Subfossils suggest worse-than-realised losses of small-bodied mammals in northern Australia
Vikram Vakil A * , Jonathan Cramb B , Gilbert Price A , Julien Louys C , John Stanisic B and Gregory E. Webb
A * , Jonathan Cramb B , Gilbert Price A , Julien Louys C , John Stanisic B and Gregory E. Webb  A
A
A
B
C
Abstract
Examining Australia’s late Quaternary subfossil record can be valuable in assessing whether the current diversity of small-bodied mammals seen across some parts of northern Australia is ‘normal’. Such records are important for establishing baselines for measuring historic changes in communities today and into the future. These datasets are becoming increasingly important, given trajectories in current global climate change, and predicted habitat losses and other potential anthropogenic impacts.
The main aim of this study is to utilise the local subfossil record from north-eastern Australia to establish a natural baseline for assessing changes in small mammal communities post-European colonisation.
Subfossils of vertebrates and other taxa were recovered from surface deposits adjacent to cave entrances at Broken River, near Greenvale in north-eastern Queensland, and were subjected to taxonomic, taphonomic and statistical analyses. These were then compared with local faunal records from modern surveys to compare differences in faunal communities between past and present.
Radiocarbon dating showed that these subfossils are geologically young, dating to approximately the time of European colonisation. We provide evidence for the former presence of extinct species of hopping mice (Notomys spp.) and rabbit rats (Conilurus spp.) in the region. Additional locally extirpated taxa such as Cape York bandicoot (Isoodon peninsulae) and Shark Bay mouse (Pseudomys gouldii) demonstrated considerable range contractions since the deposits accumulated, compared with their current distribution. Independent evidence from subfossil land snails recovered from these deposits is, with two exceptions, all modern-day vine thicket, karst-dwelling species indicating a long-term maintenance of vine thicket habitat. Thus, the loss of several mammal species is unlikely to be the result of habitat loss.
Analysis of the surface deposits showed that local historic small-mammal communities were much more diverse than are the region’s extant faunas recorded by modern surveys. Many extinctions and extirpations evidently occurred prior to such faunas being recorded as local inhabitants of the region.
Our data suggested that post-European colonisation small-mammal losses are likely to be substantially worse than previously realised.
Keywords: conservation palaeontology, limestone caves, natural baselines, northern Australia, palaeoenvironment, severe losses, small mammals, subfossils.
Introduction
Australia’s biodiversity includes a significant portion of native species: over 90% of its frogs and reptiles and over 80% of its plants and mammals occur nowhere else (Chapman 2009; Woinarski et al. 2017, 2019; Legge et al. 2023). Severe deterioration of biodiversity has occurred in the past 200 years since European colonisation (Legge et al. 2023). This is most conspicuously reflected in the nation’s terrestrial mammal extinction rates, which are the highest in the world. More than 30 species have gone extinct since European arrival in 1788, amounting to approximately 50% of global mammal extinctions since that time (Woinarski et al. 2019; Legge et al. 2023). These extinctions have been selective, being more likely in species generally characterised as the ‘critical weight range’ (CWR; 35 g – 5.5 kg, including species of bandicoots, dasyurids, and rodents among others) (Burbidge and McKenzie 1989). Many of the factors that have contributed to post-European colonisation extinctions continue to threaten extant communities and include factors such as habitat loss, changed fire regimes, over-predation and competition from introduced predators or herbivores, along with the ongoing threat of climate change (Burbidge and McKenzie 1989; Coulson 2009; Johnson and Isaac 2009; Schloss et al. 2012; Waller et al. 2017).
Owing to the multiplicity and complex interplay of the threats (Woinarski et al. 2019), it is fundamental to have a baseline understanding of how many small-bodied mammal species have been lost since European colonisation. Modern biodiversity studies address this issue through robust sampling and detailed ecological surveys of extant communities (e.g. Gibson 2011). However, several taxa have gone extinct prior to ever being recorded, such as the northern pig-footed bandicoot, Chaeropus yirratji (Travouillon et al. 2019). Additionally, extant faunal communities may represent impoverished and relict subsets of former populations, and their current numbers and environments may not necessarily reflect their ‘natural’ state (Woinarski et al. 2015). This hinders the ability of researchers, environmentalists, and conservationists (among others) to determine their true rates of loss, namely, whether faunal populations have truly declined after European arrival, have always remained low, or have simply not been documented well enough.
Northern Australian savanna (encompassing parts of Queensland, Northern Territory and northern Western Australia) was a region previously thought to be relatively secure in biodiversity, compared to other parts of Australia (Woinarski et al. 2011; Ziembicki et al. 2013; Murphy et al. 2016). However, recent camera-trap studies showed an unexpectedly low diversity of terrestrial mammals (specifically small-bodied mammals) in many regions of northern Australia (e.g. Waller 2019). Additional trapping and monitoring studies also yielded fewer species of mammals than do survey data from other parts of Australia (Woinarski et al. 2004, 2010; Russell-Smith et al. 2009). The low capture success of these mammals (e.g. Davies et al. 2018), combined with lack of long-term surveys and resurveys, makes it difficult to assess whether this is an accurate baseline state of northern Australian ecosystems. It is possible that northern Australian faunal communities have always been low in diversity or, perhaps, they are diverse but have not yet been accurately measured. A longer temporal perspective of these faunas needs to be considered to assess the true rates of their losses.
Price et al. (2020) examined fossil vertebrate communities from cave deposits in the Broken River region, Greenvale, north-eastern Queensland, and showed that early late Quaternary fossil assemblages from the region were markedly more diverse (>25 species of small-bodied mammals) than were modern communities (eight species). However, the existing fossil record of Broken River lacks late Holocene faunal assemblages, i.e. those immediately prior to European colonisation.
A geologically recent (subfossil) record for these faunas is the only source that can help establish such a baseline to assess biodiversity change through recent time (Morton and Baynes 1985; Baynes and Jones 1993; Baynes and McDowell 2010; Fusco et al. 2016; Price et al. 2019; Louys et al. 2023). Knowledge of diversity immediately around the time of European colonisation is therefore fundamental for determining rates of loss, identifying priorities for conservation as well as for understanding geographic range changes and ecological tolerances of extant taxa prior to significant disruption brought about by European colonialism.
Here, we report previously undescribed late Quaternary subfossil assemblages from the geologically recent surface deposits located in cave entrances (Millennium, Three-Avenue and Swiftlet) in the Broken River region (Fig. 1). Preliminary examination showed that the subfossil deposits are rich in vertebrates as well as invertebrates (karst-dwelling land snails). The latter are not cave-dwelling species but are useful for inferring the external environment of the caves at the time of accumulation of the fossils. The fossils are inferred to date to approximately the time of European colonisation (see Materials and methods below). We aim to use the local subfossil record to establish a baseline to examine whether diversity of modern small-mammal communities seen across present-day northern Australia have been low since around the time of European colonisation (e.g. Murphy et al. 2016; Waller 2019).
Geology and geography
The fossil samples examined in this study come from the Broken River region in north-eastern Queensland (Fig. 1), whereby the Broken River, along with its tributary, the Clarke River, flows through a complexity of faulted and folded landforms composed of a variety of rock types (Withnall 1989). The climate has been described as semi-arid and hot, with a summer concentration of rainfall, followed by a dry winter, with an estimated annual rainfall of 623–675 mm (Williams et al. 2005). Fossils described from Broken River come from cave deposits located in limestones of the Graveyard Creek Group (Withnall 1989; Price et al. 2020; Vakil et al. 2023). All three caves sampled for this study (Millennium, Three-Avenue and Swiftlet) occur in the extensively karstified upper Silurian to Lower Devonian Jack Limestone, which is tilted ~90° (i.e. strata are vertical) and contains extensive rillenkarren at the surface (Price et al. 2020; Vakil et al. 2023). All three caves are joint controlled, containing tall, narrow passages and caverns. The habitat around each of the caves is open, dominated primarily by eucalypt/gum woodlands and open forests and grasslands (Queensland Globe database, accessed 22 February 2024). The vegetation shows a mosaic (heterogeneous) habitat dominated by open forests, woodlands and open woodlands. Grasslands additionally comprise plants that form the subcanopy shrub layer within these regions (Neldner et al. 2023).
Materials and methods
Fossil samples were recovered from unlithified sands and muds on the floor directly beneath the current main entrances of the caves (Fig. 2a, b). The sediment and fossils are likely to have entered each cave through their respective main entrances because there are no other openings in any of the caves. Larger clasts are rare in the surface deposits and the deposits themselves are mostly fragmentary remains of small-bodied vertebrates and invertebrates such as gastropod shells (Fig. 2c). Each deposit is adjacent to a limestone wall, at floor level, and lacks any associated speleothems. The fossiliferous sediments were exhumed using hand tools and were bagged on site. They were later wet-sieved using mesh of 10 and 1 mm, and vertebrate fossils were hand-sorted under microscopes and magnifier lamps.
Surface deposits examined for this research. (a) Tall, narrow cave walls of Three-Avenue Cave enclosing the cave floor, with tree roots growing around the surface fossils. (b) The surface deposit of Millennium Cave, with a human for scale. (c) Plan view of the surface deposit of Swiftlet Cave fossils, with gastropod shells visible in the upper-central portion of the image.

The deposits examined here appear to be geologically younger than the youngest fossil deposit previously documented in the region (early Holocene Beehive Cave ~8500 years; Price et al. 2020), given that the subfossils were collected from the ground surface adjacent to cave entrances. They are likely to date before or approximately the time of European colonisation, given that they lack introduced non-native small-bodied mammal species such as Mus musculus (house mouse) and Rattus rattus (black rat), i.e. taxa that are frequently reported in modern owl pellet studies (e.g. Domínguez García et al. 2019; Schoenefuss et al. 2024). Radiocarbon dating has also demonstrated the presumed age of the subfossils (see below).
Identification of fossil taxa was accomplished using standard comparative morphological techniques (using comparative specimens and published descriptions) (e.g. Merrilees and Porter 1979; Hocknull 2002; Baynes and McDowell 2010; Cramb and Hocknull 2010; Beck et al. 2022). Minor but critical diagnostic comments about species identification are provided in Supplementary material. Owing to their higher relative abundance, mammal fossils are given more emphasis, although other vertebrates, including frogs, squamates and birds, are also represented in the deposits and were resolved to the lowest taxonomic rank possible (see Supplementary material). Fossil specimens were photographed using an iPhone 12 Pro MAX camera system with an ultra-wide f/2.4 aperture, 120° field of view (Apple, California, US).
Number of identifiable specimens (NISP) and minimum number of individuals (MNI) were calculated following Lyman (1994), for each taxon resolved to the lowest taxonomic rank possible. Different parts of the vertebrate skeleton were used for identifying different taxa. For frogs, portions of their pelvises (ilia) were used for their identification; for snakes, teeth and vertebrae were used; for birds, thigh bones (femora) were used (upper arm bones/humeri are normally diagnostic of birds, but the deposits lacked any well-preserved humerus specimen); for dragon lizards, skinks and mammals, teeth, upper jaw (maxillary) and lower jaw (mandibular) elements were used, and in the case of the Capricorn rabbit rat (Conilurus capricornensis), certain postcranial elements (such as limb bones) were also useful (owing to the relatively large size of the species) (see Cramb and Hocknull 2010); for land snails, their shells were used. For complete taxonomic descriptions of mammals, refer to AMTC (2022), for amphibians, refer to Knight and Tyler (2020), for birds, to Chesser (2009), for reptiles, to Wilson and Swan (2020), and for land snails, to Stanisic et al. (2010).
It must be mentioned that within each major group of vertebrates (e.g. anurans sauropsids, mammals, aves), and invertebrates (land snails), all taxa identified above the species level were described (e.g. Table 1, Figs S1–S6) only if they were determined to be taxonomically distinct from those that were identified to the species level. For example, Sminthopsis sp. indet. was described and utilised in taphonomic and taxonomic analyses once it had been identified as being taxonomically distinct from S. murina and S. macroura.
| Sample ID | Internal ID | F14C | ± | 14C age | ± | Yield (mg) | Yield (%) | %N | %C | d13C (VPDB) | d15N (AIR) | C:N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-R-BR | 26018 | 0.9825 | 0.0020 | 142 | 7.3 | 7.30 | 6.6 | 16.22 | 44.36 | −7.13 | 3.83 | 3.19 |
The d13C values are the machine-quoted values, and these were used to correct the age. The quoted age in radiocarbon years was given using the Libby half-life of 5568 years and following the conventions of Stuiver and Polach (1977). The radiocarbon concentration is given as per cent F14C and conventional radiocarbon age.
Taphonomic analysis was undertaken to understand the origin of the vertebrate fossil assemblages inside each cave. This was accomplished to account for any major bias that could have been introduced by different accumulation mechanisms in the caves (e.g. pitfall trap versus predator assemblage), aiding meaningful comparisons among the cave sites. This was primarily achieved by comparing for the activity patterns of fauna (e.g. diurnal versus nocturnal) and their adult bodyweight category (small-bodied versus medium-/large-bodied). Additionally, bones were analysed for signs of digestive corrosion because digestion is the most direct indication of predation (Marin-Monfort et al. 2021). Protocols for identifying digestion grades of murid incisors and molars are given in Fernández-Jalvo et al. (2016) and Marin-Monfort et al. (2021). Four grades of digestion (light, moderate, heavy, and extreme) were recorded for this. A detailed analysis of digestion grades and traits on the teeth of non-rodent Australian small mammals (such as dasyurids, bandicoots, bilbies etc.) is currently lacking.
Rarefaction analysis was performed to compare relative diversity of assemblages under bias owing the differential modes of accumulation as well as for testing sampling frequency. MNIs for each cave site were individually plotted against the number of taxa, with samples considered to be reworked (on the basis of their differing colour and texture) being excluded from the analysis. Following taphonomic and rarefaction analyses, taxonomic abundance was calculated for each fossil assemblage to account for relative abundance (Ri) of a given taxon within that assemblage (Lyman 1994). Additionally, taxic richness (S) and Simpson’s diversity Index (1-D) were computed for fossil assemblages in Millennium and Three-Avenue caves. The former is a measure of taxic (species) richness, whereas the latter is a measure of both taxic richness and relative abundance (see also Rhodes et al. 2018). NISP/MNI data for Beehive Cave (from Price et al. 2020) were added to the diversity analyses to assess any change in faunal diversity in the pre-European Broken River region. Rarefaction and diversity analyses were performed using PAST (ver. 3.26; see Hammer et al. 2001). Finally, faunal diversity of cave fossil vertebrates was compared with that of extant faunal diversity in the region by accessing the extant species list from the Queensland Government’s Wildnet database (https://www.qld.gov.au/environment/plants-animals/species-information/species-list) (accessed 9 April 2023). Owing to the absence of numerical counts of species/individuals recorded in modern surveys, the comparison was limited to qualitative analysis.
For radiocarbon dating, bones and teeth from all three cave deposits were selected. These included an isolated murid dentary (Millennium Cave) and two mammalian femora (each from Three-Avenue and Swiftlet caves). Sample preparation background, including an ultrafiltration-specific background, was subtracted, on the basis of the measurements of samples of 14C-free CO2. Collagen was extracted from the bones and teeth and purified using an ultrafiltration protocol based on Brock et al. (2010). Sample preparation and radiocarbon isotope analysis were conducted at the Australian National University (ANU) Radiocarbon Dating Centre (Radiocarbon Facility), following protocols given in Wood et al. (2023) and Fallon et al. (2010) respectively. Isotope-ratio mass spectrometry (IRMS) stable isotope results were measured at the ANU by using a Sercon 20-22 mass spectrometer coupled to an ANCA-GSL operating in continuous flow mode. Typical errors are <0.2 per mille, for both carbon and nitrogen isotopes. Samples were measured against an in-house gelatine reference and corrected against USGS-40, USGS-41 and ANU sucrose. Indicators of well-preserved collagen for terrestrial C3 fauna generally are as follows: %yield, >1; d15N, 2–12 per mille; d13C, 26 per mille; %C, 30–50%; and C:N, 2.9–2.4 (following van Klinken 1999). All fossil samples are accessioned into the collections of the Queensland Museum, Brisbane, Australia (Table S1, Figs S1–S5).
Results
Dating
The results for radiocarbon isotope analysis are given in Table 1. Only the murid dentary from Millennium Cave yielded a reliable date among all examined deposits. Samples from Three-Avenue and Swiftlet caves did not return sufficient collagen for dating.
The radiocarbon age for the Millennium Cave specimen is 142 ± 7.3 years (Table 1). Converting the radiocarbon age to calendar years is challenging owing to the geologically very young age and difficulties and uncertainties with the 14C calibration curve (Hogg et al. 2020). However, calibration was performed on the basis of the SHcal20 calibration curve in the OxCal 4.4 programme. The calibrated median calendar age for the specimen is ~1810 AD, with uncertainty (95.4% confidence interval) of the age being between 1698 and 1906 AD. Despite the uncertainty, the specimen clearly dates to the time approximately of European colonisation. It may date to the century before, and possibly as recently as the early 1900s.
Fauna
Presence/absence data for small-bodied vertebrates of Millennium, Three-Avenue and Swiftlet caves are given in Table 2. In total, 36 taxa were identified from the caves (excluding a possible barn owl and gastropod shells), with 25 taxa identified to species level and seven to genus level only. The taxa recovered contain six globally extinct species, six species recorded in modern surveys within 50 km of the caves and 24 species not seen at Broken River today. Pertinent remarks for the taxonomic identification of all taxa examined are given in Supplementary material, Figs S1–S5 and associated text. The list of NISP and MNI for each taxon from the three caves is also given in Table 2. It is immediately evident that Swiftlet Cave is highly impoverished in the total number of taxa (only five taxa are recorded from it against 28 and 20 from Millennium and Three-Avenue caves respectively). Three taxa (all mammals) co-occur in all three deposits, whereas 14 are unique to Millennium, six to Three-Avenue and two to Swiftlet caves. A further 11 taxa are shared between Millennium and Three-Avenue caves.
| Taxon | Millennium | Three-Avenue | Swiftlet | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NISP | MNI | Ri | NISP | MNI | Ri | NISP | MNI | Ri | ||
| Litoria sp. | 1 | 1 | 0.6 | |||||||
| Diporiphora Group 2 cf. D. australis | 4 | 2 | 1.2 | |||||||
| Agamidae gen. et sp. indet. | 1 | 1 | 0.6 | |||||||
| Egernia sp. indet. | 1 | 1 | 0.6 | 1 | 1 | 0.7 | ||||
| Scincidae sp. indet. | 12 | 7 | 4.2 | 1 | 1 | 0.7 | ||||
| Gekkonidae gen. et sp. indet. | 11 | 9 | 5.4 | |||||||
| D. geoffroii | 9 | 2 | 1.2 | |||||||
| Sminthopsis murina | 19 | 9 | 5.4 | 1 | 1 | 0.7 | 1 | 1 | 16.7 | |
| Sminthopsis macroura | 1 | 1 | 0.7 | |||||||
| Sminthopsis sp. indet. | 2 | 1 | 0.7 | |||||||
| Antechinus sp. indet. | 3 | 1 | 0.6 | |||||||
| Phascogale tapoatafa | 24 | 7 | 4.2 | 1 | 1 | 0.7 | ||||
| Planigale ingrami | 2 | 1 | 0.6 | |||||||
| Isoodon peninsulae | 29 | 8 | 4.8 | 5 | 2 | 1.4 | ||||
| Pseudocheirus peregrinus | 34 | 11 | 6.6 | |||||||
| Petaurus norfolcensis | 11 | 4 | 2.9 | 1 | 1 | 0.7 | ||||
| Trichosurus sp. indet. | 1 | 1 | 0.6 | |||||||
| Petrogale sp. | 2 | 1 | 0.6 | |||||||
| Macropodidae indet. | 2 | 1 | 0.7 | |||||||
| Aepyprymnus rufescens | 1 | 1 | 0.7 | |||||||
| Rattus lutreola | 31 | 17 | 10.1 | |||||||
| Rattus sordidus/tunneyi/villosissimus/colletti group. | 43 | 26 | 15.5 | 54 | 28 | 20.1 | 2 | 1 | 16.7 | |
| Conilurus capricornensis | 44 | 9 | 5.4 | |||||||
| Conilurus albipes | 4 | 2 | 1.4 | |||||||
| Zyzomys argurus | 13 | 8 | 4.8 | |||||||
| Melomys cervinipes | 5 | 2 | 1.2 | 2 | 1 | 0.7 | ||||
| Pseudomys australis | 7 | 5 | 3.0 | 55 | 28 | 20.1 | 5 | 2 | 33.3 | |
| Pseudomys gracilicaudatus | 33 | 17 | 10.1 | 69 | 43 | 30.9 | ||||
| Pseudomys sp. cf. P. delicatulus | 17 | 9 | 5.4 | 6 | 4 | 2.9 | ||||
| Pseudomys gouldii | 14 | 8 | 5.8 | |||||||
| Leggadina forresti | 2 | 1 | 0.6 | 10 | 6 | 4.3 | ||||
| Notomys magnus | 1 | 1 | 16.7 | |||||||
| Notomys longicaudatus | 10 | 6 | 3.6 | 12 | 7 | 5.0 | ||||
| Notomys sp. 1 | 1 | 1 | 16.7 | |||||||
| Miniopterus australis | 1 | 1 | 0.6 | 1 | 1 | 0.7 | ||||
| Macroderma gigas | 1 | 1 | 0.6 | |||||||
| Total | 371 | 168 | 243 | 139 | 10 | 6 | ||||
Additionally, most of the land snail species are known and described species. Two species (Punctid sp. 1, Charopid sp. 2) are undescribed species and new records for the Greenvale limestones (see Supplementary material).
Taphonomy
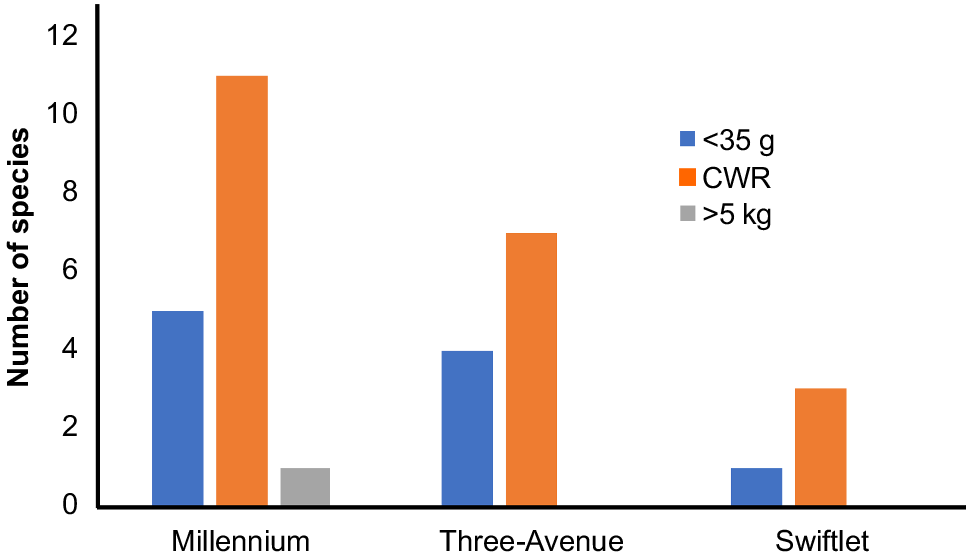
The taphonomic signatures across all three caves, Millenium, Three-Avenue and Swiftlet, are similar in that they all contain faunal remains of small-bodied vertebrates, primarily nocturnal, non-cave dwelling mammals, all within the CWR category (~35 g – 5.5 kg) (Fig. 3). A few mammals of <35 g were also recovered and these are usual components of fossil owl roost assemblages (see also Fusco et al. 2016; Price et al. 2020; Louys et al. 2023).
Plots of weight bins of mean body mass versus number of specimens per weight bin for taxa examined at Millennium, Three-Avenue and Swiftlet caves, showing that most taxa are well within the CWR limit (~35 g – 5.5 kg) (see text). Data for all taxa (except Notomys magnus) were obtained from Silva and Downing (1995). Data on N. magnus were obtained from Vakil et al. (2023).
The bones are moderately fragmented and the broken ends on most of them lack evidence of abrasion. All the bones in the deposits were randomly oriented at the time of collection and the long bones showed no preferential alignment. None of the examined bones showed any visible tooth markings and showed low digestive corrosion (Table 3). Additionally, a few isolated bones from Three-Avenue Cave appear to have been reworked, owing to their darker stain and smooth texture. These were few (n = 8) and have been omitted from statistical analysis.
| Cave | Isolated | % | In situ n | % | Total | % | |
|---|---|---|---|---|---|---|---|
| Millennium | |||||||
| Incisors | 178 | 14 | 192 | ||||
| None | 166 | 93.26 | 12 | 85.71 | 178 | 92.7 | |
| Light | 10 | 5.62 | 1 | 7.14 | 11 | 5.73 | |
| Moderate | 2 | 1.12 | 1 | 7.14 | 3 | 1.56 | |
| Heavy | 0 | 0 | 0 | 0 | 0 | 0 | |
| nDigested | 12 | 6.74 | 2 | 14.29 | 14 | 7.29 | |
| Molars | 128 | 50 | 178 | ||||
| None | 123 | 96.1 | 48 | 96 | 171 | 96.1 | |
| Light | 2 | 1.56 | 1 | 2 | 3 | 1.69 | |
| Moderate | 3 | 2.34 | 1 | 2 | 4 | 2.25 | |
| Heavy | 0 | 0 | 0 | 0 | 0 | 0 | |
| nDigested | 5 | 3.91 | 2 | 4 | 7 | 3.93 | |
| Three-Avenue | |||||||
| Incisors | 377 | 7 | 384 | ||||
| None | 315 | 83.55 | 6 | 85.71 | 321 | 83.6 | |
| Light | 53 | 14.06 | 1 | 14.29 | 54 | 14.06 | |
| Moderate | 9 | 2.39 | 0 | 0 | 9 | 2.34 | |
| Heavy | 0 | 0 | 0 | 0 | 0 | 0 | |
| nDigested | 62 | 16.45 | 1 | 14.29 | 63 | 16.4 | |
| Molars | 193 | 99 | 292 | ||||
| None | 186 | 96.37 | 96 | 96.97 | 282 | 96.58 | |
| Light | 5 | 2.59 | 1 | 1.01 | 6 | 2.05 | |
| Moderate | 2 | 1.04 | 2 | 2.02 | 4 | 1.37 | |
| Heavy | 0 | 0 | 0 | 0 | 0 | 0 | |
| nDigested | 7 | 3.63 | 3 | 3.03 | 10 | 3.42 | |
| Swiftlet | |||||||
| Incisors | 5 | 6 | 11 | ||||
| None | 5 | 100 | 5 | 83.33 | 10 | 90.91 | |
| Light | 0 | 0 | 1 | 16.67 | 1 | 9.09 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | |
| Heavy | 0 | 0 | 0 | 0 | 0 | 0 | |
| nDigested | 0 | 0 | 1 | 16.67 | 1 | 9.09 | |
| Molars | 2 | 15 | 17 | ||||
| None | 2 | 100 | 15 | 100 | 17 | 100 | |
| Light | 0 | 0 | 0 | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | |
| Heavy | 0 | 0 | 0 | 0 | 0 | 0 | |
| nDigested | 0 | 0 | 0 | 0 | 0 | 0 | |
Table format follows Andrews (1990) and Fernández-Jalvo et al. (2016).
Scores of bone corrosion effects on craniodental elements (in situ and isolated incisors and molars) (Table 3) highlighted the low frequency of digested dental elements and the light grades of digestion on bones. The digestion signatures (percentages and grades) of all three assemblages are similar (X2 test P = 0.1) and are congruent with a Category 1 predator (barn owls), according to conventional classification (Andrews 1990; Fernández-Jalvo et al. 2016).
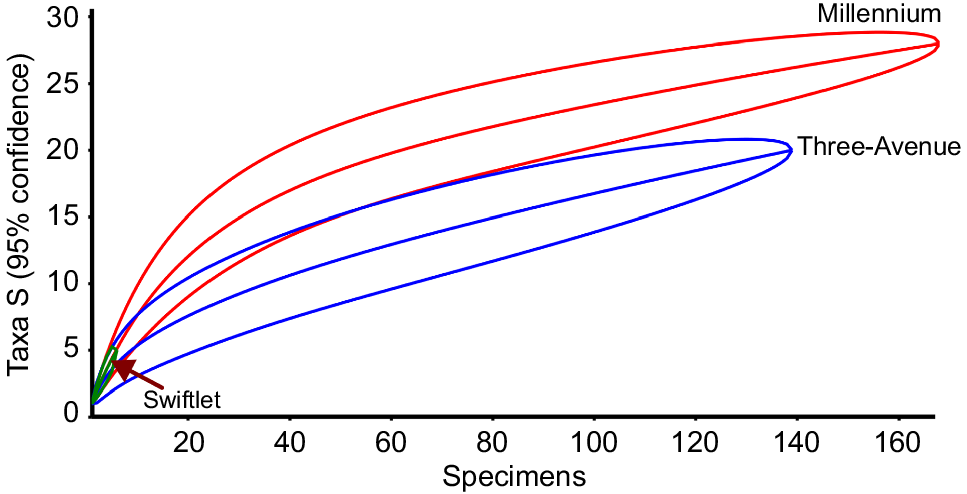
When compared against a common number of specimens, i.e. 140, the rarefaction curve for Millennium predicts a presence of approximately 25 taxa whereas that of Three-Avenue predicts 20, at 95% confidence intervals (Fig. 4). Although it is likely that further collection would yield additional taxa for both Millennium and Three-Avenue caves, the two curves are likely to attain the asymptote condition without overlapping, suggestive of real differences in taxic richness and abundance between the two caves. As expected, (owing to impoverished NISP and MNI counts), the confidence interval of Swiftlet Cave falls well within those of the other two caves, and the linear trajectory followed by its rarefaction curve clearly suggests that more sampling would most likely yield additional specimens. Swiftlet Cave is not included in the subsequent diversity analyses, below; however, its importance lies in its possession of the probable youngest known record (see Materials and methods above) of Notomys magnus, and the first record of a possibly new large-bodied Notomys species.
Diversity
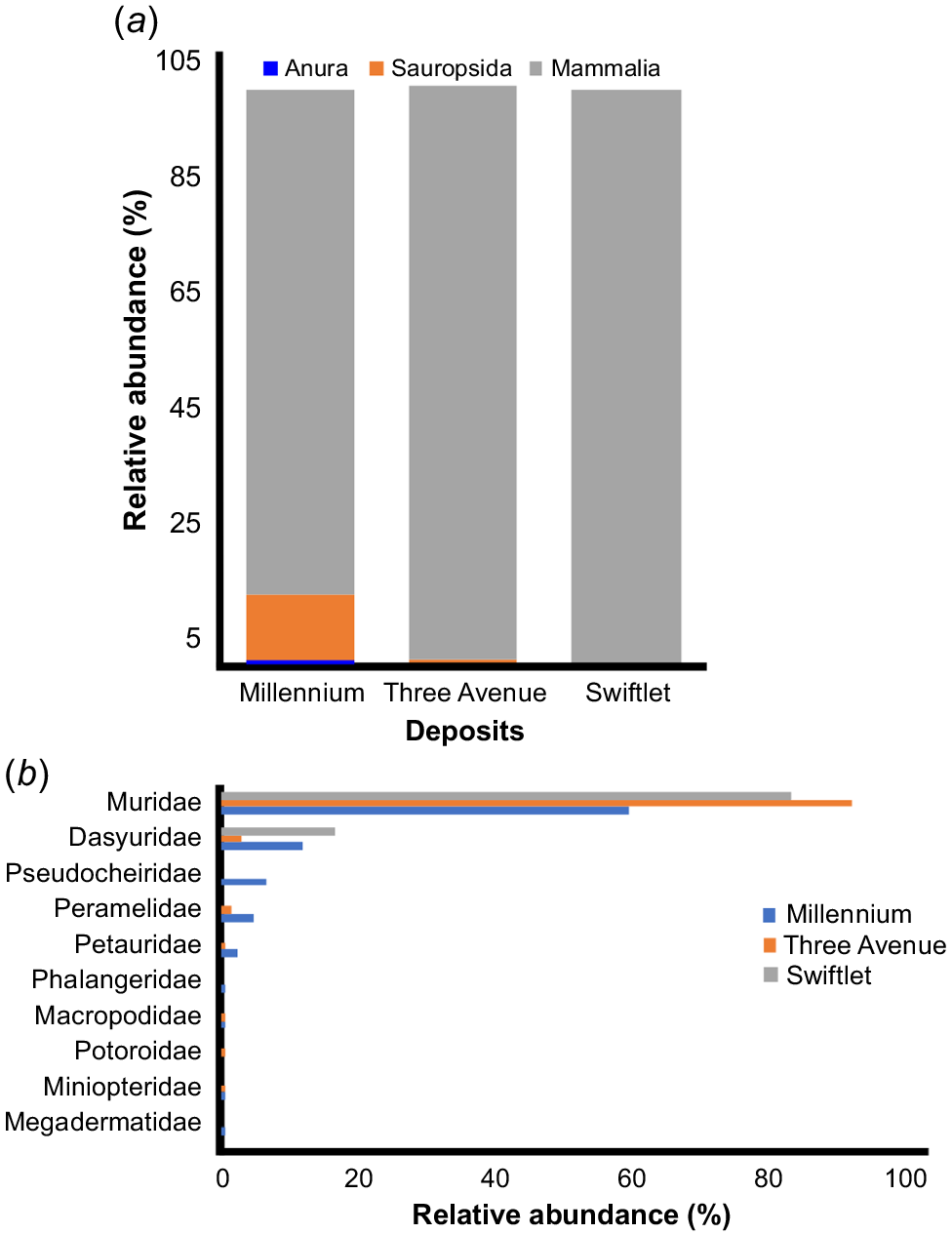
Mammals are the most abundant group in all three caves (87.5% in Millennium Cave, 99.3% in Three-Avenue Cave and 100% in Swiftlet Cave) (Fig. 5a). Murid rodents are by far the most abundant mammals in all three deposits (59.5% in Millennium Cave, 92% in Three-Avenue Cave and 83.3% in Swiftlet Cave) (Fig. 5b). These results are similar to those reported from Beehive Cave where murids dominated the assemblage (Price et al. 2020). The diversity indices (taxonomic richness and Simpson’s diversity index) were broadly similar across all three caves (Millennium, Three-Avenue and Beehive) (Table 4). Additionally, the indices of evenness and equitability across these three caves have varied only slightly during the past 8500 years, since the Beehive Cave deposits accumulated (see Price et al. 2020), with slightly higher values of each for Millennium Cave (Table 4). Conversely, the lower evenness of the assemblage at Three-Avenue Cave (Table 4) is congruent with its high relative abundance of only three taxa, namely, Rattus spp. (20.1%,), Pseudomys australis (20.1%) and P. gracilicaudatus (30.9%) (Table 2; see also Price et al. 2020 for Beehive Cave), compared with others.
Discussion
Taphonomy
The lack of evidence for abrasion on the broken ends of bones precludes the likelihood of reworking. Furthermore, the random orientation of the bones in the deposits suggests that there is no evidence of any major fluvial deposition and/or sediment slumping. The lack of visible tooth markings further precludes the possibility of denning by predatory mammals (including bats) (see also Andrews 1990). Additionally, the opening within each cave is situated directly above the site of bone accumulation and, hence, accumulation by non-volant mammalian predators seems unlikely, because this would have required a passageway to enter the caves. The overall absence of any large-bodied taxa precludes the caves acting as pitfall traps for the most part, although a few animals (such as Petrogale sp., >4 kg) (Fig. 3) may have entered that way. However, the caves do bear the appearance of typical owl roost deposits seen elsewhere (e.g. Price et al. 2020). The absence of owl pellets from the fossil sites precluded identification of the owl, but the low pH digestive acid of diurnal, predatory birds such as owls was clear (Table 3) (see also Fusco et al. 2016).
Geochronology
The calibrated radiocarbon age of Millennium Cave (~150 years before present) accords well with our inference of it being historic in age (dating to approximately the time of European colonisation), from independent geological and palaeontological observations. For the remainder of the discussion, therefore, all three cave deposits have been collectively referred to as being historic.
Establishing a ‘natural’ baseline
The data presented in this study can be used to test whether the current low diversity seen in northern Australian small-bodied mammalian communities is ‘normal’. The results above show that historic fossil assemblages of Broken River are markedly diverse in small-bodied mammals and that their present low diversity is not commensurate with the subfossil record. In total, 26 non-volant mammal species were recovered from the surface deposits at Broken River (Table 2). When compared with the presence/absence data from local species list obtained from Wildnet Database (accessed 7 February 2024), for a 5 km radius (the average hunting range for owls) (see Comay and Dayan 2018), only two small-/medium-sized non-volant mammals were recovered that had a fossil record in the region (Table S1), emphasising the severity of potential losses. The northern greater glider (Petauroides volans minor) was known only from extant surveys and not from fossils. The remaining mammalian fauna in the modern species list were either large-bodied mammals such as macropodids or introduced animals such as pig, sheep and cattle. When the search was extended to a radius of 50 km (10 times the average hunting range for an owl), the results yielded a total of only six small-/medium-bodied non-volant taxa with a fossil record at Broken River (Table S1). Furthermore, an additional four species of small-bodied non-volant mammals were known only from surveys, without a fossil record. Of these four, the water rat (Hydromys chrysogaster), although found in the region today and absent from both Beehive Cave and the surface deposits, was nevertheless recovered from the older Big Ho Cave (see Price et al. 2020). Additionally, introduced small-bodied mammals such as the house mouse (Mus musculus) and black rat (Rattus rattus) were also recorded in surveys (but not as subfossils). The remaining extant mammals were once again large-bodied macropodids and koalas and non-native taxa such as rabbits, cats and red foxes.
By examining the habitat preferences of subfossil taxa, Broken River historically sported a mosaic of local habitats comprising primarily open grassland (e.g. Pseudomys spp., Leggadina forresti, Sminthopsis murina) and woodland (e.g. Phascogale tapoatafa, Petaurus norfolcensis). The presence of vine thickets is deduced from the region’s karst-dwelling land snail fauna (see Supplementary material). Local permanent waterbodies were likely to be present as indicated by the presence of Rattus lutreola. Overall, open habitat-adapted taxa such as Pseudomys australis, P. gracilicaudatus, P. delicatulus, Leggadina forresti and Notomys longicaudatus are present in all three caves (see Table 2). The subfossils, therefore, include species representative of environments found in the region today.
Furthermore, the overall number of non-volant, small-bodied mammalian taxa of the surface deposits are comparable to the early Holocene Beehive Cave (26 mammals for surface deposits and 23 for Beehive) (Fig. 4; see also Price et al. 2020). In total, 19 non-volant, small-bodied mammalian taxa are shared between the surface deposits and Beehive Cave. Four mammalian taxa were exclusive to Beehive cave, whereas seven were exclusive to the surface deposits (Table S1). These differences are likely to be due to chance (i.e. not all the region’s faunas will be represented in a given fossil assemblage) or local fluctuations in populations across time since these taxa have been recovered from the older Big Ho Cave (Price et al. 2020). Additionally, the broadly taxic richness and Simpson’s diversity index between those of Millennium and Three-Avenue caves on one hand and Beehive deposit on the other hand suggest that the overall diversity of taxa across the pre-European Holocene of Broken River has remained relatively stable (Table 4). Furthermore, the overall similarity between the surface deposits (Millennium and Three-Avenue caves) and Beehive Cave in the indices of evenness and equitability (Table 4) suggest broadscale similarities in species distributions, in the vicinities of the caves, throughout this time (~8500 years to ~150 years before present).
This comparison between Broken River’s recent past and present demonstrates the contrast in the numbers and diversity of the region’s non-volant native small-bodied mammals. Their diversity appears to have remained relatively high and stable in the region’s geologically recent past. This is followed by a steep decline in diversity in comparison to modern day. It is unknown if the small-bodied mammals absent in modern surveys (but present in the fossil record) have all been subjected to extinctions and extirpations; more comprehensive modern surveys are needed to test that. Nevertheless, the subfossil record highlights the potential severity of their losses. The absence of comprehensive modern-day surveys of the region’s land snails makes it difficult to determine whether the recovery of two previously undescribed species within two families (Punctidae and Charopidae) (see Supplementary material) is the result of localised extinctions or lack of survey effort. The presence of an otherwise robust, locally extant vine thicket land snail community among the deposits would strongly suggest the latter. Local extirpations at Broken River appear to be significantly greater than previously recognised (Wildnet Database, accessed 7 February 2024), despite broadly similar environments between the region’s past and present. The current low diversity of the region is therefore unusual. The subfossils have therefore been a valuable source in establishing a new natural baseline for the region’s native small-bodied mammalian fauna.
Notable extinctions and range contractions
It seems probable that post-European colonisation factors led to the global extinction of taxa such as Notomys longicaudatus, Notomys magnus, Notomys sp. 1, Conilurus capricornensis and Conilurus albipes, all of which (except for Notomys sp.) have been implicated to have gone extinct shortly after European arrival (Cramb and Hocknull 2010; Louys et al. 2023; Vakil et al. 2023). Notomys sp. 1 is described here for the first time, and although it is morphologically distinct from other Notomys specimens, it could very well be an aberrant N. longicaudatus (see Supplementary material). The discovery of the probable youngest specimen of N. magnus in pre-European surface deposit of Swiftlet Cave further reinforces the possibility of its geologically recent extinction, as suggested by Vakil et al. (2023).
The current distributions of extant taxa reflect severe range contractions, since the subfossils accumulated (Fig. 6). For instance, the extant plains mouse, Pseudomys australis Gray, has not been recorded locally in the Broken River region but occurs as fossils in the surface deposits and older deposits (see Price et al. 2020). Today, it has a much more central distribution in the arid interior of the continent. Post-European range contraction was observed in P. australis (see Breed and Ford 2007; Fusco et al. 2016; Fig. 6a). It is formerly known to have occurred from as far west as the Nullarbor Plain, through the Lake Eyre Basin and across to the Great Dividing Range in northern New South Wales and Queensland (Brandle et al. 2023). Fossils of this taxon in the surface deposits of Broken River extend this range contraction in recent times. Elevated predator populations, resource depletion and destruction of warren systems by livestock are all known to limit the distribution of the plains mouse today (Brandle et al. 2023). The very high abundance (~20%) (Table 2) of P. australis in the pre-European Three-Avenue Cave and its total absence in the region today further elucidates the severity of its susceptibility to environmental stressors.
Comparison of extant, historical, subfossil and fossil distributions to demonstrate range shifts for (a) Pseudomys australis, (b) Isoodon peninsulae and (c) P. gouldii.
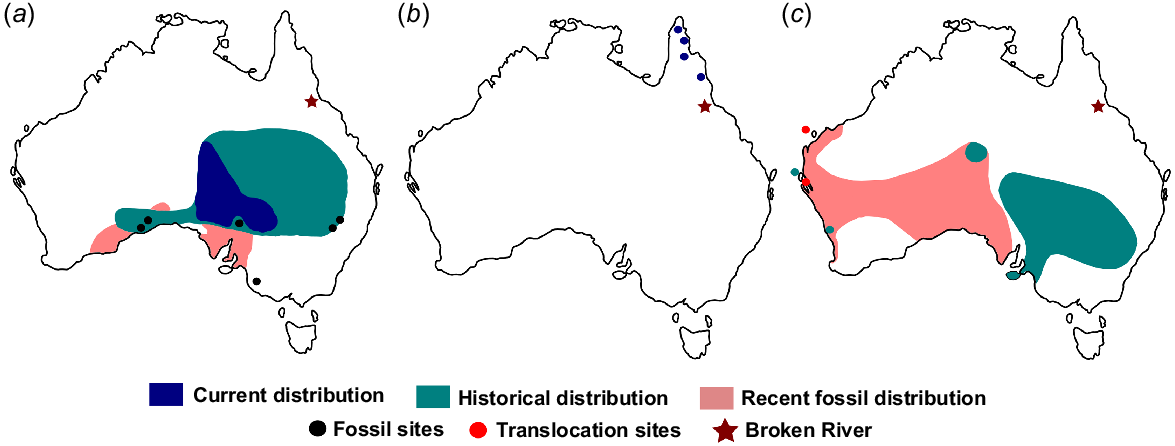
The Cape York bandicoot, Isoodon peninsulae Thomas, is currently restricted to two isolated patches of rainforest near Cape York and near Cairns (Warburton and Travouillon 2016; Fig. 6b). In Queensland, its fossils are known from older Broken River deposits (Price et al. 2020) and the Rockhampton cave deposits (Louys et al. 2023). It is clear that its geographical range contracted sometime close to European colonisation. Its presence at Broken River and Rockhampton sites suggests that it had a more continuous distribution in the recent past, but has undergone severe range contraction to result in the isolated populations of today.
Pseudomys gouldii Waterhouse was recorded alive in mainland Australia in historical times (Gould 1863) but was presumed to have gone extinct globally in the 19th century. It was only recently confirmed as being extant in the Shark Bay region of Western Australia (see Roycroft et al. 2021), where it survives in an isolated, offshore population; Fig. 6c). It is a common component of Queensland fossil fauna and was reported previously from older Pleistocene deposits at Broken River (Price et al. 2020) and the caves of Rockhampton (Louys et al. 2023). Its presence in the surface deposits at Broken River indicates that it may have been alive in Queensland until sometime recently (see also Louys et al. 2023), although it remains unclear as to what exactly led to its eventual extirpation at Broken River.
Conclusions
The subfossils presented here inform a historic natural faunal baseline for the Broken River region that can serve as a reference point for future studies examining changes in the region’s small-bodied mammalian faunas. This study fills a gap in knowledge of the ‘original’ fauna of the region and highlights the need to have at our disposal better distributional and ecological records of these taxa, especially in regions such as northern Australian savanna, where survey intensities have been minimal. Such records (both palaeontological and ecological) will help elucidate the cause(s) of the apparent contrast in diversity of the past and present, i.e. whether they are truly extirpated from the area or whether surveys are yet to document them, or a combination of both.
Data availability
The data used to generate results in this paper are available in the Supplementary material section.
Declaration of funding
This work was funded by a UQ PhD scholarship to V. Vakil and Australian Research Council grants DE120101533 and DP120101752.
Acknowledgements
We acknowledge the Traditional Custodians of the Gugu Badhun Country and the Broken River region. We thank Paul Osborne and the Chillagoe Caving Club for facilitating access to the field areas, as well as local landowners. Additional on-ground support came from Douglas and Mary Ann Irvin, Paco Murray, and numerous members of the local caving community. We also thank the following people: Scott Hocknull of Queensland Museum for help with sauropsid identification; Roy Farman of University of New South Wales for help with anuran identification; and Kenny Travouillon of Western Australian Museum for help with bandicoot identification. We appreciate the constructive criticisms and feedback from Dr Sean Tomlinson and an anonymous reviewer in improving the paper. The Australian Research Council supported the research under Grants DE120101533 and DP120101752.
References
AMTC (2022) The AMTC Australian Mammal Species List. Version 1.0. Australasian Mammal Taxonomy Consortium. Available at https://australianmammals.org.au/publications/amtc-species-list [accessed 19 April 2024]
Baynes A, Jones B (1993) The mammals of Cape Range peninsula, north-western Australia. Records of the Western Australian Museum 45, 207-225.
| Google Scholar |
Baynes A, McDowell MC (2010) The original mammal fauna of the Pilbara biogeographic region of north-western Australia. Records of the Western Australian Museum 78, 285-298.
| Crossref | Google Scholar |
Beck RMD, Voss RS, Jansa SA (2022) Craniodental morphology and phylogeny of marsupials. Bulletin of the American Museum of Natural History 457, 1-352.
| Crossref | Google Scholar |
Brock F, Higham T, Ditchfield P, Ramsey CB (2010) Current pretreatment methods for AMS radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (Orau). Radiocarbon 52, 103-112.
| Crossref | Google Scholar |
Burbidge AA, McKenzie NL (1989) Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143-198.
| Crossref | Google Scholar |
Comay O, Dayan T (2018) What determines prey selection in owls? Roles of prey traits, prey class, environmental variables, and taxonomic specialization. Ecology and Evolution 8, 3382-3392.
| Crossref | Google Scholar | PubMed |
Coulson G (2009) Behavioural ecology of red and grey kangaroos: Caughley’s insights into individuals, associations and dispersion. Wildlife Research 36, 57-69.
| Crossref | Google Scholar |
Cramb J, Hocknull S (2010) New Quaternary records of Conilurus (Rodentia: Muridae) from eastern and northern Australia with the description of a new species. Zootaxa 2634, 41-56.
| Crossref | Google Scholar |
Davies HF, McCarthy MA, Firth RSC, Woinarski JCZ, Gillespie GR, Andersen AN, Rioli W, Puruntatameri J, Roberts W, Kerinaiua C, Kerinauia V, Womatakimi KB, Murphy BP (2018) Declining populations in one of the last refuges for threatened mammal species in northern Australia. Austral Ecology 43, 602-612.
| Crossref | Google Scholar |
Domínguez García ÁC, Laplana C, Sevilla P, Blain H-A, Zumajo NP, Benítez de Lugo Enrich L (2019) New data on the introduction and dispersal process of small mammals in southwestern Europe during the Holocene: Castillejo del Bonete site (southeastern Spain). Quaternary Science Reviews 225, 106008.
| Crossref | Google Scholar |
Fallon SJ, Fifield LK, Chappell JM (2010) The next chapter in radiocarbon dating at the Australian National University: status report on the single stage AMS. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 268(7–8), 898-901.
| Crossref | Google Scholar |
Fernández-Jalvo Y, Andrews P, Denys C, Sesé C, Stoetzel E, Marin-Monfort D, Pesquero D (2016) Taphonomy for taxonomists: implications of predation in small mammal studies. Quaternary Science Reviews 139, 138-157.
| Crossref | Google Scholar |
Fusco DA, McDowell MC, Prideaux GJ (2016) Late-Holocene mammal fauna from southern Australia reveals rapid species declines post-European settlement: implications for conservation biology. The Holocene 26, 699-708.
| Crossref | Google Scholar |
Gibson LA (2011) The importance of incorporating imperfect detection in biodiversity assessments: a case study of small mammals in an Australian region. Diversity and Distributions 17, 613-623.
| Crossref | Google Scholar |
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4, 1-9.
| Google Scholar |
Hocknull SA (2002) Comparative maxillary and dentary morphology of the Australian dragons (Agamidae: Squamata): a framework for fossil identification. Memoirs of the Queensland Museum 48, 125-145.
| Google Scholar |
Hogg AG, Heaton TJ, Hua Q, Palmer JG, Turney CSM, Southon J, Bayliss A, Blackwell PG, Boswijk G, Ramsey CB, Pearson C, Petchey F, Reimer P, Reimer R, Wacker L (2020) SHCal20 Southern Hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 62, 759-778.
| Crossref | Google Scholar |
Johnson CN, Isaac JL (2009) Body mass and extinction risk in Australian marsupials: the ‘Critical Weight Range’ revisited. Austral Ecology 34, 35-40.
| Crossref | Google Scholar |
Legge S, Rumpff L, Garnett ST, Woinarski JCZ (2023) Loss of terrestrial biodiversity in Australia: magnitude, causation, and response. Science 381, 622-631.
| Crossref | Google Scholar | PubMed |
Louys J, Cramb J, Ferguson K, Kemp J, Wood R, Miszkiewicz JJ, Dias Guimarães NR, Higgins P, Travouillon KJ, Hocknull SA, Webb GE, Price GJ (2023) Interim report on the vertebrate deposits recovered from the Capricorn Caves, Rockhampton, Queensland. Alcheringa: An Australasian Journal of Palaeontology 47, 562-589.
| Crossref | Google Scholar |
Lyman RL (1994) Quantitative units and terminology in zooarchaeology. American Antiquity 59, 36-71.
| Crossref | Google Scholar |
Marin-Monfort MD, Fagoaga A, García-Morato S, Ruíz Sánchez FJ, Mallol C, Hernández C, Galván B, Fernández-Jalvo Y (2021) Contribution of small mammal taphonomy to the last Neanderthal occupations at the El Salt site (Alcoi, southeastern Spain). Quaternary Research 103, 208-224.
| Crossref | Google Scholar |
Morton SR, Baynes A (1985) Small mammal assemblages in arid Australia: a reappraisal. Australian Mammalogy 8, 159-169.
| Crossref | Google Scholar |
Murphy BP, Andersen AN, Parr CL (2016) The underestimated biodiversity of tropical grassy biomes. Philosophical Transactions of the Royal Society B: Biological Sciences 371, 20150319.
| Crossref | Google Scholar |
Price GJ, Louys J, Smith GK, Cramb J (2019) Shifting faunal baselines through the Quaternary revealed by cave fossils of eastern Australia. PeerJ 6, e6099.
| Crossref | Google Scholar |
Price GJ, Cramb J, Louys J, Travouillon KJ, Pease EMA, Feng Y-X, Zhao J-X, Irvin D (2020) Late Quaternary fossil vertebrates of the Broken River karst area, northern Queensland, Australia. Records of the Australian Museum 72, 193-206.
| Crossref | Google Scholar |
Rhodes SE, Ziegler R, Starkovich BM, Conard NJ (2018) Small mammal taxonomy, taphonomy, and the paleoenvironmental record during the Middle and Upper Paleolithic at Geißenklösterle Cave (Ach Valley, southwestern Germany). Quaternary Science Reviews 185, 199-221.
| Crossref | Google Scholar |
Roycroft E, MacDonald AJ, Moritz C, Moussalli A, Portela Miguez R, Rowe KC (2021) Museum genomics reveals the rapid decline and extinction of Australian rodents since European settlement. Proceedings of the National Academy of Sciences 118(27), e2021390118.
| Crossref | Google Scholar |
Russell-Smith J, Edwards AC, Woinarski JCZ, McCartney J, Kerin S, Winderlich S, Murphy BP, Watt FA (2009) Fire and biodiversity monitoring for conservation managers. In ‘Culture, ecology and economy of fire management in north Australian savannas: rekindling the wurrk tradition’. (Eds J Russell-Smith, P Whitehead, P Cooke) pp. 247–285. (CSIRO Publishing: Melbourne, Vic, Australia)
Schloss CA, Nunez TA, Lawler JJ (2012) Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proceedings of the National Academy of Sciences 109, 8606-8611.
| Crossref | Google Scholar |
Schoenefuss P, Kutt AS, Kern PL, Moffatt KA, Bon J, Wardle GM, Dickman CR, Hurwood DA, Baker AM (2024) An investigation into the utility of eastern barn owl pellet content as a tool to monitor small mammal diversity in an arid ecosystem. Austral Ecology 49, e13503.
| Crossref | Google Scholar |
Silva M, Downing JA (1995) The allometric scaling of density and body mass: a nonlinear relationship for terrestrial mammals. The American Naturalist 145(5), 704-727.
| Crossref | Google Scholar |
Stuiver M, Polach HA (1977) Discussion reporting of 14C data. Radiocarbon 19, 355-363.
| Crossref | Google Scholar |
Travouillon KJ, Simoes BF, Miguez RP, Brace S, Brewer P, Stemmer D, Price GJ, Cramb J, Louys J (2019) Hidden in plain sight: reassessment of the pig-footed bandicoot, Chaeropus ecaudatus (Peramelemorphia, Chaeropodidae), with a description of a new species from Central Australia, and use of the fossil record to trace its past distribution. Zootaxa 4566, 1-69.
| Crossref | Google Scholar |
Vakil V, Cramb J, Price GJ, Webb GE, Louys J (2023) Conservation implications of a new fossil species of hopping-mouse, Notomys magnus sp. nov. (Rodentia: Muridae), from the Broken River Region, northeastern Queensland. Alcheringa: An Australasian Journal of Palaeontology 47, 590-601.
| Crossref | Google Scholar |
van Klinken GJ (1999) Bone collagen quality indicators for palaeodietary and radiocarbon measurements. Journal of Archaeological Science 26, 687-695.
| Crossref | Google Scholar |
Waller NL, Gynther IC, Freeman AB, Lavery TH, Leung LK-P (2017) The Bramble Cay melomys Melomys rubicola (Rodentia: Muridae): a first mammalian extinction caused by human-induced climate change? Wildlife Research 44, 9-21.
| Crossref | Google Scholar |
Warburton NM, Travouillon KJ (2016) The biology and palaeontology of the Peramelemorphia: a review of current knowledge and future research directions. Australian Journal of Zoology 64, 151-181.
| Crossref | Google Scholar |
Withnall IW (1989) Revision of the stratigraphy of the Broken Rive area, north Queensland – Ordovician and Silurian units. Queensland Government Mining Journal 90, 213-218.
| Google Scholar |
Woinarski JCZ, Risler J, Kean L (2004) Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecology 29, 156-176.
| Crossref | Google Scholar |
Woinarski JCZ, Armstrong M, Brennan K, Fisher A, Griffiths AD, Hill B, Milne DJ, Palmer C, Ward S, Watson M, Winderlich S, Young S (2010) Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildlife Research 37, 116-126.
| Crossref | Google Scholar |
Woinarski JCZ, Legge S, Fitzsimons JA, Traill BJ, Burbidge AA, Fisher A, Firth RSC, Gordon IJ, Griffiths AD, Johnson CN, McKenzie NL, Palmer C, Radford I, Rankmore B, Ritchie EG, Ward S, Ziembicki M (2011) The disappearing mammal fauna of northern Australia: context, cause, and response. Conservation Letters 4, 192-201.
| Crossref | Google Scholar |
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences of the United States of America 112, 4531-4540.
| Crossref | Google Scholar | PubMed |
Woinarski JCZ, Garnett ST, Legge SM, Lindenmayer DB (2017) The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species. Conservation Biology 31, 13-23.
| Crossref | Google Scholar | PubMed |
Woinarski JCZ, Braby MF, Burbidge AA, Coates D, Garnett ST, Fensham RJ, Legge SM, McKenzie NL, Silcock JL, Murphy BP (2019) Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Crossref | Google Scholar |
Wood RE, Esmay R, Usher E, Fallon SJ (2023) Sample preparation methods used at the Australian National University Radiocarbon facility. Radiocarbon 65, 573-589.
| Crossref | Google Scholar |
Ziembicki MR, Woinarski JCZ, Mackey B (2013) Evaluating the status of species using Indigenous knowledge: novel evidence for major native mammal declines in northern Australia. Biological Conservation 157, 78-92.
| Crossref | Google Scholar |