They’re sicker than we think: an exploratory study profiling the cardio-metabolic health in a sample of adults with pre-diabetes in Aotearoa New Zealand
Christine Barthow 1 * , Sue Pullon
1 * , Sue Pullon  2 , Mark Weatherall 1 , Jeremy Krebs 1
2 , Mark Weatherall 1 , Jeremy Krebs 1
1 Department of Medicine, University of Otago, Wellington, PO Box 7343, Wellington South 6242, New Zealand.
2 Department of Primary Health Care & General Practice, University of Otago, Wellington, PO Box 7343, Wellington South 6242, New Zealand.
Journal of Primary Health Care 14(3) 221-228 https://doi.org/10.1071/HC22068
Published: 30 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of The Royal New Zealand College of General Practitioners. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Introduction: Type 2 diabetes mellitus (T2DM) is a highly prevalent and potentially preventable condition associated with significant health, social, and economic costs. The detection and management of pre-diabetes is an important opportunity to prevent or delay the onset of T2DM and associated morbidities; however, its importance is controversial as the health risks associated with pre-diabetes are poorly understood.
Aim: To understand the cardio-metabolic health profile of a sample of adults with pre-diabetes in Aotearoa New Zealand.
Methods: Secondary analyses of baseline data from all 153 adults recruited to an intervention trial for adults with pre-diabetes were carried out. A profile of cardio-metabolic risk was measured by describing the proportion with metabolic syndrome (MetS) calculated using Adult Treatment Panel III criteria, which includes blood pressure, lipids, and obesity in addition to glycaemic measures. The severity of MetS was calculated as MetS Z-scores. Subgroup analyses for sex, ethnicity and glycated haemoglobin (HbA1c) were performed.
Results: Overall, 74% of this study population had MetS, and the proportion varied according to ethnicity and HbA1c level. The severity of MetS was highly variable, with MetS-Z-scores ranging from −1.0 to 2.8. Although mean MetS Z-scores differed according to ethnicity and HbA1c level, all subgroups included individuals with widely differing severity of MetS, suggesting likely quite different risks for progression to diabetes or cardiovascular disease across the range of pre-diabetes defined by HbA1c.
Discussion: Single biochemical markers of glycaemia are insufficient to ascertain overall cardio-metabolic risk when prioritising clinical efforts for those with pre-diabetes, particularly in primary care, where the potential for preventing or delaying the onset of type 2 diabetes mellitus (T2DM) is significant. Findings indicate the importance of attending to all cardio-metabolic risk factors when caring for people with pre-diabetes. The development of tools using multiple relevant variables and predicting a comprehensive range of outcomes would improve timely risk stratification and treatment effect monitoring of pre-diabetes populations.
Keywords: cardiometabolic risk factors, cardiovascular disease, glycated haemoglobin, metabolic syndrome, prediabetic state, primary health care, progression, renal disease, risk assessment, type 2 diabetes mellitus.
| WHAT GAP THIS FILLS |
| What is already known: Pre-diabetes detected by elevated glycated haemoglobin (HbA1c) is common and indicates an increased risk of progression to Type 2 diabetes mellitus (T2DM) and related comorbidities. Not everyone with pre-diabetes will progress to T2DM or develop related comorbidities; however, considerable uncertainty exists around which individuals with pre-diabetes face the greatest health risks. |
| What this study adds: Single biochemical markers of glycaemia are insufficient to ascertain overall cardio-metabolic risk for those with pre-diabetes. Evaluating pre-diabetes-related health risks by relying on HbA1c alone does not identify those who most need interventions to prevent (or at least delay the onset of) future T2DM and/or cardiovascular disease – additional robust, clinically applicable measurement tools are required to inform comprehensive care. |
Introduction
Type 2 diabetes mellitus (T2DM) is a largely preventable, highly prevalent chronic condition associated with comorbidities, premature mortality, and significant personal, social, and economic costs. The care of people with T2DM and pre-diabetes in Aotearoa New Zealand (NZ) rests largely in primary care, with GPs and primary care nurses (primary care clinicians) responsible for detection and follow up. In NZ, high rates of T2DM and disparities among Māori, Pacific Peoples, Asians, and socioeconomically disadvantaged populations persist.1 A concerning trend is the increasingly earlier onset of T2DM.2,3 The long-term health burden for people with established T2DM is significant, and clinical care is expensive and resource intensive. Optimisation of diabetes prevention is critical to improving outcomes for individuals, whānau and the health system.
Pre-diabetes identifies individuals who are likely to be at risk for developing T2DM; however, its importance is controversial,4–6 as not all with pre-diabetes will develop T2DM.7 The primary care health professionals interviewed as part of a qualitative component of an intervention trial8 repeatedly expressed uncertainty about who with pre-diabetes would best benefit from active intervention and at what stage. NZ uses a glycated haemoglobin (HbA1c) of 41–49 mmol/mol to classify pre-diabetes. Past estimates suggest that >25% of the NZ adult population has pre-diabetes;9 therefore, a critical issue for primary care clinicians tasked with diabetes prevention work is determining which individuals with pre-diabetes will most benefit from active intervention and how the effect of this care can be monitored. Similarly, ensuring healthcare resourcing is appropriately directed to providing effective preventative care for high-risk groups is essential to reduce health disparities and ensure the sustainability of healthcare services.
Those with pre-diabetes may also have non-glycaemic abnormalities such as dyslipidaemia, hypertension and renal impairment. Recent data relating to HbA1c-defined pre-diabetes indicate risks for cardiovascular disease (CVD) and chronic kidney disease increase even when HbA1c levels are below the diagnostic threshold for diabetes, and highlight the need for a broad characterisation of pre-diabetes risk.10,11
In NZ, cardiovascular risk screening in the general population is commonly completed for adults using multivariable-derived risk equations (known as PREDICT equations), based on a nationally representative sample of the NZ population.12 A strength of models like PREDICT is their use of multiple relevant variables to predict risk for CVD; however, although closely related, PREDICT does not assess the independent risk of developing T2DM.
In contrast to PREDICT, the metabolic syndrome (MetS) tool defines a cluster of abnormalities demonstrated to increase the risk of developing T2DM and, to a lesser extent, CVD.13 Commonly used MetS criteria from the US National Cholesterol Education Programme Adult Treatment Programme III include waist circumference (WC), elevated blood pressure (BP), fasting plasma glucose (FPG), blood triglycerides and reduced high-density lipoprotein cholesterol (HDL-C). The prevalence of MetS varies by age, gender and ethnicity, and criteria for central adiposity can be adjusted to account for ethnic differences.13,14
MetS Z-scores are a further refinement, which are calculated from MetS components and can include body mass index (BMI) in place of WC.13 There is no ideal or threshold MetS Z-score. A zero score represents the mean score of the population from which the score was derived. Scores may range from negative to positive values, and each unit represents one standard deviation (SD) away from the mean. Higher values identify more severe MetS, and changes over time have correlated with changing cardio-metabolic health risks and provide early prediction of treatment responses in those with pre-diabetes.15,16 Therefore, the further development of tools like PREDICT or MetS Z-scores for those with pre-diabetes, which would ideally give an absolute risk of disease, may be a promising way to identify those with considerably higher health risks than their HbA1c alone would indicate, and may be helpful for the assessment of treatment response.
The analysis reported here is part of a larger randomised controlled trial of the effects of probiotic and cereal interventions in NZ adults with pre-diabetes (defined by HbA1c 41–49 mmol/mol).17,18 In this paper, we explore and profile the cardio-metabolic characteristics of this study population. We also sought to quantify the proportions and severity of cardio-metabolic abnormality in this study population with pre-diabetes, using the tools of MetS and MetS Z-scores.
Methods
Design, participants and setting
This study is an exploratory secondary analysis of baseline data from all participants recruited to an intervention trial for adults with pre-diabetes. The original study was registered at www.anzctr.org.au ACTRN12617000990325, Universal Trial Number U1111-1195-7561.
The design and methods of the original study are reported elsewhere.18 Briefly, English-speaking adults aged 18–80 years classified as having pre-diabetes by HbA1c 41–49 mmol/mol who were not receiving glucose-lowering medications were recruited from the Wellington urban region to a randomised controlled trial. Participants were predominantly recruited from primary care practices after being sent a study invitation letter if they were coded on practice management systems as having pre-diabetes. Additionally, some were recruited following responses to study advertising in news media and through organisational email lists. Enrolment occurred between February 2018 and March 2019. Ethnicity was coded based on the individual’s self-defined ethnicity, and where multiple ethnicities were selected, the order of priority was Māori, Pacific, Asian (including East and South Asian), and then European. Blood sample analyses were performed in research laboratories using standardised procedures. HbA1c, FPG, and lipids were analysed on a Roche Hitachi Cobas c331 analyser using Roche reagents, and insulin was analysed by Human/Canine/Porcine Insulin DuoSet R&D systems Inc. Insulin resistance was assessed using the homeostatic model assessment for insulin resistance (HOMA-IR) and calculated using the formula proposed by Matthews et al.19 The assessment of such a comprehensive range of biomarkers in a population with pre-diabetes provides a valuable data set, particularly in NZ, where Māori and Pacific peoples constitute one-quarter of the population20 and have disproportionately high rates of diabetes and pre-diabetes.1,9 Although this study population is not necessarily representative of all people with pre-diabetes in NZ, data generated proved valuable for initial exploration of the utility (or otherwise) of a multivariable tool – providing an assessment of the presence and severity of MetS.
Definitions and measurements
The proportions of those with MetS according to the National Cholesterol Education Programme Adult Treatment Panel III 2009 definition14 were calculated using the criteria and ethnic-specific WC thresholds provided in Supplementary Table S1. The relationship between WC, BMI and body fat mass differs between Māori and Pacific peoples and Europeans; however, the relationship between these measures and cardiovascular and metabolic risk also differs by ethnicity. As there are no universally accepted specific WC or BMI thresholds for Māori and Pacific peoples,21–25 recognising this limitation, for this analysis, we applied the same thresholds to these groups as Europeans.
MetS Z-scores based on WC (MetS-Z-WC)26 and BMI (MetS-Z-BMI)15 were calculated using published gender-specific formulae detailed in Supplementary Table S1.
Statistical analysis
Data descriptions use counts and proportions, as percentages, for categorical variables, and mean, standard deviation (s.d.), median, interquartile range (IQR), and minimum and maximum for continuous variables. Our primary interest was to describe the cardio-metabolic health of people with pre-diabetes in this sample, using the MetS-Z-score and to explore the association between MetS-Z-scores and sex; ethnicity (described as Māori/Pacific, Asian, or European); and HbA1c. Although HbA1c is a continuous variable, for illustrative purposes, a cut-off point of HbA1c ≥45 versus <45 mmol/mol was also used based on clinical observations that those in the upper range are more at risk of progression to T2DM.27 Linear regression and ANOVA were used to explore these associations. An overall P-value is shown for the difference in mean values in the ANOVA with ethnicity, and the two pre-specified comparisons were made with European as a reference ethnicity. Although the data set is small and includes limited numbers of Māori and Pacific, we felt that some statistical evaluation of differences was relevant to achieve the exploratory purposes of this work. Summary descriptive data are presented for these variables by sex, ethnicity, and HbA1c cut-off points. Analyses used SAS 9.4 (SAS Institute Inc.).
Ethics approval
Ethical approval was granted by the Central Health and Disability Ethics Committee, New Zealand (17/CEN/88). All participants gave informed written consent.
Results
Participant characteristics
Of the 427 individuals screened for entry into the main study, 245 (57.4%) were ineligible, and 29 (6.8%) declined to participate. The characteristics of all 153 participants enrolled in the study (48% female, 20% Māori/Pacific) are shown in Table 1 and additional baseline data is supplied in Supplementary Table S2. Over 50% of this study population had a family history of T2DM, relatively high proportions were receiving antihypertensive and lipid-lowering medications, and a few had a history of angina, myocardial infarction, congestive heart failure, or stroke.
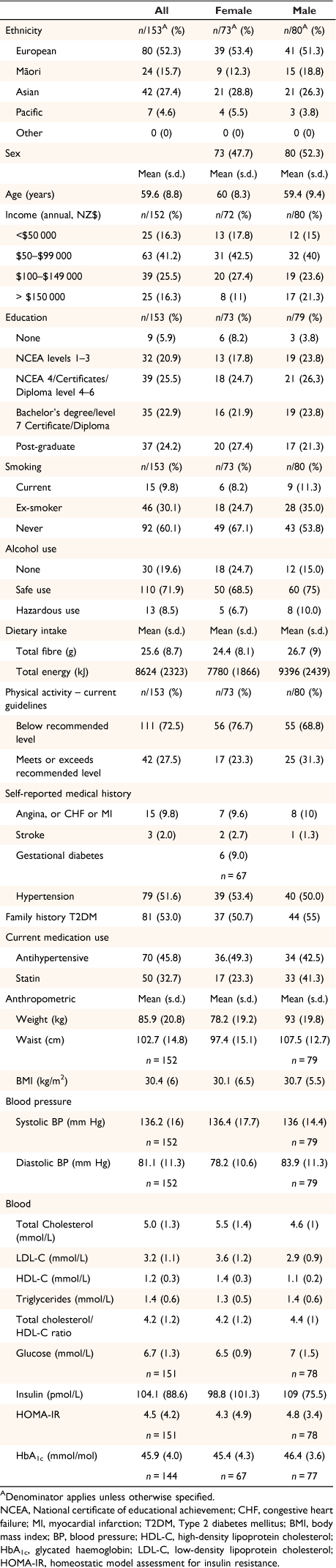
|
Metabolic syndrome
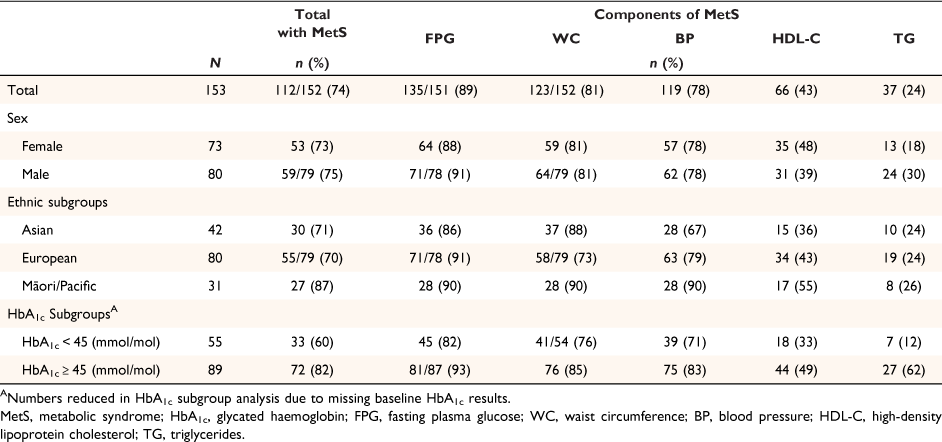
As shown in Table 2, MetS was present in 74% of this sample. Eighty-seven per cent of Māori/Pacific peoples had MetS, which was larger than that for Europeans or Asians, both at about 70%. The proportion of this study sample with MetS varied by HbA1c ≥45 versus <45 mmol/mol, 82% vs 60%.
|
As expected, and shown in Table 2, the most common MetS component present was an elevated FPG. Central adiposity measured by WC was the second most common MetS component, followed by hypertension, HDL-C, and TG. Of the whole study population, 78% (119/153) met the criteria for hypertension.14 Notably, among Māori and Pacific Peoples, elevated FPG, WC and hypertension were present in 90% of those with MetS, and the numbers reaching thresholds for MetS HDL-C levels were at least 12% higher than that in other ethnic groups.
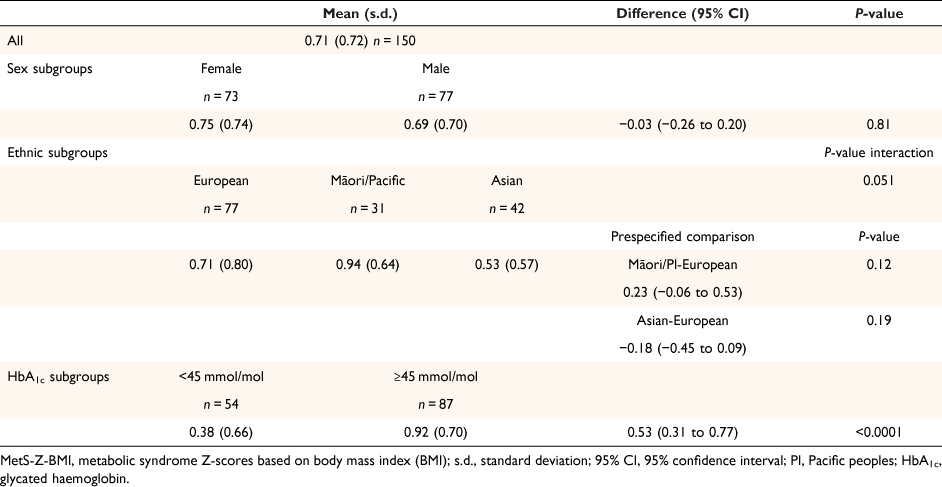
The distribution of MetS Z-scores was similar when calculated by WC or BMI (Supplementary Figs S1, S2). The more clinically relevant MetS-Z-BMI results are shown in Table 3, and full descriptive statistics are available in Supplementary Table S3. Notably, there appear to be some differences in mean MetS-Z-BMI according to ethnicity (P = 0.051) and HbA1c (P < 0.0001) subgroups; however, the range of scores in each group was relatively wide (−1.0 to 2.8) and consistent across the sub-groups (Supplementary Table S3).
|
There was strong evidence of a linear relationship between HbA1c and MetS-Z-BMI; however, HbA1c explained only 17% of the variance in MetS-Z-BMI (Supplementary Fig. S3). For every 5 mmol/mol increase in HbA1c, the Mets-Z-BMI score increased by 0.37 [95% CI, 0.23 to 0.51], P < 0.0001.
Discussion
In this group of people with pre-diabetes who were predominantly recruited from primary care practices into a randomised controlled trial based on HbA1c-defined pre-diabetes, we found a high proportion (74%) had MetS. The proportion of this study sample with MetS appears to differ according to ethnicity and HbA1c level. The severity of MetS was highly variable, and although mean MetS Z-scores appear to differ according to ethnicity and HbA1c level, all subgroups included individuals with widely differing severity of MetS.
Among this study population, elevated blood glucose, waist circumference and hypertension frequently occurred, whereas abnormal HDL-C and triglycerides were less common. Although 33% of the study population were receiving statins, this is unlikely to have impacted HDL-C and triglyceride levels as statins predominantly act on LDL-C.28 Importantly, a key clinical consideration is that lifestyle interventions resulting in weight loss are the most effective interventions that will simultaneously reduce all these abnormalities.29,30 In contrast, although medications also help minimise cardio-metabolic risk, they individually act on fewer risk factors (MetS components) and obviously have potential side-effects and compliance issues. The proportions of individuals with MetS were roughly 17% higher among Māori/Pacific peoples than in other ethnic subgroups, reflecting the different rates of T2DM and CVD found in NZ populations.1 Although these initial findings need further confirmation locally, they strongly suggest that emphasis on weight loss initiatives (as well as optimal prescribing) are critical interventions.31–35 There is a central role for Māori and Pacific primary health providers in leading culturally appropriate lifestyle and other interventions.36–38
Assessment of the severity of MetS using MetS-Z-BMI provided additional helpful insights into this group’s cardio-metabolic health. Māori/Pacific peoples generally had more severe MetS, with mean MetS-Z-BMI scores of approximately 0.9 compared with Europeans 0.7 and Asians 0.5. There was some evidence of a difference in MetS-Z-BMI related to ethnicity (P = 0.051); however, our pre-specified comparisons with Europeans were not individually statistically significant; and may reflect the small sample size.
MetS-Z-BMI increased by 0.37 for every 5 mmol/mol increase in HbA1c, indicating that as HbA1c levels rise, clinical concerns should be directed to reducing hyperglycaemia in conjunction with reducing the full range of CVD risk factors. Our results suggest that those with a HbA1c <45 mmol/mol have MetS less commonly than those with a HbA1c ≥45 mmol/mol. However, notably, even for those with a HbA1c <45 mmol/mol, there was a large range in the severity of MetS (−1.0 to 2.8). This finding illustrates the variability of cardio-metabolic risk found across the spectrum of HbA1c values and underlines the clinical importance of attending to all cardio-metabolic risks and the importance of finding better ways to understand pre-diabetes-associated health risks.
Several things stand out when these results are considered overall. Rather than pre-diabetes being solely an indication of potential future T2DM, our findings suggest that many with this condition are at greater risk of cardio-metabolic disease than is immediately apparent. Although the severity of MetS generally worsened with increasing HbA1c, this was not uniform. The differences in proportions with MetS and variable severity of MetS found for Māori/Pacific peoples emphasise the underlying heterogeneity of pre-diabetes populations.
HbA1c alone appears to be a blunt tool for understanding the cardio-metabolic health of individuals with pre-diabetes, and this is critically important in primary care practice, given the high incidence of pre-diabetes and persistent health disparities. These findings suggest that assessment and therapeutic decision-making need to consider multiple aspects of cardio-metabolic health in those with pre-diabetes. Robust and clinically applicable tools to synthesise this information are required to inform comprehensive care. Any tool proposed for clinical use needs to be easily undertaken and applied – further work needs to be undertaken to create a fit-for-purpose model that could be utilised at scale in primary care. Tools incorporating HbA1c rather than FPG and predicting risk for T2DM, CVD, and microvascular disease, including chronic kidney disease, could be beneficial.
A limitation of this study is the small and potentially unrepresentative sample. Notably, our study population did not experience high levels of deprivation, and given that populations with high deprivation are two and a half times more likely to develop T2DM than non-deprived populations,1 our results likely underestimate the proportions with, and the severity of, MetS in some groups. There are key limitations related to MetS-Z-scores. Notably, these scores were derived from US populations and use FPG rather than the more commonly used HbA1c. Furthermore, development of such tools requires analysis of comprehensive and longitudinal datasets, which are fully representative of NZ populations. Nevertheless, this analysis illustrates the importance of greater emphasis on the overall health of people with pre-diabetes, and explores the use of a possible multivariable, easily applied tool.
Conclusion
Single biochemical markers of glycaemia are insufficient to ascertain overall cardio-metabolic risk when prioritising clinical efforts for pre-diabetes patients, particularly in primary care, where the potential to prevent or delay T2DM and associated morbidities is significant. This exploratory study demonstrated a more nuanced understanding of pre-diabetes cardio-metabolic risks when assessed using MetS-Z-BMI rather than HbA1c or MetS alone. Our findings indicate the importance of attending to all cardio-metabolic risks by primary care clinicians when addressing pre-diabetes. More research is required to develop tools using NZ data, multiple relevant variables and predicting a comprehensive range of outcomes including the development of diabetes, CVD and renal disease for those with pre-diabetes. This could improve timely risk stratification and enhance monitoring of treatment effects in pre-diabetes populations.
Data availability
The datasets analysed during the current study are not publicly available, but reasonable requests to the corresponding author will be considered on a case-by-case basis.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Data used in this study came from the Food 4 Health– He Oranga Kai research programme funded by the Health Research Council of New Zealand, the Ministry of Health New Zealand, and the Healthier Lives National Science Challenge [grant number 16/724]. CB was supported by a University of Otago PhD scholarship.
Supplementary material
Supplementary material is available online.
Acknowledgements
The authors thank study participants for providing data for the Food 4 Health – He Oranga Kai research programme, the other members of the Food 4 Health – He Oranga Kai research team, including J. Crane, F. Hood, E. McKinlay, B. Gray, J. Hilder, C. Cleghorn, M. Huthwaite (University of Otago, Wellington), for their advice and contributions to study design, and research assistants and administrative staff who supported the Food 4 Health – He Oranga Kai research.
References
[1] Ministry of Health. Annual Data Explorer 2019/20: New Zealand health survey. 2020. Available at https://minhealthnz.shinyapps.io/nz-health-survey-2019-20-annual-data-explorer/ [Accessed 10 November 2021][2] Gu Y, Warren J, Kennelly J, et al. Incidence rate of prediabetes: an analysis of New Zealand primary care data. In: Georgiou A, Grain H, Schaper LK, editors. Driving Reform: Digital Health Is Everyone’s Business. IOS Press, Inc.; 2015. pp. 81–86.
| Crossref |
[3] Ministry of Health. Living well with diabetes: a plan for people at high risk of or living with diabetes 2015-2020. 2015. Available at https://www.health.govt.nz/publication/living-well-diabetes
[4] Messina J, Campbell S, Morris R, et al. A narrative systematic review of factors affecting diabetes prevention in primary care settings. PLoS One 2017; 12 e0177699
| A narrative systematic review of factors affecting diabetes prevention in primary care settings.Crossref | GoogleScholarGoogle Scholar |
[5] Kandula NR, Moran MR, Tang JW, et al. Preventing diabetes in primary care: providers’ perspectives about diagnosing and treating prediabetes. Clin Diabetes 2018; 36 59–66.
| Preventing diabetes in primary care: providers’ perspectives about diagnosing and treating prediabetes.Crossref | GoogleScholarGoogle Scholar |
[6] Piller C. Dubious diagnosis. Science 2019; 363 1026–1031.
| Dubious diagnosis.Crossref | GoogleScholarGoogle Scholar |
[7] Richter B, Hemmingsen B, Metzendorf M-I, et al. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev 2018; 10 CD012661
| Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia.Crossref | GoogleScholarGoogle Scholar |
[8] McKinlay E, Hilder J, Hood F, et al. Uncertainty and certainty: perceptions and experiences of prediabetes in New Zealand primary care – a qualitative study. J Prim Health Care 2022; 14 138–145.
| Uncertainty and certainty: perceptions and experiences of prediabetes in New Zealand primary care – a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[9] Coppell KJ, Mann JI, Williams SM, et al. Prevalence of diagnosed and undiagnosed diabetes and prediabetes in New Zealand: findings from the 2008/09 adult nutrition survey. N Z Med J 2013; 126 23–42.
[10] Honigberg MC, Zekavat SM, Pirruccello JP, et al. Cardiovascular and kidney outcomes across the glycemic spectrum. J Am Coll Cardiol 2021; 78 453–464.
| Cardiovascular and kidney outcomes across the glycemic spectrum.Crossref | GoogleScholarGoogle Scholar |
[11] Yahyavi SK, Snorgaard O, Knop FK, et al. Prediabetes defined by first measured HbA1c predicts higher cardiovascular risk compared with HbA1c in the diabetes range: a cohort study of nationwide registries. Diabetes Care 2021; 44 2767–2774.
| Prediabetes defined by first measured HbA1c predicts higher cardiovascular risk compared with HbA1c in the diabetes range: a cohort study of nationwide registries.Crossref | GoogleScholarGoogle Scholar |
[12] Pylypchuk R, Wells S, Kerr A, et al. Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: a derivation and validation study. Lancet 2018; 391 1897–1907.
| Cardiovascular disease risk prediction equations in 400 000 primary care patients in New Zealand: a derivation and validation study.Crossref | GoogleScholarGoogle Scholar |
[13] DeBoer MD, Gurka MJ. Clinical utility of metabolic syndrome severity scores: considerations for practitioners. Diabetes, Metab Syndr Obes Targets Ther 2017; 10 65–72.
| Clinical utility of metabolic syndrome severity scores: considerations for practitioners.Crossref | GoogleScholarGoogle Scholar |
[14] Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. Circulation 2009; 120 1640–1645.
| Harmonizing the metabolic syndrome.Crossref | GoogleScholarGoogle Scholar |
[15] Gurka MJ, Filipp SL, Musani SK, et al. Use of BMI as the marker of adiposity in a metabolic syndrome severity score: derivation and validation in predicting long-term disease outcomes. Metabolism 2018; 83 68–74.
| Use of BMI as the marker of adiposity in a metabolic syndrome severity score: derivation and validation in predicting long-term disease outcomes.Crossref | GoogleScholarGoogle Scholar |
[16] DeBoer MD, Filipp SL, Gurka MJ. Use of a metabolic syndrome severity Z score to track risk during treatment of prediabetes: an analysis of the diabetes prevention program. Diabetes Care 2018; 41 2421–2430.
| Use of a metabolic syndrome severity Z score to track risk during treatment of prediabetes: an analysis of the diabetes prevention program.Crossref | GoogleScholarGoogle Scholar |
[17] Barthow C, Hood F, Crane J, et al. A randomised controlled trial of a probiotic and a prebiotic examining metabolic and mental health outcomes in adults with pre-diabetes. BMJ Open 2022; 12 e055214
| A randomised controlled trial of a probiotic and a prebiotic examining metabolic and mental health outcomes in adults with pre-diabetes.Crossref | GoogleScholarGoogle Scholar |
[18] Barthow C, Hood F, McKinlay E, et al. Food 4 Health - He Oranga Kai: assessing the efficacy, acceptability and economic implications of Lactobacillus rhamnosus HN001 and β-glucan to improve glycated haemoglobin, metabolic health, and general well-being in adults with pre-diabetes: study protocol for a 2 × 2 factorial design, parallel group, placebo-controlled randomized controlled trial, with embedded qualitative study and economic analysis. Trials 2019; 20 464
| Food 4 Health - He Oranga Kai: assessing the efficacy, acceptability and economic implications of Lactobacillus rhamnosus HN001 and β-glucan to improve glycated haemoglobin, metabolic health, and general well-being in adults with pre-diabetes: study protocol for a 2 × 2 factorial design, parallel group, placebo-controlled randomized controlled trial, with embedded qualitative study and economic analysis.Crossref | GoogleScholarGoogle Scholar |
[19] Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28 412–419.
| Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man.Crossref | GoogleScholarGoogle Scholar |
[20] Statistics New Zealand. Ethnicity. 2018. Available at http://nzdotstat.stats.govt.nz/wbos/Index.aspx?DataSetCode=TABLECODE8338 [Accessed 27 October 2021]
[21] Rush EC, Crook N, Simmons D. Optimal waist cutpoint for screening for dysglycaemia and metabolic risk: evidence from a Maori cohort. Br J Nutr 2009; 102 786–791.
| Optimal waist cutpoint for screening for dysglycaemia and metabolic risk: evidence from a Maori cohort.Crossref | GoogleScholarGoogle Scholar |
[22] Taylor RW, Brooking L, Williams SM, et al. Body mass index and waist circumference cutoffs to define obesity in indigenous New Zealanders. Am J Clin Nutr 2010; 92 390–397.
| Body mass index and waist circumference cutoffs to define obesity in indigenous New Zealanders.Crossref | GoogleScholarGoogle Scholar |
[23] Halim AA, Basu A, Kirk R. The prevalence of body mass index-associated chronic diseases in diverse ethnic groups in New Zealand. Asia Pac J Public Health 2019; 31 84–91.
| The prevalence of body mass index-associated chronic diseases in diverse ethnic groups in New Zealand.Crossref | GoogleScholarGoogle Scholar |
[24] Meredith-Jones K, Taylor R, Brown R, et al. Age and sex-specific visceral fat reference cutoffs and their association with cardio-metabolic risk. Int J Obes 2021; 45 808–817.
| Age and sex-specific visceral fat reference cutoffs and their association with cardio-metabolic risk.Crossref | GoogleScholarGoogle Scholar |
[25] Cervantes A, Singh RG, Kim JU, et al. Relationship of anthropometric indices to abdominal body composition: a multi-ethnic New Zealand magnetic resonance imaging study. J Clin Med Res 2019; 11 435–446.
| Relationship of anthropometric indices to abdominal body composition: a multi-ethnic New Zealand magnetic resonance imaging study.Crossref | GoogleScholarGoogle Scholar |
[26] Gurka MJ, Lilly CL, Oliver MN, et al. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism 2014; 63 218–225.
| An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score.Crossref | GoogleScholarGoogle Scholar |
[27] Teng A, Blakely T, Scott N, et al. What protects against pre-diabetes progressing to diabetes? Observational study of integrated health and social data. Diabetes Res Clin Pract 2019; 148 119–129.
| What protects against pre-diabetes progressing to diabetes? Observational study of integrated health and social data.Crossref | GoogleScholarGoogle Scholar |
[28] Simons LA. An updated review of lipid‐modifying therapy. Med J Aust 2019; 211 87–92.
| An updated review of lipid‐modifying therapy.Crossref | GoogleScholarGoogle Scholar |
[29] Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary. J Am Coll Cardiol 2019; 73 3168–3209.
| AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: executive summary.Crossref | GoogleScholarGoogle Scholar |
[30] Grundy SM. Metabolic syndrome. In: Bonora E, DeFronzo RA, editors. Diabetes Complications, Comorbidities and Related Disorders, 2nd edn. Springer; 2020, pp. 71–107.
| Crossref |
[31] Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346 393–403.
| Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin.Crossref | GoogleScholarGoogle Scholar |
[32] Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344 1343–1350.
| Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance.Crossref | GoogleScholarGoogle Scholar |
[33] Pan X-R, Li G-W, Hu Y-H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. Diabetes Care 1997; 20 537–544.
| Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance.Crossref | GoogleScholarGoogle Scholar |
[34] Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29 2102–2107.
| Effect of weight loss with lifestyle intervention on risk of diabetes.Crossref | GoogleScholarGoogle Scholar |
[35] Kriska AM, Rockette-Wagner B, Edelstein SL, et al. The impact of physical activity on the prevention of type 2 diabetes: evidence and lessons learned from the diabetes prevention program, a long-standing clinical trial incorporating subjective and objective activity measures. Diabetes Care 2021; 44 43–49.
| The impact of physical activity on the prevention of type 2 diabetes: evidence and lessons learned from the diabetes prevention program, a long-standing clinical trial incorporating subjective and objective activity measures.Crossref | GoogleScholarGoogle Scholar |
[36] Wild CEK, Rawiri NT, Willing EJ, et al. Determining barriers and facilitators to engagement for families in a family-based, multicomponent healthy lifestyles intervention for children and adolescents: a qualitative study. BMJ Open 2020; 10 e037152
| Determining barriers and facilitators to engagement for families in a family-based, multicomponent healthy lifestyles intervention for children and adolescents: a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[37] Wild CEK, Rawiri NT, Willing EJ, et al. What affects programme engagement for Māori families? A qualitative study of a family‐based, multidisciplinary healthy lifestyle programme for children and adolescents. J Paediatr Child Health 2021; 57 670–676.
| What affects programme engagement for Māori families? A qualitative study of a family‐based, multidisciplinary healthy lifestyle programme for children and adolescents.Crossref | GoogleScholarGoogle Scholar |
[38] Tane T, Selak V, Hawkins K, et al. Māori and Pacific peoples’ experiences of a Māori-led diabetes programme. N Z Med J 2021; 134 79–89.


