Climate drying reduces serotinous seedbanks and threatens persistence in two fire-killed shrubs
N. J. Enright A * and M. C. Agne B CA
B
C Present address:
Abstract
Mediterranean-type ecosystems (MTEs) are experiencing declining rainfall, increasing temperature, and shifting fire regimes as climate changes. While changes in fire regimes and post-fire recruitment are widely reported, evidence for changing plant demographic rates is limited.
We hypothesised increased time to maturity and decreased serotinous seed stores available for post-fire recruitment due to declining rainfall over recent decades for two fire-killed serotinous shrubs of south-east Australian MTEs, Hakea decurrens and Banksia ornata.
Fruit and cone production for populations across time since fire chronosequences were measured in the same regions in the 1990s and in 2017.
Estimated time to 50% maturity increased from 3–15 years and 6–15 years for H. decurrens and B. ornata, respectively, while estimated canopy seed stores were 90% and 50% lower in 2017 than in the 1990s.
Delayed reproductive maturity and decreased total seed stores were significantly related to decreasing rainfall received by 2017 populations over their lifetimes (5–17% less than for stands in the 1990s).
Shifts in inter-fire rates of seed production and storage, combined with changes to fire regimes and post-fire recruitment conditions due to climate change may already threaten the persistence of some species.
Keywords: Banksia, chronosequence, climate change, fire, Hakea, seed storage, serotiny, shrublands.
Introduction
Global warming as a consequence of anthropogenic climate change has been occurring over at least the past three decades and is projected to continue through the 21st Century (IPCC 2022). In many parts of the world, and particularly in Mediterranean type climate regions, the climate is also becoming drier (Diffenbaugh and Field 2013). Fire regimes are changing in response to these climate changes, with fire season length and severity of fire weather conditions increasing, and estimated fire return intervals decreasing (Moritz et al. 2012; Jolly et al. 2015; Jones et al. 2022). A number of studies in the past decade have illustrated a growing immaturity risk for fire-killed tree species (i.e. fire occurring before populations have produced sufficient seeds for self-replacement; Keeley et al. 1999) based on a shortening of fire intervals due to a combination of global environmental change drivers, including for Californian conifers (Agne et al. 2022), Mediterranean Basin pines (Espelta et al. 2008), western USA conifers (Turner et al. 2019), boreal conifers (Brown and Johnstone 2012; Whitman et al. 2019), and Australian eucalypts (Bassett et al. 2015; von Takach Dukai et al. 2018). More recently, evidence of increased recruitment failure after fire has emerged, with increasing frequency of post-fire drought implicated as a major driver, particularly in the western USA (Harvey et al. 2016; Stevens-Rumann et al. 2018; Davis et al. 2019; Hansen and Turner 2019).
Enright et al. (2015) hypothesised potential decline in populations of many woody plant species in fire-prone regions where climate is drying as well as warming, based on the combined impacts of shortened fire intervals, reduced post-fire recruitment and changes to demographic rates during the inter-fire intervals available for plant growth (i.e. reduced rates of growth, survival and seed production, and increased time to reproductive maturity). Under their ‘interval squeeze’ hypothesis, affected plant populations would require longer fire-free periods to reach a demographic state compatible with self-replacement after fire, while climate change impacts on fire regimes and post-fire recruitment conditions would make this increasingly unlikely. A growing body of evidence supports the first two of these interval squeeze drivers as described above, but evidence for shifts in demographic rates directly resulting from climate change remains sparse. Selwood et al. (2015) reviewed the effects of climate change on species demographic rates based on 147 studies published between 1970 and 2012, finding an overall negative effect of changes in rainfall and temperature on reproduction and survival across a range of fauna (birds, mammals, fish), but noted that there were few published studies for plants. More recently, Iler et al. (2021) reviewed the literature on phenology shifts associated with climate change that also had demographic rate consequences, reporting on 238 studies published to 2020. The review showed a continued strong focus on fauna (61% of studies), while of the studies on plants (39% of studies), 77% were experimental, with 73% primarily focused on the effects of shifts in the (typically earlier) onset of flowering. Most did not address demographic rate change consequences and the authors emphasised the need for more studies on plants (Iler et al. 2021).
If shifts in key demographic variables are occurring, changes might be seen most clearly in regions with evidence of climate warming and drying over recent decades, such as those with Mediterranean type climates (Diffenbaugh and Field 2013; Vicente-Serrano et al. 2014). Mediterranean-type ecosystem (MTE) woodlands/shrublands of southern Australia provide an ideal ‘field laboratory’ for studying this question. These plant communities are woody-fuel dominated and characterised by a crown fire regime with short to intermediate fire return intervals of 10–30 years (Keith et al. 2014; Enright et al. 2015). Short to intermediate longevity (15–50 years) fire-killed shrub and small tree species are prominent and many accumulate a (serotinous) canopy seed store that is readily quantifiable in relation to plant age. This region also shows evidence of ongoing warming since the 1950s and drying over at least the past 30 years (Head et al. 2014).
Here, we capitalise on the rare opportunity to compare estimated seed production and storage for two fire-killed serotinous shrub species of MTE woodlands/shrublands in south-east Australia, Hakea decurrens subsp. physocarpa W.R. Barker and Banksia ornata (F.Muell.) (both Proteaceae) based on data collected in the 1990s (Shaw 1997; Enright and Goldblum 1999; N. J. Enright, unpubl. data) and again in 2017 in the same or nearby field locations and using the same methods. Our study seeks to provide evidence of a decline in seed production and storage in response to climate change over the past 20–30 years that may threaten species persistence. For both species, we hypothesise that: (1) time to reproductive maturity has increased and fruit/cone production (and estimated seed production and storage) has decreased since the 1990s; and (2) this decline is associated with decreased rainfall under a changing climate.
Materials and methods
Study species and sites
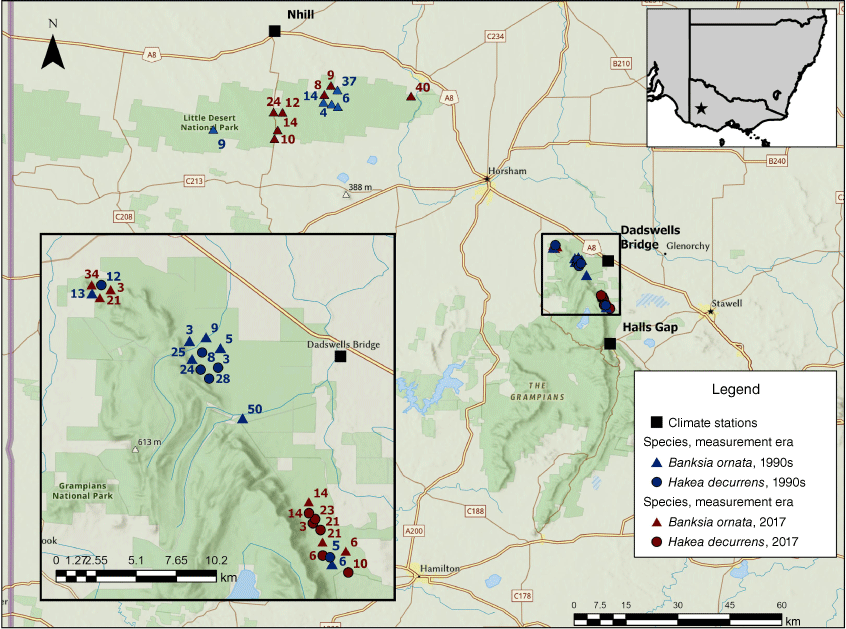
Data on number of fruits per plant by plant age, seeds per fruit and plant height collected in the 1990s were available for Hakea decurrens subsp. physocarpa at sites in the northern part of Grampians (Gariwerd) National Park (NP) (Enright and Goldblum 1999), and on number of cones per plant by plant age, seeds per cone and plant height collected in the 1990s for Banksia ornata at sites in the northern Grampians and in Little Desert NP (N. J. Enright, unpubl. data; Shaw 1997) (Fig. 1). Summary demographic data were also available from the 1980s for B. ornata at sites in Little Desert NP, as reported in Gill and McMahon (1986).
The Grampians and Little Desert National Park study areas, south-east Australia (inset top right, study region indicated by star), showing the location and age (numbers next to site location symbols are time since last fire in years) of all populations of Hakea decurrens (circles) and Banksia ornata (triangles) sampled in the 1990s (blue) and in 2017 (red). Sample site locations for the Grampians are shown in more detail in the inset (bottom left). Some overlapping sample locations have been separated for clarity. For details on their more precise (latitude/longitude) locations, see Supplementary Table S1. Locations of the Nhill, Dadswells Bridge, and Halls Gap climate stations (black squares) referred to in the text are also shown.
H. decurrens is a common understorey shrub that grows to 3.5 m height in open Eucalyptus baxteri (Myrtaceae)–Callitris rhomboidea (Cupressaceae) shrubby woodlands on unconsolidated, nutrient-poor sands. Historically, first seed set occurred around 3 years of age, with seeds retained in serotinous woody fruits in the plant canopy for at least 10 years (Enright and Goldblum 1999). Each fruit contains up to two seeds, and Enright and Goldblum (1999) reported an overall seed set and viability of 95.5% (2% aborted, and 97% of the 98% firm seeds viable). Seeds are primarily released as a result of fire and germinate in the first winter after fire (or otherwise perish), establishing single-aged (post-fire recruitment) stands with age equal to time since fire. In the absence of fire, plants begin to senesce by age 25–30 years. At stand ages >20 years, some inter-fire establishment may occur as old fruits desiccate and release their seeds following plant or branch death, so that old stands become increasingly multi-aged and seed stores decline (Enright and Goldblum 1999). However, in stands <30 years, inter-fire recruits are readily identifiable as younger and smaller than the original post-fire cohort individuals. Data reported in Enright and Goldblum (1999) were collected in 1991 and 1995 and sampled across a time since fire (TSF) chronosequence of stands ranging in age from 3 years to 28 years, representing cohorts established after fires in the period 1967–1992. The raw (individual plant) data for H. decurrens reported in Enright and Goldblum (1999) were re-analysed here.
B. ornata is a prominent species of MTE type shrublands in Little Desert NP, and of open Eucalyptus (Myrtaceae)–Callitris (Cupressaceae) woodlands in Grampians NP. It grows to 2.5 m height, and matures at age 5–6 years, retaining viable seeds in serotinous cones in the plant canopy for at least 30 years (Gill and McMahon 1986). Each cone contains ~13 follicles on average, with up to two viable seeds per follicle (Gill and McMahon 1986; Shaw 1997). Follicles open primarily in response to the heat of fire, and released seeds germinate in the first winter after fire (or otherwise perish), establishing single-aged post-fire stands. Gill and McMahon (1986) found <1% of follicles open on plants up to 50 years old, so that opportunities for inter-fire recruitment are rare. In the absence of fire, plants begin to senesce around age 50 years with most seed likely perishing within rotting cones. Germination rate data from the 1980s and 1990s indicates an average viable seed set of 60% (±s.e. 4–6%) for plants spanning ages 6–50 years and sites spanning both Grampians and Little Desert NP (Gill and McMahon 1986; Shaw 1997).
Individual-level plant data (N. J. Enright, unpubl. data) for a chronosequence of TSF sample populations of B. ornata in the northern Grampians from the 1990s was available for comparison with data collected in 2017. In addition, site-level summary data from two previous demographic studies in Little Desert NP were available for comparison with 2017 data. Gill and McMahon (1986) reported data for a TSF chronosequence of sites in and around Little Desert NP (centred on 36.54°S, 141.70°E) spanning stand ages from 1 year to 38 years, representing stands established after fires from 1946 to 1977, and Shaw (1997) for a TSF chronosequence of five stands ranging in age from 4 years to 37 years in the same area.
We used total counts of fruits (H. decurrens) and cones (B. ornata) per plant as a measure of lifetime reproduction. For B. ornata, follicle rupture in the absence of fire (i.e. inter-fire seed release) was rarely observed and we used total cone counts to estimate seed production and viable seeds per plant. For H. decurrens, open fruits were more commonly encountered, so we recorded both open and closed fruits per plant for this species to estimate total canopy seed store at a given stand age. Estimates of viable seeds per plant by species and stand age in 2017 were based on fruit and cone count data from 2017 and germination rate data reported for the earlier time-period by Enright and Goldblum (1999) for H. decurrens, and by Gill and McMahon (1986) and Shaw (1997) for B. ornata.
Plant surveys
Data for B. ornata from the 1990s were collected from a TSF chronosequence of sample populations in the northern sector of Grampians NP, overlapping the burn areas described in Enright and Goldblum (1999) for H. decurrens, (fire age data sourced from the Department of Natural Resources and Environment of Victoria, Horsham Office, and our own field observations) (Fig. 1). We also included one long-unburned site of unknown TSF that we assigned a notional age of 50 years (so assigned burn year of 1946) based on the large size of individual plants and strong evidence of senescence. TSF maps used for field site selection in 2017 were obtained from the Arthur Rylah Institute, State Government of Victoria (A. Muir, pers. comm.; Little Desert NP fire history maps downloaded on 7 April 2017; Grampians NP fire history maps downloaded on 8 May 2017). Field locations in the same parts of Little Desert NP (B. ornata only) and Grampians NP (both species) as used in the earlier studies by Gill and McMahon (1986), Shaw (1997) and Enright and Goldblum (1999) were stratified by TSF and each fire age area was searched for individuals of the target species.
We measured plant height and counted total number of open and closed fruits per plant for H. decurrens on a minimum of 50 plants at each TSF chronosequence site in Grampians NP (see Supplementary Table S1). We used quadrats of 10 m × 10 m to 30 m × 30 m depending on target plant density and measured all target species individuals within the plots. Plots were placed a random x–y distance (20–1000 m) from the most readily accessible corner of each mapped burn area and located by GPS. Where plant density was too low to make quadrats a viable method, a random walk to nearest neighbour search approach was used until the minimum sample size was achieved.
The same methods were used to collect total cones data for B. ornata at both Grampians NP and Little Desert NP (Table S1). For this species, sample size in low density or small burn area sites was sometimes <50. A sample of 30 burned cones was collected (two per plant from 15 plants) from the skeletons of 24-year-old plants killed by fire in 2015 at the Nhill-Harrow Rd West site, Little Desert NP (Table S1) and the number of follicles per cone counted. This assessment was made only on post-fire plants because cones on living plants retain their florets for many years so that follicles are not visible and cannot readily be counted.
No viability tests were conducted on seeds for either species in 2017. There were insufficient fruits on individuals of H. decurrens to make seed collection feasible (see results below). In B. ornata, there was no difference in seed viability in the 1980s vs 1990s based on results reported by Gill and McMahon (1986) and Shaw (1997), and follicle set per cone was no different from the 1980s to 2017 inclusive (Gill and McMahon 1986; Shaw 1997, and data collected for this study in 2017; see results below). Given this, we assume no change in seed viability over the time-period examined in this study. While we acknowledge that seed viability could have changed, such changes are not considered likely to affect the overall conclusions drawn from our study.
Other than for wildfires in 1983 and 2014 in the northern Grampians, and 1983 and 2015 in Little Desert, all samples were located in sites last burned by managed (fuel reduction) fires (Table S1). Fire frequency spanning our Grampians study region over the period 1950–2010 is reported by Coates et al. (2010) to correlate with rainfall and fuel accumulation rates, with wetter areas requiring more frequent management burns. They found that most areas in the drier northern Grampians experienced 1–2 fires over this 50-year time-period while those in the wetter south experienced 3–4 fires.
Climate data
Monthly rainfall records for Australian Bureau of Meteorology climate stations surrounding our two field regions, ranging from Halls Gap in the south (climate station 079074; 37°14′S, 142°52′E) to Nhill in the north (climate station 078040; 36°31′S, 141°65′E) (Fig. 1) were downloaded (http://www.bom.gov.au/) and checked for length of record and missing data; i.e. months with no rainfall entry. Our protocol was to use rainfall records for the years 1946–1990, starting from the estimated establishment year of the oldest stand censused in this study, as the ‘baseline’ for comparison with rainfall from 1991 to 2016 (based on our data collection in May 2017). Where there were rainfall data for at least 11 of the 12 calendar months, the missing month was interpolated using the mean rainfall for that month to obtain an estimated annual value. Where there were 2 or more months missing within 1 year, then that year was deemed to be missing and was not used in any analyses. At Halls Gap, the closest climate station to our southern-most Grampians field sites, there were 15 years deemed missing across the available records from 1958 (start of records) to 2016, with nine of these missing in the period after 1990, making it unsuitable for comparison with the baseline 1946–1990 time-period. In addition, a further 5 years used interpolated values. At Dadswells Bridge, the closest climate station to our Grampians field sites overall, the available record was even shorter (1969–2016) and had five missing and nine interpolated years. By contrast, the Nhill climate station (closest to our Little Desert sites) had almost complete monthly rainfall data from 1946 to 2016, with just one missing and two interpolated years. We ran pairwise correlation analyses for these climate stations across all available years and found highly significant relationships: Nhill vs Dadswells Bridge r = 0.852, n = 40; Nhill vs Halls Gap r = 0.843, n = 44; and Dadswells Bridge vs Halls Gap r = 0.874, n = 27 (P < 0.001 in all cases).
To further determine the best source of rainfall data for use in our analyses, we obtained estimated annual rainfall (1960–2016) for the specific latitude–longitude locations of our Little Desert and Grampians field sites from two global climate estimation models, Terraclimate (Abatzoglou et al. 2018) and ANUclim-WorldClimate (Xu and Hutchinson 2013). Both were highly correlated with each other and with the Nhill, Dadswells Bridge, and Halls Gap climate station annual rainfall data (r > 0.90 in all cases). The TerraClimate mean annual rainfall estimates for our sites in Grampians NP for the baseline rainfall period ranged from 595 mm to 630 mm (cf. Dadswells Bridge mean of 580 mm, and Halls Gap mean of 984 mm), and across the full data period to 2016 showed a near-perfect correlation among sites within the Grampians (r > 0.999, n = 57), as well as between the Grampians and Little Desert (r = 0.989, n = 57; Fig. S1). Given these strongly correlated patterns in actual and estimated annual rainfall across the whole study region, we used the Nhill climate station annual rainfall data for comparison with B. ornata demographic data from Little Desert NP sites, and estimated annual rainfall values using the TerraClimate northern Grampians mean of 630 mm per year and regression relationship to Nhill rainfall for B. ornata and H. decurrens demographic data for Grampians NP sites (y = 1.172x + 139.4, where y is predicted northern Grampians annual rainfall and x is Nhill annual rainfall; r = 0.92, n = 57, P < 0.001; Fig. S2).
To characterise the rainfall history for our censused populations, we determined the rainfall difference from the 1946 to 1990 mean for each H. decurrens and B. ornata population based on the span of years for which it was extant. For example, for a stand that was 28 years old when censused in 1995, having established and grown over the post-fire period from 1967 to 1995, growing conditions are expressed as the average deviation from mean 1946–1990 rainfall for each of those 28 years. These data were then used in statistical and descriptive analyses as detailed below.
Statistical analysis
To determine if the reproductive rates for H. decurrens and B. ornata have changed over time (1990s vs 2017), we fit generalised linear mixed models for the plant-level response variables probability of reproductive maturity and total fruit/cone production for each species in Grampians NP. Statistical analyses were not possible for B. ornata populations from Little Desert NP as we did not have individual-level data for the 1990s, but summary comparisons are reported here based on mean values available from the earlier studies. Probability of reproductive maturity was measured as fruit/cone presence or absence on a live plant. Total fruit/cone production was measured as the total count of open and closed fruits (H. decurrens) or cones (B. ornata) on a live plant. For each response variable and species combination, we fit two sets of candidate models that considered the effect of stand age (years since last stand-replacing fire) and either: (1) the continuous effect of rainfall change during the plant’s life-time (hereafter, rain differential); or (2) the categorical effect of measurement era (1990s vs 2017), representing a broader measure of combined global environmental change impacts that includes warming.
For each species and response variable, we fit models that included the focal predictor (rain differential or measurement era) and stand age, both with and without an interaction term. To compare the magnitude of the effects of predictors, models were fit using standardised predictors (mean-centred per two standard deviations). For each of the eight relationships considered (two species × two response variables × two sets of predictor variables), we evaluated models in the set for outliers, uniform residuals and over-dispersion (count variables only) and selected the model form with an interaction term unless assumptions were violated. Collinearity of predictor variables was assessed prior to model fitting and all models included a random effect of site. Models of probability of reproductive maturity were fit with a binomial error structure while models of total fruit/cone production were fit with a negative binomial error structure. We note that the response variable total fruit/cone is defined differently for each species due to modelling constraints. We modelled H. decurrens total fruits using only individuals with <100 fruits (representing 99.2% of sampled plants) and B. ornata total cones using only individuals with at least one cone. We interpreted P ≤ 0.01 as strong, P ≤ 0.05 as moderate, and P ≤ 0.10 as suggestive evidence of effects (Ramsey and Schafer 2012). Models were fit in glmmTMB (Brooks et al. 2017), diagnostics conducted in DHARMa (Hartig 2021), and effects visualised in dotwhisker (Solt and Hu 2024), ggeffects (Lüdecke 2018), ggplot (Wickham 2016), and ggpubr (Kassambara 2020). Analyses were conducted in R version 4.2.2 (R Core Team 2022).
Results
Hakea decurrens
We surveyed 842 H. decurrens plants in Grampians NP; 444 individuals from six stand ages in the 1990s, and 398 from six stand ages in 2017. There was no evidence of change in plant height in relation to plant age between the 1990s and 2017 (Table S2). Stands measured in the 1990s received an average of 8 mm per year (1.2%) less than the 1946–1990 mean annual rainfall during their lifetimes, while stands measured in 2017 received 73 mm per year (11.6%) less (Table 1). Stands measured in the 1990s showed a strong trend of increasing fruit production with age. Reproductive maturity was first evident at 3 years and serotinous fruits accumulated in the plant canopy mostly remained closed until individuals were >20 years old (by 28 years ~20% of fruits had opened and released their seeds in the absence of fire). In contrast, populations measured in 2017 showed very low levels of fruit production at all ages <20 years and higher rates of fruit opening in the absence of fire (Table 1).
| Sampling year | TSF (years) | n | Closed fruits per plant (mean ± s.e.) | Open fruits per plant (mean ± s.e.) | Total fruits per plant(mean ± s.e.) | Estimated viable seeds per plant | Mean rain differential (mm per year, %) | |
|---|---|---|---|---|---|---|---|---|
| 1990s | 3 | 63 | 1.4 ± 0.3 | 0 | 1.4 ± 0.3 | 0 | −8.1 (−1.3) | |
| 5 | 74 | 2.6 ± 0.4 | 0 | 2.6 ± 0.4 | 0 | −17.3 (−2.7) | ||
| 8 | 64 | 4.7 ± 0.6 | 0.5 ± 0.1 | 5.2 ± 0.7 | 9.0 | −5.6 (−0.9) | ||
| 12 | 111 | 15.6 ± 1.5 | 0.7 ± 0.2 | 16.3 ± 1.5 | 29.8 | 4.4 (0.7) | ||
| 24 A | 67 | 17.8 ± 4.1 | 1.1 ± 0.5 | 18.9 ± 4.3 | 34.0 | −10.1 (−1.6) | ||
| 28 | 65 | 31.8 ± 4.1 | 8.6 ± 1.7 | 40.5 ± 4.4 | 60.7 | −8.5 (−1.3) | ||
| 2017 | 3 | 52 | 0.1 ± 0.1 | 0 | 0.1 ± 0.1 | 0.2 | −113.1 (−17.8) | |
| 6 | 65 | 0.5 ± 0.2 | 0 | 0.5 ± 0.2 | 1.0 | −68.3 (−10.7) | ||
| 10 | 80 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.6 | −53.2 (−8.4) | ||
| 14 | 50 | 1.3 ± 0.3 | 0.3 ± 0.1 | 1.6 ± 0.3 | 2.5 | −61.6 (−9.7) | ||
| 21 | 100 | 0.3 ± 0.1 | 2.7 ± 0.4 | 3.0 ± 0.4 | 0.6 | −71.4 (−11.2) | ||
| 23 | 51 | 0.1 ± 0.1 | 15.4 ± 1.8 | 15.4 ± 1.8 | 0.2 | −74.2 (−11.7) |
Mean rain differential, difference in mm per year (percentage in parentheses) between mean annual rainfall for the period when the population was extant and baseline (1946–1990) estimated mean annual rainfall as detailed in the text.
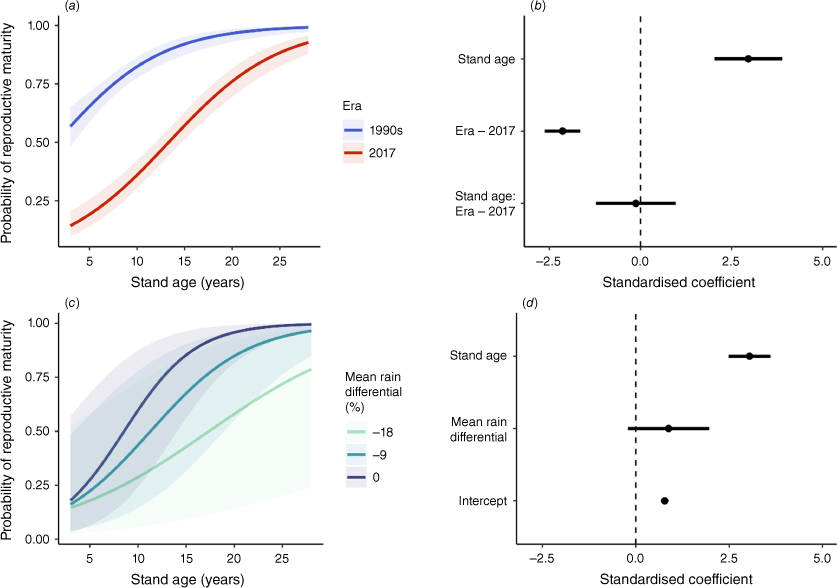
Across the TSF chronosequence, there was strong evidence of decreased and delayed reproductive output for H. decurrens in 2017 compared with the 1990s (Table 1, Figs 2, 3). Although cones were first present at 3 years TSF for both periods, probability of reproductive maturity was 50% at 3 years and 90% by 14 years TSF in the 1990s but did not reach comparable levels until 15 years and 26 years TSF, respectively, in 2017, representing a 12-year delay (Fig. 2a, b). Estimates of total fruit production in 2017 ranged from 5% to 30% of 1990s estimates for stands of the same age, with differences declining as stand age increased. Modelled total fruit production was seven fruits per plant at 10 years and 20 fruits per plant at 20 years TSF for plants in the 1990s compared with less than one fruit per plant and five fruits per plant, respectively, at the same plant ages in 2017 (Fig. 3a, b).
Modelled probability of reproductive maturity for populations of Hakea decurrens as a function of (a, b) stand age and measurement era, and (c, d) mean rain differential [average% deviation from mean 1946–1990 rainfall]. Prediction plots (a, c) show change in probability of reproductive maturity across gradients of covariates. Solid lines are median estimates, shaded areas are 95% confidence intervals. Coefficient plots (b, d) show estimated effect sizes of predictor variables. Dots represent medians and horizontal lines represent 95% confidence intervals. Effects for each predictor are per two s.d.
Modelled total fruit production for populations of Hakea decurrens as a function of (a, b) stand age and measurement era, and (c, d) stand age and mean rain differential [average% deviation from mean 1946–1990 rainfall]. Prediction plots (a, c) show change in total fruit production across gradients of covariates. Coefficient plots (b, d) show estimated effect sizes of predictor variables. Symbols are described in Fig. 2.
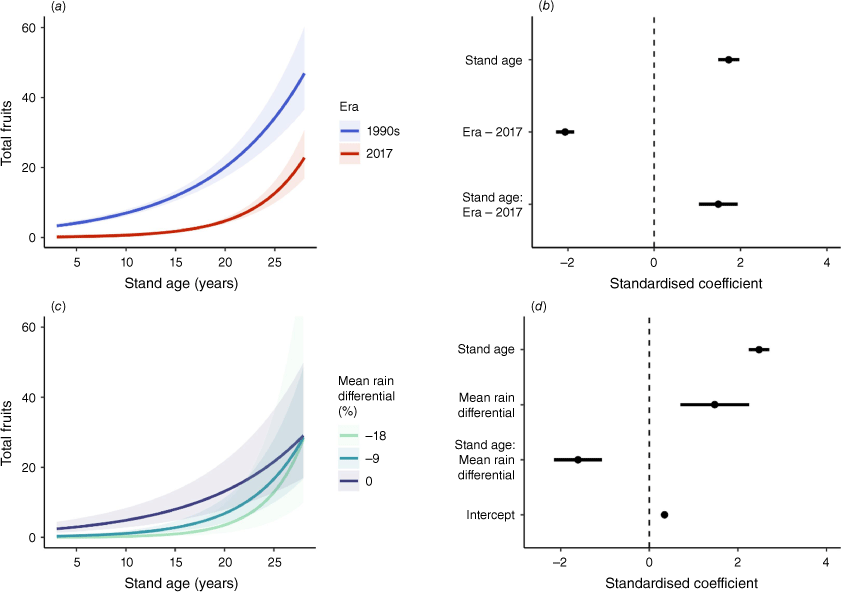
There was strong evidence for an effect of rainfall on total fruit production across the chronosequence of stand ages for H. decurrens (Fig. 3c, d). This effect was especially pronounced at younger stand ages; at age 10 years, 9% and 18% decreases from pre-1990s rainfall were associated with 77% and 95% decreases in estimated total seed store per plant. By age 20 years, the same decreases in rainfall were associated with 48% and 73% reductions in estimated seed stores compared with individuals in stands that experienced mean rainfall (Fig. 3c, d). Although probability of reproductive maturity did not significantly differ with mean rain differential due to wide variation around estimates (Fig. 2c, d), estimated time to produce one fruit per plant was 3 years for plants that experienced mean rainfall, compared with 9 years and 15 years for plants that experienced a 9% and 18% decrease from mean rainfall, respectively (Fig. 3c, d).
Banksia ornata
We surveyed 469 B. ornata plants in Grampians NP; 224 individuals from seven stand ages in the 1990s (N. J. Enright, unpubl. data), and 245 from six stand ages in 2017. Maximum plant height was greater in Grampians than in Little Desert populations (~2.5 m vs 1.7 m), but there was no evidence of change in plant height in relation to plant age between the 1990s and 2017 measurements (Table S2). Stands measured in the 1990s received an average of 1.3 mm per year (0.2%) less than the 1946–1990 mean annual rainfall during their lifetimes, while stands measured in 2017 received 71 mm per year (11.2%) less (Table 2). Data for B. ornata in Little Desert for the 1990s were taken from Shaw (1997) who sampled 250 plants across five TSF sites (50 per site) and compared with data for 439 plants measured in 2017. Stands measured in the 1990s received an average of 8 mm per year (1.8%) less than the 1946–1990 mean annual rainfall during their lifetimes, while stands measured in 2017 received an average of 38 mm per year (8.4%) less.
| Sampling year: 1990s A | Sampling year: 2017 A | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TSF | n | Total cones per plant (mean ± s.e.) | Estimated viable seeds per plant | Mean rain differential (mm per year, %) | TSF | n | Total cones per plant (mean ± s.e.) | Estimated viable seeds per plant | Mean rain differential (mm per year, %) | |
| Grampians National Park | ||||||||||
| 3 | 6 | 0 | 0 | 14.0 (2.2) | 3 | 50 | 0 | 0 | −113.1 (−17.8) | |
| 5 | 30 | 0.1 ± 0.1 | 2 | −4.1 (−0.6) | 6 | 43 | 0.1 ± 0.1 | 2 | −68.3 (−10.7) | |
| 6 | 72 | 3.3 ± 0.4 | 51 | −12.2 (−1.9) | 14 | 45 | 5.1 ± 1.0 | 72 | −61.6 (−9.7) | |
| 9 | 15 | 12.3 ± 2.1 | 192 | −3.5 (−0.5) | 21 | 40 | 3.3 ± 0.7 | 51 | −71.4 (−11.2) | |
| 13 | 88 | 25.5 ± 3.3 | 398 | 5.1 (0.8) | 21 | 42 | 12.5 ± 2.4 | 195 | −71.4 (−11.2) | |
| 25 | 2 | 58.0 ± 3.0 | 905 | −8.6 (−1.3) | 34 | 25 | 29.5 ± 4.7 | 460 | −42.2 (−6.6) | |
| 50 | 11 | 100.7 ± 16.9 | 1571 | 0.3 (0.0) | ||||||
| Little Desert National Park | ||||||||||
| 4 | 50 | 0 | 0 | −28.8 (−6.4) | 8 | 81 | 1.8 ± 0.2 | 28 | −21.6 (−4.8) | |
| 6 | 50 | 3.5 ± 0.5 | 55 | 9.6 (2.1) | 9 | 80 | 0.6 ± 0.1 | 9 | −32.8 (−7.3) | |
| 9 | 50 | 5.9 ± 0.8 | 90 | −6.8 (−1.5) | 10 | 70 | 1.9 ± 0.4 | 30 | −31.1 (−6.9) | |
| 14 | 50 | 23.7 ± 1.7 | 290 | −5.9 (−1.3) | 12 B | 45 | 5.7 ± 0.8 | 89 | −44.7 (−10.0) | |
| 37 | 50 | 71.7 ± 5.8 | 736 | −9.0 (−2.0) | 14 | 53 | 9.1 ± 1.5 | 142 | −41.5 (−9.3) | |
| 24 B | 40 | 29.1 ± 3.9 | 454 | −54.8 (−12.2) | ||||||
| 40 | 70 | 14.3 ± 1.7 | 223 | −38.0 (−8.5) | ||||||
Mean rain differential is the difference in mm per year (percentage in parentheses) between mean annual rainfall for the period when the population was extant and baseline (1946–1990) mean annual rainfall at Nhill climate station for Little Desert sites, and estimated rainfall for Grampians sites as detailed in the text.
First onset of cone production in Grampians NP was at 5–6 years, and while cone production and accumulation increased rapidly for populations in the 1990s, it was much lower and slower for populations measured in 2017 (Table 2). There was no difference in mean (±s.e.) number of follicles per cone between time-periods in Little Desert NP (13.6 in Gill and McMahon (1986), 12.9 ± 0.6 in Shaw (1997), and 14.0 ± 1.1 in 2017). Past studies reported that ~70% of seeds were firm, and ~80% of these viable (Gill and McMahon 1986; Shaw 1997). Based on these values, estimated viable seed store per plant in Grampians NP in the 1990s increased rapidly with age following the onset of reproductive maturity, exceeding 100 by age 10 years and 500 by around age 20 years (Table 2). A similar pattern of seed accumulation was evident for stands in Little Desert NP (Table 2). Maximum seed stores in the 1990s exceeded 1500 seeds per plant in the Grampians and 700 seeds per plant in Little Desert, while Gill and McMahon (1986) found even higher seed stores of around 2000 seeds per plant for stands in Little Desert in the 1980s (Table S3). For stands measured in 2017, seed stores were generally less than half the size at the same age with maximum seed stores of less than 500 per plant (Table 2).
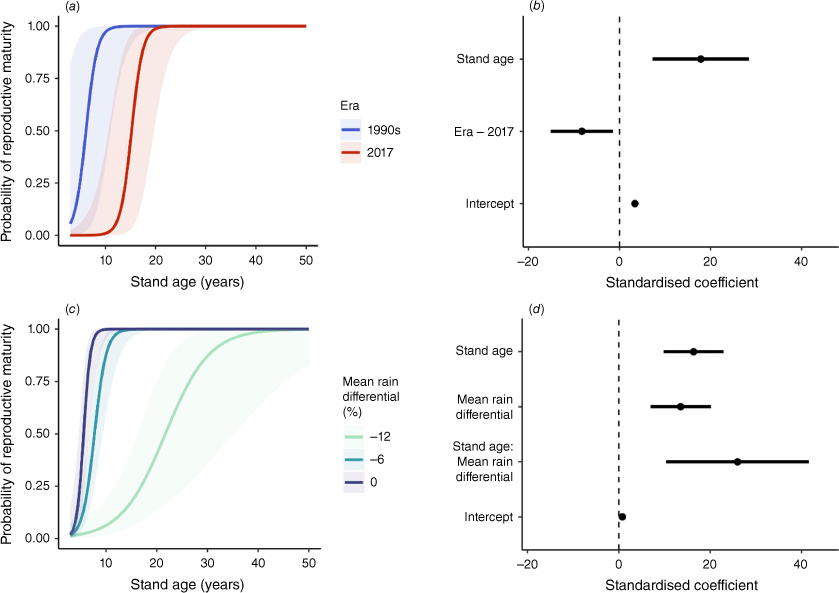
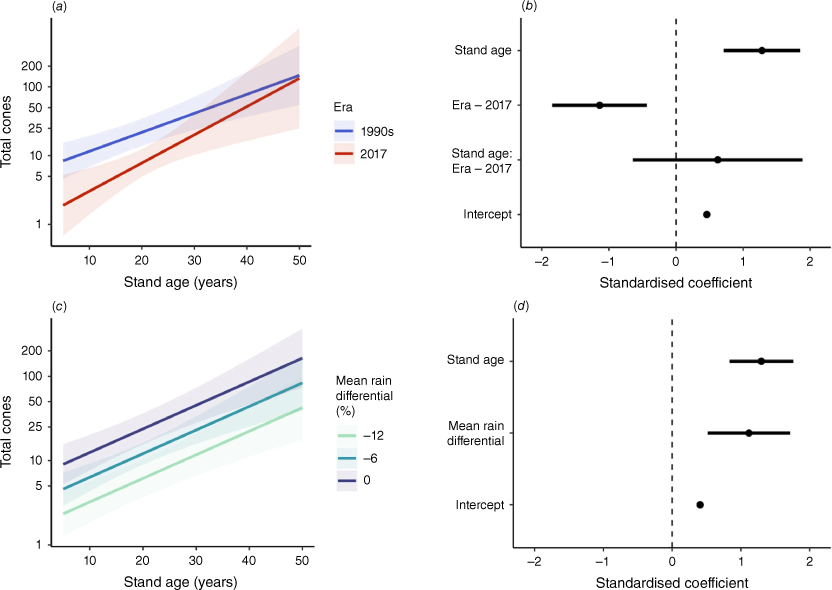
Across the chronosequence of stand ages, there was strong evidence of decreased and delayed reproductive output for Grampians NP populations of B. ornata in 2017 compared with the 1990s (Figs 4, 5). Although cones were first present by 6 years TSF in both periods, there was a 9-year delay in reaching the same probability of reproductive maturity in 2017 compared with the 1990s (Fig. 4a, b). There was a 50% probability of reaching reproductive maturity by 6 years TSF in the 1990s, a level not reached until 15 years TSF in 2017. Modelled total cone production also differed markedly, with 2017 estimates 43–77% lower than those in the 1990s for stands of the same age (Fig. 5a, b). There was strong evidence for an effect of mean rain differential on reproductive output (Figs 4, 5). For stands between 5 years and 24 years TSF, probability of reproductive maturity declined strongly as the mean rain differential increased (Fig. 4c, d). Estimated probability of reproductive maturity reached 50% by 6 years TSF for individuals that experienced mean rainfall (Fig. 4c, d). There was 50% probability of reaching reproductive maturity by 8 years TSF under a 6% decrease from mean rainfall, but this took until 22 years TSF under a 12% decrease (Fig. 4c, d). Mean rain differential had a strong effect on cone production, with 6% and 12% decreases from pre-1990s mean rainfall associated with 49% and 74% reductions in estimated total cone production, respectively (Fig. 5c, d).
Modelled probability of reproductive maturity for populations of Banksia ornata in Grampians National Park as a function of stand age and (a, b) measurement era, or (c, d) mean rain differential [average% deviation from mean 1946–1990 rainfall]. Prediction plots (a, c) show change in probability of reproductive maturity across gradients of covariates. Coefficient plots (b, d) show estimated effect sizes of predictor variables. Symbols are described in Fig. 2.
Modelled total cone production for populations of Banksia ornata in Grampians National Park as a function of stand age and (a, b) measurement era, or (c, d) mean rain differential [average% deviation from mean 1946–1990 rainfall]. Prediction plots (a, c) show change in total fruit production across gradients of covariates. Coefficient plots (b, d) show estimated effect sizes of predictor variables. Symbols are described in Fig. 2.
Discussion
Reported climate change impacts on the demographic rates of plant species of fire-prone ecosystems, and their potential population dynamic consequences, are rare relative to impacts relating to fire regimes (Jones et al. 2022) and post-fire recruitment (Davis et al. 2019). We investigated the hypothesis that there would be decreases in rates of seed production and storage due to climate drying for populations of two fire-killed shrub species from MTE woodlands/shrublands in south-east Australia. We found markedly slowed time to reproductive maturity and reduced levels of seed production and storage at comparable plant ages for populations of both species measured in 2017 compared with the 1990s and related to decreased rainfall. The consistent significance of time period of study (1990s vs 2017; referred to as ‘Era’ in our results) also suggests a role for increasing temperature (most likely exacerbating water stress) in our findings. These results are similar to those reported for B. hookeriana in MTE shrublands in south-west Australia where rainfall has declined by ~20% since the mid-1970s (Keith et al. 2014; Enright et al. 2015), providing strong evidence of a demographic shift that, in combination with increasing fire activity, may threaten persistence of some species.
Time to maturity
Time to reproductive maturity has variously been defined as the plant age when reproduction (flowering or fruiting) is first observed in a single individual within a population of known age, or when a specified percentage (e.g. 50%) of a population is observed to have produced flowers/fruits (Kraaij et al. 2013; Gosper et al. 2022). In fire-prone ecosystems, it is an important indicator of immaturity risk and of minimum fire intervals compatible with population persistence. Delayed time to reproductive maturity with increased aridity has been shown for several species in relation to variations in available soil moisture associated with landscape position (von Takach Dukai et al. 2018) and rainfall gradients and history (Burrows et al. 2008; Agne et al. 2022), with mean delays of up to 10 years attributed to increased moisture stress. These delays represent an expanded zone of immaturity risk where short interval fires might result in compromised rates of post-fire recruitment. In the present study, we document markedly lengthened estimated times for individuals to reach 50% probability of maturity in both H. decurrens (from 3 years to 15 years) and B. ornata (from 6 years to 15 years), representing a massive increase in immaturity risk in relation to the historical, and projected future shorter-interval, fire regime in this region.
Demographic data relating to time to maturity can be used to inform fire management policy, especially the setting of minimum fire return intervals for management (e.g. fuel reduction) burns compatible with biodiversity conservation. For example, in southern (temperate and MTE) Australia, a ‘2× years to first reproduction rule of thumb’ (Gill and Nicholls 1989) has been considered by fire management agencies where sufficient demographic data are available. For Banksia hookeriana in south-west Australia and B. ornata in south-east Australia, time to first reproduction was historically 4 years and 6 years, respectively (Gill and McMahon 1986; Enright et al. 1996), indicating minimum fire return intervals of 8 years and 12 years. In both cases, individuals at these ages were estimated to have canopy seed stores of ~100 seeds per plant, sufficient for self-replacement under average rainfall conditions (Enright et al. 1996). However, our results show that delayed time to reproductive maturity in B. ornata associated with declining rainfall from the 1990s to 2017 suggests a minimum fire return interval of 30 years in Grampians NP, more than double the historical minimum interval. Although our results are limited to a subset of the species range, they are similar to recent findings across the range by Morgan et al. (2021) who have reported data on density, height, and cones per plant in relation to plant age for six populations spanning the full geographic range of the species in western Victoria and south-eastern South Australia.
Seed production and storage
The massive decrease in seed production and storage in H. decurrens associated with declining rainfall suggests a climate threshold has been crossed for the northern Grampians populations of this species. The study populations are near the geographic range limit for the species and it does not occur in nearby Little Desert NP (Walsh and Entistle 1996) which receives ~200 mm per year less rainfall. Seed storage declined by 90% in tandem with the ~12% decline in rainfall across this region over the past three decades. Low or no seed store means that populations of this species are likely to decline quickly after fire and may become locally extinct. Under prevailing conditions, recruitment may be limited to short windows in which high rainfall years conducive to fruit production are closely followed by fire. Inter-fire seedling recruitment may act as a bet-hedging strategy against senescence risk (sensu Keeley et al. 1999) when occasional long fire intervals exceed plant longevity (Enright and Goldblum 1999). However, the probability of seedling survival is lower for inter-fire versus post-fire recruits due to competition with established vegetation for nutrients, moisture and light (Enright et al. 1998), and a drying climate will likely further reduce survivorship. Seed availability and suitable microsites for germination are likely to severely constrain responses of plant species to climate change, and this will apply both to inter- and post-fire recruitment environments (Kroiss and HilleRisLambers 2015). Low levels of seed production and storage, increased loss to inter-fire seed release, and hotter, drier conditions for seedling establishment and survival suggest there may be no management regime that is compatible with the long-term persistence of H. decurrens in this region.
Decreases in maximum seed store size for B. ornata of at least 50% over the past few decades are apparent across both the Little Desert and Grampians study areas. Historical estimates in Little Desert NP ranged from 1000 seeds per plant to 2600 seeds per plant (Gill and McMahon 1986; Shaw 1997). In 2017, the maximum seed store was 375 seeds per plant, and another contemporary estimate based on cones per plant data reported in Morgan et al. (2021) equates to ~260 seeds per plant. In the north of Grampians NP, the maximum estimated seed store was ~1000 seeds per plant in 1997 but was just 419 seeds per plant in 2017. A recent estimate from the wetter southern Grampians NP of ~600 seeds per plant (estimated from Morgan et al. 2021) is higher, but still low relative to the 1990s.
Enright et al. (1996) modelled seed production and accumulation for B. hookeriana in MTE shrublands in south-west Australia with similar rainfall to that reported here. Individuals began to store seeds by age 5 years, reached maximum seed production per year at around age 15 years, and maximum canopy seed store at age 25 years before it then slowly declined due to seed leakage from the increasing proportion of old cones. They estimated that individuals required a canopy store of 100 seeds to ensure self-replacement after fire in average rainfall years, and 200 seeds when dry years (specified as 20% below average) followed fire. Based on these values, they calculated that optimum population growth rate occurred at a mean fire interval of 16–17 years under average post-fire rainfall, increasing to 18 years under drier conditions.
Applying the same principles to B. ornata, maximum seed store occurs at age ~40 years and has not changed over time (Gill and McMahon 1986; Morgan et al. 2021, Table 2). However, lower rates of seed production and accumulation will have lowered the potential rate of natural increase and lengthened the optimum fire interval in 2017 relative to the 1990s. These results create the management conundrum that populations require much longer minimum inter-fire intervals and more seeds per plant to ensure that self-replacement after fire is feasible, but are increasingly likely to have a smaller seed store and experience more rather than less frequent fire. Potentially exacerbating this conundrum for some parts of the Grampians region is the higher frequency of fuel reduction burns required to treat the more rapidly accumulating fuel loads associated with higher rainfall areas as reported by Coates et al. (2010). While their analyses found that time since fire (stand age) showed a stronger effect on key species presence than did fire frequency, the evidence presented here of declining seed production and storage suggests that longer fire intervals may be critical for persistence of obligate seeder species such as H. decurrens and B. ornata.
Evidence of reduced rates of seed production and levels of seed storage over the lifetime of individuals as presented here clearly intersects with explanations for post-fire recruitment failure (sensu Enright et al. 2015), which is becoming increasingly common, especially for western USA conifer forests. Ponderosa pine (Pinus ponderosa) and Douglas fir (Pseudotsuga menziesii) forests in the western USA show reduced rates of post-fire recruitment due to drying climate over the past 20 years, particularly towards the drier ends of the species ranges where recruitment failure thresholds were more likely to be crossed (Davis et al. 2019). While a direct causal link could be made to drought after fire, which reduces rates of germination, establishment, and survival of seedlings, they noted that declining seed availability could contribute to recruitment failure but was not assessed. Similarly, post-fire recruitment levels for subalpine forests dominated by Engelmann spruce (Picea engelmannii) and subalpine fir (Abies lasiocarpa) declined sharply under drier conditions and with increasing distance from seed sources (Harvey et al. 2016). In context of this study, the effect of distance from seed source can be regarded as a surrogate gradient of how reduced seed store size at the time of fire impacts recruitment levels. These examples highlight that most studies reporting on post-fire recruitment failure are confounded by the lack of investigation of change in seed availability for stands of a given age. More evidence is needed regarding seed availability at the time of fire to address questions about the drivers of recruitment failure and their relative importance.
In the present study, low seed availability clearly emerges as a factor that may limit post-fire recruitment for H. decurrens, with no measured stands in the northern sector of Grampians NP showing sufficient seed stores for population self-replacement. The 3-year-old stand censused here (burned in 2014, censused in 2017) showed very low plant density, with a large search area (~1 ha) required to obtain the reported sample of 52 individuals (Table 2). In stands established after fires in the 1990s and earlier, sample sizes of n ≥ 50 were readily obtained within plots of 100–400 m2 (Enright and Goldblum 1999). In B. ornata, current seed stores are around half what they were in the 1980s (Gill and McMahon 1986) and 1990s (Shaw 1997; N. J. Enright, unpubl. data) but may be sufficient for self-replacement where stands have had at least 20 years since fire to accumulate a seed bank, and drought does not cause post-fire recruitment failure. In our Little Desert site burned in 2015 (24 years at time of fire), 2-year-old seedlings were present at a mean rate of 10 seedlings per pre-fire parent (Table S4). Roughly similar recruitment rates of 14 1-year-old seedlings per parent were estimated from data in Gill and McMahon (1986) for a stand of indeterminate age burned in 1977, and of 4–23 1-year-old seedlings per parent by Shaw (1997) for a 13-year-old stand in Grampians NP burned in 1996.
The evidence for major reductions in cone and fruit production in Grampians and Little Desert populations of the two fire-killed shrub species examined here lends strong empirical support for the greater future consideration of demographic shifts as climate changes. Changes in the inter-fire rates of plant survivorship, growth and seed production must be addressed as a third critical driver, in combination with deleterious changes to fire regime and increased likelihood of post-fire recruitment failure, which act together to threaten population persistence of fire-killed woody plant species of fire-prone ecosystem types as climate changes (Enright et al. 2015). These data will also provide a better platform for the analysis and interpretation of post-fire recruitment failure, allowing separation of the effects of changes in seed availability from those of declining rates of post-fire recruitment.
Demographic data for other plant species and regions are sorely needed to further understand the incidence and magnitude of demographic shifts in biomes around the globe and their possible consequences for species persistence as climate changes. Our study suggests that re-measurement of demographic rates for plant species at intervals of ~20 years may be sufficient for identifying climate-driven shifts in rates where recurrent fire (both planned and unplanned) provides suitable chronosequences for study. The long timeframes required for such analyses also highlights the need for researchers to ensure that relevant data are placed in data repositories and/or are passed on to next generation researchers.
Data availability
The data that support this study are available in the Murdoch University Research Portal, available at https://doi.org/10.60867/00000027.
Declaration of funding
This research was funded by Australian Research Council (ARC) Discovery Project grant DP170101288 held by NJE at Murdoch University.
Acknowledgements
We thank Dr Philip Ladd and Dr Joseph Fontaine from Murdoch University and Dr Annette Muir from the Arthur Rylah Institute for Environmental Research (State Government of Victoria) for help with collection of the 2017 field data, and Dr Muir also for arranging field collection permits and access to the relevant fire history information.
Author contributions
NJE devised the research, collected the field data, conducted preliminary data analyses and led the writing of the manuscript. MCA determined and conducted the statistical analyses and shared in the interpretation of results and writing of the manuscript.
References
Abatzoglou JT, Dobrowski SZ, Parks SA, Hegewisch KC (2018) TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Scientific Data 5, 170191.
| Crossref | Google Scholar | PubMed |
Agne MC, Fontaine JB, Enright NJ, Harvey BJ (2022) Fire interval and post-fire climate effects on serotinous forest resilience. Fire Ecology 18, 22.
| Crossref | Google Scholar |
Bassett OD, Prior LD, Slijkerman CM, Jamieson D, Bowman DMJS (2015) Aerial sowing stopped the loss of alpine ash (Eucalyptus delegatensis) forests burnt by three short-interval fires in the Alpine National Park, Victoria, Australia. Forest Ecology and Management 342, 39-48.
| Crossref | Google Scholar |
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378-400.
| Crossref | Google Scholar |
Brown CD, Johnstone JF (2012) Once burned, twice shy: repeat fires reduce seed availability and alter substrate constraints on Picea mariana regeneration. Forest Ecology and Management 266, 34-41.
| Crossref | Google Scholar |
Burrows ND, Wardell-Johnson G, Ward B (2008) Post-fire juvenile period of plants in south-west Australia forests and implications for fire management. Journal of the Royal Society of Western Australia 91, 163-174.
| Google Scholar |
Davis KT, Dobrowski SZ, Higuera PE, Holden ZA, Veblen TT, Rother MT, Parks SA, Sala A, Maneta MP (2019) Wildfires and climate change push low-elevation forests across a critical climate threshold for tree regeneration. Proceedings of the National Academy of Sciences 116, 6193-6198.
| Crossref | Google Scholar | PubMed |
Diffenbaugh NS, Field CB (2013) Changes in ecologically critical terrestrial climate conditions. Science 341, 486-492.
| Crossref | Google Scholar | PubMed |
Enright NJ, Goldblum D (1999) Demography of a non-sprouting and resprouting Hakea species (Proteaceae) in fire-prone Eucalyptus woodlands of southeastern Australia in relation to stand age, drought and disease. Plant Ecology 144, 71-82.
| Crossref | Google Scholar |
Enright NJ, Lamont BB, Marsula R (1996) Canopy seed bank dynamics and optimum fire regime for the highly serotinous shrub, Banksia hookeriana. Journal of Ecology 84, 9-17.
| Crossref | Google Scholar |
Enright NJ, Marsula R, Lamont BB, Wissel C (1998) The ecological significance of canopy seed storage in fire-prone environments: a model for non-sprouting shrubs. Journal of Ecology 86, 946-959.
| Crossref | Google Scholar |
Enright NJ, Fontaine JB, Bowman DM, Bradstock RA, Williams RJ (2015) Interval squeeze: altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Frontiers in Ecology and the Environment 13, 265-272.
| Crossref | Google Scholar |
Espelta JM, Verkaik I, Eugenio M, Lloret F (2008) Recurrent wildfires constrain long-term reproduction ability in Pinus halepensis Mill. International Journal of Wildland Fire 17, 579.
| Crossref | Google Scholar |
Gill A, McMahon A (1986) A postfire chronosequence of cone, follicle and seed production in Banksia ornata. Australian Journal of Botany 34, 425-433.
| Crossref | Google Scholar |
Gosper CR, Miller BP, Gallagher RV, Kinloch J, van Dongen R, Adams E, Barrett S, Cochrane A, Comer S, McCaw L, Miller RG, Prober SM, Yates CJ (2022) Mapping risk to plant populations from short fire intervals via relationships between maturation period and environmental productivity. Plant Ecology 223, 769-787.
| Crossref | Google Scholar |
Hansen WD, Turner MG (2019) Origins of abrupt change? Postfire subalpine conifer regeneration declines nonlinearly with warming and drying. Ecological Monographs 89, e01340.
| Crossref | Google Scholar |
Hartig F (2021) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.0. Available at https://CRAN.R-project.org/package=DHARMa
Harvey BJ, Donato DC, Turner MG (2016) High and dry: post-fire tree seedling establishment in subalpine forests decreases with post-fire drought and large stand-replacing burn patches. Global Ecology and Biogeography 25, 655-669.
| Crossref | Google Scholar |
Head L, Adams M, McGregor HV, Stephanie T (2014) Climate change and Australia. WIREs Climate Change 5, 175-197.
| Crossref | Google Scholar |
Iler AM, CaraDonna PJ, Forrest JRK, Post E (2021) Demographic consequences of phenological shifts in response to climate change. Annual Review of Ecology, Evolution, and Systematics 52, 221-245.
| Crossref | Google Scholar |
IPCC (2022) Climate change 2022: impacts, adaptation, and vulnerability. In ‘Contribution of Working Group II to the Sixth Assessment of the Intergovernmental Panel on Climate Change’. (Eds H-O Pörtner, DC Roberts, M Tignor, ES Poloczanska, K Mintenbeck, A Alegría, M Craig, S Langsdorf, S Löschke, V Möller, A Okem, B Rama). PP 37–118. (Cambridge University Press)
Jolly WM, Cochrane MA, Freeborn PH, Holden ZA, Brown TJ, Williamson GJ, Bowman DM (2015) Climate-induced variations in global wildfire danger from 1979 to 2013. Nature Communications 6, 7537.
| Crossref | Google Scholar | PubMed |
Jones MW, Abatzoglou JT, Veraverbeke S, Andela N, Lasslop G, Forkel M, Smith AJP, Burton C, Betts RA, Van Der Werf GR, Sitch S, Canadell JG, Santín C, Kolden C, Doerr SH, Le Quéré C (2022) Global and regional trends and drivers of fire under climate change. Reviews of Geophysics 60, e2020RG000726.
| Crossref | Google Scholar |
Kassambara A (2020) ggpubr: “ggplot2” Based Publication Ready Plots. R package version 0.4.0. Available at https://CRAN.R-project.org/package=ggpubr
Keeley JE, Ne’eman G, Fotheringham CJ (1999) Immaturity risk in a fire-dependent pine. Journal of Mediterranean Ecology 1, 41-48.
| Google Scholar |
Keith D, Lindenmayer D, Lowe A, Russell-Smith J, Barrett S, Enright N, Fox B, Guerin G, Paton D, Tozer M, Yates C (2014) Heathlands. In ‘Biodiversity and environmental change: monitoring, challenges and direction’. (Eds D Lindenmayer, E Burns, N Thurgate, A Lowe) pp. 283–334. (CSIRO Publishing: Melbourne, Australia)
Kraaij T, Baard JA, Cowling RM, van Wilgen BW, Das S (2013) Historical fire regimes in a poorly understood, fire-prone ecosystem: eastern coastal fynbos. International Journal of Wildland Fire 22, 277.
| Crossref | Google Scholar |
Kroiss SJ, HilleRisLambers J (2015) Recruitment limitation of long-lived conifers: implications for climate change responses. Ecology 96, 1286-1297.
| Crossref | Google Scholar | PubMed |
Lüdecke D (2018) ggeffects: tidy data frames of marginal effects from regression models. Journal of Open Source Software 3, 772.
| Crossref | Google Scholar |
Morgan JW, McCarthy MA, Willocks E (2021) Does intraspecific variation in demography have implications for fire management of an obligate-seeder shrub across its geographic range? Austral Ecology 46, 315-323.
| Crossref | Google Scholar |
Moritz MA, Parisien M-A, Batllori E, Krawchuk MA, Dorn JV, Ganz DJ, Hayhoe K (2012) Climate change and disruptions to global fire activity. Ecosphere 3, art49.
| Crossref | Google Scholar |
Selwood KE, McGeoch MA, MacNally R (2015) The effects of climate change and land‐use change on demographic rates and population viability. Biological Reviews 90, 837-853.
| Crossref | Google Scholar | PubMed |
Solt F, Hu Y (2024) dotwhisker: Dot-and-Whisker Plots of Regression Results. Available at the Comprehensive R archive Network (CRAN). https://cran.r-project.org/
Stevens-Rumann CS, Kemp KB, Higuera PE, Harvey BJ, Rother MT, Donato DC, Morgan P, Veblen TT (2018) Evidence for declining forest resilience to wildfires under climate change. Ecology Letters 21, 243-252.
| Crossref | Google Scholar | PubMed |
Turner MG, Braziunas KH, Hansen WD, Harvey BJ (2019) Short-interval severe fire erodes the resilience of subalpine lodgepole pine forests. Proceedings of the National Academy of Sciences 116, 11319-11328.
| Crossref | Google Scholar | PubMed |
Vicente-Serrano SM, Lopez-Moreno J-I, Beguería S, Lorenzo-Lacruz J, Sanchez-Lorenzo A, García-Ruiz JM, Azorin-Molina C, Morán-Tejeda E, Revuelto J, Trigo R, Coelho F, Espejo F (2014) Evidence of increasing drought severity caused by temperature rise in southern Europe. Environmental Research Letters 9, 044001.
| Crossref | Google Scholar |
von Takach Dukai B, Lindenmayer DB, Banks SC (2018) Environmental influences on growth and reproductive maturation of a keystone forest tree: Implications for obligate seeder susceptibility to frequent fire. Forest Ecology and Management 411, 108-119.
| Crossref | Google Scholar |
Whitman E, Parisien M-A, Thompson DK, Flannigan MD (2019) Short-interval wildfire and drought overwhelm boreal forest resilience. Scientific Reports 9, 18796.
| Crossref | Google Scholar | PubMed |
Xu T, Hutchinson MF (2013) New developments and applications in the ANUCLIM spatial climatic and bioclimatic modelling package. Environmental Modelling & Software 40, 267-279.
| Crossref | Google Scholar |


