Invasive pneumococcal disease and serotype emergence in the Auckland region during the vaccine era 2009–16
Nick Eichler 1 , Edwin Reynolds 1 2 , Catherine Jackson 1 , Simon Thornley 1 , Julia Peters 11 Auckland Regional Public Health Service, Cornwall Complex Private Bag 92-605, Symonds Street, Auckland 1150, New Zealand
2 Corresponding author. Email: edwinr@adhb.govt.nz
Journal of Primary Health Care 11(1) 24-31 https://doi.org/10.1071/HC17080
Published: 3 April 2019
Journal Compilation © Royal New Zealand College of General Practitioners 2019.
This is an open access article licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Abstract
INTRODUCTION: There is a deficit of knowledge in New Zealand as the epidemiology of invasive pneumococcal disease varies significantly between countries.
AIM: Time trends and sociodemographic characteristics of cases of invasive pneumococcal disease (IPD) in the Auckland region are reviewed after the introduction of a conjugate vaccination, to provide evidence for future vaccine policy and to ensure Auckland region analysis is representative of national trends for subsequent IPD analysis.
METHODS: Data on all cases of IPD occurring in Waitemata, Auckland and Counties Manukau District Health Boards between 2009 and 2016 were extracted from EpiSurv. Denominator data were drawn from mid-year estimates supplied by Statistics New Zealand. Descriptive epidemiology and time-series regression was performed to analyse trends.
RESULTS: Rates of IPD have fallen in the Auckland region over the past 8 years by 32%. While absolute rates in the elderly have reduced by 12%, they have the highest disease burden at 32/100,000. The ethnic disparity continues with Pacific people (33/100,000) and Māori (14/100,000) over represented compared to European (10/100,000). In the elderly, the 19A serotype has increased from an incidence of 0 in 2008 to 8.2/100,000.
DISCUSSION: Large ethnic and age-related disparities are observed in the Auckland region, consistent with the rest of the country, since the start of the pneumococcal vaccination era. Extending immunisation to the elderly may help close these gaps. As with other countries, there is 19A serotype replacement occurring following conjugate vaccine introduction.
KEYWORDS: Invasive pneumococcal disease; descriptive epidemiology; ethnic disparity; vaccine policy
| WHAT GAP THIS FILLS |
| What is already known: The occurrence of invasive pneumococcal disease can be controlled by vaccine policy. |
| What this study adds: This study describes the epidemiology of the disease in the Auckland region as a snapshot of the New Zealand situation. |
Introduction
Streptococcus pneumoniae (S. pneumoniae) is a ubiquitous gram-positive bacterium with 96 serotypes identified by their capsular polysaccharide.1,2 Infection with S. pneumoniae is often asymptomatic, due to nasopharyngeal carriage, especially in young children, where up to 75% can be carriers.3 When disease progresses, it can range from mild mucosal infections such as acute otitis media and sinusitis, to more serious infections including pneumonia, meningitis, empyema and septic arthritis. These severe infections are collectively referred to as invasive pneumococcal disease (IPD).2 It can occur both as a complication of mucosal infection or as a primary infection without an identifiable source.1
Globally, certain groups are particularly at risk from IPD, including the young (aged <2 years), the elderly (aged ≥65 years), the immunocompromised and specific ethnic minorities.4 The mechanism of increased susceptibility is likely immature immune responses in infants, insufficient immune responses in the immunocompromised and immunosenescence in the elderly.1 In New Zealand (NZ), certain groups such as Māori and Pacific people are more frequently exposed to disease through socioeconomic deprivation and crowded living conditions.4
The capsular polysaccharide is a determinant of virulence and invasiveness, thus certain serotypes are associated with more invasive potential and anti-microbial resistance.5 With the introduction of worldwide vaccine programmes, the phenomenon of serotype replacement has resulted where vaccine strain serotypes are replaced with non-vaccine serotypes.6,7 The emergence of non-vaccine serotypes, which are potentially more virulent, is a selective pressure on vaccine programmes.8 The 19A serotype is particularly concerning, given its rapid appearance and propensity to cause more severe disease.9
The types of vaccines and the serotypes they protect against are summarised in Table 1.10 Two types of vaccine against S. pneumoniae, conjugate and polysaccharide, have been developed. Pneumococcal conjugate vaccines (PCVs) use bacterial serotype antigens attached to a relatively immunogenic carrier protein. The bystander effect of the carrier protein induces strong antibody responses and immunological memory to 7–13 attached vaccine serotypes (PCV7, PCV10 and PCV13). Pneumococcal polysaccharide vaccines (PPSVs such as Pneumovax-23©) use purified bacterial surface sugar antigens for immunogenicity with 23 serotypes.11 PPSVs produce a short-lived antibody response with poor effective immunological memory and are not suitable for children aged <2 years.12
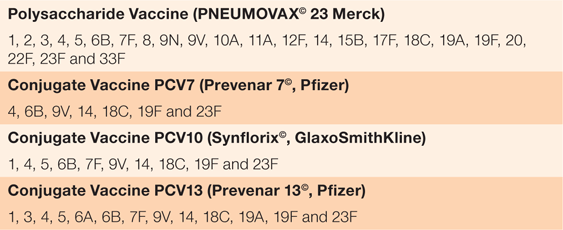
|
The NZ schedule is four doses, given at ages 6 weeks, 3 months, 5 months and 15 months.10 Pneumococcal conjugate vaccine-7 (PCV7 or Prevenar-7©), the first conjugate vaccine for S. pneumoniae, was introduced to the NZ immunisation schedule in June 2008.10 This was replaced by PCV10 (Synflorix©) in 2011, which in turn replaced by PCV13 (Prevenar-13©) in 2014. Each new conjugate vaccine has increased the number of S. pneumoniae serotypes included (from seven to 13), focusing on those that most commonly cause severe disease. From 1 July 2017, PCV10 replaced PCV13 as the primary vaccination for the prevention of IPD on the immunisation schedule. Research in NZ and elsewhere shows that addition of the scheduled PCV vaccine in the <4 years age cohort results in indirect herd reductions in vaccine strain IPD incidence in the elderly.13
Because outbreaks of IPD are rare; public health action to address the disease is largely dependent on vaccination policy and reducing population exposure to determinants of disease.14 IPD has been notifiable since October 2008, with information on each case collated in the Institute of Environmental Science and Research (ESR) EpiSurv database. While countrywide descriptive epidemiology reports are readily available, this study analysed IPD in the Auckland region to confirm similar trends in the NZ-wide data, and further examines the characteristics in the context of the types of vaccine available and their strategic use.
Methods
Setting
Approximately 1.5 million people live in the Auckland region, which has three district health boards (DHBs) – Waitemata, Auckland and Counties Manukau. Auckland Regional Public Health Service is the public health unit for the whole region. The three DHBs each have approximately one-third of the total population, with Waitemata the largest and Auckland the smallest. The three areas together are representative of the NZ population because the age, ethnic and socioeconomic demographic distributions of these regions vary considerably. Counties Manukau has a relatively young population (one-third of its population is aged <20 years), with a higher proportion of Pacific ethnic groups than the national average and a higher proportion of people in the most socioeconomically deprived quintile. Waitemata has the least deprivation, a low proportion of Māori and an age structure that closely matches the national average. Immunisation rates for the 2-year-old cohort are 93.4% for Auckland, 93.5% for Counties Manukau and 90.3% for Waitemata DHB, compared with the national average of 90.9% of children fully immunised at age 2 years.15
Case definition and notification
A case of invasive pneumococcal disease is defined as: ‘the isolation of S. pneumoniae from cerebrospinal fluid (CSF), blood or other normally sterile site; or the detection by nucleic acid amplification test of pneumococcal DNA in CSF, blood or other normally sterile site; or a positive newer generation S. pneumoniae antigen test on CSF or pleural fluid’.16
Data collection
No ethics review was required to analyse these data as no information can identify cases. EpiSurv is the national notifiable disease database maintained by the ESR (Wellington, New Zealand). IPD is notified directly by laboratories. Details sent to EpiSurv also populate the Auckland Regional Public Health Service’s notification system. No public health action is taken in response to a notification; the purpose of notification is passive surveillance only.
Analysis
The EpiSurv database was queried for all cases of IPD occurring in the Auckland region between 1 January 2009 and 31 December 2016. Where incidence rates are reported, denominator data are taken from Statistics New Zealand’s population estimates prepared for the Ministry of Health, based on the 2013 census. Data were grouped by sex, age (0–4, 5–64 and ≥65 years), ethnicity and DHB of residence. Incidence rates are reported for the total number of cases reported in each 3-month period (one quarter) of the calendar year, divided by the denominator Census population and divided by four to yield an incidence per 100,000 person-years. The period between 1 January and 31 March represents the first quarter, and so on. Information on S. pneumoniae serotype (determined by ESR at its National Reference Laboratory) was also analysed to determine trends in relative proportions of serotypes causing cases of IPD. Ethnicity extracted from both EpiSurv and the Statistics New Zealand data is prioritised, which tends to increase representation of Māori, while decreasing representation of Pacific people.17 Where ethnicity was not recorded in EpiSurv, this was cross-matched using the unique National Health Index identifier numbers (NIHs) in the electronic patient database (Concerto) at the Auckland DHB. These cases were not included in analyses where ethnicity was a variable.
Statistical analysis was performed in Microsoft Excel©, with Microsoft Analysis ToolPak© add-in and using the OpenEpi version 3.01 Byar approximation when calculating incidence rate ratios.18 For incidence rate ratios, the numerator was the total number of confirmed cases notified to the Auckland Regional Public Health Service, available on Episurv. The denominator was the estimated resident population for Auckland from the corresponding year, which was calculated as the sum of people living in the Auckland, Waitemata and Counties Manukau DHBs, available from Statistics New Zealand. Incidence rates were summarised into counts per 100,000 person-years. To assess the long-term trend while adjusting for seasonality, the third-order polynomial smoothing function was used.
Results
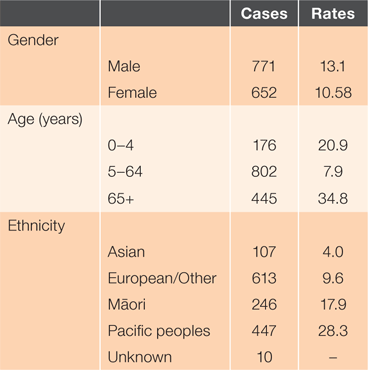
Between January 2009 and December 2016, there were 1,423 cases of IPD in Auckland. Serotype characteristics are presented in Table 2.
|
From 2009 to mid-2016, there was a trend of gradually decreasing annual incidence rates from 14.2 to 9.6/100,000 person-years. The winter of 2016 had a spike in incidence rates. As illustrated in Figure 1, a seasonal pattern occurred with increased incidence over winter. The average number of cases was 178 per year, with an overall incidence rate of 11.8/100,000 person-years. There was a significant difference in incidence rates by sex, with an incidence rate ratio of 0.81 for females compared to males (95% CI 0.72–0.90, P < 0.05).
|
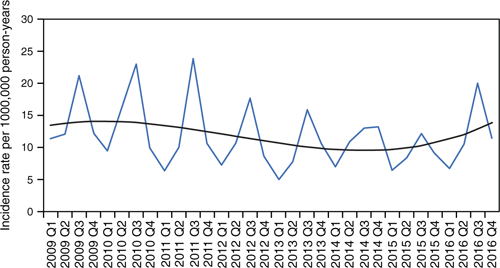
Figure 2 shows the age distribution of cases in Auckland, with the two major at-risk groups (people aged 0–4 and ≥65 years) over-represented in rates of IPD. There was a decline in IPD rates of 12% and 32% over the 8 years for the ≥65 and 0–4 years age groups respectively. Cases increased in the winter quarter of 2016, mainly in the 0- to 4-year age group.
|
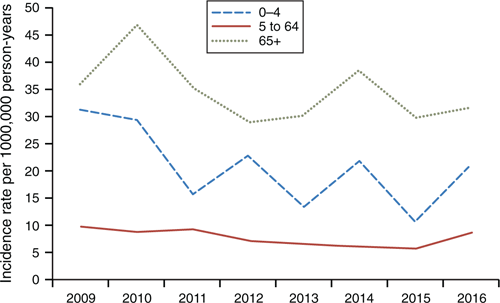
Figure 3 shows the differential rates when analysed by ethnicity. Through the study period, European/Other and Asian rates remain consistently low, at 3.8 and 10/100,000 person-years respectively. The Māori incidence rate rapidly declined between 2009 and 2013, before increasing in 2014–15, then dropping again in 2016. The highest incidence rates were found in Pacific people, peaking at 36/100,000 person-years. Before 2016, the Pacific people incidence rate of IPD was decreasing more quickly than the European/Other and Māori rates. However, the IPD incidence rate for Pacific people increased noticeably in late 2016. Between 2009 and 2016, there was an overall incidence rate ratio of 1.86 (95% CI 1.61–2.16, P < 0.01) between Māori and European/Other, and 2.9 (95% CI 2.61–3.34) between Pacific people and European/Other (P < 0.05).
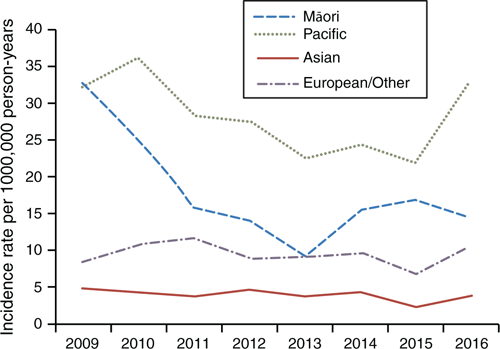
|
Figure 4 demonstrates that socioeconomic deprivation is also positively associated with rates of IPD. There was an incidence ratio of 3.53 (95% CI 2.80–4.44, P < 0.01) for people in the most deprived quintile compared with people in the least deprived quintile. This association is exponential, becoming more pronounced with increasing deprivation.
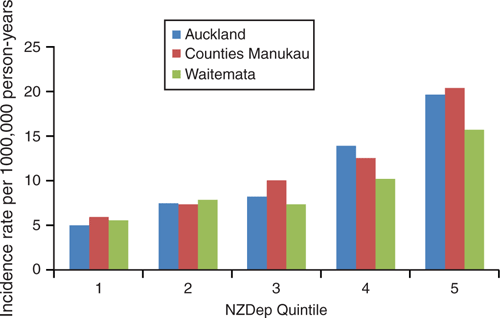
|
In Auckland children aged 0–4 years, there is evidence for both an increasing proportion of IPD cases caused by serotype 19A after introduction of the conjugate vaccine (PCV7 – Prevenar-7©), and a reduction in this proportion from 2015 as the vaccine serotypes were widened to include 19A (PCV13) and cross reactivity to serotype 19A with 19F in PCV10. Figure 5 demonstrates the role of serotype 19A: it caused only 3% of cases in 2008, but rapidly became the dominant serotype, and now causes one-third of IPD cases in this age group.
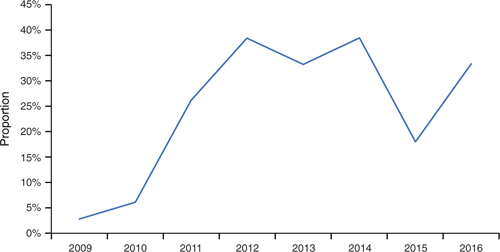
|
Figure 6 demonstrates the emergence of 19A-caused IPD since 2009 between age groups. It shows a sharp rise in the 0- to 4-year age group from 2011, with a gradual and continuing rise in the elderly, which became the group with the highest rates by 2016. Serotypes 1 and 14 were prominent causative types between 2008 and 2011, but have since disappeared, while 15B has recently emerged as a major variant, particularly in the 0- to 4-year age group.
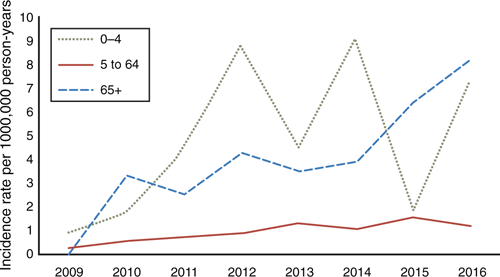
|
Discussion
This study examines the descriptive epidemiology of IPD in the Auckland region.
Like other regions in NZ, IPD rates are cyclical with peaks in winter and troughs in summer.4 Since the vaccine era started, there has been a gradual decrease in disease in the 0- to 4-year age group and no trend in the 5- to 64-year age group. Results show a dominance of IPD disease burden in the elderly – a shift from the pre-conjugate vaccine era where infants had the highest incidence for all cause serotypes. We found a significant difference in disease burden by socioeconomic status, with high rates in NZ Deprivation Index 4 and 5 groups. Ethnically, there were substantially higher rates in Pacific people and Māori than in NZ European/Other and Asian ethnic groups. In the second half of 2016, there was an increase in the rate of IPD in Auckland, mostly in children aged 0–4 years and people of Pacific ethnicity. Most of these extra cases were caused by serotype 19A, despite 90–95% PCV coverage in this population.15 The reason for this is uncertain and may be a result of greater exposure to other determinants of IPD infection not captured by these data.
This study was carried out through the vaccine era and shows a reduction of cases in the 0- to 4-year age group, and herd immunity for older age groups. PCVs have been shown to reduce carriage of S. pneumoniae in the nasopharynx and inner ear, thus conferring herd immunity because the overall prevalence of the organism is reduced in the vaccinated population.19 This is evident in the significant drop in rates of IPD following introduction of the first conjugate vaccine, even in unvaccinated children.20 PCVs also produce herd immunity for adults, as children are the main reservoir of infection.21 Immunisation of children with PCVs translates to reduced nasopharyngeal carriage in adult populations22 and an overall decreased rate of IPD in the elderly.23 PPSV23 is considered inferior due to reduced effectiveness against more aggressive serotypes and inability to prevent pneumonia and otitis media in children.10
The emergence of the 19A serotype has been cause for concern in vaccinated populations. This variant is associated with more serious invasive disease, and with high proportions (30% in the US) of multi-drug resistance.8 PCVs have well-demonstrated vaccine efficacy of up to 97% for vaccine-type serotype invasive infections, but can also have some efficacy against vaccine-related serotypes (i.e. non-vaccine strains), due to cross-reactivity of related antigens.24 A 2014 case-control study of 316 IPD cases demonstrated that PCV10 (Synflorix©) has a vaccine efficacy of 77.9% against 19A, due to cross-reaction with the 19F serotype included in the 10-valent vaccine.25 Prevenar-13© (PCV13) contains a specific 19A antigen, but in recent studies, its vaccine efficacy against IPD due to 19A has been shown to be similar to that of PCV10 (Synflorix©), ranging from 70%26 to 73%.24 This evidence has resulted in NZ replacing PCV13 (Prevenar-13) with PCV10 (Synflorix©) on the National Immunisation Schedule in 2017. New Zealand is the first developed nation to make this change, so it is important that surveillance mechanisms closely follow developments in serotype dynamics.
This analysis shows the emergence of the 19A serotype in Auckland, from being the causative organism in very few cases in 2009 to accounting for one-third of all cases in 0- to 4-year-olds in 2016. We show a reduction in the proportion of 19A serotype in the 0- to 4-year-old age group after the introduction of the PCV10–13 valent vaccine in 2011 and 2014. This was an expected trend due to cross reactivity of 19F in PCV10 vaccine (introduced 2011) and the appearance of 19A in PCV13 in 2014. Following the emergence of 19A in the 0- to 4-year-old age group, the serotype has become a major cause of IPD in the ≥65-year age group as well, with a steadily rising incidence despite a fall in overall incidence. There may be a case for PCV vaccination for the elderly due to this shift in serotypes and the associated increased disease severity of 19A. This conclusion is supported by the CAPITA study,27 a randomised controlled trial that compared immunisation with the 13-valent conjugate vaccine with placebo vaccination in vaccine-naïve adults aged ≥65 years, using a sample of 84,496 study participants. The trial demonstrated statistically significant efficacy in preventing vaccine-type community-acquired pneumonia (VE 45.6%) and vaccine-type IPD (VE 75%) in this cohort, but it did not reduce overall mortality or rates of pneumococcal pneumonia and IPD. Subsequent analysis suggested the intervention was highly cost-effective for high-risk individuals.28 If rates of 19A disease remain high, there may be a role to control this serotype by vaccinating the elderly.
This study did not examine the role of pre-existing comorbidities on the incidence of IPD. Whether iatrogenic or pathological, immunosuppression is a major risk factor for IPD, and PCV is indicated for these patients as well as people with asplenia, cochlear implants or those receiving dialysis.14 Further investigating the comorbidities and vaccination rates of IPD cases could reveal whether PCVs are being adequately delivered to high-risk populations. Studies investigating the correlation of comorbidities with IPD and the rise in the serotype 19A non-vaccine strain replacement are ongoing.
Conclusion
NZ has made considerable progress towards the control of IPD with the introduction of the conjugate vaccine. However, large ethnic and age-based differences in disease incidence remain. Vaccine and surveillance policy must now use epidemiological evidence to implement further measures to protect people at greatest risk of the disease. As well as childhood vaccination, it might be useful to fund a conjugate vaccine for the most at-risk groups – the elderly and immunocompromised people.
FUNDING
This research did not receive any specific funding.
COMPETING INTERESTS
The authors declare no competing interests.
References
[1] O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009; 374 893–902.| Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates.Crossref | GoogleScholarGoogle Scholar | 19748398PubMed |
[2] Randle E, Ninis N, Inwald D. Invasive pneumococcal disease. Arch Dis Child Educ Pract Ed. 2011; 96 183–90.
| Invasive pneumococcal disease.Crossref | GoogleScholarGoogle Scholar | 21555595PubMed |
[3] García-Rodríguez JA, Fresnadillo Martinez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother. 2002; 50 59–73.
| Dynamics of nasopharyngeal colonization by potential respiratory pathogens.Crossref | GoogleScholarGoogle Scholar | 12556435PubMed |
[4] Borman A, Heffernan, H. Invasive Pneumococcal Disease Quarterly Report January–March 2015. Prepared for Ministry of Health. Porirua, NZ: Institute for Environmental Science and Research Ltd; 2015
[5] Kalin M. Pneumococcal serotypes and their clinical relevance. Thorax. 1998; 53 159–62.
| Pneumococcal serotypes and their clinical relevance.Crossref | GoogleScholarGoogle Scholar | 9659348PubMed |
[6] Lynch JP, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med. 2010; 16 217–25.
| 20375783PubMed |
[7] Myint TTH, Madhava H, Balmer P, et al. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther. 2013; 30 127–51.
| The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review.Crossref | GoogleScholarGoogle Scholar |
[8] Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011; 378 1962–73.
| Serotype replacement in disease after pneumococcal vaccination.Crossref | GoogleScholarGoogle Scholar | 21492929PubMed |
[9] Reinert R, Jacobs MR, Kaplan SL. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine. 2010; 28 4249–59.
| Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development.Crossref | GoogleScholarGoogle Scholar | 20416266PubMed |
[10] Ministry of Health. Immunisation Handbook 2014. Wellington: Ministry of Health; 2014.
[11] Pletz MW, Welte T. Community-Acquired Pneumonia. Pneumococcal and influenza vaccination. Euro Resp Soc. Mono 2014; 63 266–84.
[12] Douglas RM, Paton JC, Duncan SJ, et al. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983; 148 131–7.
| Antibody response to pneumococcal vaccination in children younger than five years of age.Crossref | GoogleScholarGoogle Scholar | 6886479PubMed |
[13] Griffin MR, Zhu Y, Moore MR, et al. US hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013; 369 155–63.
| US hospitalizations for pneumonia after a decade of pneumococcal vaccination.Crossref | GoogleScholarGoogle Scholar | 23841730PubMed |
[14] Ministry of Health. Communicable Disease Control Manual 2012. Wellington: Ministry of Health; 2012.
[15] Ministry of Health. National and DHB immunization data. Wellington: Ministry of Health; 2018. [Cited 30 November 2018]. Available from: www.health.govt.nz/our-work/preventative-health-wellness/immunisation/immunisation-coverage/national-and-dhb-immunisation-data
[16] Lopez L, Heffernan H. Invasive ESR: Pneumococcal Disease Quarterly Report. Wellington: Ministry of Health; 2018.
[17] Cormack D. The practice and politics of counting: ethnicity data in official statistics in Aotearoa/New Zealand. Wellington: Te Ropu Rangahau Hauora a Eru Pomare; 2010.
[18] Breslow NE, Day NE. Statistical methods in Cancer Research, The Design and Analysis of Cohort Studies. II. IARC scientific publication no. 82. Lyon: International Agency for Research on Cancer; 1986.
[19] Pletz MW, Maus U, Krug N, et al. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008; 32 199–206.
| Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species.Crossref | GoogleScholarGoogle Scholar | 18378430PubMed |
[20] Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003; 348 1737–46.
| Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine.Crossref | GoogleScholarGoogle Scholar | 12724479PubMed |
[21] Bogaert D, de Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004; 4 144–54.
| Streptococcus pneumoniae colonisation: the key to pneumococcal disease.Crossref | GoogleScholarGoogle Scholar | 14998500PubMed |
[22] Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005; 294 2043–51.
| Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine.Crossref | GoogleScholarGoogle Scholar | 16249418PubMed |
[23] Hammitt LL, Bruden DL, Butler JC, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis. 2006; 193 1487–94.
| Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease.Crossref | GoogleScholarGoogle Scholar | 16652275PubMed |
[24] Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014; 14 839–46.
| Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study.Crossref | GoogleScholarGoogle Scholar | 25042756PubMed |
[25] Domingues CM, Verani JR, Renoiner EI, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014; 2 464–71.
| Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study.Crossref | GoogleScholarGoogle Scholar | 24726406PubMed |
[26] Miller E, Andrews NJ, Waight PA, et al. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine. 2011; 29 9127–31.
| Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine.Crossref | GoogleScholarGoogle Scholar | 21983361PubMed |
[27] Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015; 372 1114–25.
| Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults.Crossref | GoogleScholarGoogle Scholar | 25785969PubMed |
[28] Mangen MJ, Rozenbaum MH, Huijts SM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015; 46 1407–16.
| Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands.Crossref | GoogleScholarGoogle Scholar | 26160871PubMed |


