pilot of alternative arrangement underway; ▲, Training involves refresher on the medicine/indication and specific guidance regarding any exclusions/referral points. Training reviewed periodically; pharmacists only need complete it once unless there are significant changes; *, Additional contextual notes.
, State level trial/pilot of alternative arrangement underway; ▲, Training involves refresher on the medicine/indication and specific guidance regarding any exclusions/referral points. Training reviewed periodically; pharmacists only need complete it once unless there are significant changes; *, Additional contextual notes.
Respiratory
Short-acting beta2-agonist inhalers containing either salbutamol or terbutaline have been available under the pharmacist-only medicine classification in Australia since 1976 but remain classified as prescription medicines in NZ (see Table 1).6,9,12 Harmonisation with Australia has been considered and declined by the NZ MCC, most recently in 2021, citing the existing general provision for emergency supply within Medicines Act 1981 and non-alignment with New Zealand asthma guidelines.18 Pharmacists in NZ may provide an ‘emergency supply’ of any medicine (excluding controlled drugs) if a person has previously had a medicine on prescription. This emergency allowance enables 3 days supply to be provided by the pharmacist, or a minimum pack where a smaller quantity is not practicable as in the case of inhalers.7
Infection
There are several examples where medicines to treat either viral or bacterial infections are classified to be accessible in NZ via consultation with a pharmacist but not in Australia (see Table 1).
Pharmacist supply of trimethoprim for the treatment of urinary tract infection (UTI) in women has been allowed in NZ since 2012.9,12 Although it remains in the prescription category, the classification statement has an exception enabling supply by a pharmacist who has successfully completed the required training (see Table 1). Pharmacist supply of a modified-release preparation of nitrofurantoin for UTIs in women was enabled in NZ in October 2022 with the same approach.9 Adding this option allowed pharmacist management of UTIs to be brought into line with recently changed NZ guidelines.19 Although not classified for pharmacist supply at a national level in Australia, individualised approaches are developing at state level. Queensland Health have used an ‘Extended Practice Authority’ to enable pharmacist supply of trimethoprim (first-line), nitrofurantoin (second-line) or cefalexin (third-line) for UTI management.20 This started as a pilot in 2021 and now continues as usual practice.20,21 The Western Australia Department of Health issued a ‘Structured Administration and Supply Arrangement’ in 2023 authorising trained pharmacists to supply certain Schedule 4 (prescription) antibiotics without a prescription for the treatment of UTIs in low-risk patients (nitrofurantoin first-line and trimethoprim second-line).22 The South Australian State Government has established a Community Pharmacy Urinary Tract Infection Services Scheme enabling trained pharmacists to supply certain prescription medicines without a prescription (the same antibiotics and order of treatment as Queensland) in accordance with an approved protocol beginning March 2024.23
Other states have pilots or trials underway with the same antibiotics and order of treatment as Queensland. New South Wales (NSW) Health commissioned a pilot study investigating pharmacist provision of UTI treatment which began in August 2023.24 The study is enabled by a NSW Health ‘Authority for Supply of Specified Restricted Substances by Pharmacists’ and includes funding for consultation.24 Victoria commenced a 12-month state-wide pilot of community pharmacist management of UTIs in October 2023.25 In the pilot, accordingly trained community pharmacists are able to provide certain antibiotics that are classified as prescription-only under a ‘structured prescribing model.’ The Tasmanian Government is taking a similar approach with a 12-month pilot programme beginning in March 2024.26 Pharmacist consultations are funded within these pilot programmes.
The antiviral oseltamivir is classified as a pharmacist-only medicine in NZ for the treatment or prophylaxis of influenza in people 13 years and older in 2012.27 Reclassification was declined in Australia due to concern about potential for increased resistance, misdiagnosis and reduced laboratory surveillance and it remains prescription-only (see Table 1).28
In July 2022, two antiviral preparations for the treatment of COVID-19 were reclassified as pharmacist-only in NZ: molnupiravir and the combination preparation Paxlovid™ (containing nirmatrelvir plus ritonavir) (see Table 1).29 There were several unique aspects to the model of care associated with the reclassification of these antivirals including that both medicine and pharmacist consultation were fully funded by the NZ Government and there were contractual requirements around pharmacist access to clinical information/dispensing history and an electronic consultation record that was shared with the patient’ GP and local COVID-19 community care hub (see Table 2).
| Unique feature | Details 55 | |
|---|---|---|
| Funded consultation | The consultation with the patient, time to analyse interactions and liaise with patient’s GP and medicine delivery were all funded by the Government (via contract administered by local health authorities). | |
| Funded medicine | The medicines were both fully funded by the Government for eligible patients. Pharmac, the New Zealand medicine buying agency, and the Ministry of Health set eligibility criteria. Molnupiravir also available for private supply, but not Paxlovid. | |
| Communication | Must have access to the COVID-19 Care in the Community IT platform to document the consultation and outcome and generate automatic communication to the patient’s GP and the local COVID-19 Care in the Community Hub. | |
| Access to laboratory test results | Must have access to laboratory test results or information to enable assessment of renal/hepatic function. | |
| Access to dispensing history | Must have access to medication dispensing history to help confirm current medicines and assess interactions. | |
| Telehealth | Consultation undertaken by the pharmacist via telehealth because only people who had tested positive for COVID-19 (or were probably cases) were eligible for medicine supply and were legally required to be in isolation. |
Sexual and reproductive health
An emergency contraceptive pill (ECP) is available via the pharmacist-only classification in both countries. Australia has two options: levonorgestrel and ulipristal, whereas NZ only has levonorgestrel.6,9 Neither Australia nor NZ have training requirements included in the classification statements for ECPs. However, the Pharmacy Council of NZ (pharmacist regulatory authority) standards require pharmacists to successfully undertake a training course accredited by them prior to being able to supply this medicine without a prescription.17 Ulipristal remains classified as prescription-only in NZ. Harmonisation with Australia was declined by the MCC citing insufficient information, although they encouraged the sector to make a formal reclassification submission.30 This never occurred likely due to no product being approved in NZ, even on prescription.31
Selected oral contraceptives may be supplied in NZ by pharmacists who have successfully completed specific training (see Table 1). Likewise, sildenafil has been available for supply by pharmacists in NZ for erectile dysfunction since 2014 but remains a prescription medicine in Australia.9,32 Both were considered for reclassification at a national level in Australia and declined. Sildenafil was declined due to due to concern that the proposed approach did not mitigate risks adequately and that erectile dysfunction is a symptom of other conditions that require medical practitioner diagnosis, monitoring and treatment.33 Oral contraceptives were declined due to concern about significant adverse effects, interactions and contraindications warranting regular review by a doctor.34 Continuation supply of oral contraceptives by pharmacists is part of the NSW Health pharmacy pilot that began in September 2023, the 12-month pilot in Victoria that began in October 2023 and a community pharmacy service due to begin in South Australia in May 2024.24,33–36
Discussion
The pharmacist-only classification allows controlled public access to a range of medicines for the general communities of both countries. Although there is broad similarity in the items with this classification in Australia and NZ, there are several key differences. Overall, based on classification, NZ is less restrictive than Australia meaning New Zealanders generally have increased access to medicines via a pharmacist in two key therapeutic areas: sexual and reproductive health and infection. This finding is consistent with previous research covering the period until 2013.37 It confirms that over the past decade NZ has continued to be more progressive than Australia using medicine classification to enable the pharmacist workforce to support care in the community by expanding the number of medicines they can supply without a prescription.
Rather than full down-classification of prescription-only medicines, this greater openness has often been facilitated through adaption of the prescription medicine category by adding exception criteria, such as specific training. This enables the incorporation of additional eligibility or referral criteria to support the optimal use of these medicines. A similar approach was facilitated in Australia in 2019 with the addition of Appendix M to the Poisons Standard which provides for Schedule 3 pharmacist-only medicines to be given additional requirements including patient eligibility criteria, pharmacist training, record-keeping and information sharing.38 To date, there are no medicines listed in Appendix M.6 A multitude of factors affect medicines reclassification and reasons that have been cited for the differences in progressiveness between the two countries include greater opposition from other health professional bodies, complexity of the reclassification application process and risk averseness of the medicines scheduling committee in Australia.8,13 It may also be that the health system focus on responding to the COVID-19 pandemic affected uptake of this new mechanism. The emergence of alternative arrangements at state level for provision of antibiotics in the management of UTIs by community pharmacists suggests that challenges of reclassification at a national level have led to other methods being sought to improve access for the public and enhance the use of the community pharmacist workforce in the provision of primary care in Australia.22,23,39 This approach is leading to variation in medicine availability for Australians depending on which state they are in.
Most medicines that have been reclassified to enable pharmacist supply in either country to date are for conditions that benefit from timely access to treatment such as infection, bronchospasm and emergency contraception. Several factors contribute to ready patient access to pharmacists, including not requiring an appointment and the long opening hours of many pharmacies. The most recent example of a progressive reclassification decision in NZ with timely access as a factor was for the COVID-19 antivirals Paxlovid™ and molnupiravir. The need to increase access was cited by the NZ Minister of Health as the impetus for this rapid reclassification.40 We are only aware of pharmacist-initiated supply of COVID-19 antivirals being instigated in two other jurisdictions – parts of Canada and the United States (US).41,42 The Food and Drug Administration extended their Emergency Use Authorisation for Paxlovid™ so it could be prescribed by state-licensed pharmacists in the US, but only if they had access to sufficient information to assess renal and hepatic function, and sufficient information about an individual’s regular medicines to assess for potential drug interactions.41 They did not enable this for molnupiravir. In Canada, a small number of provinces enabled pharmacist prescribing of Paxlovid™.42
The COVID-19 antiviral reclassification in NZ was implemented under Section 106 of the Medicines Act 1981 which allows the Minister of Health to declare a classification by Gazette publication without the usual MCC processes. This clause has been used previously to support the role of pharmacists in the vaccination response to the measles outbreak in 2019.43 This pathway means the Government can rapidly act on policy intention when required.
There are several unique components to the model of care associated with the COVID-19 antiviral reclassification in NZ that are relevant to pharmacist-initiated supply of medicines and pharmacist contribution to primary care. This was the first time that pharmacy in NZ had been funded at a national level for a primary care consultation. Local funding arrangements for some consultations existed in a small number of the geographic areas in NZ (eg ECP consultations for certain patient groups in the Bay of Plenty44); otherwise, the patient may be charged a consultation fee (in addition to the cost of the medicine) at the discretion of the pharmacy. Funding for consultation has been included in the Australian trials or pilots underway for pharmacist UTI management but this is not the case in the states where pharmacist supply is already fully implemented.25,26 Funded consultations help to reduce barriers to access, especially where alternative options are not associated with cost to the patient, eg hospital emergency departments in both countries. Considering the wider context, however, paying for a pharmacist consultation may be less expensive than a GP consultation in many cases in both countries.
Government funding of the medicine when supply has been initiated by a pharmacist is also unusual. There are only two other examples in NZ of funded ‘direct provision’ – the ECP and nicotine replacement therapy.16 We are not aware of funding for pharmacist-initiated medicine supply in Australia outside the continuation supply provisions known as Continued Dispensing or the recent state-level trial/pilot programmes.24–26,45 Consideration of equal funding for medicines, regardless of where they are obtained, is necessary to fully benefit from the increased access afforded by reclassification to enable pharmacist supply.
Cost to the patient is a factor in equity of access and may affect the achievement of equitable outcomes from medicines and services available in community pharmacies. The national level funding for a pharmacist consultation regardless of outcome has similarities with funded common ailments services commissioned in other jurisdictions such as the United Kingdom (UK).46,47 Little is yet known about whether such services achieve equitable health outcomes.48 NZ based researchers have explored Māori experiences of minor ailments care and the pharmacists’ role.49 They identified factors considered important for designing services to deliver equitable care, with potential international application. NZ ran a pilot-funded minor ailments service in certain priority areas during winter 2023 based on emergency department capacity and pressure at key hospital locations.50 The evaluation is not available at the time of writing although certain areas have opted to continue the service (Northland, Hawkes Bay and the Hutt Valley).51 In Canada, all provinces have pharmacist prescribing for minor ailments in place, with evidence of improving patient access to timely and cost-effective health care.52 A new more formalised ‘pharmacist primary care clinic’ model is emerging in some with quantitative and qualitative evaluation pending.53 Overall, there is limited published literature on clinical and economic outcomes of medicine reclassifications, although what is available is promising in terms of safety and effectiveness.54
A key requirement built into the COVID-19 antiviral service enabled by the reclassification of the antivirals in NZ was communication and integration with the wider primary care team. Pharmacists initiating supply have access to a shared electronic portal where they document and communicate the outcome of their consultation to the patient’s GP and others involved in COVID-19 community care.55 Although protocols for other pharmacist-only medicines in NZ encourage communication with the patient’s GP, a digital portal to specifically facilitate this is novel. It is unclear whether the particular tool used worked well in practice, yet it indicates a step towards a future where a multidisciplinary approach to primary care is supported and enabled by digital tools helping to connect various providers. Development of this concept is crucial in addressing concerns around the potential for fragmentation when multiple providers are involved in care.56
Approaches other than medicines reclassification have been used in various jurisdictions to enable extended use of the pharmacist workforce in primary care. Standing orders1 underpin the community pharmacy anticoagulant management and gout management services in NZ where pharmacists undertake monitoring and dose adjustment of the prescribed medicines warfarin and allopurinol respectively.57,58 Other similar examples include Collaborative Practice Agreements in the US59 and Patient Group Directions in the UK.60
A significant upcoming change in the UK intended to support optimal use of the pharmacy workforce in clinical care is amendment to pharmacy education standards so that all pharmacy graduates will be able to be qualified as independent prescribers on entry to the practice register by 2026.61 Research will be needed to fully understand the impact of this change on future pharmacist roles and patient access to medicines in primary care.62 Digital integration and information sharing with the wider primary care team will be just as important in this model to support coordination and continuity of care.63
Despite timely access for acute conditions or symptoms being a traditional driver of reclassifications, the current findings show some medicines used more chronically, selected oral contraceptives and sildenafil for erectile dysfunction, have been reclassified in NZ to enable supply by a pharmacist. Resupply of oral contraceptives is also emerging in some Australian states.24,25,36 The NZ COVID-19 antiviral example of digital integration and information sharing within the primary care team lays a foundation for further expansion of this type of service.64,65 In NZ work is underway on a national digital health record that will provide patients and health providers with access to a range of health information such as prescribed and dispensed medicines, immunisation records, diagnostic test results and summary primary care information.66 A digital health record such as this is already in existence in Australia and sharing to it is mandatory in the trials and pilots underway.24,26,67
This analysis provides a comparison of medicines classified for access via community pharmacists in Australia and NZ as of 1 February 2024 to aid health policymakers and planners considering medicines access and optimal use of the primary care workforce. It shows a continuing trend of NZ making use of medicines classification to enable pharmacist contribution to help meet the growing demand on primary care and highlights the emergence of alternative state level legislative arrangements in Australia. A strength of the analysis is that information has been collated from several credible sources including classification databases, committee meeting notes, government websites, policy documents and published literature. Limitations include that it has been conducted with a cross-sectional lens in a dynamic environment. Several Australian states have pilots or trials underway for community pharmacist management of UTI and resupply of oral contraceptives using mechanisms other than national level reclassification.24–26 A further pilot on a major expansion of community pharmacist scope of practice is beginning in Queensland.68 The legislation regulating therapeutic products in NZ is also in the midst of change which will potentially impact medicines access and supply going forward. Furthermore, the underpinning structure of the whole NZ health system was reformed in 2022, with the implications still unfolding.14,15
Medicines reclassification has a role in enabling pharmacist contribution to primary care. However, commissioning of services and policy that facilitates integration of pharmacy-provided care within the wider health care environment are also required. Digital tools that support information sharing, collaboration and communication will be key in future models of care.
Data availability
Data sharing is not applicable as no new data were generated; data are available in the public domain.
References
1 Bader L, Duggan C. FIP’s Commitment to Action on the WHO Astana Declaration: transforming pharmacy for better health for all. Res Social Adm Pharm 2020; 16(5): 724-6.
| Crossref | Google Scholar | PubMed |
2 Aggarwal P, Woolford SJ, Patel HP. Multi-morbidity and polypharmacy in older people: challenges and opportunities for clinical practice. Geriatrics 2020; 5(4): 85.
| Crossref | Google Scholar | PubMed |
3 Ministry of Health. Pharmacy Action Plan 2016 to 2020. Available at https://www.health.govt.nz/publication/pharmacy-action-plan-2016-2020 [accessed 12 February 2024].
4 Australian Government. Australia’s Long Term National Health Plan. 2019. Available at https://www.health.gov.au/resources/publications/australias-long-term-national-health-plan [accessed 1 February 2024].
5 Isenor JE, Cossette B, Murphy AL, et al. Community pharmacists’ expanding roles in supporting patients before and during COVID-19: an exploratory qualitative study. Int J Clin Pharm 2023; 45(1): 64-78.
| Crossref | Google Scholar | PubMed |
6 Therapeutic Goods Administration. Therapeutic Goods (Poisons Standard - February 2024) Instrument. 2024. Available at https://www.legislation.gov.au/F2024L00095/asmade/text [accessed 11 February 2024].
7 Medicines Regulations 1984. Available at https://www.legislation.govt.nz/regulation/public/1984/0143/latest/DLM95668.html [accessed 12 February 2024].
8 Gauld NJ, Kelly FS, Emmerton LM, et al. Widening consumer access to medicines: a comparison of prescription to non-prescription medicine switch in Australia and New Zealand. PLoS One 2015; 10(3): e0119011.
| Crossref | Google Scholar | PubMed |
9 Medsafe. Medicines Classification Database. Available at https://www.medsafe.govt.nz/profs/class/classintro.asp [accessed 1 February 2024].
10 Medsafe. How to change the legal classification of a medicine in New Zealand: Guidance document. 2019. Available at https://www.medsafe.govt.nz/downloads/how_to_change_medicine_classification.pdf [accessed 12 February 2024].
11 Bessell TL, Hiller JE, Sansom LN. Pharmacist only’ medicines. Aust N Z J Public Health 1999; 23(6): 661-2.
| Crossref | Google Scholar | PubMed |
12 Gauld NJ, Kelly FS, Kurosawa N, et al. Widening consumer access to medicines through switching medicines to non-prescription: a six country comparison. PLoS One 2014; 9(9): e107726.
| Crossref | Google Scholar | PubMed |
13 Hope DL, Woods P, Mey A, et al. Australian pharmacists: ready for increased non-prescription medicines reclassification. Int J Pharm Pract 2020; 28(3): 246-54.
| Crossref | Google Scholar | PubMed |
14 Pae Ora (Healthy Futures) Act 2022. Available at https://www.legislation.govt.nz/act/public/2022/0030/latest/LMS575405.html [accessed 12 February 2024].
15 Therapeutic Products Act 2023. Available at https://www.legislation.govt.nz/act/public/2023/0037/latest/whole.html [accessed 13 February 2024].
16 Pharmac. Pharmaceutical Schedule Rules - Rule 1.3.7. 2024. Available at https://schedule.pharmac.govt.nz/ScheduleOnline.php [accessed 12 February 2024].
17 Pharmacy Council of New Zealand. Standards for the supply by pharmacists of the emergency contraceptive pill. 2019. Available at https://pharmacycouncil.org.nz/pharmacist/medicines-reclassification/ [accessed 13 February 2024].
18 Medicines Classification Committee. Minutes of the 67th Meeting of the Medicines Classification Committee. 2021. Available at https://www.medsafe.govt.nz/profs/class/Minutes/2021-2025/mccMin26Oct2021.htm [accessed 11 February 2024].
19 Medicines Classification Committee. Minutes of the 68th Meeting of the Medicines Classification Committee. 2022. Available at https://www.medsafe.govt.nz/profs/class/Minutes/2021-2025/mccMin26Apr2022.htm [accessed 12 February 2024].
20 Queensland Government. Extended Practice Authority - Pharmacists - Version 4. 2023. Available at https://www.health.qld.gov.au/__data/assets/pdf_file/0027/1108944/epa-pharmacists.pdf [accessed 11 February 2024].
21 Queensland Government. Extended Practice Authority - Pharmacists - Version 1. 2021. Available at https://documents.parliament.qld.gov.au/tableoffice/tabledpapers/2021/5721T1437.pdf [accessed 11 February 2024]
22 Government of Western Australia Department of Health. Treatment of urinary tract infection by pharmacists. 2023. Available at https://www.health.wa.gov.au/Articles/S_T/Treatment-of-urinary-tract-infection-by-pharmacists [accessed 11 February 2024].
23 South Australia Health. South Australia Community Pharmacy Urinary Tract Infection (UTI) Services UTI Management Protocol. 2024. Available at https://www.sahealth.sa.gov.au/wps/wcm/connect/b52bbef1-4fed-4502-96ed-3ebf97ebdee1/Community+Pharmacy+UTI+Service+SA+Management+Protocol+-+Final1.pdf
24 New South Wales Health. NSW Pharmacy Trial. 2024. Available at https://www.health.nsw.gov.au/pharmaceutical/Pages/community-pharmacy-pilot.aspx [accessed 11 February 2024].
25 Victoria State Government Department of Health. Victorian Community Pharmacist Statewide Pilot. 2024. Available at https://www.health.vic.gov.au/primary-care/victorian-community-pharmacist-statewide-pilot [accessed 1 March 2024].
26 Tasmanian Government Department of Health. Protocol for Management of Urinary Tract Infections - Tasmanian Community Pharmacist Pilot Program Version 1.0 - January 2024. 2024. Available at https://www.health.tas.gov.au/sites/default/files/2024-01/protocol_for_management_of_urinary_tract_infections.pdf [accessed 1 February 2024].
27 Medicines Classification Committee. Minutes of the 48th Meeting of the Medicines Classification Committee. 2012. Available at https://www.medsafe.govt.nz/profs/class/Minutes/2011-2015/mccMin30Oct2012.htm [accessed 11 February 2024].
28 Therapeutic Goods Administration. Final decisions and reasons for decisions by delegates of the Secretary to the Department of Health and Ageing - 2.6 Oseltamivir. 2013. Available at https://www.tga.gov.au/sites/default/files/scheduling-decisions-1306-final.pdf [accessed 12 February 2024].
29 New Zealand Government. Classification of Medicines Gazette Notice 2022-go2856. 2022. Available at https://gazette.govt.nz/notice/id/2022-go2856 [accessed 11 February 2024].
30 Medicines Classification Committeee. Minutes of the 58th Meeting of the Medicines Classification Committee. 2017. Available at https://www.medsafe.govt.nz/profs/class/Minutes/2016-2020/mccMin16May2017.htm [accessed 12 February 2024].
31 Medsafe. Product/Application Search. Available at https://www.medsafe.govt.nz/regulatory/dbsearch.asp [accessed 1 February 2024].
32 Braund R, Ratnayake K, Tong K, et al. Pharmacist supply of sildenafil: pharmacists’ experiences and perceptions on training and tools for supply. Int J Clin Pharm 2018; 40(3): 650-8.
| Crossref | Google Scholar | PubMed |
33 Therapeutic Goods Administration. Final decisions amending or not amending the Poisons Schedule - 1.1 Sildenafil. 2018. Available at https://www.tga.gov.au/resources/publication/scheduling-decisions-final/final-decisions-amending-or-not-amending-current-poisons-standard-november-2018/11-sildenafil [accessed 13 February 2024].
34 Therapeutic Goods Administration. Final decisions amending or not amending the Poisons Schedule - 2.4 Oral contraceptive substances. 2021. Available at https://www.tga.gov.au/resources/publication/scheduling-decisions-final/notice-final-decisions-amend-or-not-amend-current-poisons-standard-acms-34-joint-acms-accs-28-accs-31 [accessed 13 February 2024].
35 Victoria State Government Department of Health. Protocol for Resupply of the Oral Contraceptive Pill - Victorian Community Pharmacist Statewide Pilot - October 2023. 2023. Available at https://www.health.vic.gov.au/sites/default/files/2023-10/clinical-protocol-oral-contraceptive-pill.pdf [accessed 1 March 2024].
36 South Australia Health. UTI treatment and contraceptive pill access. 2024. Available at https://www.sahealth.sa.gov.au/wps/wcm/connect/Public+Content/SA+Health+Internet/About+us/Department+for+Health+and+Wellbeing/Office+of+the+Chief+Pharmacist/Community+pharmacy+initiatives/UTI+treatment+and+contraceptive+pill+access+new/UTI+treatment+and+contraceptive+pill+access [accessed 1 March 2024].
37 Gauld N, Bryant L, Emmerton L, et al. Why does increasing public access to medicines differ between countries? Qualitative comparison of nine countries. J Health Serv Res Policy 2015; 20(4): 231-9.
| Crossref | Google Scholar | PubMed |
38 Therapeutic Goods Administration. Submissions received and TGA response: Proposed criteria for Appendix M of the Poisons Standard to support rescheduling of substances from Schedule 4 (Prescription only) to Schedule 3 (Pharmacist only). 2019. Available at https://www.tga.gov.au/resources/publication/publications/submissions-received-and-tga-response-proposed-criteria-appendix-m-poisons-standard-support-rescheduling-substances-schedule-4-prescription-only-schedule-3-pharmacist-only [accessed 12 February 2024].
39 Queensland Government. Extended Practice Authority - Pharmacists - Version 2. 2022. Available at https://documents.parliament.qld.gov.au/tp/2022/5722T1345-36EC.pdf [accessed 12 February 2024].
40 Minister of Health. New measures to tackle COVID-19 and flu. 2022. Available at https://www.beehive.govt.nz/release/new-measures-tackle-covid-19-and-flu [accessed 11 February 2024].
41 Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes pharmacists to prescribe Paxlovid with certain limitations. 2022. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pharmacists-prescribe-paxlovid-certain-limitations [accessed 7 October 2023].
42 Traynor K. Québec authorizes pharmacists to prescribe Paxlovid. Am J Health Syst Pharm 2022; 79(14): 1126-7.
| Crossref | Google Scholar | PubMed |
43 New Zealand Government. Classification of Medicines Gazette Notice 2021-go1534. 2021. Available at https://gazette.govt.nz/notice/id/2021-go1534 [accessed 14 February 2024].
44 Bay of Plenty Community Pharmacy Group. Pharmacy programmes and services assisted by Bay of Plenty Community Pharmacy Group. 2024. Available at https://bopcpg.co.nz/services [accessed 12 February 2024].
45 Australian Government. PBS Continued Dispensing Arrangements. 2024. Available at https://www.pbs.gov.au/info/general/continued-dispensing [accessed 11 February 2024].
46 Aly M, García-Cárdenas V, Williams K, et al. A review of international pharmacy-based minor ailment services and proposed service design model. Res Social Adm Pharm 2018; 14(11): 989-98.
| Crossref | Google Scholar | PubMed |
47 NHS England. Community pharmacy advanced service specification: NHS Pharmacy First Service - November 2023. 2023. Available at https://www.england.nhs.uk/publication/community-pharmacy-advanced-service-specification-nhs-pharmacy-first-service/accessed [11 February 2024].
48 Hikaka J, Haua R. Examining equity in a void of evidence - pharmacist minor ailments services and the role of systematic reviews. Explor Res Clin Soc Pharm 2022; 7: 100174.
| Crossref | Google Scholar | PubMed |
49 Hikaka J, Haua R, Parore N, et al. Designing for health equity: a mixed method study exploring community experiences and perceptions of pharmacists’ role in minor ailment care. Res Social Adm Pharm 2023; 19(4): 643-52.
| Crossref | Google Scholar | PubMed |
50 Te Whatu Ora Health New Zealand. Community Pharmacy Minor Ailments Service Frequently Asked Questions - June 2023. 2023. Available at https://www.tewhatuora.govt.nz/assets/For-the-health-sector/Community-pharmacy/Minor-ailments-service/Community-Pharmacy-Minor-Ailments-Service-FAQ.pdf [accessed 11 February 2024].
51 Burling NJ. Pharmacists still funded for minor-ailments in Northland, Hawke’s Bay and Upper Hutt. 2023. Available at https://www.pharmacytoday.co.nz/article/news/pharmacists-still-funded-minor-ailments-northland-hawkes-bay-and-upper-hutt [accessed 12 February 2024].
52 Nakhla N, Taylor J. Self-care and minor ailments: the view from Canada. Explor Res Clin Soc Pharm 2024; 13: 100412.
| Crossref | Google Scholar | PubMed |
53 Gysel SC, Tsuyuki RT. The pharmacist primary care clinic: the evolution of pharmacy practice? Can Pharm J 2024; 157(2): 47-9.
| Crossref | Google Scholar | PubMed |
54 Swart A, Benrimoj SI, Dineen-Griffin S. The clinical and economic evidence of the management of urinary tract infections by community pharmacists in women aged 16 to 65 years: a systematic review. Int J Clin Pharm 2024; 46(3): 574-89.
| Crossref | Google Scholar | PubMed |
55 Pharmaceutical Society of New Zealand. Covid-19 oral antivirals for pharmacist only supply - training programme. 2022. Available at https://www.psnz.org.nz/Product?Action=View&Product_id=810 [accessed 13 September 2023].
56 Thai T, Chen G, Lancsar E, et al. Beyond dispensing: better integration of pharmacists within the Australian primary healthcare system. SSM Qual Res Health 2022; 2: 100109.
| Crossref | Google Scholar |
57 Te Whatu Ora Health New Zealand. Integrated Community Pharmacy Services Agreement. 2023. Available at https://www.tewhatuora.govt.nz/for-the-health-sector/community-pharmacy/community-pharmacy-agreement/ [accessed 13 February 2024].
58 Andrews S, Gasparini J, Henderson G. Evaluation of Gout Stop and Owning My Gout management programmes - A final report for Arthritis New Zealand and its partners. 2020. Available at https://www.arthritis.org.nz/research/reports/ [accessed 13 February 2024].
59 Pollack S, Skillman S, Frogner B. Assessing the size and scope of the pharmacist workforce in the U.S. 2020. Available at https://familymedicine.uw.edu/chws/publications/assessing-the-size-and-scope-of-the-pharmacist-workforce-in-the-us/ [accessed 13 February 2024].
60 National Institute for Health and Care Excellence. Patient Group Directions. 2013. Available at https://www.nice.org.uk/guidance/mpg2/resources/patient-group-directions-pdf-1779401941189 [accessed 12 February 2024].
61 General Pharmaceutical Council. Standards for the initial education and training of pharmacists. 2021. Available at https://www.pharmacyregulation.org/education/education-standards [accessed 13 February 2024].
62 Walpola RL, Issakhany D, Gisev N, et al. The accessibility of pharmacist prescribing and impacts on medicines access: a systematic review. Res Social Adm Pharm 2024; 20(5): 475-86.
| Crossref | Google Scholar | PubMed |
63 Akman M, Ayhan Başer D, Usanma Koban B, et al. Organization of primary care. Prim Health Care Res Dev 2022; 23: e49.
| Crossref | Google Scholar | PubMed |
64 Keller ME, Kelling SE, Cornelius DC, et al. Enhancing practice efficiency and patient care by sharing electronic health records. Perspect Health Inf Manag 2015; 12(Fall): 1b.
| Google Scholar | PubMed |
65 Mercer K, Burns C, Guirguis L, et al. Physician and pharmacist medication decision-making in the time of electronic health records: mixed-methods study. JMIR Hum Factors 2018; 5(3): e24.
| Crossref | Google Scholar | PubMed |
66 Te Whatu Ora Health New Zealand. Hira: Connecting health information. 2024. Available at https://www.tewhatuora.govt.nz/our-health-system/digital-health/hira-connecting-health-information/ [accessed 12 February 2024].
67 Australian Digital Health Agency. My Health Record. 2024. Available at https://www.digitalhealth.gov.au/initiatives-and-programs/my-health-record [accessed 8 February 2024].
68 Queensland Health. Queensland Community Pharmacy Scope of Practice Pilot. 2024. Available at https://www.health.qld.gov.au/clinical-practice/guidelines-procedures/community-pharmacy-scope-of-practice-pilot [accessed 29 February 2024].


 Prescription-only medicine
Prescription-only medicine Pharmacist-only medicine S3
Pharmacist-only medicine S3 Prescription-only medicine
Prescription-only medicine Pharmacist-only medicine S3
Pharmacist-only medicine S3 Prescription only except when for oral use containing 300 mg or less per dose unit when sold in a pack of 3 solid dosage units to a woman aged 16–65 years for the treatment of an uncomplicated UTI by a registered pharmacist who has successfully completed the New Zealand College of Pharmacists’ training ▲ in the treatment of UTIs.
Prescription only except when for oral use containing 300 mg or less per dose unit when sold in a pack of 3 solid dosage units to a woman aged 16–65 years for the treatment of an uncomplicated UTI by a registered pharmacist who has successfully completed the New Zealand College of Pharmacists’ training ▲ in the treatment of UTIs. Prescription-only medicine S4
Prescription-only medicine S4 Queensland: Supply for uncomplicated UTI (1st-line) in female 18–65 years by trained pharmacist enabled via Extended Practice Authority in 2021.
Queensland: Supply for uncomplicated UTI (1st-line) in female 18–65 years by trained pharmacist enabled via Extended Practice Authority in 2021. Western Australia: Supply for uncomplicated UTI (2nd-line) in females 18–65 years by trained pharmacist enabled via Structured Administration and Supply Arrangement in 2023.
Western Australia: Supply for uncomplicated UTI (2nd-line) in females 18–65 years by trained pharmacist enabled via Structured Administration and Supply Arrangement in 2023. South Australia: Supply for uncomplicated UTI (1st-line) in females 18–65 years by trained pharmacist enabled via Community Pharmacy Urinary Tract Infection Services Scheme and Protocol from March 2024.
South Australia: Supply for uncomplicated UTI (1st-line) in females 18–65 years by trained pharmacist enabled via Community Pharmacy Urinary Tract Infection Services Scheme and Protocol from March 2024. New South Wales: Trial of uncomplicated UTI management by trained community pharmacists for females 18–65 years began August 2023 – trimethoprim 1st-line.
New South Wales: Trial of uncomplicated UTI management by trained community pharmacists for females 18–65 years began August 2023 – trimethoprim 1st-line. Victoria and Tasmania: Statewide pilot programme of uncomplicated UTI management by trained community pharmacists for females 18–65 years began October 2023 and March 2024 respectively – trimethoprim 1st-line.
Victoria and Tasmania: Statewide pilot programme of uncomplicated UTI management by trained community pharmacists for females 18–65 years began October 2023 and March 2024 respectively – trimethoprim 1st-line. Prescription-only except when supplied for oral use containing 100 mg per dose unit when sold in a pack of 10 solid dosage units to a woman aged 16–65 years for the first-line empiric treatment of an uncomplicated UTI by a registered pharmacist who has successfully completed the Pharmaceutical Society of New Zealand training ▲ in the treatment of UTIs.
Prescription-only except when supplied for oral use containing 100 mg per dose unit when sold in a pack of 10 solid dosage units to a woman aged 16–65 years for the first-line empiric treatment of an uncomplicated UTI by a registered pharmacist who has successfully completed the Pharmaceutical Society of New Zealand training ▲ in the treatment of UTIs. Prescription-only medicine S4
Prescription-only medicine S4 Queensland: Supply for uncomplicated UTI (2nd-line) is enabled via Extended Practice Authority in 2021.
Queensland: Supply for uncomplicated UTI (2nd-line) is enabled via Extended Practice Authority in 2021. Western Australia: Supply for uncomplicated UTI (1st-line) enabled via Structured Administration and Supply Arrangement in 2023.
Western Australia: Supply for uncomplicated UTI (1st-line) enabled via Structured Administration and Supply Arrangement in 2023. South Australia: Supply for uncomplicated UTI (2nd-line) enabled via Community Pharmacy Urinary Tract Infection Services Scheme and Protocol from March 2024.
South Australia: Supply for uncomplicated UTI (2nd-line) enabled via Community Pharmacy Urinary Tract Infection Services Scheme and Protocol from March 2024. New South Wales: Trial of uncomplicated UTI management by community pharmacists began August 2023 – nitrofurantoin 2nd-line.
New South Wales: Trial of uncomplicated UTI management by community pharmacists began August 2023 – nitrofurantoin 2nd-line. Victoria and Tasmania: Statewide pilot programme of uncomplicated UTI management by community pharmacists began October 2023 and March 2024 respectively – nitrofurantoin 2nd-line.
Victoria and Tasmania: Statewide pilot programme of uncomplicated UTI management by community pharmacists began October 2023 and March 2024 respectively – nitrofurantoin 2nd-line. Prescription-only medicine
Prescription-only medicine Prescription-only medicine S4
Prescription-only medicine S4 Queensland: Supply for uncomplicated UTI (3rd-line) enabled via Extended Practice Authority in 2021.
Queensland: Supply for uncomplicated UTI (3rd-line) enabled via Extended Practice Authority in 2021. South Australia: Supply for uncomplicated UTI (3rd-line) enabled via Community Pharmacy Urinary Tract Infection Services Scheme and Protocol from March 2024.
South Australia: Supply for uncomplicated UTI (3rd-line) enabled via Community Pharmacy Urinary Tract Infection Services Scheme and Protocol from March 2024. New South Wales: Trial of uncomplicated UTI management by community pharmacists began August 2023 – cefalexin 3rd-line.
New South Wales: Trial of uncomplicated UTI management by community pharmacists began August 2023 – cefalexin 3rd-line. Victoria and Tasmania: Statewide pilot programme of uncomplicated UTI management by community pharmacists began October 2023 and March 2024 respectively – cefalexin 3rd-line.
Victoria and Tasmania: Statewide pilot programme of uncomplicated UTI management by community pharmacists began October 2023 and March 2024 respectively – cefalexin 3rd-line. Western Australia: Cefalexin NOT included in UTI Structured Administration and Supply Arrangement.
Western Australia: Cefalexin NOT included in UTI Structured Administration and Supply Arrangement. Pharmacist-only medicine
Pharmacist-only medicine Prescription-only medicine S4
Prescription-only medicine S4 Pharmacist-only medicine
Pharmacist-only medicine Prescription-only medicine S4
Prescription-only medicine S4 Pharmacist-only medicine
Pharmacist-only medicine Prescription-only medicine S4
Prescription-only medicine S4 Pharmacist-only medicine
Pharmacist-only medicine Pharmacist-only medicine S3
Pharmacist-only medicine S3 Prescription-only medicine
Prescription-only medicine Pharmacist-only medicine S3
Pharmacist-only medicine S3 Prescription-only except when supplied for oral contraception to women who meet the clinical and eligibility criteria of the Pharmacy Council and The Pharmaceutical Society of New Zealand approved training programme ▲ on oral contraception when sold in Medsafe approved manufacturer’s original pack containing not more than 6 months’ supply by a registered pharmacist who has successfully completed the approved training programme.
Prescription-only except when supplied for oral contraception to women who meet the clinical and eligibility criteria of the Pharmacy Council and The Pharmaceutical Society of New Zealand approved training programme ▲ on oral contraception when sold in Medsafe approved manufacturer’s original pack containing not more than 6 months’ supply by a registered pharmacist who has successfully completed the approved training programme. Prescription-only medicine S4
Prescription-only medicine S4 New South Wales: Trial of resupply of lower risk oral contraceptives by trained community pharmacists began September 2023.
New South Wales: Trial of resupply of lower risk oral contraceptives by trained community pharmacists began September 2023. Victoria: Statewide pilot of resupply of certain oral contraceptives by trained community pharmacists began October 2023.
Victoria: Statewide pilot of resupply of certain oral contraceptives by trained community pharmacists began October 2023. Prescription-only except when in medicines for oral use containing 100 mg or less per dose unit when sold in the manufacturer’s original pack containing not more than 12 solid dosage units for the treatment of erectile dysfunction in males aged 35–70 years by a registered pharmacist who has successfully completed a training programme ▲ endorsed by the Pharmaceutical Society of New Zealand.
Prescription-only except when in medicines for oral use containing 100 mg or less per dose unit when sold in the manufacturer’s original pack containing not more than 12 solid dosage units for the treatment of erectile dysfunction in males aged 35–70 years by a registered pharmacist who has successfully completed a training programme ▲ endorsed by the Pharmaceutical Society of New Zealand. Prescription-only medicine S4
Prescription-only medicine S4