Primary care experiences in the ‘Let’s test for HPV’ study: a qualitative analysis
Kayden Borchowsky 1 , Morgan Rush 2 , Thomas Mullally 3 , Lynn McBain 1 , Ben Hudson 3 , John McMenamin 2 , Debra Smith 2 , Peter Sykes 4 , Susan Garrett
3 , John McMenamin 2 , Debra Smith 2 , Peter Sykes 4 , Susan Garrett  1 *
1 *
1 Department of Primary Health Care and General Practice, University of Otago, Wellington, PO Box 7343, Wellington South 6242, New Zealand.
2 Health and Research Collaborative, Wicksteed Street, Whanganui, New Zealand.
3 Department of Primary Care and Clinical Simulation, University of Otago, Christchurch, New Zealand.
4 Department of Obstetrics and Gynaecology, University of Otago, Christchurch, New Zealand.
Journal of Primary Health Care 15(2) 147-154 https://doi.org/10.1071/HC23038
Published: 27 June 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: The National Cervical Screening Programme (NCSP) will switch from cervical cytology to Human Papillomavirus (HPV) testing as the primary cervical screening modality in 2023. To prepare for rollout an implementation study of HPV testing in primary care commenced in August 2022 in three different geographic regions in NZ.
Aims: This study explores Primary Care Staff’s experience of using the HPV testing pathway in the ‘Let’s test for HPV’ study so that recommendations can be made to improve the testing process before nationwide roll-out.
Method: Thirty-nine primary care staff were interviewed across all 17 practices in the Capital and Coast, Canterbury and Whanganui region participating in the ‘Let’s Test For HPV’ study. In total 19 interviews took place which followed a semi-structured approach. These interviews were recorded and transcribed. Template analysis was carried out on transcripts to aid in identifying themes.
Results: Three key themes, with additional subthemes, were identified. Staff were strongly supportive of the new testing regime. Interviewees identified some issues with the new pathway. Educational needs for both patients and clinicians were identified.
Conclusion: Primary care staff described the experience of using the HPV testing pathway positively; however, there were requests for ongoing additional support and nationwide rollout to be supported by practitioner and patient education programmes. With the right support this new pathway for cervical cancer screening has the potential to improve access for previously underserved and unserved groups.
Keywords: cervical cancer, cervical screening, health services research, HPV, human papillomavirus, self‐sampling, self‐testing, primary care.
| WHAT GAP THIS FILLS |
| What is already known: Evidence shows that HPV testing has a greater sensitivity for early detection of precancerous lesions than cytology. HPV tests can be delivered as vaginal self-swabs, which are more acceptable for under-screened women than clinician-taken samples and therefore can increase screening participation, especially for Māori and Pasifika. |
| What this study adds: Clinicians favoured this new method of screening for cervical cancer and reported high levels of patient enthusiasm for self-swabbing. Lessons for nationwide roll-out included providing more clarity around some points in the pathway, providing an education package for the wider community and ongoing support for clinicians when problems arise. |
Introduction
Cervical cancer is a largely preventable disease, however in Aotearoa New Zealand 170 women are diagnosed every year, and 50 die from it annually with the incidence and mortality being higher in Māori and Pasifika.1,2 Of those diagnosed in New Zealand, half had not been screened within five years prior to diagnosis.3 Many (62%) of those who had a screen within five years prior to diagnosis had their cytology reported as ‘normal’.3 This suggests that cervical screening in New Zealand can be improved through both increasing participation and improving the sensitivity of the screening test.
The National Cervical Screening Programme (NCSP) will switch from cervical cytology to Human Papillomavirus (HPV) testing as the primary cervical screening modality in 2023. Over 95% of cervical cancer is caused by preceding HPV infections, a large proportion being attributable to the 16/18 subtypes (‘high risk’ HPV).4,5 HPV testing aims to detect the presence and subtype of HPV. Evidence suggests HPV testing has a greater sensitivity (89.9% cf. 62.5% for CIN 2+) for early detection of precancerous lesions than cytology.5
HPV tests can be delivered as vaginal self-swabs, whereas cytological testing cannot. Evidence shows that self-swabs are more acceptable for under-screened women than clinician-taken samples and therefore can increase screening participation,5–7 especially for Māori and Pasifika.8–10 Self-swabs are as accurate as clinician-taken samples.11 Therefore, HPV self-testing has the potential to reduce the incidence and inequities surrounding cervical cancer in New Zealand.
An implementation study, funded by the NCSP and led by Associate Professor Peter Sykes (University of Otago, Christchurch) was undertaken to provide the NCSP and the NZ public with information to assist the nationwide implementation of HPV, particularly focusing on uptake, efficiency and equity. The implementation trial of HPV testing in primary care commenced in August 2022 and involved 17 general practices across three NZ regions (five from Canterbury, seven from Capital & Coast and five from Whanganui), with 3000 participants expected over six months. The implementation trial did not include research into primary care staff experiences.
The study reported here was undertaken as a summer student research project and aimed to assess how primary care staff are finding using the screening pathway in the implementation trial. In particular what aspects primary care staff considered were working well, challenges, managing results and recommendation for changes.
Methods
Recruitment
Seventeen practices were recruited for the larger implementation trial across three regions: Canterbury, Capital and Coast and Whanganui. Eight practices were recruited by selecting four practices at random from two regions (Canterbury and Capital and Coast). An additional practice from each of the two regions was chosen (at random) from practices with a higher than population level of eligible enrolled Pacific women. Due to the small size of two of the practices randomly selected from Capital and Coast, two additional practices were selected randomly to increase the potential pool of women eligible for recruitment from this region. In Whanganui, following discussion with iwi representatives (and to achieve oversampling for Māori), five practices were selected which represent the six iwi in the region. For the current study an email was sent out to lead trial clinicians at each of the 17 practices. The lead clinicians suggested which staff were most involved with study processes (recalls, appointments and follow up) and interviews were scheduled with these staff.
Data collection
Interview schedules were drafted by the student researcher in each region based on local nuances in the way the main study was run. These were reviewed by study supervisors, then by the Principal Investigator of the larger implementation study. Interview questions were tested on supervisors and between researchers in each region. The schedules were iterative, involving revision to ensure questions were appropriately worded, considering responses from previous interviewees.
The student researcher in each region interviewed all practices within that region. Participants were sent an information sheet, consent form and list of questions ahead of the interview. Interviews were carried out in person where possible, but via zoom or phone in some situations. If possible, participants from the same practice were interviewed together (dyad/triad) to provide a collective experience of the new pathway. Individual interviews were undertaken if participants’ availability did not match. Interviews explored experiences of primary care staff using the screening pathway, what was and wasn’t working well and what could be improved prior to nationwide roll-out. Interviews ranged from 25 min to over an hour. Interviews were audio-recorded, transcribed using ‘Otter.ai’ or the Zoom transcription function and necessary correction was undertaken by the interviewer.
Analysis
Template analysis was carried out on the interview transcripts by the interviewer. Template analysis differs from other methods of qualitative analysis (eg Braun and Clark’s approach) by encouraging development of the coding template based on only a subset of the data and defining themes at the initial template phase rather than further on in the analysis process. Although similar to Framework analysis, the iterative redevelopment of the coding structure is a much more central aspect in Template Analysis. Transcripts were imported into and managed using NVivo.12 Template analysis involves developing a coding template based on only a subset of the data, which helps to identify themes in textual data.13 Each interviewer developed an initial template through coding a subset of their transcripts, and this template was used and revised to code subsequent transcripts. On completion of coding, themes and sub-themes emerged which differed slightly between regions/interviewers.
Results were then discussed between interviewers/regions and similar themes were found across regions. The first author (KB) was responsible for integrating themes from the three regions into one set of collective results. All authors reviewed the collective findings to ensure themes reflected findings from all three regions.
Ethical approval was obtained from the University of Otago Human Ethics Committee in November 2022, ref: D22/285.
Results
Thirty-nine individuals were interviewed from all 17 practices comprising 19 different interviews. Table 1 describes the participants by region, practice identifier and discipline.
| Practice identifierA | Interviewee discipline | |||||
|---|---|---|---|---|---|---|
| Practice nurse lead | Practice nurse | GP | Healthcare assistant | Nurse practitioner | Practice manager | |
| Capital and Coast | ||||||
| WG-1 triad | 1 | 1 | 1 | |||
| WG-2 | 1 | |||||
| WG-3 | 1 | 1 | ||||
| WG-4 dyad | 1 | 1 | ||||
| WG-5 dyad | 1 | 1 | ||||
| WG-6 dyad* | 1* | 1* | 1 | |||
| WG-7 dyad | 1 | 1 | ||||
| Whanganui | ||||||
| WNG-1 | 1 | 1 | ||||
| WNG-2 dyad | 1 | 1 | ||||
| WNG-3 | 1 | |||||
| WNG-4 | 2 | 1 | ||||
| WNG-5 four people | 1 | 1 | 1 | 1 | ||
| WNG-6 | 1B | |||||
| WNG-7 | 1 | |||||
| Canterbury | ||||||
| CH-1 triad | 2 | 1 | ||||
| CH-2 dyad | 1 | 1 | ||||
| CH-3 | 1 | |||||
| CH-4 | 1 | |||||
| CH-5 triad | 3 | |||||
*Staff with an asterisk were part of the dyad, the GP was interviewed individually.
AAssume individual interviews unless dyad or triad is noted, with the exception of WNG5 where four people were interviewed together.
BCervical screening nurse, not practice based.
Twelve interviews took place in person, six interviews took place over Zoom and three interviewees were interviewed over the phone.
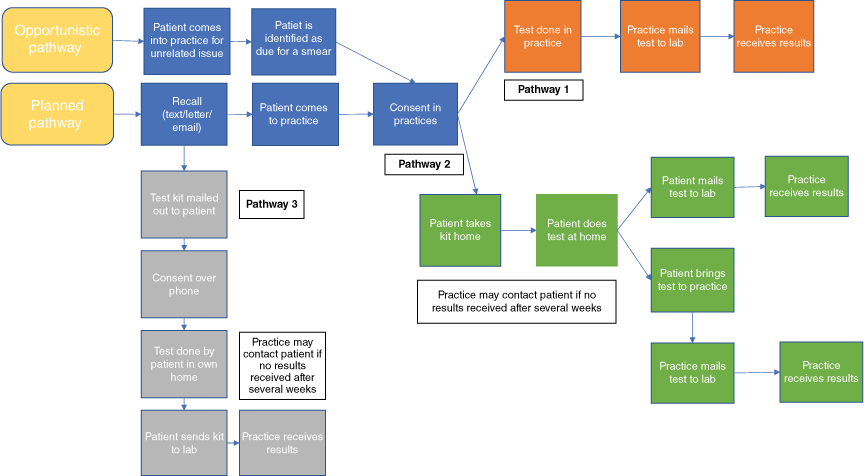
Practices described four distinct pathways which were used to carry out the screening programme, up to the point of receiving results. Each practice was given liberty as to which way/s they delivered this part of the programme. A summary of pathways is available in Fig. 1.
In addition to the pathways described in Fig. 1, Whanganui had a centralised screening coordinator responsible for recall invitations (by letter and/or text), in addition to opportunistic invitation within clinics. Priority patients, based on ethnicity and socioeconomic status, were referred to the PHO outreach service for follow up after one month if they remained unscreened and had no appointment scheduled. Follow up could involve bringing testing kits to the patient’s home or other community locations. After the initial invitation from the screening coordinator, Whanganui practices could then choose to follow pathway one or two and could also initiate screening through the opportunitistic pathway if appropriate.
Following notification of a test result, the pathway was more prescribed (P. Sykes, J. Nip, C. Innes, L. McBain, J. McMenamin, et al. Study protocol: an intervention study investigating the impact of HPV‐based cervical screening in New Zealand, unpubl.). See Supplementary File S1 for the process to follow once results are received.
Three key themes (with subthemes) were identified across the three regions. Anonymised identifiers follow each quote, which include region followed by role held by the interviewee (GP = general practitioner; NL = nurse lead; N = nurse; HCA = health care assistant; NP = nurse practitioner).
Theme one – clinicians were strongly positive towards the pathway
Clinicians noted that most patients were very enthusiastic about the new pathway and prefer it over traditional ‘smears’. Reasons clinicians cited for this positivity included ‘it’s not as invasive’ [WG – 1, NL], ‘it’s not uncomfortable’ [WG – 4, NL], and ‘younger women… are used to doing their own STI swabs, so for them it’s easy’ [CH – 2, N]. Clinicians were pleased to be able to offer this test, as they believed it is superior to cytology alone and felt they were offering the most acceptable care.
During interviews, the concept of mana motuhake (self-determination) was identified by the interviewers. Mana motuhake envisions returning control of health back to the patient. Clinicians believe ‘if people own their body, and own their health, they’re gonna look after it better’ [WG – 5, N]. They see this test as a way of returning power to patients, describing the self-swab as ‘Mana enhancing’ and therefore believe that it is a better test. Clinicians also commented that Māori patients felt positively about the decreased invasion of Tapu (restricted) bodily spaces.
I think it gives people more options and it’s more empowering, they’re taking responsibility for their own health [CH – 3, N]
Increased acceptability and uptake by patients were the main reasons for the positive reaction from clinicians. Key groups in NZ who are often missed in the current cervical screening programme include: Māori, Pasifika, Rural, Asian, more deprived and Transgender individuals (Female to Male).14–16 Many practices interviewed found it was women in these groups whose uptake was most positively impacted.
We’ve picked up patients who we would never get for a smear, ever. And we’ve been trying for 20 years to get them in. [CH – 5, N]
In Whanganui, the community outreach service was key in increasing screening uptake as they were able to locate patients and deliver screening kits directly to them at their residence (which could not be done as easily with cervical cytology).
Some clinicians in this study cited whakamā (shame) as a barrier preventing Māori from participating in the current cervical screening programme. The root of this shame was having to expose oneself to clinicians, especially in smaller communities where patients may know their clinicians outside of a clinical setting. Clinicians believe the self-swab nature of this new screening tool has ‘removed that whakamā’ [WG – 6, NL] and therefore will increase uptake for Māori.
All clinicians have found this test less time consuming, easier, and puts less pressure on them compared to a smear. With this test being less time consuming than cervical cytology, some practices have been able to offer more opportunistic screening, further increasing uptake in the cervical screening programme.
HPV testing in itself is less time consuming, less onerous, less of a demand on resources - whether it’s clinician time, or appointment time… than conventional cytology screening. [WG – 6, GP]
Theme two: issues with the pathway
Many practices cited some difficulty with implementing this programme. Issues were found in areas including understanding the pathway, patient explanation and administration. However, as more tests were processed, familiarity with the process increased and difficulties decreased. This theme of, ‘the pathway is clunky because it’s new’ was found in almost all practices.
It’s literally just because it’s new, it’s clunky, but it won’t be. [WG – 2, N]
We’re getting better. The more you do it, the more you improve on it. [WNG – 6, N]
The re-testing recommendations included in the HPV lab reports were not in line with the new HPV protocol but instead followed the old cervical screening programme recall recommendations. This was particularly evident with the test of cure pathway where the lab would note on a positive HPV result that a patient didn’t need to be tested for another 3 years when they needed re-testing in a year.
Most clinicians mentioned needing to spend considerable time explaining the nuances of the HPV test and required follow up. This included the difference between cytology and HPV testing and convincing some patients that this was a ‘better’ test. Some patients were doubtful that a swab was going to be superior to a ‘smear’: ‘they doubted that that [a swab] would be adequate, they’re like shouldn’t you just have a smear?’ [WNG – 6, CSN]. Explaining that a positive HPV test does not necessarily mean cancer, but rather that further investigation is required was also problematic.
We’re screening them and talking to them and they’re saying, so what if it comes back positive? What’s wrong? Do I have cancer? Like it’s an immediate, straight to cancer. [WNG – 5, NL]
An additional complicating factor was providing an adequate explanation to patients whose first language was not English: ‘I think if anything’s the hardest it’s probably where English isn’t the first language’ [WNG – 5, PN].
Most clinicians reported having trouble reaching some patients who had performed swabs at home, and extra work was required to follow up and ensure swabs were processed. Most results were eventually received by the practices, although this did sometimes take weeks. Some clinicians stated that they don’t trust patients to take the swabs home and therefore strongly encouraged in-clinic appointments. A small number of clinics noted the lack of guidance regarding what level of follow up to undertake if patients couldn’t be contacted as difficult.
I just think they need to tighten up around … sending things out and how that’s gonna look when they don’t respond. So, tighten up that non-responder thing - What’s the next step if they’ve already got the swab? [WG – 2, N]
Most of them do them in the clinic because I don’t trust them to go away…Otherwise, if it’s taken away, and they’ve dropped off and you think, oh, gosh, I’ve got to pull out all that paperwork and do all of that did they sign? [WNG – 4, NP]
Many clinicians believed that patients didn’t view telephone appointments as seriously as they viewed in-person appointments, and therefore were less likely to attend them or set aside sufficient time. Patients were also less receptive to being charged for telehealth appointments.
A lot of the time [patients are] like… ‘Oh can you call me back later? And then when I try and call back later something’s cropped up’ [CH – 4, N]
When asked about the option for patients to take swabs home or have them mailed out to them, there was still support for this option. Removing the barrier of in-person visits for rural dwellers or those who work non-standard hours was seen as positive; however, in-person appointments were seen as likely to support more timely processing of tests and less requirement for follow up.
I think it works better when it’s done in the clinic. And that should be the first way [WNG – 1, N]
Issues with patients having to find a post-box (seen as an issue in more deprived areas), and delays in postal services in rural areas were also cited as issues with home-collected swabs.
Several issues were raised regarding negative consequences of moving to the new pathway. Concern was raised in one practice about the potential for decreased sexual/gynecological health and domestic violence screening which is often included in a traditional cervical screening appointment. The appropriateness of raising these issues during a telehealth consultation was questioned.
You sort of do miss a little bit of the face to face contact, and as part of cervical screening we would often ask family violence questions, and sexual health questions, and I think over the phone you can’t really…[CH – 4, N]
For clinicians to become qualified cervical cytology sample takers, they must complete a set number of cervical cytology sampling procedures under supervision. With the new pathway potentially reducing the number of cervical cytology samples collected, there was concern it would be much harder to meet these requirements.
Theme three – educational requirements for nationwide rollout
Clinicians highlighted the need for the information which patients receive to be at the correct health literacy level, culturally appropriate, accessible, and concise. Common feedback was that information supplied was ‘quite wordy’ [WG – 6, N]. Clinicians found when a language barrier existed, patients’ experiences suffered.
Some patients had concerns relating to the test. They believed they might perform the swab incorrectly or didn’t believe that it was as accurate as a ‘smear’. Clinicians suggested a resource on how to explain HPV testing to patients would be helpful, especially around the differences between what is analysed in cervical cytology compared to an HPV test and why this is a better test.
There was a strong belief among clinicians that a national advertising campaign was necessary to maximise uptake. If ‘the general public don’t even know this is an option’ [WG – 7, N] uptake may be poor, and therefore, clinicians wished to get the word out.
Just so that people understand, and then you’re not sitting there trying to spend a whole consult just explaining why we’re doing things this way now. [CH – 1, N].
It was suggested that this message includes answers to frequently asked questions, what HPV testing involves and direction to resources where patients can find more information. A focus on how HPV is different from other STIs would be helpful, as some patients who had a positive result felt as if ‘they were dirty’ [WNG – 1, N] or had concerns that their ‘partner has cheated’ [WNG – 1, N].
To inform key populations (such as Māori and Pasifika) the need to use culturally appropriate sources of information was suggested such as Māori TV, Pasifika Radio and Churches.
Some clinicians reported, prior to the study commencing, they believed HPV testing was a ‘second line thing …[that] wasn’t as good’ [WG – 5, GP] as cervical cytology, with its implementation aimed at increasing uptake of cervical screening but sacrificing accuracy. Once they had undergone training and education prior to the study their views were changed; however, some clinicians found this belief was still held by their colleagues with one interviewee reporting that a large challenge for them was dealing with colleagues’ misinformation. This also extended to other professions, for example a midwife recommending a patient delay her HPV swab due to being pregnant.
Training regarding the new pathway provided to practices was viewed as sufficient, but its delivery was suboptimal. Training was delivered through a series of Zoom calls which clinicians reported were spaced too far apart and too early before HPV testing started. Clinicians were busy and felt as if they couldn’t give their full attention to the Zoom training. Some clinicians suggested that an online training module with a questions and answers session would better suit them. The use of exemplars in teaching was also suggested.
Clinicians felt they were given too much information to read. A desire was expressed for a summary document that would allow clinicians to quickly refresh their knowledge of the pathway without having to re-read through all the information provided.
Some interviewees reported confusion when dealing with positive results. ‘The process is not clear cut when it comes to a positive result’[WNG – 5, N] particularly when a patient had a complex cervical screening history. Some confusion stems from seemingly contradicting information regarding whether cytology is required or recommended after a positive HPV result prior to colposcopy. The need for ongoing expert support for non-standard presentations was noted by a number of clinicians.
Discussion
Clinicians in this study described using the HPV testing pathway positively and found patients to be equally enthusiastic. Issues included confusion surrounding the appropriate referral and follow up pathway when receiving a positive result, communicating positive results to the patient and uncertainty about the extent of follow up required with patient home-testing. For smooth transition to nationwide roll-out participants had useful suggestions for both clinician and patient education.
Comparison with other studies
Clinician reports in this study mirror the findings of previous NZ patient studies with regards to the acceptability of HPV testing over clinician taken samples, especially for Māori.8–10 The removal of barriers such as lessening whakamā and a desire for bodily autonomy was also raised by participants and has been cited in previous research.8
Mailing out self-test kits is considered pro-equity as it is seen as more acceptable in those who are under- or never-screened.6,17 However, the issue with loss to follow up was present in both of these studies6,17 and has also played out in this study with clinicians being unsure about what level and method of follow up to employ.
Both clinicians and women cited poor health literacy surrounding HPV and cervical cancer as barriers to screening in these studies, with education being an important component in successful testing.8 Recent recommendations for HPV testing implementation include education packages for healthcare providers, culturally appropriate patient resources and an appropriate public communications strategy.18 This was also noted in our study, with the suggestion of a national promotional campaign to increase patient awareness of HPV testing nuances along with more tailored education for primary care clinicians.
Strengths and weaknesses
All practices involved in the larger implementation study were included in this study with a diverse mix of different clinical and non-clinical roles participating. Each practice used a slightly different combination of pathways to deliver the programme, therefore providing a diverse range of views on the strengths and weaknesses of each delivery pathway. Those interviewed had direct involvement with either: carrying out HPV testing, testing oversight or administration relating to testing. This means the most relevant clinician experiences have been included.
The study involved pooling data from three regions, with one individual tasked with integrating themes. Although coding may have varied slightly between the three researchers, it was important that each researcher became familiar with, undertook interviews, analysed data and be based in their region. The benefits gained by becoming familiar with regional nuances outweighed any variation in coding.
The interviews were carried out early in the life of the implementation study (eg one practice had not processed any positive results so was unable to comment on utilising this part of the pathway). If the interviews had been carried out later, we may have uncovered more difficulties with the pathway, although we also may have interviewed people after many teething problems had been resolved.
Implications
Findings from this study have identified training and information needs both for patients and providers and will help inform national roll-out of the programme both for practices and for the NCSP. The inclusion of 17 different practices, across three geographical regions using slightly different methods of delivery means findings will be relevant for a range of practices within NZ.
There was some dissatisfaction noted with incorrect information noted on lab result forms with regards to follow up recommendations. When rolled out nationally lab reporting will comply with the new pathway protocol.
This screening pathway is different from the one NZ currently offers. The interval between testing increases, there is more nuance surrounding positive results and the way positive results are dealt with is different. Due to these changes, effective education is required to help patients understand what is being done and the reasons behind the changes. Clinician education should be broad to allow all primary health care providers to be on board with the new testing regime and its superiority as a screening test.
Conclusion
HPV testing has the potential to increase cervical cancer screening uptake and, in this study, clinicians were very supportive of the new pathway. To ensure smooth roll-out, education for both patients and clinicians about the benefits and processes of the new test were seen as key.
Clinicians requested ongoing expert support for non-standard presentations, more clarity about when cytology should be performed prior to colposcopy, clarification on the intervals between testing if someone has had a previous positive HPV result or abnormal cytology results and clear guidelines regarding follow up for patients who are home-testing.
Acknowledgements
The authors would like to thank the Primary Care staff who participated in these interviews. Thanks also to the Implementation study team for their guidance and advice, and to the Implementation study nurse Rebecca Bell who helped facilitate recruitment for interviews and provided valuable information to the researchers.
References
1 World Health Organisation. Eliminating Cervical Cancer. World Health Organisation; 2022. Available at https://www.who.int/health-topics/cervical-cancer#tab=tab_2 [cited 15 November 2022].
2 Ministry of Health. National Cervical Screening Programme Annual Report. Ministry of Health; 2017. Available at https://www.nsu.govt.nz/system/files/page/national-cervical-screening-programme-annual-report-2017-oct20.pdf [cited 15 November 2022].
3 Hider P, Dempster-Rivett K, Williman J, et al. A review of cervical cancer occurrences in New Zealand 2008–2012. N Z Med J 2018; 131(1472): 53-63 Available at https://journal.nzma.org.nz/https://pubmed.ncbi.nlm.nih.gov/29565936/https://journal.nzma.org.nz/.
| Google Scholar |
4 WHO. Cervical Cancer. World Health Organization; 2022. Available at https://www.who.int/news-room/fact-sheets/detail/cervical-cancer [cited 9 January 2023].
5 Koliopoulos G, Nyaga VN, Santesso N, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev 2017; 2018(8): CD008587.
| Crossref | Google Scholar |
6 Tranberg M, Bech BH, Blaakær J, et al. Preventing cervical cancer using HPV self-sampling: direct mailing of test-kits increases screening participation more than timely opt-in procedures - a randomized controlled trial. BMC Cancer 2018; 18(1): 273.
| Crossref | Google Scholar |
7 Nelson EJ, Maynard BR, Loux T, et al. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. 2017; 93: 56-61 .
| Crossref | Google Scholar |
8 Adcock A, Cram F, Lawton B, et al. Acceptability of self‐taken vaginal HPV sample for cervical screening among an under‐screened Indigenous population. Aust N Z J Obstet Gynaecol 2019; 59(2): 301-7.
| Crossref | Google Scholar |
9 Brewer N, Bartholomew K, Grant J, et al. Acceptability of human papillomavirus (HPV) self-sampling among never- and under-screened Indigenous and other minority women: a randomised three-arm community trial in Aotearoa New Zealand. Lancet Reg Health West Pac 2021; 16: 100265.
| Crossref | Google Scholar |
10 MacDonald EJ, Geller S, Sibanda N, et al. Reaching under‐screened/never‐screened indigenous peoples with human papilloma virus self‐testing: a community‐based cluster randomised controlled trial. Aust N Z J Obstet Gynaecol 2021; 61(1): 135-41.
| Crossref | Google Scholar |
11 Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ 2018; 363: k4823.
| Crossref | Google Scholar |
13 Brooks J, McCluskey S, Turley E, et al. The utility of template analysis in qualitative psychology research. Qual Res Psychol 2015; 12(2): 202-22.
| Crossref | Google Scholar |
14 Brewer N, Pearce N, Jeffreys M, et al. Does screening history explain the ethnic differences in stage at diagnosis of cervical cancer in New Zealand? Int J Epidemiol 2010; 39(1): 156-65.
| Crossref | Google Scholar |
15 Carroll R, Tan KKH, Ker A, et al. Uptake, experiences and barriers to cervical screening for trans and non-binary people in Aotearoa New Zealand. Aust N Z J Obstet Gynaecol 2023;
| Crossref | Google Scholar |
17 Bartholomew K, Grant J, Maxwell A, et al. Feasibility and acceptability of telehealth and contactless delivery of human papillomavirus (HPV) self-testing for cervical screening with Māori and Pacific women in a COVID-19 outbreak in Aotearoa New Zealand. N Z Med J 2021; 135(1565): 83-94 Available at https://journal.nzma.org.nz/https://pubmed.ncbi.nlm.nih.gov/36356272/https://journal.nzma.org.nz/.
| Google Scholar |
18 Bartholomew K, Lawton B, Sherman S, et al. Recommendations for implementing HPV self-testing in Aotearoa. N Z Med J 2021; 134(1535): 11-6.
| Google Scholar |