Adapting the Auckland Sleep Screening Tool for pharmacy: pharmacists’ experience and feedback
Natalie Gauld 1 4 , Crystal Braganza 2 , Bruce Arroll 31 School of Pharmacy, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand.
2 Natalie Gauld Ltd, PO Box 9349, Newmarket, Auckland 1023, New Zealand.
3 Department of General Practice and Primary Health Care, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand.
4 Corresponding author. Email: n.gauld@auckland.ac.nz
Journal of Primary Health Care 11(2) 170-177 https://doi.org/10.1071/HC19003
Published: 18 July 2019
Journal Compilation © Royal New Zealand College of General Practitioners 2019.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: Insomnia has negative health effects and may indicate underlying serious conditions, but is underdiagnosed and often not discussed with a doctor.
AIM: This study aimed to explore the utility and workability in New Zealand community pharmacies of a 23-question sleep-screening tool adapted from the Short Auckland Sleep Questionnaire.
METHODS: A multidisciplinary advisory group (sleep specialist, general practitioner and pharmacists) discussed the tool, pharmacists’ capability in managing insomnia and training needs for pharmacists, and recommended management strategies, including referral points. Twelve community pharmacists piloted the tool with people with insomnia who presented in pharmacies, recording the time it took to administer the tool. The pharmacists were then surveyed about their experiences with the tool and possible improvements.
RESULTS: Ten pharmacists took an average of 12.4 min (range 4–35 min) for each use of the screening tool with 62 people with insomnia. Most pharmacists found the screening tool easy to administer, organised and easy to follow and nine of 10 said it provided better information than their usual consultation. Seven of 10 pharmacists would use it again. Time limitations and low recruitment were potential barriers to usage especially for pharmacy owners.
DISCUSSION: The screening tool could provide a useful addition to pharmacists’ toolkits, aiding information gathering and better than usual practice. The tool was acceptable to most pharmacists, but its use takes time and remuneration needs consideration.
KEYwords: Community pharmacists; community pharmacy services; consultation and referral; insomnia; screening; sleep disorders.
| WHAT GAP THIS FILLS |
| What is already known: A short screening tool for insomnia is available for general practice in New Zealand. |
| What this study adds: This study shows pharmacists’ experiences with and their views of this tool adapted for pharmacy. The tool was found to be workable and useful, but often time-consuming. |
Introduction
Insomnia negatively affects daily functioning, work performance and quality of life.1–4 It is associated with an increased likelihood of cardiovascular disease, diabetes, obesity, injury and depression.4 Insomnia can also be a symptom of important underlying conditions, such as depression, anxiety and sleep apnoea.5 Despite this importance to health, insomnia remains underdiagnosed,4 probably because many patients with insomnia do not discuss it with their doctor.6 However, people commonly present to pharmacies for sleep aids.7
In New Zealand, pharmacy staff triage patients presenting with sleep problems and sometimes supply short-term sedating antihistamines, herbal remedies, advice on sleep hygiene and referrals where considered necessary. Although pharmacists use screening tools for activities such as supplying trimethoprim, sildenafil and vaccinations, they have not used one for helping patients manage insomnia.
Concerns about pharmacists’ ability to find sufficient time for patient consultations to diagnose insomnia contributed to rejection by the New Zealand Medicines Classification Committee of the melatonin reclassification from prescription to pharmacist-only medicine in 2012.8,9 The Committee considered pharmacists needed a screening tool to help ascertain underlying conditions. When melatonin was proposed for pharmacist-only supply, a condensed tool to aid in diagnosing primary insomnia was proposed.9 However, the Medicines Classification Committee was concerned that even with this tool pharmacists may miss important underlying diagnoses and would have insufficient time to use it in their pharmacies.
In Australia, the Pharmacy Tool for Assessment of Sleep Health was developed from four validated instruments and found to be user friendly and feasible.10 Questions related to sleep apnoea, medications taken, shift work, restless legs and sleep health factors. However, the tool did not identify possible depression or anxiety, the most common causes of insomnia in general practice5 and bidirectionally related to insomnia.11 The tool also did not identify problems with alcohol and drugs, bruxism or delayed sleep phase. Fuller et al.12 later used three tools, namely the Insomnia Severity Index (ISI), Dysfunctional Beliefs About Sleep and the Depression, Anxiety and Stress Scales, in a cluster randomised study to assess the feasibility and efficacy of pharmacists’ intervention regarding sleep.
An online-based 68-item screening tool (o-SQ) designed by the Swiss Federation of Pharmacists13,14 combines the Epworth Sleepiness Scale (ESS) and Stanford Sleep Disorders Questionnaire with input from experts. When used online with the results available to the treating pharmacist, 23% of pharmacy clients had possible obstructive sleep apnoea, 15% had restless leg syndrome and 1% potentially had a psychiatric condition causing their insomnia. However, the o-SQ would probably be too long to use in community pharmacies, and self-completion may be challenging where health literacy is low.
A condensed insomnia screening tool has been developed for use in New Zealand general practice (the Short Auckland Sleep Questionnaire) based on the longer ‘gold-standard’ Auckland Sleep Questionnaire.15 The gold-standard Auckland Sleep Questionnaire had at least 53 questions: some affirmative responses (eg sleep walking or menopause) triggered further questions. The Short Auckland Sleep Questionnaire considers a more complete range of potential underlying conditions than either pharmacy questionnaire from Australia,10,12 and should be more manageable than the seven-page gold-standard tool or the 68-item o-SQ from Switzerland, but it has not been tested in pharmacy use.
The present study adapted a 23-question (two-page) sleep-screening tool and investigated its utility and workability in community pharmacies. This paper reports on the number of screenings the pharmacists completed, how long they took, their views of using the adapted screening tool and the improvements they recommended.
Methods
This study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617001077358p) and received ethics approval from the Central Health and Disability Ethics Committee (17/CEN/67).
Tool development
The Short Auckland Sleep Questionnaire was adapted for pharmacy by the authors, and feedback on it sought from pharmacists, general practitioners (GPs), a sleep doctor and a nurse working in sleep disorders. Before piloting, the adapted tool was finalised, pharmacists’ training needs were ascertained and decisions made as to how to use the tool and when to refer patients for medical review.
A multidisciplinary advisory group finalised the screening tool for pharmacy and recommended training and referral points. The advisory group included a GP, sleep doctor and four pharmacist participants purposively chosen for varied and extensive community pharmacy experience (rural and city, lower-socioeconomic through to high socioeconomic areas, and a pharmacy serving a high Māori population). Participants provided informed consent and worked through key discussion points. The advisory group discussions were audio recorded and transcribed verbatim. The key points arising from the group’s discussion are shown in Box 1. Box 2 shows the areas investigated in the Short Auckland Sleep Questionnaire adapted for pharmacy.
| Box 1. Key points from the advisory group |
|
| Box 2. Areas investigated in the condensed screening tool adapted for pharmacy |
| Identification of insomnia |
| Depression |
| Anxiety |
| Delayed sleep phase |
| Obstructive sleep apnoea |
| Shift work |
| Parasomnias |
| Alcohol misuse |
| Recreational drugs |
| Chronic condition causing insomnia, eg chronic pain or breathing difficulties |
| Additional questions in the tool for pharmacists: |
| • Duration of insomnia |
| • Patient-attributed cause of insomnia |
| • Medication taken |
| • Caffeine use |
| • Whether they have discussed their insomnia with their doctor |
Pharmacist recruitment and training
Pharmacists invited to participate in the pilot study were purposively chosen for variety in ages and pharmacy type. Two pharmacists were also chosen for having a special interest in insomnia. Pharmacists were trained to use the adapted tool in a 1 hour online training course provided by the Goodfellow Unit.16 A supplementary 1 hour webinar included a sleep doctor reviewing when to refer patients, providing an opportunity to ask questions and covering study logistics. Affirmative responses indicating underlying disorders were identified as potential referral points. Pharmacists used these answers and their clinical judgement to help make decisions about whether referral was necessary, asking further questions as needed. Pharmacists were given additional tools for depression (Patient Health Questionnaire, PHQ-9; https://www.phqscreeners.com/select-screener/41), anxiety (Generalised Anxiety Disorder Assessment, GAD-7; https://www.phqscreeners.com/select-screener/41) and alcohol misuse (CAGE; https://psychology-tools.com/test/cage-alcohol-questionnaire) and the Epworth Sleepiness Survey (ESS; https://epworthsleepinessscale.com/about-the-ess/). Pharmacists could decide whether to use these tools for individual patients.
At the end of the study, participating pharmacists who recruited patients were asked nine questions about the tool and to consider possible improvements. Five questions were rated using a five-point Likert scale (from strongly disagree to strongly agree). One question asked about likely future use of the questionnaire and the reason for the answer. Three other questions were open ended, seeking opinions on the screening tool’s questions and potential improvements. Pharmacists in the study who had not used the tool in any consultations were asked why they had not.
Patient participant recruitment and screening
Pharmacists were asked to recruit up to 12 participants each, with a goal of 100 consumer participants in total. The screening was performed between October 2017 and March 2018. Patients could be included in the pilot study if they were aged ≥18 years and requesting a product for sleep or asking for advice on sleep or responding to a sign in the pharmacy about the study. Exclusion criteria included the regular use of prescription sedatives (≥2 days per week; based on advice from the sleep doctor) or difficulty with English.
After patient participants had provided written informed consent, they completed the 23-question screening tool and the ISI with the study pharmacists, who could ask other questions as required. Pharmacists recorded how long this screening took. Patient participants then self-completed the gold standard seven-page tool from which the 23-question tool was derived and returned it, sealed, to their pharmacist (findings about use of the seven-page tool will be presented elsewhere). Pharmacists gave advice based on their initial screening, briefly documented their encounter and recorded how long they took to complete this process. Pharmacists followed-up with patient participants by telephone approximately 2 weeks after the initial consultation, rerunning the ISI and asking six questions for a further part of the study. This paper reports on the pharmacists’ views and experiences of piloting the 23-question tool.
Payment for participation
Advisory group participants were given a gift. Pharmacists were given NZ$40 per participant towards their time. Patient participants received NZ$20.
Results
Pharmacists
Sixteen pharmacists were invited to participate in the study; three declined and one withdrew without recruiting patients. Thus, 12 pharmacists participated from 11 pharmacies located in large and small cities, two from rural areas, one from a central business district (CBD) and a range of socioeconomic areas. The pharmacists represented a range of experience, with five having <5 years experience and four having >20 years experience. Pharmacists also identified with various ethnicities: six were New Zealand European or European, two were South African, and one each were Korean, Indian, Chinese and part-Māori. Four were male and five were owners. The pharmacies were open 40–80.5 h per week, with most open 50–53 h per week. Four pharmacies had a single pharmacist, four had two pharmacists, three had three pharmacists and one had five pharmacists. Three pharmacists serviced areas with high Māori populations. Three pharmacists had participated in the advisory group and accounted for a total of four consumer participants screened.
Patient participation
Ten pharmacists completed between one and 12 consultations each with patients, having an average of 5.3 consultations each over a seven-month period. A total of 62 consultations was available for analysis. Most of the patient participants were female (n = 39; 65.0%), and a range of ages was seen (Figure 1). New Zealand European was the most common patient ethnicity (n = 36; 58.0%), with Indian (n = 6; 10.0%) and Māori (n = 4; 6.4%) the next most common. Just over half the participants reported having had insomnia for over a year, this time. Three pharmacists (including one who withdrew) did not recruit any consumers.
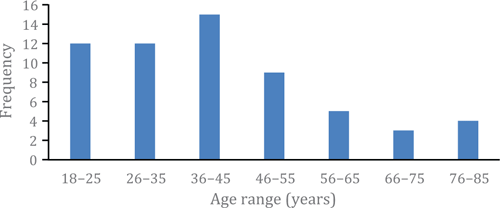
|
Time to complete each screening
Pharmacists reported spending an average of 12.4 minutes (range 4–35 minutes) working through the screening tool with participants, with one pharmacist not answering the questions on the time taken. Two pharmacists (one owner and one employee) typically took under 10 minutes for screenings. One of these was a CBD sole pharmacist (employee) with 12 screenings, who took 4–7 minutes each time. An employee pharmacist (with a special interest in sleep) averaged 22.1 minutes. In low socioeconomic areas one pharmacist averaged 16.7 minutes and another 8.3 minutes. Efficiency did not increase with more screenings.
Advice provision averaged 13.6 minutes (range 4.5–30 minutes), with one pharmacist not answering. The pharmacist with the lowest screening times (4–7 minutes) also had low advice times (4.5–10 minutes; mean 6.2 minutes). The pharmacist with a special interest in sleep averaged 18.6 minutes for advice time.
The five pharmacists with most recruitments (7–12 participants each) were employees. Five of the seven pharmacists recruiting the fewest (0–5 participants) were owners or partners, and three of these five were in the advisory group.
Pharmacists’ views following the pilot
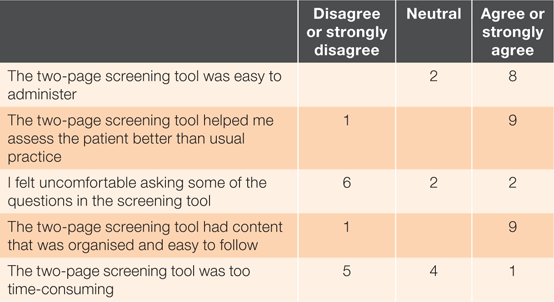
Ten pharmacists who conducted between one and 12 consultations provided feedback on the screening tool (Table 1). Most found the screening tool reasonably easy and more helpful in assessing patients than their usual consultations. Two pharmacists (a female qualified <5 years, and a male qualified for 10–20 years) reported discomfort asking some of the questions: they both reported that the drug and alcohol questions were awkward. However, they had conducted seven and nine screenings, respectively.
|
…the 2-page questionnaire and screening tool were well designed and easy to follow. (Pharmacist 11; female CBD, practising <5 years)
Although half disagreed the tool was too time-consuming (Table 1), owner pharmacists’ other responsibilities hindered recruitment, including one whose pharmacy was relocated during the study period. A pharmacist working alone in a rural pharmacy who used the tool three times also considered it too time-consuming.
A pharmacist noted the importance of training alongside the tool:
I don’t think this is a tool an untrained pharmacist can pick up and use. Training plus experience in applying cognitive behavioural techniques would be necessary. (Pharmacist 4; male, 10–20 years experience, practising in a suburban mall pharmacy)
Seven pharmacists would use the screening tool again, five because it was easy and useful:
…it was easy to administer in a busy setting. The questions had a logical order to them and patients seemed comfortable with the language. (Pharmacist 6; female, <5 years experience, practising in a suburban mall pharmacy in a low socioeconomic area)
Despite the mostly positive findings in Table 1, three pharmacists would not use the screening tool again. Two with ownership responsibilities and sole pharmacist periods valued the tool but noted their time limitations:
For a sole charge pharmacist it was too long. (Pharmacist 7; male pharmacy owner, 5–10 years experience, sole pharmacist in a high-needs rural area)
An employee pharmacist specially interested in insomnia found the tool frustrating and over-simplistic, wanting more sleep hygiene questions, bedtime and rising time, and more room to document findings and advice. He considered other tools were better, but did not suggest which ones.
Seven participants identified sometimes conflicting improvements, with no common theme. Two wanted the tool to be shorter, despite one of them considering it valuable. Suggestions from one person each were: more questions, alcohol and drug questions combined, space for comments after each question and making it computer enabled.
One pharmacist with no recruitments noted ownership responsibilities took priority, and the other without recruitments noted that the pharmacy had virtually no retail business, making recruitment too difficult. Another pharmacist with only one recruitment cited ownership responsibilities and difficulties with being the sole-charge pharmacist over the weekend when she often worked.
Discussion
Pharmacists often reported lengthy screenings, suggesting they had conversations beyond asking the screening questions, as recommended by the advisory group. Almost all pharmacists agreed that the screening tool helped them assess patients better than their usual practice. Increasing efficiency with more screenings was not evident, suggesting that pharmacists found the conversation valuable, although there was considerable variation. The shortest consultation times occurred in a CBD pharmacy with a sole pharmacist, possibly because participants had greater health literacy or participants and the pharmacist were time poor. The longest screening times were from a pharmacist with a special interest in insomnia, who wanted more questions in the tool.
This study did not reach the target 100 participants. Most pharmacists did not reach their target 12 participants, despite an extended study period, with three pharmacists (one who withdrew early) recruiting no patients. Three pharmacists would not use the tool again. Although most pharmacists found the tool easy to administer and more thorough than their usual insomnia consultations, pharmacy owners and some with sole-pharmacist periods struggled with time. The advisory group observed that the tool would not be needed for every person with insomnia, and possibly it was not deemed necessary for many consultations where insomnia was occasional, not too much of a problem, or quickly identified as needing referral without using the tool. The study requirements meant pharmacists had to find extra time to explain the study, obtain informed consent, wait while participants completed a questionnaire in a private room, do the ISI and to later follow-up. This process would have burdened both pharmacists and consumers. We do not know whether consumer lack of interest caused low recruitment as no information was collected on decline rates or numbers presenting with insomnia.
The advisory group and pilot pharmacists recommended few tool changes. Both shorter and longer tools were desired after the pilot, reflecting differences in needs and practices.
Problems with finding time and low recruitment have arisen with other sleep studies in pharmacy,10,12,14 even where participating pharmacists were interested in sleep research and have previous involvement in sleep screening.12 In the Swiss sleep study14 participants completed an online tool and received a summary of results, but screening and counselling still took 15–30 minutes. Tran et al.,10 piloting a screening tool in five Australian pharmacies with participants ‘at risk of sleep disorders’ reported a median 10 minutes to screen (range 2–33 minutes). These participants may have differed from those in the present study because the Australian study excluded patients with a diagnosed sleep disorder, and people taking cardiovascular or endocrine medications, who may not have had insomnia, were included. Furthermore, the completed screening tool was sent to the researchers to decide on action rather than pharmacists needing to decide on referral. The pharmacists in the present study probably needed longer conversations with patients to decide whether referral was necessary.
We found that pharmacists mostly liked the screening tool, but some did not use it or would not use it again. The Swiss online tool was largely liked by pharmacists, but 28% of pharmacies that agreed to participate in the study did not conduct any screenings and only 53% of pharmacist respondents wanted to continue with it.14 Australian pharmacists found their tool relatively easy to complete, but also reported time challenges, frustration about waiting for the researchers to respond with the necessary action and had mixed views about repeating the service as an online form.10
Our advisory group noted the need for a conversation and to interpret questions for some people, preferring face-to-face completion if possible. In addition, high motivation and health and computer literacy may be needed to self-complete a long tool online. The Swiss study14 did not indicate how many people declined or did not complete the online tool, or whether any had difficulty answering questions.
Given the time needed for the tool, it may not be financially feasible without charging the patient. In Australia, 50% of patients were willing to pay for the sleep service they trialled, on average AU$63 (€39),17 and some pharmacies charge for their time providing sleep apnoea advice and devices.18 Extended consultations increasingly occur in New Zealand pharmacies (eg government-funded warfarin International Normalised Ratio (INR) testing).19 In New Zealand, trimethoprim20 and sildenafil21 dispensing requires a consultation (usually charged for) using a tool and is largely viewed positively. A move to more lengthy consultations needs to be associated with benefit for patients, and this will be considered in other papers from this study.
Strengths and weaknesses
A strength of this study was that it used a tool intended to identify a range of underlying concerns developed for general practice and adapted for pharmacy, with advice from a multidisciplinary group on tool content, use and referrals. The study engaged pharmacists from a range of pharmacies, although only 10 pharmacists provided feedback on the tool.
Recorded screening time likely included ISI questions and possibly time explaining the study and collecting consent, overestimating screening time in this study. The ISI would not be required if the screening tool was used outside the study because this was a research tool.
No information was collected about consumers who were eligible but not recruited. Participants selected by study pharmacists or wanting to go into the study may have had more complex needs than consumers declining or not approached, potentially lengthening the screening time. Many screening times were round numbers, suggesting estimation rather than accurate recording. The low recruitment numbers suggest that the participants are not representative of consumers with insomnia presenting at pharmacies.
Implications for practice
Since the completion of this study, melatonin has been reclassified in New Zealand using this tool and there is mandatory pharmacist training, informed by this study.
Pharmacy owners and some (but not all) sole pharmacists reported difficulty finding time to use the tool. Multipharmacist pharmacies may be better placed to provide insomnia consultations and other extended services. The low recruitment to the present study may also reflect the busy-ness of pharmacy and that not everyone wanting a sleep remedy or advice needs such formal screening, as noted by the advisory group. Time may be saved through consumer self-completion, or additional self-completed sleep hygiene questions, an area for further research. A consultation fee may also be needed.
Because this tool will be mandatory for melatonin provision in NZ, it will be interesting to see how it works in real life, where pharmacists could become more accustomed to the tool and screening process over time. Although some pharmacists did not use the screening tool at all, some had low recruitment and some did not want to continue using the tool. Where pharmacists consider melatonin may be appropriate, the tool will be used, whereas in the present study pharmacists had to find time in their day for a task that was researcher led rather than led by patient need. The tool has had slight revision and a little shortening following this study. However, given the desire of the Medicines Classification Committee to ensure screening for important underlying causes,9 further shortening is unlikely unless some underlying causes are omitted.
Implications for research
For future research, more employee pharmacists and fewer pharmacy owners may better aid recruitment. However, the higher recruitment by employee pharmacists may reflect that they were more driven by the payment of NZ$40 per recruitment than owners. Further research into real-life use and feedback from a wider range of pharmacists is warranted. Comparison between a self-completed tool and face-to-face completion with pharmacists could also be helpful.
Conclusions
The screening tool is useful and workable for some pharmacists in assessing sleep disorders, with training and guidance, subjectively improving their consultations. Being time-consuming could limit its use or need a consultation charge to be viable.
References
[1] Sateia MJ. Update on sleep and psychiatric disorders. Chest 2009; 135 1370–9.| Update on sleep and psychiatric disorders.Crossref | GoogleScholarGoogle Scholar | 19420207PubMed |
[2] Wilson JF. In the clinic. Insomnia. Ann Intern Med 2008; 148 ITC13–1-ITC-6.
| 18166757PubMed |
[3] Falloon K, Arroll B, Elley CR, et al. The assessment and management of insomnia in primary care. BMJ 2011; 342 d2899
| The assessment and management of insomnia in primary care.Crossref | GoogleScholarGoogle Scholar | 21622505PubMed |
[4] Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press; 2006.
[5] Arroll B, Fernando Iii A, Falloon K, et al. Prevalence of causes of insomnia in primary care: a cross-sectional study. Br J Gen Pract 2012; 62 e99–103.
| Prevalence of causes of insomnia in primary care: a cross-sectional study.Crossref | GoogleScholarGoogle Scholar | 22520782PubMed |
[6] Ramsawh HJ, Bloom HG, Ancoli-Israel S. Chapter 111: Sleep, aging, and late-life insomnia. In Fillit H, Rockwood K, Woodhouse K, editors. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 7th ed. Philadelphia: W. B. Saunders; 2010. p. 943–8.
[7] Hamann J, Linde K, Schweiger HD, et al. Over-the-counter-drugs for the treatment of mood and anxiety disorders – the views of German pharmacists. Pharmacopsychiatry 2014; 47 84–8.
| Over-the-counter-drugs for the treatment of mood and anxiety disorders – the views of German pharmacists.Crossref | GoogleScholarGoogle Scholar | 24652700PubMed |
[8] Medsafe. Minutes of the 47th meeting of the Medicines Classification Committee, 1 May 2012. [Cited 22 July 2012]. Available from: http://www.medsafe.govt.nz/profs/class/mccMin1May2012.htm
[9] Medsafe. Minutes of the 48th meeting of the Medicines Classification Committee, 30 October 2012. [Cited 22 December 2012]. Available from: http://www.medsafe.govt.nz/profs/class/mccMin30Oct2012.htm
[10] Tran A, Fuller JM, Wong KK, et al. The development of a sleep disorder screening program in Australian community pharmacies. Pharm World Sci 2009; 31 473–80.
| The development of a sleep disorder screening program in Australian community pharmacies.Crossref | GoogleScholarGoogle Scholar | 19462256PubMed |
[11] Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res 2008; 64 443–9.
| A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population.Crossref | GoogleScholarGoogle Scholar | 18374745PubMed |
[12] Fuller JM, Wong KK, Hoyos C, et al. Dispensing good sleep health behaviours not pills – a cluster-randomized controlled trial to test the feasibility and efficacy of pharmacist-provided brief behavioural treatment for insomnia. J Sleep Res 2016; 25 104–15.
| 26306418PubMed |
[13] Schwegler K, Klaghofer R, Nirkko AC, et al. Sleep and wakefulness disturbances in Swiss pharmacy customers. Swiss Med Wkly 2006; 136 149–54.
| 16633960PubMed |
[14] Hersberger KE, Renggli VP, Nirkko AC, et al. Screening for sleep disorders in community pharmacies – evaluation of a campaign in Switzerland. J Clin Pharm Ther 2006; 31 35–41.
| Screening for sleep disorders in community pharmacies – evaluation of a campaign in Switzerland.Crossref | GoogleScholarGoogle Scholar | 16476118PubMed |
[15] Arroll B, Fernando A, Falloon K, et al. Development, validation (diagnostic accuracy) and audit of the Auckland Sleep Questionnaire. J Prim Health Care 2011; 3 107–13.
| Development, validation (diagnostic accuracy) and audit of the Auckland Sleep Questionnaire.Crossref | GoogleScholarGoogle Scholar | 21625658PubMed |
[16] Goodfellow Unit. Insomnia: diagnosis and treatment. Auckland, NZ: Goodfellow Unit; 2012. [Cited 6 February 2019]. Available from: https://www.goodfellowunit.org/courses/insomnia-diagnosis-treatment
[17] Fuller JM, Wong KK, Krass I, et al. Sleep disorders screening, sleep health awareness, and patient follow-up by community pharmacists in Australia. Patient Educ Couns 2011; 83 325–35.
| Sleep disorders screening, sleep health awareness, and patient follow-up by community pharmacists in Australia.Crossref | GoogleScholarGoogle Scholar | 21640541PubMed |
[18] Hanes CA, Wong KKH, Saini B. Clinical services for obstructive sleep apnea patients in pharmacies: the Australian experience. Int J Clin Pharm 2014; 36 460–8.
| Clinical services for obstructive sleep apnea patients in pharmacies: the Australian experience.Crossref | GoogleScholarGoogle Scholar | 24562977PubMed |
[19] Harper P, McMichael I, Griffiths D, et al. The community pharmacy-based anticoagulation management service achieves a consistently high standard of anticoagulant care. N Z Med J 2015; 128 31–41.
| 26411845PubMed |
[20] Braund R, Henderson E, McNab E, et al. Pharmacist-only trimethoprim: pharmacist satisfaction on their training and the impact on their practice. Int J Clin Pharm 2016; 38 1357–61.
| Pharmacist-only trimethoprim: pharmacist satisfaction on their training and the impact on their practice.Crossref | GoogleScholarGoogle Scholar | 27832404PubMed |
[21] Braund R, Ratnayake K, Tong K,, et al. Pharmacist supply of sildenafil: pharmacists’ experiences and perceptions on training and tools for supply. Int J Clin Pharm 2018; 40 650–8.
| Pharmacist supply of sildenafil: pharmacists’ experiences and perceptions on training and tools for supply.Crossref | GoogleScholarGoogle Scholar | 29605946PubMed |


