Deprivation and inequalities lead to worse outcomes with dabigatran etexilate
Bryan H. Simpson 1 , David Reith 2 , Natalie J. Medlicott 1 , Alesha Smith 11 University of Otago, School of Pharmacy Dunedin, New Zealand
2 University of Otago, School of Medicine, Department of Women’s and Children’s Health, Dunedin, New Zealand
Correspondence to: Bryan H. Simpson, University of Otago, School of Pharmacy Dunedin, New Zealand. Email: bryan.simpson@postgrad.otago.ac.nz
Journal of Primary Health Care 10(4) 303-311 https://doi.org/10.1071/HC17081
Published: 7 December 2018
Journal Compilation © Royal New Zealand College of General Practitioners 2018.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: Dabigatran etexilate is now prescribed for 51% of the patients receiving oral anticoagulation treatment in New Zealand. Its prescribing trends in relation to patient outcomes are, however, largely unknown for these patients.
AIM: To describe patient characteristics, effectiveness and safety of treatment with dabigatran etexilate in the New Zealand population.
METHODS: This retrospective cohort study used administrative health data for patients dispensed dabigatran etexilate between 1 July 2011 and 31 December 2015. Adverse events (haemorrhage) and treatment failure (thromboembolism or cerebrovascular accident) data were extracted and linked to patient-specific demographic data. Baseline patient characteristics were analysed with descriptive statistics to examine trends in dabigatran etexilate prescribing. Raw and adjusted hazard ratios (HRs), including covariates, were derived using Cox proportional hazard models.
RESULTS: In total, 52,413 patients were dispensed dabigatran etexilate. Multivariate analysis indicated the risk of haemorrhagic events were significantly increased for Māori (HR and 95% Confidence Interval (CI): 2.10 (1.54–2.86)) and Pacific Peoples (HR = 2.20 (1.49–3.24)); those aged >80 years (HR = 1.25 (1.08–1.43)); and more deprived populations in quintile 4 (HR = 1.24 (1.08–1.43)) and quintile 5 (HR = 1.30 (1.12–1.50)). There was an increased risk of thromboembolism and cerebrovascular accident among people aged >80 years (HR = 1.79 (1.49–2.15)).
DISCUSSION: Demographic factors are associated with adverse outcomes in patients treated with dabigatran etexilate. Targeted strategies are needed to prescribe dabigatran etexilate more appropriately in these populations.
KEYWORDS: Dabigatran etexilate; Deprivation; Haemorrhage
| WHAT GAP THIS FILLS |
| What is already known: There has been rapid adoption of dabigatran etexilate in New Zealand since its subsidy in 2011, and it is now prescribed for 51% of the patients receiving oral anticoagulation. |
| What this study adds: Real world evidence that demographic factors, such as ethnicity, deprivation and age, directly affect outcomes for people treated with dabigatran etexilate. People with Māori and Pacific Peoples ethnicities are at greater risk of harm than people of other ethnicities when treated with dabigatran etexilate. |
Introduction
Dabigatran, a direct thrombin inhibitor, has been available in New Zealand and fully subsided since July 2011. Following its introduction, it has been rapidly adopted, with 51% of patients receiving oral anticoagulation now being treated with dabigatran. 1 This rapid adoption can be partly attributed to dabigatran having very few interactions with other medications and food, along with minimal requirement for routine laboratory monitoring.2
Treatment with dabigatran is complicated because of the limited knowledge of its real-world safety and efficacy, such as its use for the prevention of thromboembolic events in different patient populations. 3 Depending on clinical risk factors, the dose of dabigatran can be affected by several patient variables. Currently, the New Zealand-approved medicines datasheet recommends a dose of 150 mg twice daily for all indications, except for the treatment of non-valvular atrial fibrillation in patients aged >75 years.4 For patients aged 75–80 years, the dose may be reduced to 110 mg twice daily if the perceived thromboembolic risk is low while the risk of haemorrhage is high.4 For patients aged >80 years, the recommended dose is 110 mg twice daily.4
Although there are some international data on the use of dabigatran,5–8 New Zealand prescribing trends for dabigatran are largely unknown. There is limited data relating to adverse outcomes such as haemorrhage or thromboembolism in patients prescribed dabigatran in New Zealand.9 Also, there are no reported investigations of outcomes from dabigatran use and patients’ demographic characteristics at regional or national levels.
The aim of this population study was to describe current use, effectiveness and safety of treatment with dabigatran in the New Zealand population.
Methods
Identification of study cohort
A retrospective cohort study using administrative health data from New Zealand was conducted. The study population included all patients aged ≥18 years who had at least one dispensing of dabigatran during the study period between 1 July 2011 and 31 December 2015. Data from different datasets were linked using patients’ encrypted National Health Index code (NHI; a life-long unique identifier for all interactions with the health system) to ensure patient anonymity. Ethical approval was obtained from the University of Otago Ethics Committee (Reference number HD15/054).
Patient information
Patient data were extracted from the Pharmaceutical Collection,10 which contains prescription details about community pharmaceutical dispensing claims for all nationally funded medicines. It also provides information on patients’ gender, date of birth, age, ethnicity, deprivation index score, geographic location via District Health Board (DHB), prescriber type and frequency and quantity of dispensed medicines. Patients were categorised into the following age groups: <65 years, 65–74 years, 75–80 years and >80 years to align to both regulatory and commonly used clinical stratifications.4,11,12 Continuous dabigatran use was defined as one or more prescriptions recorded in the Pharmaceutical Collection, with less than 120 days between dispensings (prescriptions in New Zealand for dabigatran typically provide 90 days’ supply and are provided in 30-day amounts). When 120 days or more elapsed between dabigatran prescriptions, participants were recorded as having intermittent treatment with dabigatran.
Patient outcomes
The outcomes of interest were any admission to hospital for a possible adverse event of haemorrhage, or treatment failure of thromboembolism or cerebrovascular accident (CVA); this information was extracted from the National Minimum Dataset (NMDS). 13 Unspecified CVAs were included for any diagnosis without a classification of haemorrhagic or thromboembolic event. The NMDS is the national record of all public and private hospital discharge information, including coded clinical data for admissions of >4 h duration. Recorded diagnoses are coded using the International Classification of Diseases and Related Health Problems Tenth Revision, Australian Modification (ICD-10-a.m.).14 Participants were followed from their first dispensing of dabigatran until the date of hospitalisation, cessation of dabigatran treatment or study end.
Statistical analyses
Baseline patient characteristics were analysed with descriptive statistics to examine trends in dabigatran prescribing. Continuous variables were tested for normal distribution by the skewness and kurtosis test. Normally distributed data are presented as the mean (standard deviation (s.d.)) and non-normally distributed data as the median (interquartile range (IQR)).
Differences between groups were analysed using t-tests, Kruskal–Wallis tests and pairwise correlations, as appropriate. Hazard ratios (95% confidence intervals (CI)), for adverse patient outcomes, comparing different covariates were derived from Cox proportional hazard models. An adjusted hazard ratio (95% CI) was obtained using bidirectional backwards then forwards stepwise Cox proportional hazard models regression. The variables considered were geographic location, ethnicity, gender, age, deprivation and dispensed formulation. The level of significance adopted for the variables was P < 0.05 for inclusion and P < 0.01 for re-inclusion. Outcomes with a P < 0.05 were considered statistically significant.
Statistical analyses were performed using Stata/IC (V.14.2; StatCorpLP, College Station, TX, USA).
Results
Patient demographics
Between 1 July 2011 and 31 December 2015, a total of 52,413 patients were initiated on dabigatran treatment. Of these patients, 5321 (10.2%) had intermittent treatment and 31,040 (59.2%) were male. The median age was 72 years (63–79 years) and most patients (38,020; 72.5%) were aged ≥65 years.
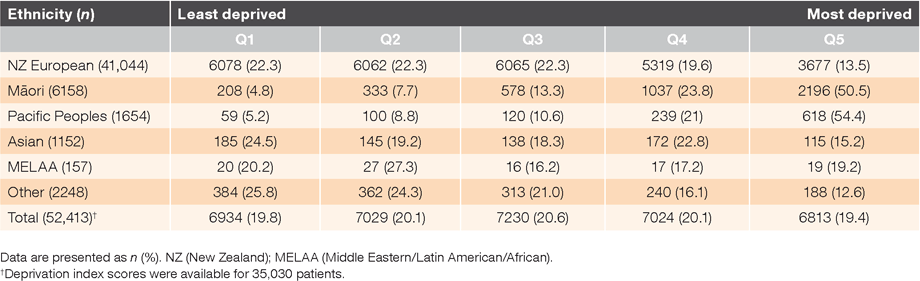
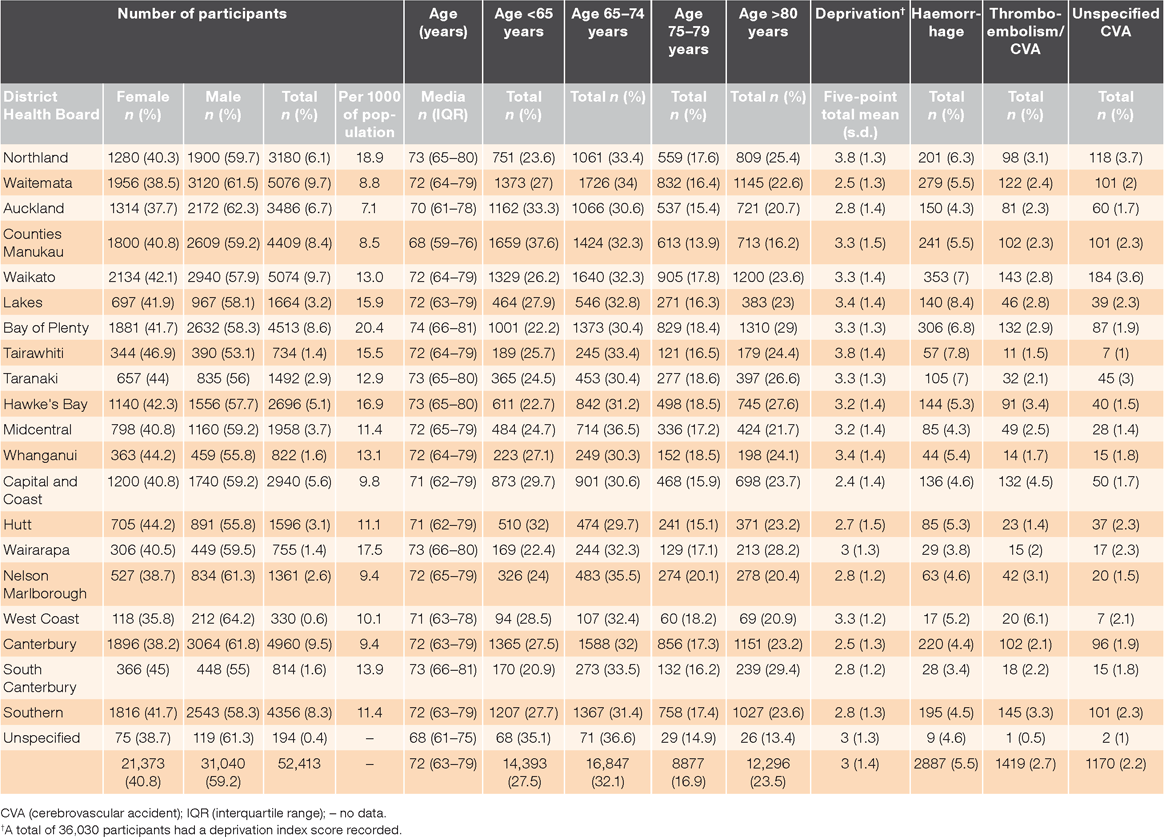
Table 1 summarises the ethnic distribution of patients prescribed dabigatran, with most being New Zealand Europeans. Māori and Pacific Peoples were disproportionately represented in the most deprived quintiles (Q4 and Q5), while New Zealand Europeans were represented more in the less deprived quintiles (Q1, Q2 and Q3). Asian, Middle Eastern, Latin American and African (MELAA) and other ethnicities were more evenly distributed across all the quintiles. A Kruskal–Wallis test showed that there was a statistically significant difference in deprivation index score between the different geographic locations; χ2 (20) = 2685.4, P = 0.001. Geographic locations with higher deprivation index scores had greater use of dabigatran per 1000 of population (P = 0.004). A summary of the demographic characteristics of the patients by geographic location is presented in Table 2.
|
|
Prescriber type
Primary health-care physicians initiated dabigatran for 31,384 (59.9%) patients, with this increasing to 42,237 (80.6%) for ongoing treatment. Secondary health-care physicians initiated dabigatran treatment for 7316 (14.0%) patients, with this decreasing to 3692 (7.0%) for ongoing treatment. Other health-care providers initiated dabigatran treatment for 13,713 (26.2%) and provided ongoing treatment for 6482 (12.4%) patients.
Adverse events requiring hospitalisation
The median follow-up time for participants was 240 days (IQR: 90–690 days). Approximately 1 in 10 patients required hospitalisation due to an adverse event, which was possibly related to dabigatran; there were 2887 (5.5%) haemorrhagic events, 1419 (2.7%) thromboembolic/CVA events and 1170 (2.2%) unspecified CVAs. Stratification of adverse events by geographic location is presented in Table 2.
The median time to a haemorrhagic event was 240 days (IQR: 65–630 days); median time to thromboembolic/CVA events was 267 days (IQR: 78–613 days); and an unspecified CVA was 280.5 days (IQR: 81–640 days).
The median age for a haemorrhagic event was 76 years (IQR: 68–82 years); thromboembolism/CVA 78 years (IQR: 70–83 years); and an unspecified CVA was 78 years (IQR: 69–83 years).
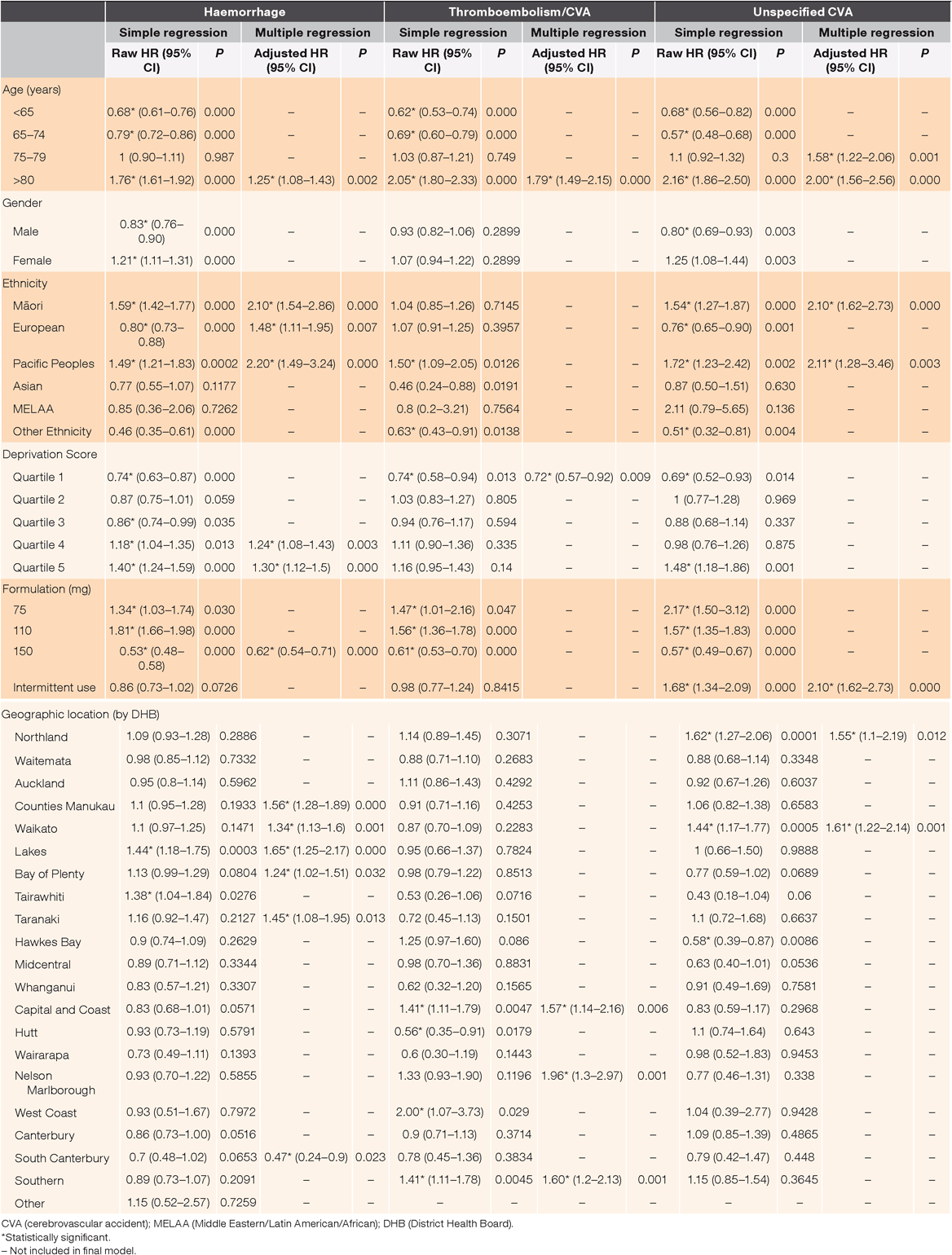
Multivariate analysis indicated that the variables significantly associated with an increase in haemorrhagic events were: geographic location, age >80 years, ethnicities of Māori, Pacific Peoples and New Zealand European, deprivation quintiles 4 and 5 and receiving 150 mg formulation (Table 3). The variables significantly associated with an increase in thromboembolism/CVA were: geographic location, age >80 years and deprivation quintile 5 (Table 3). The variables significantly associated with an increase in unspecified CVA were geographic location, age 75–79 years, age >80 years, ethnicities of Māori and Pacific Peoples and intermittent use (Table 3). None of the covariates used in the final models were correlated.
|
Discussion
This study aimed to produce a more complete picture of the current use, effectiveness and safety of treatment with dabigatran in the New Zealand population. The study highlights that dabigatran is mainly prescribed in primary care to the elderly, and areas with a higher deprivation score have significantly higher usage.
Adverse events
The rate of haemorrhagic adverse events in the current study of 5.5% is lower than reported in the New Zealand Medicines Datasheet. 4 However, we found a higher rate of treatment failure with a thromboembolism/CVA rate of 2.7% in the current study. The association of demographic factors with dabigatran treatment outcomes has not been previously reported for New Zealand patients. This study showed that living in certain geographical locations, living in higher deprivation areas, being aged >80 years and identifying as Māori or Pacific Peoples were all associated with an increased risk for adverse outcomes. While previous studies have shown a relationship between geographic deprivation and poor health outcomes in general for New Zealanders,15–18 none have investigated this in relation to dabigatran treatment. This study specifically showed that patients living in more deprived areas are more likely to experience haemorrhagic events that can be attributed to dabigatran. This is consistent with the findings of a New Zealand national health survey that found Māori and Pacific Peoples and people living in the most deprived areas of New Zealand generally report greater unmet health needs than others. 19 To address this issue, additional resources and strategies need to be targeted at these populations during treatment initiation and maintenance.
The time to either adverse event or treatment failure occurs mainly in the first 2 years of treatment. A median time of 120 days (IQR: 30–340 days) to haemorrhagic event has been previously reported only for gastrointestinal haemorrhage.20 The current study combined all forms of haemorrhagic events and showed that most of these occur in the first 2 years of treatment. This indicates the importance of adhering to recommended patient monitoring at clinically appropriate time points. For example, it has been reported that patients with atrial fibrillation receiving oral anticoagulation exhibit a decline in renal function over time, indicating the need for renal function monitoring at regular intervals.21 There are varying reports indicating different levels of compliance to recommended monitoring timeframes,1,22 making it important to increase the awareness of clinicians and patients regarding appropriate clinical monitoring. This could involve development of consistent education programmes, such as national continuing medical education modules.
The limitations of this study include the NMDS capturing patient data only for people who required hospitalisation for a duration of >4 h. Therefore, outcomes of interest that did not meet this criterion, for example, a haemorrhage or CVA that resulted in death, would not be included in the dataset, resulting in possible under estimations. Also, there can be errors in the clinical codes entered in the NMDS, resulting in possible inclusion or exclusion of the clinical outcomes of interest. As comorbidities vary between population groups of different ethnicities and as their interaction with dabigatran treatment is not accounted for in this study, there is possible bias in outcome profiles. For example, Māori have a higher prevalence of diabetes and this may attribute to CVA at younger ages.23 As the NMDS contains no diagnostic information, it was not possible to investigate disease-specific dose regimens by indication. The main strength of this study is the inclusion of a large national cohort of patients, with sufficient sample size to provide adequate information about the effects of demographics on dabigatran outcomes for an entire population.
The findings of this study demonstrate that dabigatran patients of Māori and Pacific Peoples ethnicity are at greater risk of harm than people of other ethnicities.
Conclusion
We have provided new evidence that demographic factors can directly affect outcomes for people treated with dabigatran and real-world evidence for when these events occur. Improvement of outcomes could be achieved by increased vigilance of clinical monitoring, especially in the Māori and Pacific Peoples populations, the elderly and people living in more deprived areas.
COMPETING INTERESTS
None.
ACKNOWLEDGEMENTS
The authors wish to thank the School of Pharmacy, University of Otago, Dunedin, for providing a PhD fee stipend for B. H. Simpson.
References
[1] bpacNZ. Dabigatran monitoring—an update. Dunedin: bpacNZ; 2017. [cited 2017 September]. Available from: http://www.bpac.org.nz/ report/2017/September/2017Dabigatran_report_Sample.pdf[2] Schulman S. Advantages and limitations of the new anticoagulants. J Intern Med. 2014; 275 1–11.
| Advantages and limitations of the new anticoagulants.Crossref | GoogleScholarGoogle Scholar |
[3] Alexander GC, O’Connor AB, Stafford RS. Enhancing prescription drug innovation and adoption. Ann Intern Med. 2011; 154 833–7.
| Enhancing prescription drug innovation and adoption.Crossref | GoogleScholarGoogle Scholar |
[4] Boehringer Ingelheim (N.Z.) Limited. Pradaxa: New Zealand Datasheet (2017). Auckland: Boehringer Ingelheim; 2017. [cited 2017 November]. Available from: http://www.medsafe.govt.nz/profs/datasheet/p/Pradaxacap.pdf
[5] Weitz JI, Semchuk W, Turpie AGG, et al. Trends in prescribing oral anticoagulants in Canada, 2008–2014. Clin Ther. 2015; 37 2506–2514.e4.
| Trends in prescribing oral anticoagulants in Canada, 2008–2014.Crossref | GoogleScholarGoogle Scholar |
[6] European Medicines Agency. European Medicines Agency updates on safety of Pradaxa. London: European Medicines Agency; 2011. [cited 2016 April]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/ 2011/11/WC500117818.pdf
[7] Loo SY, Dell’Aniello S, Huiart L, Renoux C. Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol. 2017; 83 2096–106.
| Trends in the prescription of novel oral anticoagulants in UK primary care.Crossref | GoogleScholarGoogle Scholar |
[8] Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016; 5 e003725
| Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation.Crossref | GoogleScholarGoogle Scholar |
[9] Metcalfe S, Moodie P. National prescribing data for dabigatran. N Z Med J. 2012; 125 97–105.
[10] New Zealand Ministry of Health. Pharmaceutical Collection. Wellington: Ministry of Health; 2016.
[11] U.S. Department of Health and Human Services, Food and Drug Administration, Guidance for Industry. E7 studies in support of special populations: geriatrics. Questions and answers. Silver Spring: U.S. Food and Drug Administration; 2012. [cited 2017 October]. Available from: https://www.fda.gov/downloads/Drugs/ GuidanceComplianceRegulatoryInformation/Guidances/UCM189544.pdf
[12] Committee for Human Medicinal Products (CHMP). Adequacy of guidance on the elderly regarding medicinal products for human use. European Medicines Agency. London: European Medicines Agency; 2011. [cited 2017 October]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/ Scientific_guideline/2010/01/WC500049541.pdf
[13] New Zealand Ministry of Health, National Minimum Dataset (Hospital Events) data dictionary. Wellington: Ministry of Health; 2015.
[14] Australian Consortium for Classification Development. The International statistical classification of diseases and related health problems. Tenth revision, Australian modification (ICD-10-AM). 6th edn. Lidcombe, NSW: National Centre for Classification in Health; 2008.
[15] Abas MA, Vanderpyl J, Robinson E, et al. Socio-economic deprivation and duration of hospital stay in severe mental disorder. Br J Psychiatry 2006; 188 581–82.
| Socio-economic deprivation and duration of hospital stay in severe mental disorder.Crossref | GoogleScholarGoogle Scholar |
[16] Atkinson J, Salmond C, Crampton P. NZDep2013 Index of Deprivation. Wellington, New Zealand: Ministry of Health; 2014.
[17] Barnett R, Lauer G. Urban deprivation and public hospital admissions in Christchurch, New Zealand, 1990–1997. Health Soc Care Community. 2003; 11 299–313.
| Urban deprivation and public hospital admissions in Christchurch, New Zealand, 1990–1997.Crossref | GoogleScholarGoogle Scholar |
[18] Exeter DJ, Zhao J, Crengle S, et al. The New Zealand Indices of Multiple Deprivation (IMD): a new suite of indicators for social and health research in Aotearoa, New Zealand. PLoS One. 2017; 12 e0181260
| The New Zealand Indices of Multiple Deprivation (IMD): a new suite of indicators for social and health research in Aotearoa, New Zealand.Crossref | GoogleScholarGoogle Scholar |
[19] New Zealand Ministry of Health. Annual update of key results. 2015/16 New Zealand Health Survey. 2016. Wellington: Ministry of Health; 2016. [cited 2017 October]. Available from: http://www.health.govt.nz/publication/annual-update-key-results-2015-16-new-zealand-health-survey
[20] Abraham NS, Noseworthy PA, Yao X, et al. Gastrointestinal safety of direct oral anticoagulants: a large population-based study. Gastroenterology. 2017; 152 1014–1022.e1.
| Gastrointestinal safety of direct oral anticoagulants: a large population-based study.Crossref | GoogleScholarGoogle Scholar |
[21] Böhm M, Ezekowitz MD, Connolly SJ, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. 2015; 65 2481–93.
| Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial.Crossref | GoogleScholarGoogle Scholar |
[22] Thorne K, Dee S, Jayathissa S. Prescriber compliance with renal function monitoring in patients taking dabigatran (Pradaxa). N Z Med J. 2015; 128 83–7.
[23] Wong SW, McGrath NM. Screening, prevalence and ethnic variation of diabetes mellitus in people with acute stroke and transient ischaemic attack: a cross-sectional study in Northland, New Zealand. N Z Med J. 2016; 129 62–6.


