Sagebrush steppe recovery after fire varies by development phase of Juniperus occidentalis woodland
Jonathan D. Bates A C , Robert N. Sharp B and Kirk W. Davies AA United States Department of Agriculture, Agricultural Research Service, Eastern Oregon Agricultural Research Center, Burns, OR 97720, USA.
B United States Department of Interior, Bureau of Land Management, Burns District Office, Burns, OR 97720, USA.
C Corresponding author. Email: jon.bates@oregonstate.edu
International Journal of Wildland Fire 23(1) 117-130 https://doi.org/10.1071/WF12206
Submitted: 4 December 2012 Accepted: 19 June 2013 Published: 10 September 2013
Abstract
Woodland ecosystems of the world have been changed by land use demands, altered fire regimes, invasive species and climate change. Reduced fire frequency is recognised as a main causative agent for Pinus–Juniperus L. (piñon–juniper) expansion in North American woodlands. Piñon–juniper control measures, including prescribed fire, are increasingly employed to restore sagebrush steppe communities. We compared vegetation recovery following prescribed fire on Phase 2 (mid-succession) and Phase 3 (late-succession) Juniperus occidentalis Hook. (western juniper) woodlands in Oregon. The herbaceous layer on Phase 2 sites was comprised of native perennial and annual vegetation before and after fire. On Phase 3 sites the herbaceous layer shifted from native species to dominance by invasive Bromus tectorum L. (cheatgrass). After fire, shrubs on Phase 2 sites were comprised of sprouting species and Ceanothus velutinus Dougl. (snowbrush). On Phase 3 woodland sites the shrub layer was dominated by C. velutinus. The results suggest that Phase 2 sites have a greater likelihood of recovery to native vegetation after fire and indicate that sites transitioning from Phase 2 to Phase 3 woodlands cross a recovery threshold where there is a greater potential for invasive weeds, rather than native vegetation, to dominate after fire.
Additional keywords: Artemisia tridentata, Bromus tectorum, Great Basin, mountain big sagebrush, state-and-transition, threshold.
Introduction
Woodland ecosystems have undergone substantial change in most regions of the world as a result of land use demands, herbivore effects, altered fire regimes, invasive species and climate change. Woodlands are categorised as those being reduced or degraded (Zerihun and Backleus 1991; Yates and Hobbs 1997; Bucher and Huszar 1999; Angassa and Baars 2000; Breshears et al. 2005) and those that have expanded in range and in-filled (Brown and Archer 1989; Macdonald and Wissel 1992; Miller and Rose 1995; Holmes and Cowling 1997; Van Auken 2000; Ansley et al. 2001). Reduced fire frequency is recognised as a main causative agent for woodland expansion in North American woodlands (Brown and Archer 1989; Archer 1994; Miller and Wigand 1994). Where woodlands have expanded and in-filled, active management using fire or mechanical treatments has been employed to kill trees to maintain or restore grassland and shrubland ecosystems (Burrows et al. 1990; Angassa 2002; Owens et al. 2002; Smit 2004; Miller et al. 2005; Peterson et al. 2007; Teague et al. 2010).
In the western United States expansion and in-filling of Pinus–Juniperus L. (piñon–juniper) has caused widespread conversion of big sagebrush steppe (Artemisia tridentata Nutt.) to coniferous woodland. A main cause of piñon–juniper expansion has been a lack of fire, a likely consequence of the grazing of fine fuels by livestock and, since the 1940s, more effective fire suppression (Burkhardt and Tisdale 1976; Miller and Rose 1999; Soulé et al. 2004; Miller et al. 2008). Pre-settlement mean fire return intervals (MFRI) in mountain big sagebrush steppe (A. t. Nutt. ssp. vaseyana (Rydb.) Beetle), a main area of piñon–juniper expansion, are estimated to have been <20 to <100 years (Miller et al. 2005, 2008; Miller and Heyerdahl 2008). Woodland development has a range of adverse effects on structural and functional properties of A. t. ssp. vaseyana steppe communities, including increased soil erosion and reduced water infiltration (Buckhouse and Mattison 1980; Reid et al. 1999; Pierson et al. 2007; Petersen et al. 2009), loss of steppe wildlife habitat (Schaefer et al. 2003; Noson et al. 2006; Reinkensmeyer et al. 2007), elimination of the shrub layer (Miller et al. 2000) and reduced herbaceous diversity and productivity (Clary and Jameson 1981; Bates et al. 2005, 2006, 2011). Thus, woodland control using fire or mechanical treatments to maintain or restore A. t. ssp. vaseyana steppe has been a major restoration focus in the western United States.
However, forecasting vegetation recovery following prescribed fire appears to become less predictable as piñon–juniper woodlands develop (Miller et al. 2008). Woodland development varies across landscapes (Johnson and Miller 2006) and has been categorised into three phases (Miller et al. 2005, 2008): in Phase 1 woodlands, shrubs and herbaceous species are the dominant vegetation with few trees present; in Phase 2 woodlands, trees co-dominate with shrubs and herbaceous plants; and in Phase 3 woodlands, trees are dominant and shrubs and herbaceous layers are reduced. The transition from Phase 2 to Phase 3 woodlands causes a shift from shrub and herbaceous fuels to a predominance of tree canopy fuels, which influences fire behaviour and severity (Tausch 1999; Miller et al. 2008; Dicus et al. 2009; Romme et al. 2009). The increase in canopy fuels generates fires of greater severity than under the historic regime, and results in post-fire weed dominance because of high mortality of herbaceous perennials (Tausch 1999; Bates et al. 2006, 2011; Condon et al. 2011). This indicates that many Phase 3 woodlands may have crossed a threshold, where natural recovery is uncertain and additional inputs, seeding and weed control may be required to restore A. t. ssp. vaseyana steppe communities.
The ability to forecast vegetation succession and dynamics as a result of management actions or natural disturbance events is central to natural resource professionals. To assist in predicting potential vegetation changes and identifying the driving factors, state-and-transition models (STMs), proposed by Westoby et al. (1989), have been increasingly refined and employed in ecological research. In the United States federal land agencies have accepted STMs for vegetation and habitat management where they serve as integral parts in the development of ecological site descriptions (ESDs) (Papanastasis and Chouvardas 2005; Chartier and Rostagno 2006; Briske et al. 2008; Petersen et al. 2009; Holmes and Miller 2010). STMs describe alternative plant community states and community transitions resulting from disturbance or management, for related vegetation associations or ESDs that can occur over time. Thus, ecological sites support multiple states or phases, comparable to successional or seral stages. Changes in disturbance regimes and introduction of exotic species can cause plant communities to decline in resilience and become unstable, with the potential for crossing a threshold to a new state(s) that differs in plant composition, structure and function. Once a threshold is crossed, a return to the former state is often difficult because of changes in species composition and site attributes (Westoby et al. 1989; Laycock 1991; Briske et al. 2008).
The development of threshold and resilience concepts and STMs for categorising woodland expansion and evaluating natural disturbances or management applications has been a building process since the late 1980s (Archer 1994; Milton et al. 1994; Briske et al. 2008; Petersen et al. 2009). Several detailed STMs have been proposed for describing the expansion of J. occidentalis Hook. (western juniper) woodlands (Miller et al. 2005; Briske et al. 2008; Petersen et al. 2009). Miller et al. (2005) developed STMs for A. t. ssp. vaseyana steppe with increasing western juniper dominance including multiple transitions and thresholds. However, the point at which thresholds are crossed during woodland development is yet to be specifically identified or tested (Briske et al. 2008). Identifying thresholds is important for applying appropriate methods of piñon–juniper control to recover A. t. ssp. vaseyana steppe vegetation. Information on vegetation recovery following prescribed fire treatments remains limited and there have been few integrated studies comparing A. t. ssp. vaseyana steppe recovery after fire among different phases in expanding J. occidentalis and piñon–juniper woodlands.
Our objective was to compare recovery of mountain big A. t. ssp. vaseyana steppe after prescribed fire in Phase 2 and Phase 3 J. occidentalis woodlands. We hypothesised that recovery of herbaceous and shrub species would occur earlier after fire in Phase 2 than in Phase 3 sites because initial shrub and herbaceous cover and densities are often greater in Phase 2 than Phase 3 woodlands (Miller et al. 2000), and because fire may result in high mortality of these life forms in Phase 3 woodlands (Bates et al. 2006, 2011). Because of lower levels of herbaceous cover and density, we expected there would be a greater potential for invasive weeds to increase after treating Phase 3 woodlands. One of our goals was to propose a revised STM for J. occidentalis woodlands based on woodland phases if warranted by our results.
Methods
Study area and treatment
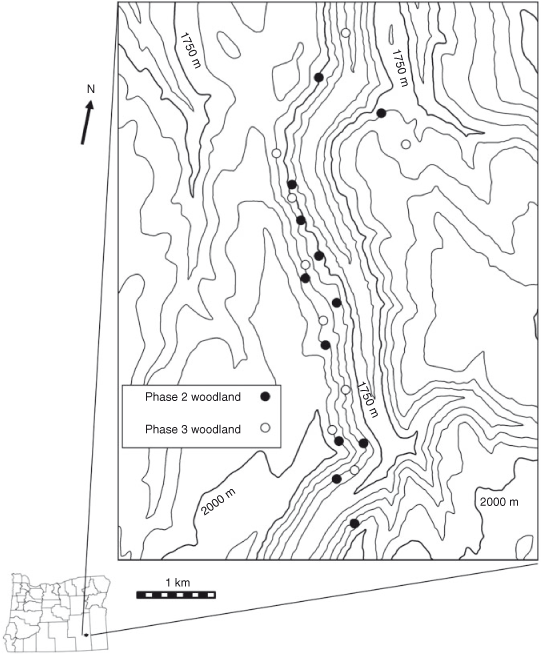
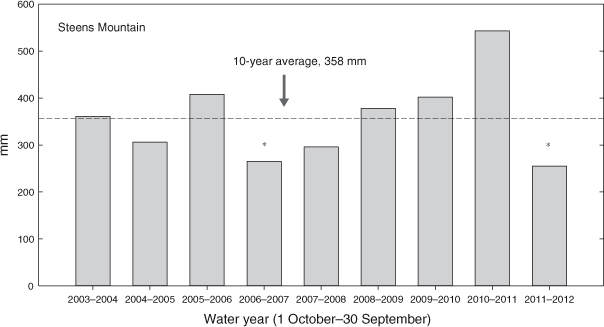
The study was located in Kiger Canyon, Steens Mountain, south-eastern Oregon (45°54′N, 118°40′W). Elevation of the study sites ranged from 1700 to 1990 m, and aspects were from east to north. The ecological sites (ESD) were Loamy (12–16 Precipitation Zone, PZ (304–406 mm)) and Deep Loamy (12–16 PZ) (NRCS 2010a) and all study sites were A. t. ssp. vaseyana–Festuca idahoensis Elmer (Idaho fescue) plant associations. Soils at the sites are a complex of Westbutte–Lambring (Loamy-skeletal, mixed, frigid Pachic Haploxerolls) series formed in residuum and colluvium derived from basalt, andesite, rhyolite and welded tuff and are moderately deep and well drained (NRCS 2006). Twelve Phase 2 and nine Phase 3, 0.63-ha plots were established in May 2003. Criteria for determining woodland phase (cover of herbaceous, shrub and tree life forms) were taken from Miller et al. (2000, 2005). Phase 2 and Phase 3 woodlands were intermixed within an area of 15 km2 and were independent of each other (Fig. 1). Nine of the Phase 2 plots were located adjacent to Phase 3 woodland plots, with others located randomly within the study area. Phase 2 woodland sites had greater initial cover and density of herbaceous and shrub species than did Phase 3 woodland sites. Herbaceous cover was 2.5 times, and shrub cover 3.5 times, greater in the Phase 2 woodlands (19.0 ± 1.0%; 19.7 ± 1.9%) than in Phase 3 woodlands (7.4 ± 1.2%; 5.5 ± 1.6%). Bromus tectorum L. (cheatgrass) was present in trace amounts on half of the sites for each woodland phase. Cover and density of J. occidentalis were 1.8 times and 1.5 times greater in the Phase 3 (47.0 ± 1.3%; 240 ± 25 trees ha–1) than in the Phase 2 woodlands (25.2 ± 1.8%; 168 ± 21 trees ha–1). The closest weather station, located at the Otley Brothers Ranch (1550-m elevation), is 7.2–11.3 km north-west of the sites. Annual precipitation (1 October–30 September) averaged 386 mm in the 10 years (Fig. 2).
|
|
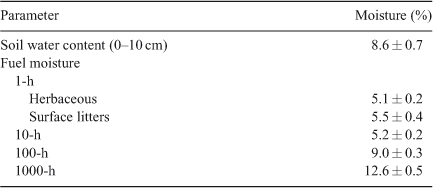
Cutting involved felling ~1/3 of the dominant and sub-canopy J. occidentalis trees (>3 m tall) and cutting was evenly distributed in stands. Trees were cut May–June 2003 and dried over the summer. On Phase 2 woodlands, an average of 47 ± 8 trees (range 8–23) were cut per hectare. On Phase 3 woodlands, an average of 71 ± 9 trees (range 43–110) were cut per hectare. Fall (autumn) burning was applied on 6 October 2003 by personnel of the Bureau of Land Management, Burns District. The prescribed fire technique used was a spot head fire using a heli-torch. Weather conditions were typical for fall burning in the northern Great Basin. Air temperatures were 18–26°C, relative humidity was 20–28% and winds were from the north-west at 6–15 km h–1. Soil water content (0–10 cm) and fuel moisture for 1-, 10-, 100- and 1000-h fuels were measured the day before fire application and were determined by drying samples at 100°C to a constant weight (Table 1). Recovery depended on natural succession and no post-fire seeding was undertaken. Livestock were excluded for 2 years before burning to increase fine fuel loads. Cattle grazed (<10% estimated utilisation) the area in late summer (August) following the herbaceous growing season in 2004, 2007 and 2008. The area was grazed moderately June–July (35–50% estimated utilisation) in 2010 and 2011.
|
Measurements
Vegetation characteristics were measured in June (2003–2007, 2009) and July (2012). On each plot, five 50-m transects were permanently established with transects spaced 25 m apart. Canopy cover of J. occidentalis and shrubs were estimated by line intercept along transects (Canfield 1941). Density of mature J. occidentalis (>2-m height) was estimated by counting individuals inside five 6 × 50-m belt transects. Density of shrubs and juvenile J. occidentalis (<2-m height) were estimated by counting all plants inside five, 2 × 50-m belt transects. Herbaceous canopy cover (perennials, annuals) and herbaceous perennial density were measured by species inside 0.2-m2 frames (0.4 × 0.5 m). Frames were placed every 2 m along transects. Scientific nomenclature follows the Natural Resource Conservation Service Plant Database (NRCS 2010b).
Statistical analysis
Repeated-measures analysis of variance for a completely randomised design using a mixed model (PROC MIX; SAS Institute Inc., Cary, NC) was used to test for year, woodland phase and year-by-phase interaction for herbaceous, shrub and J. occidentalis response variables. Because the study lacks woodland controls the design does not permit separation of interannual variation, thus comparisons made between pre- and post-treatment response variables should be interpreted with caution. However, the response to the treatments in this study followed patterns similar to those measured in other studies comparing vegetation dynamics between treated and untreated woodlands (controls). These studies indicate that total herbaceous and life form cover typically increases within the first 2–3 years following cutting or burning of western juniper woodlands (Vaitkus and Eddleman 1987; Rose and Eddleman 1994; Bates et al. 2000, 2005, 2006, 2011).
Response variables were J. occidentalis cover and density, shrub cover and density (species), herbaceous cover (species and life form, bare ground and surface litter) and herbaceous density (species and life form). Herbaceous life forms were grouped as Poa secunda Vasey (Sandberg’s bluegrass), perennial bunchgrasses (e.g. F. idahoensis, B. marginatus Nees ex Steud. (mountain brome), Achnatherum lemmon2 (Vasey) Barkworth (Lemmon’s needlegrass), Elymus elymoides (Raf.) Swezey (bottlebrush squirreltail)), B. tectorum, perennial forbs and annual forbs. Poa secunda was treated as a separate functional group from other perennial grasses because its phenological development occurs earlier (Link et al. 1990; Davies 2008). An auto regressive order one covariance structure was used as it provided the best fit for data analysis (Littell et al. 1996). The models included year (d.f. = 6), phase (d.f. = 1) and year-by-phase interaction (d.f. = 6; with the error term d.f. = 92). Mean separation involved comparison of least-squares using the LSMEANS statement (SAS Institute Inc., Cary, NC). All data were tested for normality using the Shapiro–Wilk test (Shapiro and Wilk 1965) and were log-transformed before analysis when necessary. Significant interactions were followed by tests of simple effects at α = 0.05.
Results
Juniper control and ground cover
The prescribed fires killed remaining uncut J. occidentalis trees in both Phase 2 and 3 woodland sites. Surface litters (herbaceous and juniper needles) were fully consumed by the fire, and shrubs were burned to the soil surface. Felled J. occidentalis were completely consumed or only trunks remained, indicating that all fuels up to and including 1000-h fuels were removed.
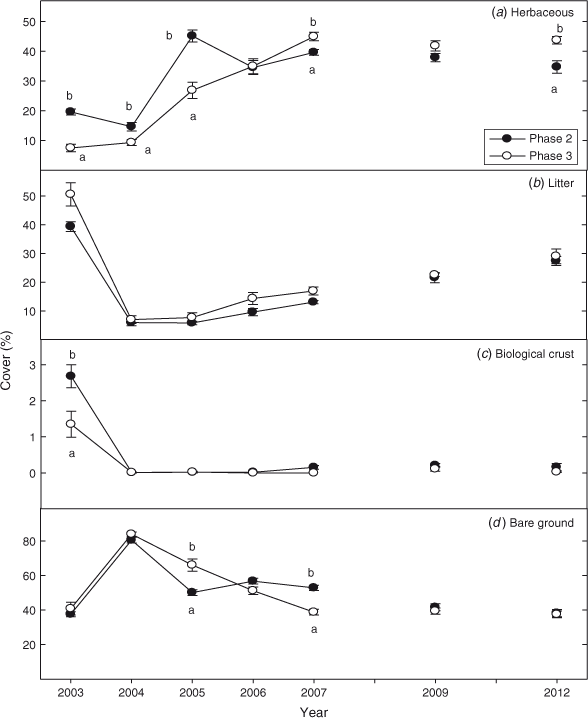
Pre-burn herbaceous cover was ~2.5 times greater in the Phase 2 than the Phase 3 sites (Fig. 3a; P < 0.0001). For the first 2 years after fire (2004–2005) herbaceous cover was 50% higher in Phase 2 than Phase 3 sites (P < 0.0001) but by 2007 (and also in 2009 and 2012) these differences had largely disappeared. Litter cover declined by 80% in the first year after fire in Phase 2 and 3 woodlands (Fig. 3b; P < 0.0001). Nine years after fire, litter was respectively 25 and 30% below pre-burn levels in Phase 2 and Phase 3 woodlands. Biological crust (moss, lichen) cover was 2 times greater in Phase 2 than Phase 3 sites before fire (Fig. 3c; P < 0.0001). The fires eliminated biological crust and there was no recovery 9 years after fire. Bare ground doubled in Phase 3 and in Phase 2 sites the year after fire (Fig. 3d; P < 0.0001). Bare ground returned to pre-burn levels respectively on Phase 2 and Phase 3 sites the sixth year (2009) and fourth year (2007) after fire.
|
Life form cover and density
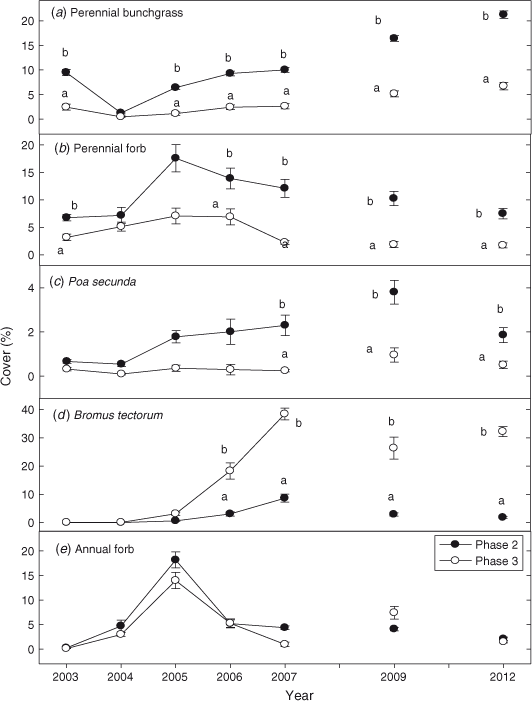
Prior to fire, perennial grass cover was 4 times (P < 0.0001) greater in Phase 2 sites than in Phase 3 sites (Fig. 4a). The first year after fire perennial grass cover decreased by 85% in Phase 2 sites and by 75% in the Phase 3 sites. Perennial grass cover returned to pre-burn levels the third year after fire and exceeded pre-burn levels the sixth year after fire in both Phase 2 and Phase 3 sites. From 2004 to 2012 perennial grass cover was 3–6 times greater (P < 0.0001) in Phase 2 than in Phase 3 sites. Perennial forb cover was 2 times (P < 0.0001) greater in Phase 2 than in Phase 3 sites before treatment (Fig. 4b). After fire, perennial forb cover was 2–10 times greater (P < 0.001) in Phase 2 than in Phase 3 sites. Cover of P. secunda was 3–7 times greater in Phase 2 sites after fire (Fig. 4c; P = 0.003). Bromus tectorum, present in trace amounts before treatment in both woodland phases, increased significantly after fire (Fig. 4d; P < 0.0001). Bromus tectorum cover was 4–16 times greater in the Phase 3 than the Phase 2 sites, in 2006–2012 (P < 0.0001). In Phase 2 and Phase 3 sites, annual forb cover increased from <1 to 16% and <1 to 20% by the second year post-fire (Fig. 4e; P < 0.0001). In subsequent years (2005–2012), annual forb cover decreased to less than 4% cover on average in Phase 2 and Phase 3 sites.
|
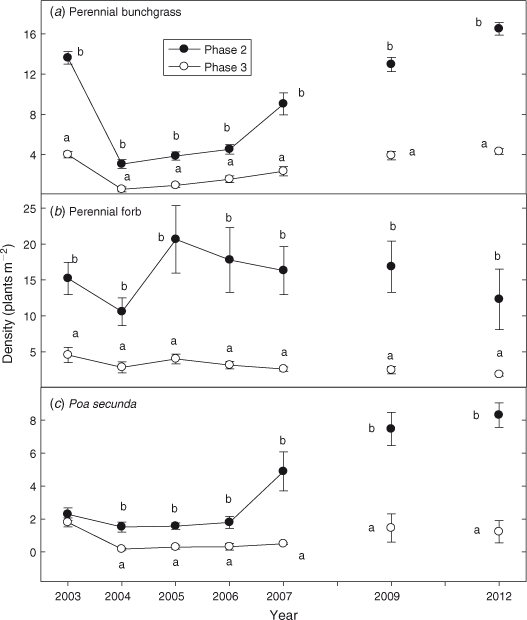
Before fire, perennial grass density was 3 times greater in Phase 2 than in Phase 3 sites (Fig. 5a; P < 0.0001). Burning decreased perennial grass density by 78% in the Phase 2 sites, from ~14 to 2–3 plants m–2. Phase 3 sites showed a decline of 95% in perennial grass density, from ~4 to < 1 plants m–2 (P = 0.004). Perennial grass densities have increased in both phases since fire, but from 2005 to 2012 densities were 4–5 times greater in the Phase 2 sites (P < 0.0001). Densities of perennial forbs were 4–5 times greater in the Phase 2 than Phase 3 sites after fire (Fig. 5b; P = 0.002). Density of P. secunda did not differ between phases before burning (Fig. 5c; P = 0.068) but after fire densities were 4–7 times greater in Phase 2 sites (P < 0.0001).
|
Shrub cover and density
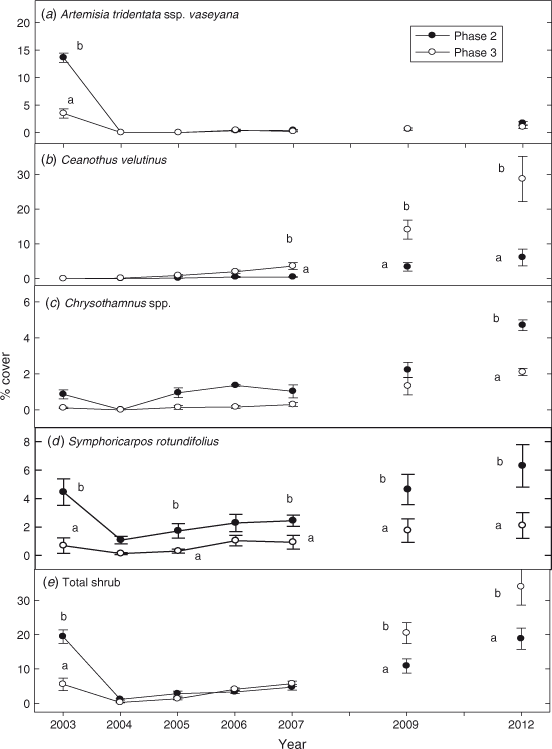
Cover of A. t. ssp. vaseyana was approximately 3 times greater on Phase 2 than Phase 3 sites before fire (P < 0.0001). A. t. ssp. vaseyana was eliminated by the fires and cover was reduced to zero in both phases (Fig. 6a). Cover of A. t. ssp. vaseyana on sites of both phases was ~1.5% in 2012 and was 75–85% below pre-burn levels. Ceanothus velutinus Dougl. (snowbrush), not present on either woodland phase before the fire, established after fire, particularly on the Phase 3 sites where it comprised 75% of total shrub cover by 2012 (P < 0.0001; Fig. 6b). Cover of C. velutinus was ~5 times greater on the Phase 3 than Phase 2 sites in 2012 (P < 0.0001). Cover of Chrysothamnus Nutt. (rabbitbrush) spp. was not affected by fire as plants rapidly re-sprouted the year after burning (Fig. 6c). Cover of Chrysothamnus spp. increased in both phase sites after fire and exceeded pre-burn levels by 2009, the sixth year after fire (P = 0.002). Chrysothamnus spp. comprised ~30 and 15% of total shrub cover in Phase 2 and Phase 3 in 2012. Cover of Symphoricarpos rotundifolius var. rotundifolius Gary (western snowberry) returned to pre-burn levels the fourth year after fire (2006) in Phase 2 sites and the third year after fire in Phase 3 sites (P = 0.0005; Fig. 6d). Cover of S. rotundifolius averaged approximately 3 times greater in Phase 2 sites than Phase 3 sites, from 2004 to 2012 (P < 0.0001). Ribes cereum Dougl. (wax currant) increased in both sites after fire, but was greater in Phase 3 than Phase 2 sites in 2012 (P = 0.006). Cover of other shrub species, Rosa woodsii Lindl. (Wood’s rose), Berberis repens Lindl. (Oregon grape), Prunus emarginata var. emarginata Dougl. (bitter cherry), Sambucus mexicana JPresl. (blue elderberry) and Amelanchier utahensis Koehne (serviceberry) (which accounted for <0 0.1% of total shrub cover), did not differ between phases or across years. Total shrub cover was 4 times greater in Phase 2 than Phase 3 sites before fire (Fig. 6e; P < 0.0001). After fire total shrub cover did not differ between phases until 2009 and 2012, when cover was ~2 times greater in the Phase 3 sites (P < 0.0001).
|
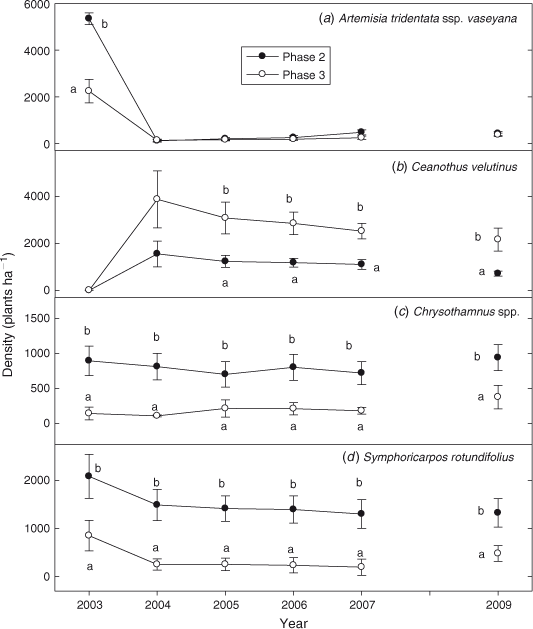
Density of A. t. ssp. vaseyana was 2.5 times greater on Phase 2 sites before burning (Fig. 7a; P < 0.0001). Because fire eliminated A. t. ssp. vaseyana there were no differences in densities between phases (P = 0.369). Ceanothus velutinus was the only species to increase in density after fire (P < 0.0001). The increase was 2–3 times greater in Phase 3 compared with Phase 2 sites between 2004 and 2012 (Fig. 7b; P = 0.001). Density of Chrysothamnus spp. increased after fire and was 4–10 times greater on Phase 2 than Phase 3 sites (Fig. 7c; P < 0.0001). S. rotundifolius density decreased by 32% in Phase 2 and 76% in Phase 3 sites after fire (Fig. 7d; P = 0.019). However, density of S. rotundifolius was 2–6 times greater in Phase 2 than Phase 3 sites (P < 0.0001). Ribes cereum density increased after fire, and was greater in Phase 3 (282 ± 56 plants ha–1) than Phase 2 (52 ± 12 plants ha–1) sites in 2012 (P < 0.0001).
|
Discussion
The combination of cutting and prescribed fire in two different phases of woodland development provided a distinct contrast in herbaceous and shrub recovery in J. occidentalis-invaded sagebrush steppe. The herbaceous layer on Phase 2 sites was dominated by native perennial and annual vegetation after fire. On Phase 3 sites the herbaceous layer shifted from native species to dominance by B. tectorum after fire. The shrub layer shifted from primarily A. t. spp. vaseyana to C. velutinus on Phase 3 sites and to an approximately equal mix of Chrysothamnus spp., S. rotundifolius and C. velutinus on Phase 2 sites. Artemisia t. ssp. vaseyana steppe recovery following fire in piñon–juniper woodlands often varies in composition and rate, as a consequence of differing fire extent and severities, seed source, abundances and competitive abilities of native and exotic species, ecological site characteristics, herbivory and environmental variation (Barney and Frischknecht 1974; Koniak 1985; Chambers et al. 2007; Rau et al. 2008; Ziegenhagen and Miller 2009; Bates et al. 2011). Our results suggest that piñon–juniper woodland phase influences post-fire recovery in the A. t. ssp. vaseyana steppe ecosystem and indicates that Phase 2 woodland sites have a greater likelihood than do Phase 3 woodlands, of recovery to A. t. ssp. vaseyana-steppe vegetation following fall prescribed fire.
Herbaceous and ground cover response
The first two years after fire herbaceous recovery was mainly comprised of perennial and annual forbs on both burned woodland phases. This successional stage is typical following fire in piñon–juniper woodlands (Barney and Frischknecht 1974; Koniak 1985; Bates et al. 2011). However, by the third year after fire, vegetation succession had diverged between phases, with B. tectorum dominating Phase 3 sites and herbaceous perennials dominating Phase 2 sites. Although perennial grasses on Phase 3 sites had returned to pre-burn levels of cover and density respectively by the fourth and sixth year after fire, this recovery was not sufficient to prevent B. tectorum dominance. Increasingly, experimental evidence indicates that the resilience of A. t. ssp. vaseyana steppe communities following fire recovery is dependent on the persistence of sufficient density of herbaceous perennial vegetation (Bates et al. 2006, 2011; Condon et al. 2011). The Phase 2 sites, after a 75% reduction in perennial grass density, retained 2–3 plants m–2 the year following fire. This level of perennial grasses was adequate for density to recover 4 years after fire and, in combination with higher densities of perennial forbs than that on Phase 3 sites, likely limited B. tectorum increases on Phase 2 sites. Others have indicated that greater presence and recovery of perennial herbaceous vegetation prevents annual grasses from dominating after fire in sagebrush steppe (Chambers et al. 2007; Davies et al. 2008; Bates and Svejcar 2009; Bates et al. 2011; Condon et al. 2011). Another element that may have supported native plant recovery on the Phase 2 sites was, potentially, a more complete soil seed bank. Koniak and Everett (1982) recorded greater seed numbers and diversity of soil seed banks in younger (Phase 1 and 2) than older (Phase 3) piñon–juniper woodlands.
The effects of fires on the understorey and early succession on Phase 3 sites were similar to those seen after high intensity and severity fires in forest and woodland ecosystems of the western United States and Canada (Tausch 1999; Brown and Smith 2000; Bauer and Weisberg 2009). High intensity wildfires in piñon–juniper woodlands of Nevada and Utah have resulted in post-fire dominance of B. tectorum and exotic weeds because of a lack of native herbaceous perennial species (Tausch 1999). Dhaemers (2006) and Condon et al. (2011) established that B. tectorum cover after fire was positively associated with pre-fire piñon–juniper cover and negatively associated with recovery of herbaceous perennials. In P. tremuloides communities invaded by J. occidentalis (Phase 3 woodlands), early-fall prescribed fire killed almost all perennial grasses, reduced perennial forbs by 60% and was followed by invasive weed dominance (Bates et al. 2006). In P. ponderosa Dougl. (ponderosa pine) forest perennial grass cover decreased and invasive species increased as fire intensity and litter consumption increased (Armour et al. 1984; Griffis et al. 2001; Bataineh et al. 2006; Sabo et al. 2009).
However, not all Phase 3 J. occidentalis woodlands that burn have responded with loss of desired perennial vegetation. In Phase 3 J. occidentalis woodlands in south-west Idaho, post-burn early succession was dominated by native forbs followed by recovery of perennial grasses 3 years after fire, despite presence of B. tectorum (Bates et al. 2011). In the study of Bates et al. (2011), where natives dominated post-fire recovery, densities of perennial grasses (0.7–2 plants m–2) and forbs (5–25 plants m–2) were greater the first year post-fire than in our study. Phase 3 sites where B. tectorum dominated after fire (Bates et al. 2006), including those in our study, had low post-fire densities of perennial grasses (<0.3–0.6 plants m–2) and forbs (<5 plants m–2).
One question, which can only be answered following extended monitoring, is whether dominance by B. tectorum is a temporary or permanent feature on the burned Phase 3 woodlands. In our region, B. tectorum is typically of concern in drier A. t. ssp. wyomingensis Beetle & Young (Wyoming big sagebrush) plant communities and sites with mesic soil temperature regimes (Miller et al. 2008; NRCS 2010a). The ecological sites in this study have frigid soil temperature regimes and are A. t. ssp. vaseyana plant communities where native perennials typically should have a competitive advantage over B. tectorum (Chambers et al. 2007). Despite dominance by B. tectorum, perennial grass density and cover continued to increase after fire. Should this trend continue, native species may, over a longer period, replace B. tectorum. A concern with the current dominance by B tectorum is the potential for this species to alter the fire regime. Mean fire return intervals can shorten to as little as every 5 years as a result of B. tectorum dominance in Artemisia communities, which can limit recovery of native species (Whisenant 1990; Wisdom et al. 2005). However, recent calculations by Balch et al. (2013) indicate that fire return intervals for many cheatgrass areas may range between 49 and 78 years.
Shrub recovery
Recovery of shrubs on both woodland phase sites was characterised by species-specific adaptations to fire. Non-sprouting A. t. ssp. vaseyana, will take longer to recover or exceed pre-fire cover and density levels because re-establishment will depend on immigration of propagules from outside the burnt areas, and plants that emerged from seed after fire (Ziegenhagen and Miller 2009). Artemisia t. ssp. vaseyana that returned the first year after fire appear to have originated from the emergence from the seed bank. These new plants produced viable seed at 2 years of age as evidenced by seedlings establishing in close proximity to parent plants between 2009 and 2012. Recovery of A. t. ssp. vaseyana canopy cover has been reported to be between 20 and 40 years (Harniss and Murray 1973; Lesica et al. 2007; Ziegenhagen and Miller 2009). Cover and density of A. t. ssp. vaseyana in 2009 was approximately the same on Phase 2 and 3 sites, so we might expect that future recovery will be similar. However, Condon et al. (2011) indicated that re-establishment of A. tridentata following fire is positively related to cover of perennial herbaceous species. This relationship may influence the rate and magnitude of A. t. ssp. vaseyana recovery on the Phase 2 sites. To speed shrub recovery managers may consider seeding A. t. ssp. vaseyana following fire (Cox and Anderson 2004).
Sprouting shrub species, S. rotundifolius and Chrysothamnus spp., had either returned to or exceeded pre-fire cover by 2009. Typically, there is little fire-caused mortality and these species increase 3–5 years after fire (Anderson and Bailey 1979; Wright et al. 1979; Sieg and Wright 1996) and are important in recovery of shrub structure on A. t. ssp. vaseyana sites (Davies et al. 2012). However, response of S. rotundifolius can be variable and recovery may require up to 15 years or longer if ungulate browsing damages regrowth (Blaisdell 1953; Bartos et al. 1994). Severe fire can reduce sprouting (Young 1983), which may explain the slight decrease in S. rotundifolius densities in our study. The emergence of C. velutinus was likely due to its presence in the soil seed bank. Ceanothus velutinus seed remains viable in soils for periods exceeding 200 years and can appear after fire where it was previously not present (Steen 1966; Kramer and Johnson 1987; Halpern 1989; Bradley et al. 1991; Tonn et al. 2000). In our study, large C. velutinus patches developed with the highest densities and cover in the Phase 3 sites. As C. velutinus is a nitrogen (N) fixer (Mozingo 1987), it is possible that increased soil available N facilitated the increase in B. tectorum on Phase 3 sites. Nitrogen fixing plants, such as Lupinus argenteus, Pursh (silvery lupine), have the potential to influence plant succession in A. t. ssp. vaseyana steppe by modifying post-fire N availability (Goergen and Chambers 2009). However, B. tectorum cover did not appear to be greater in the presence of C. velutinus, hence we concluded that the lack of perennial herbaceous vegetation on the Phase 3 sites allowed post-fire B. tectorum dominance.
State and transition models
State-and-transition models for interpreting expanding piñon–juniper woodlands using fire are essential for woodland management and sagebrush steppe restoration. Our interpretation applies to the two ecological sites used in our study (Loamy (12–16 PZ, 304–406 mm) and Deep Loamy (12–16 PZ); NRCS 2010a) and potentially other A. t. ssp. vaseyana ESDs with similar site characteristics, xeric soil moisture and frigid soil temperature regimes. The model assumes that all states except the Annual Grass state retain an intact native understorey with few or no weeds present. Our results suggest that sites in Phase 1 and Phase 2 woodland states will likely recover native herbaceous species composition following fire disturbances. Fire in Phase 3 woodlands has the potential for creating several transitional events, with surviving perennial plant density and invasive species presence becoming the prime determinants of early successional composition. We suggest that native species composition will recover when perennial grass and forb densities respectively exceed 1 and 5 plants m–2, based on results from Bates et al. (2006, 2011). Sites with herbaceous values below these levels, as in our study, may have a greater chance of becoming dominated by invasive annual grasses following fire, indicating that a threshold may have been crossed. Our interpretation is marginal and suffers from a major drawback that is common to state-transition models in that they remain mainly observational and lack adequate predictive power. To acquire greater management utility for piñon–juniper woodlands, developing probabilities for transitional events, thresholds and outcomes is needed to refine STMs.
Management implications and conclusions
Piñon–juniper species continue to expand and in-fill woodlands as a result of reduced fire frequency and anthropogenic factors, and without active management the expansion will result in continued declines and loss of A. t. ssp. vaseyana steppe communities. It is currently estimated that 40% of piñon–juniper woodlands are Phase 1 and another 40% are Phase 2 (Miller et al. 2008). In 30–50 years, Phase 3 woodlands have the potential to increase from a current 20% to 75% of total woodland area (Johnson and Miller 2006). Because of increasing canopy fuel loading, the potential for more intense fires is likely to increase in Phase 3 woodlands (Tausch 1999; Miller et al. 2008). Thus, burning in Phase 3 woodlands is less predictable because of depleted understorey components and the potential for greater fire severity effects on herbaceous vegetation, which may encourage subsequent weed dominance (Bates et al. 2006; Bates et al. 2011; Condon et al. 2011). Phase 3 woodlands that are burned by wildfire or prescribed fire in fall are more likely to require additional inputs, primarily seeding and weed control, for vegetation recovery goals to be accomplished (Cox and Anderson 2004; Miller et al. 2005; Sheley and Bates 2008). Applying alternative treatments that have less severe effects than fall fire may potentially improve community recovery in Phase 3 woodlands. Cutting all trees on Phase 3 woodland sites has recovered herbaceous and shrub vegetation without weed dominance (Vaitkus and Eddleman 1987; Bates et al. 2005). Clear cutting followed by winter or early spring burning of juniper fuels has resulted in low understorey plant mortality and earlier recovery of sagebrush and native herbaceous species (Bates et al. 2006; Bates and Svejcar 2009).
Because control efforts in Phase 3 piñon–juniper woodlands are expensive (Miller et al. 2005) and fall burning offers less predictable results, managers involved with shrub–steppe restoration should give priority to treatment of Phase 1 and Phase 2 woodlands. Phase 1 and Phase 2 woodlands, which have an intact understorey of shrubs and herbaceous species, will most likely be dominated by native vegetation after fire as our study has demonstrated. Managers should expect that it will take several decades for A. t. ssp. vaseyana to recover following burning of Phase 1 and 2 woodlands; however, there is greater potential for achieving recovery goals and preventing woodland dominance by reintroducing fire in Phase 2 and earlier stages of piñon–juniper woodland development.
Acknowledgements
Many thanks are given to Fred Otley (Otley Brothers Ranch, Diamond, Oregon) and the Bureau of Land Management (Burns District) for providing logistics or properties for conducting the study. We recognise the many summer range technicians who assisted in the field and laboratory for the study: K. Adams, J. Anderson, C. Archuleta, G. Ash, J. (Crusher) Duchene, E. Ersch, K. Haile, A. Herdrich, J. Hobbs, J. Jackson, R. Kessler, C. Lehman, K. (Spike) Mumm, R. O’Connor, E. O’Connor, J. Jackson, J. Price, K. Price, J. Pyrse, K. Ralston, E. (Edna) Rhodes, B. Smith, J. (Trapper) Svejcar, M. Villagrana, C. Williams and D. Zvirdin. Many thanks to range technicians Georjanna Pokorney and Lori Ziegenhagen who assisted in the initial stages of the study. We thank Jay Kerby, Dr Rick Miller and Dr Jeanne Chambers, and anonymous reviewers for their comments on previous drafts of this manuscript. The research was financially supported by the Agricultural Research Service and Oregon State Agricultural Experiment Station in Burns, Oregon. The Eastern Oregon Agricultural Research Center is jointly funded by the USDA-Agricultural Research Service and Oregon State Agricultural Experiment Station. USDA and Oregon State University are equal opportunity providers and employers.
References
Anderson ML, Bailey AW (1979) Effect of fire on a Symphoricarpos occidentalis shrub community in central Alberta. Canadian Journal of Botany 57, 2819–2823.| Effect of fire on a Symphoricarpos occidentalis shrub community in central Alberta.Crossref | GoogleScholarGoogle Scholar |
Angassa A (2002) The effect of clearing bushes and shrubs on range condition in Borana, Ethiopia. Tropical Grasslands 36, 69–76.
Angassa A, Baars RMT (2000) Ecological condition of encroached and nonencroached rangelands in Borana, Ethiopia. African Journal of Ecology 38, 321–328.
| Ecological condition of encroached and nonencroached rangelands in Borana, Ethiopia.Crossref | GoogleScholarGoogle Scholar |
Ansley RJ, Wu XB, Kramp BA (2001) Observation: long-term increases in mesquite canopy cover in North Texas. Journal of Range Management 54, 171–176.
| Observation: long-term increases in mesquite canopy cover in North Texas.Crossref | GoogleScholarGoogle Scholar |
Archer S (1994) Woody plant encroachment into southwestern grasslands and savannas: rates, patterns and proximate causes. In ‘Ecological Implications of Livestock Herbivory in the West’. (Eds M Vavra, WA Laycock, RD Peiper) pp. 13–68. (Society for Range Management: Denver, CO)
Armour CD, Bunting SC, Neuenschwander LF (1984) Fire intensity effects on the understory in Ponderosa Pine forests. Journal of Range Management 37, 44–49.
| Fire intensity effects on the understory in Ponderosa Pine forests.Crossref | GoogleScholarGoogle Scholar |
Balch JK, Bradley BA, D’Antonio CM, Gomez-Dans J (2013) Introduced annual grass increases regional fire activity across the arid western USA (1980–2009). Global Change Biology 19, 173–183.
| Introduced annual grass increases regional fire activity across the arid western USA (1980–2009).Crossref | GoogleScholarGoogle Scholar | 23504729PubMed |
Barney MA, Frischknecht NC (1974) Vegetation changes following fire in the pinyon–juniper type of west-central Utah. Journal of Range Management 27, 91–96.
| Vegetation changes following fire in the pinyon–juniper type of west-central Utah.Crossref | GoogleScholarGoogle Scholar |
Bartos DL, Brown JK, Booth GD (1994) Twelve years of biomass response in aspen communities following fire. Journal of Range Management 47, 79–83.
| Twelve years of biomass response in aspen communities following fire.Crossref | GoogleScholarGoogle Scholar |
Bataineh AL, Oswald BP, Bataineh MM, Williams HM, Coble DW (2006) Changes in understory vegetation of a ponderosa pine forest in northern Arizona 30 years after a wildfire. Forest Ecology and Management 235, 283–294.
| Changes in understory vegetation of a ponderosa pine forest in northern Arizona 30 years after a wildfire.Crossref | GoogleScholarGoogle Scholar |
Bates JD, Svejcar TJ (2009) Herbaceous succession after burning cut western juniper trees. Western North American Naturalist 69, 9–25.
| Herbaceous succession after burning cut western juniper trees.Crossref | GoogleScholarGoogle Scholar |
Bates JD, Miller RF, Svejcar TJ (2000) Understory dynamics in cut and uncut western juniper woodlands. Journal of Range Management 53, 119–126.
| Understory dynamics in cut and uncut western juniper woodlands.Crossref | GoogleScholarGoogle Scholar |
Bates JD, Miller RF, Svejcar TJ (2005) Long-term successional trends following western juniper cutting. Rangeland Ecology and Management 58, 533–541.
| Long-term successional trends following western juniper cutting.Crossref | GoogleScholarGoogle Scholar |
Bates JD, Miller RF, Davies KW (2006) Restoration of quaking aspen woodlands invaded by western juniper. Rangeland Ecology and Management 59, 88–97.
| Restoration of quaking aspen woodlands invaded by western juniper.Crossref | GoogleScholarGoogle Scholar |
Bates JD, Davies KW, Sharp RN (2011) Shrub-Steppe early succession following invasive juniper cutting and prescribed fire. Environmental Management 47, 468–481.
| Shrub-Steppe early succession following invasive juniper cutting and prescribed fire.Crossref | GoogleScholarGoogle Scholar | 21344252PubMed |
Bauer JM, Weisberg PJ (2009) Fire history of central Nevada pinyon–juniper woodland. Canadian Journal of Forest Research 39, 1589–1599.
| Fire history of central Nevada pinyon–juniper woodland.Crossref | GoogleScholarGoogle Scholar |
Blaisdell JP (1953) Ecological effects of planned burning of sagebrush-grass range on the Upper Snake River Plains. US Department of Agriculture, Technical Bulletin 1975. (Washington, DC)
Bradley AF, Noste NV, Fischer WC (1991) Fire ecology of forests and woodlands in Utah. USDA Forest Service, Intermountain Research Station, General Technical Report INT-287. (Ogden, UT)
Breshears DD, Kastens JH, Romme WH, Belnap J, Floyd ML, Balice RG, Rich PM, Cobb NS, Allen CD, Price KP (2005) Regional vegetation die-off in response to global-change-type drought. Proceedings of the National Academy of Sciences of the United States of America 102, 15144–15148.
| Regional vegetation die-off in response to global-change-type drought.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGqtb3I&md5=62845f3c1f386eab7ae0c4249f69b92eCAS | 16217022PubMed |
Briske DD, Bestelmeyer BT, Stringham TK, Shaver PL (2008) Recommendations for development of resilience-based state-and-transition models. Rangeland Ecology and Management 61, 359–367.
| Recommendations for development of resilience-based state-and-transition models.Crossref | GoogleScholarGoogle Scholar |
Brown JR, Archer S (1989) Woody plant invasion of grasslands: establishment of honey mesquite on sites differing in herbaceous biomass and grazing history. Oecologia 80, 19–26.
| Woody plant invasion of grasslands: establishment of honey mesquite on sites differing in herbaceous biomass and grazing history.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3svntVWhug%3D%3D&md5=9e9b3809366b8d84ae4b4a34f1cf0b3aCAS | 23494340PubMed |
Brown JK, Smith JK (2000) Wildland fire in ecosystems: effects of fire on flora. USDA Forest Service, Rocky Mountain Research Station, General Technical Report, RMRS-GTR-42, Volume 2. (Ogden, UT)
Bucher EH, Huszar PC (1999) Sustainable management of the Gran Chaco of South America: ecological promise and economic constraints. Journal of Environmental Management 57, 99–108.
| Sustainable management of the Gran Chaco of South America: ecological promise and economic constraints.Crossref | GoogleScholarGoogle Scholar |
Buckhouse JC, Mattison JL (1980) Potential soil erosion of selected habitat types in the high desert region of central Oregon. Journal of Range Management 33, 282–285.
| Potential soil erosion of selected habitat types in the high desert region of central Oregon.Crossref | GoogleScholarGoogle Scholar |
Burkhardt JW, Tisdale EW (1976) Causes of juniper invasion in southwestern Idaho. Ecology 57, 472–484.
| Causes of juniper invasion in southwestern Idaho.Crossref | GoogleScholarGoogle Scholar |
Burrows WH, Carter JO, Scanlan JC, Anderson ER (1990) Management of savannas for livestock production in north-east Australia: contrasts across the tree-grass continuum. Journal of Biogeography 17, 503–512.
| Management of savannas for livestock production in north-east Australia: contrasts across the tree-grass continuum.Crossref | GoogleScholarGoogle Scholar |
Canfield RH (1941) Application of the line interception methods in sampling range vegetation. Journal of Forestry 39, 388–394.
Chambers JC, Roundy BA, Blank RR, Meyer SE, Whittaker A (2007) What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecological Monographs 77, 117–145.
| What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum?Crossref | GoogleScholarGoogle Scholar |
Chartier MP, Rostagno CM (2006) Soil erosion thresholds and alternative states in northeastern Patagonian rangelands. Rangeland Ecology and Management 59, 616–624.
| Soil erosion thresholds and alternative states in northeastern Patagonian rangelands.Crossref | GoogleScholarGoogle Scholar |
Clary WP, Jameson DA (1981) Herbage production following tree and shrub removal in the pinyon–juniper type of Arizona. Journal of Range Management 34, 109–113.
| Herbage production following tree and shrub removal in the pinyon–juniper type of Arizona.Crossref | GoogleScholarGoogle Scholar |
Condon L, Weisberg PJ, Chambers JC (2011) Abiotic and biotic influences on Bromus tectorum invasion and Artemisia tridentata recovery after fire. International Journal of Wildland Fire 20, 597–604.
| Abiotic and biotic influences on Bromus tectorum invasion and Artemisia tridentata recovery after fire.Crossref | GoogleScholarGoogle Scholar |
Cox RD, Anderson VJ (2004) Increasing native diversity of cheatgrass-dominated rangeland through assisted succession. Journal of Range Management 57, 203–210.
| Increasing native diversity of cheatgrass-dominated rangeland through assisted succession.Crossref | GoogleScholarGoogle Scholar |
Davies KW (2008) Medusahead dispersal and establishment in sagebrush steppe plant communities. Rangeland Ecology and Management 61, 110–115.
| Medusahead dispersal and establishment in sagebrush steppe plant communities.Crossref | GoogleScholarGoogle Scholar |
Davies KW, Sheley RL, Bates JD (2008) Does prescribed fall burning Artemisia tridentata steppe promote invasion or resistance to invasion after a recovery period? Journal of Arid Environments 72, 1076–1085.
| Does prescribed fall burning Artemisia tridentata steppe promote invasion or resistance to invasion after a recovery period?Crossref | GoogleScholarGoogle Scholar |
Davies GM, Bakker JD, Dettweiler-Robinson E, Dunwiddie PW, Hall SA, Downs J, Evans J (2012) Trajectories of change in sagebrush-steppe vegetation communities in relation to multiple wildfires. Ecological Applications 22, 1562–1577.
Dhaemers JM (2006) Vegetation recovery following spring prescribed fire in pinyon–juniper woodlands of central Nevada: effects of elevation and tree cover. MSc thesis, University of Nevada, Reno.
Dicus DA, Delfino K, Weise DR (2009) Predicted fire behavior and societal benefits in three eastern Sierra Nevada vegetation types Fire Ecology Special Issue 5, 67–78.
| Predicted fire behavior and societal benefits in three eastern Sierra Nevada vegetation typesCrossref | GoogleScholarGoogle Scholar |
Goergen EM, Chambers JC (2009) Influence of a native legume on soil N and plant response following prescribed fire in sagebrush steppe. International Journal of Wildland Fire 18, 665–675.
| Influence of a native legume on soil N and plant response following prescribed fire in sagebrush steppe.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFGgsrjN&md5=4cafccd0014780618546e2fc13f5b9abCAS |
Griffis KL, Crawford JA, Wagner MR, Moir WH (2001) Understory response to management treatments in northern Arizona ponderosa pine forest. Forest Ecology and Management 146, 239–245.
| Understory response to management treatments in northern Arizona ponderosa pine forest.Crossref | GoogleScholarGoogle Scholar |
Halpern CB (1989) Early successional patterns of forest species: interactions of life history traits and disturbance. Ecology 70, 704–720.
| Early successional patterns of forest species: interactions of life history traits and disturbance.Crossref | GoogleScholarGoogle Scholar |
Harniss RO, Murray RB (1973) 30 years of vegetal change following burning of sagebrush-grass range. Journal of Range Management 26, 322–325.
| 30 years of vegetal change following burning of sagebrush-grass range.Crossref | GoogleScholarGoogle Scholar |
Holmes PM, Cowling RM (1997) The effects of invasion by Acacia saligna on the guild structure and regeneration capabilities of fynbos shrublands. Journal of Applied Ecology 34, 317–332.
| The effects of invasion by Acacia saligna on the guild structure and regeneration capabilities of fynbos shrublands.Crossref | GoogleScholarGoogle Scholar |
Holmes AL, Miller RF (2010) State-and-transition models for assessing grasshopper sparrow habitat use. The Journal of Wildlife Management 74, 1834–1840.
| State-and-transition models for assessing grasshopper sparrow habitat use.Crossref | GoogleScholarGoogle Scholar |
Johnson DD, Miller RF (2006) Structure and development of expanding western juniper woodlands as influenced by two topographic variables. Forest Ecology and Management 229, 7–15.
| Structure and development of expanding western juniper woodlands as influenced by two topographic variables.Crossref | GoogleScholarGoogle Scholar |
Koniak S (1985) Succession in pinyon–juniper woodlands following wildfire in the Great Basin. The Great Basin Naturalist 45, 556–566.
Koniak S, Everett RL (1982) Seed reserves in soils of successional stages of pinyon woodlands. American Midland Naturalist 108, 295–303.
| Seed reserves in soils of successional stages of pinyon woodlands.Crossref | GoogleScholarGoogle Scholar |
Kramer NB, Johnson FD (1987) Mature forest seed banks of three habitat types in central Idaho. Canadian Journal of Botany 65, 1961–1966.
| Mature forest seed banks of three habitat types in central Idaho.Crossref | GoogleScholarGoogle Scholar |
Laycock WA (1991) Stable states and thresholds of range condition on North American rangelands: a viewpoint. Journal of Range Management 44, 427–433.
| Stable states and thresholds of range condition on North American rangelands: a viewpoint.Crossref | GoogleScholarGoogle Scholar |
Lesica P, Cooper SV, Kudray G (2007) Recovery of big sagebrush following fire in southwest Montana. Rangeland Ecology and Management 60, 261–269.
| Recovery of big sagebrush following fire in southwest Montana.Crossref | GoogleScholarGoogle Scholar |
Link SO, Gee GE, Downs JL (1990) The effects of water stress on phenological development and ecophysiological characteristics of cheatgrass and Sandberg’s bluegrass. Journal of Range Management 43, 506–512.
| The effects of water stress on phenological development and ecophysiological characteristics of cheatgrass and Sandberg’s bluegrass.Crossref | GoogleScholarGoogle Scholar |
Littell RC, Milliken GA, Stroup WW, Wolfinger RD (1996) SAS system for mixed models. (SAS Institute: Cary, NC)
Macdonald IAW, Wissel C (1992) Determining the optimal clearing treatments for alien shrub Acacia saligna in the southwestern Cape, South Africa. Agriculture, Ecosystems & Environment 39, 169–186.
| Determining the optimal clearing treatments for alien shrub Acacia saligna in the southwestern Cape, South Africa.Crossref | GoogleScholarGoogle Scholar |
Miller RF, Heyerdahl EK (2008) Fine-scale variation of historical fire regimes in sagebrush-steppe and juniper woodland: an example from California, USA. International Journal of Wildland Fire 17, 245–254.
| Fine-scale variation of historical fire regimes in sagebrush-steppe and juniper woodland: an example from California, USA.Crossref | GoogleScholarGoogle Scholar |
Miller RF, Rose JR (1995) Historic expansion of Juniperus occidentalis southeastern Oregon. The Great Basin Naturalist 55, 37–45.
Miller RF, Rose JR (1999) Fire history and western juniper encroachment in sagebrush steppe. Journal of Range Management 52, 550–559.
| Fire history and western juniper encroachment in sagebrush steppe.Crossref | GoogleScholarGoogle Scholar |
Miller RF, Wigand PE (1994) Holocene changes in semi-arid piñon–juniper woodlands: response to climate, fire, and human activities. Bioscience 44, 465–474.
| Holocene changes in semi-arid piñon–juniper woodlands: response to climate, fire, and human activities.Crossref | GoogleScholarGoogle Scholar |
Miller RF, Svejcar TJ, Rose JR (2000) Impacts of western juniper on plant community composition and structure. Journal of Range Management 53, 574–585.
| Impacts of western juniper on plant community composition and structure.Crossref | GoogleScholarGoogle Scholar |
Miller RF, Bates JD, Svejcar TJ, Pierson FB, Eddleman LE (2005) Biology, Ecology, and Management of Western Juniper. Oregon State University, Agricultural Experiment Station, Technical Bulletin 152. (Corvallis, OR) Available at http://extension.oregonstate.edu/catalog/html/tb/tb152/ [Verified 24 July 2013]
Miller RF, Tausch RJ, McArthur ED, Johnson DD, Sanderson SC (2008) Age structure and expansion of piñon–juniper woodlands: a regional perspective in the Intermountain West. USDA Forest Service, Rocky Mountain Research Station, Research Paper RMRS-RP-69. (Fort Collins, CO)
Milton SJ, Dean WRJ, Duplessis MA, Siegfri WR (1994) A conceptual model of arid rangeland degradation. Bioscience 44, 70–76.
| A conceptual model of arid rangeland degradation.Crossref | GoogleScholarGoogle Scholar |
Mozingo HN (1987) ‘Shrubs of the Great Basin: A natural history’. (University of Nevada Press: Reno, NV)
NRCS (2006) Soil Survey of Harney County Area, Oregon. (United States Department of Agriculture, Natural Resource Conservation Service: Washington, DC)
NRCS (2010a) Ecological Site Description. (United States Department of Agriculture, Natural Resource Conservation Service: Washington, DC) Available at http://esis.sc.egov.usda.gov/Welcome/pgApprovedSelect.aspx?type=ESD [Verified 1 March 2007]
NRCS (2010b) The PLANTS database. (United States Department of Agriculture, Natural Resource Conservation Service, National Plant Data Center: Baton Rouge, LA) Available at http://plants.usda.gov/index.html [Verified 22 November 2010]
Noson AC, Schmitz RF, Miller RF (2006) Influence of fire and juniper encroachment on birds in high elevation sagebrush steppe. Western North American Naturalist 66, 343–353.
| Influence of fire and juniper encroachment on birds in high elevation sagebrush steppe.Crossref | GoogleScholarGoogle Scholar |
Owens MK, Mackey JW, Carroll CJ (2002) Vegetation dynamics following seasonal fires in mixed mesquite/acacia savannas. Journal of Range Management 55, 509–516.
| Vegetation dynamics following seasonal fires in mixed mesquite/acacia savannas.Crossref | GoogleScholarGoogle Scholar |
Papanastasis VP, Chouvardas D (2005) Applications of the state-and-transition approach to conservation management of a grazed Mediterranean landscape in Greece. Israel Journal of Plant Sciences 53, 191–202.
| Applications of the state-and-transition approach to conservation management of a grazed Mediterranean landscape in Greece.Crossref | GoogleScholarGoogle Scholar |
Petersen SL, Roundy BA, Stringham TK (2009) A process-based application of state-and-transition models: a case study of western juniper encroachment. Rangeland Ecology and Management 62, 186–192.
| A process-based application of state-and-transition models: a case study of western juniper encroachment.Crossref | GoogleScholarGoogle Scholar |
Peterson DW, Reich PB, Wrage KJ (2007) Plant functional group responses to fire frequency and tree canopy cover gradients in oak savannas and woodlands. Journal of Vegetation Science 18, 3–12.
| Plant functional group responses to fire frequency and tree canopy cover gradients in oak savannas and woodlands.Crossref | GoogleScholarGoogle Scholar |
Pierson FB, Bates JD, Svejcar TJ, Hardegree S (2007) Runoff and erosion after cutting western juniper. Rangeland Ecology and Management 60, 285–292.
| Runoff and erosion after cutting western juniper.Crossref | GoogleScholarGoogle Scholar |
Rau BM, Chambers JC, Blank RR, Johnson DW (2008) Prescribed fire, soil, and plants: burn effects and interactions in the central Great Basin. Rangeland Ecology and Management 61, 169–181.
| Prescribed fire, soil, and plants: burn effects and interactions in the central Great Basin.Crossref | GoogleScholarGoogle Scholar |
Reid KD, MacDonald L, Breshears DD, Wilcox BP (1999) Runoff and erosion in a piñon–juniper woodland: influence of vegetation patches. Soil Science Society of America Journal 63, 1869–1879.
| Runoff and erosion in a piñon–juniper woodland: influence of vegetation patches.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhsFyrsLw%3D&md5=ed501809f9098e3adf179cb6e827e077CAS |
Reinkensmeyer DP, Miller RF, Anthony RG, Marr VE (2007) Avian community structure along a mountain big sagebrush successional gradient. The Journal of Wildlife Management 71, 1057–1066.
| Avian community structure along a mountain big sagebrush successional gradient.Crossref | GoogleScholarGoogle Scholar |
Romme WH, Allen CD, Bailey JD, Baker WL, Bestelmeyer BT, Brown PT, Eisenhart KS, Floyd ML, Huffman DW, Jacobs BF, Miller RF, Muldavin EH, Swetnam TW, Tausch RJ, Weisberg PJ (2009) Historical and modern disturbance regimes, stand structures and landscape dynamics in piñon–juniper vegetation of the western United States. Rangeland Ecology and Management 62, 203–222.
| Historical and modern disturbance regimes, stand structures and landscape dynamics in piñon–juniper vegetation of the western United States.Crossref | GoogleScholarGoogle Scholar |
Rose JR, Eddleman LE (1994) Ponderosa pine and understory growth following western juniper removal. Northwest Science 68, 79–85.
Sabo KE, Hull-Sieg C, Hart SC, Bailey JD (2009) The role of disturbance severity and canopy closure on standing crop of understory plant species in ponderosa pine stands in northern Arizona, USA. Forest Ecology and Management 257, 1656–1662.
| The role of disturbance severity and canopy closure on standing crop of understory plant species in ponderosa pine stands in northern Arizona, USA.Crossref | GoogleScholarGoogle Scholar |
Schaefer RJ, Thayer DJ, Burton TS (2003) Forty-one years of vegetation change on permanent transects in northeastern California: implications for wildlife. California Fish and Game 89, 66–71.
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52, 591–611.
Sheley RL, Bates JD (2008) Restoring western juniper (Juniperus occidentalis) infested rangeland after prescribed fire. Weed Science 56, 469–476.
| Restoring western juniper (Juniperus occidentalis) infested rangeland after prescribed fire.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntVersLw%3D&md5=786c60d7e57057b549796a27708d4d41CAS |
Sieg CH, Wright HA (1996) The role of prescribed burning in regenerating Quercus macrocarpa and associated woody plants in stringer woodlands in the Black Hills of South Dakota. International Journal of Wildland Fire 6, 21–29.
| The role of prescribed burning in regenerating Quercus macrocarpa and associated woody plants in stringer woodlands in the Black Hills of South Dakota.Crossref | GoogleScholarGoogle Scholar |
Smit GN (2004) An approach to tree thinning to structure southern African savannas for long-term restoration from bush encroachment. Journal of Environmental Management 71, 179–191.
| An approach to tree thinning to structure southern African savannas for long-term restoration from bush encroachment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c3jslGrtA%3D%3D&md5=53d5e80710ac89dbaee51f34e7361e0fCAS | 15135951PubMed |
Soulé PT, Grissino-Mayer HD, Knapp PA (2004) Human agency, environmental drivers, and western juniper establishment during the late Holocene. Ecological Applications 14, 96–112.
| Human agency, environmental drivers, and western juniper establishment during the late Holocene.Crossref | GoogleScholarGoogle Scholar |
Steen HK (1966) Vegetation following slash fires in one western Oregon locality. Northwest Science 40, 113–120.
Tausch RJ (1999) Transitions and thresholds: influences and implications for management in pinyon and Utah juniper woodlands. In ‘Proceedings: Ecology and Management of Pinyon–Juniper Communities within the Interior West’, 15–18 September 1999, Provo, UT. (Eds SB Monsen, R Stevens, RJ Tausch, R Miller, S Goodrich) USDA Forest Service, Rocky Mountain Research Station Proceedings RMRS-P-9, pp. 61–65. (Ogden, UT)
Teague WR, Pinchak WE, Waggoner JA, Dowhower SL, Ansley RJ (2010) Integrated grazing and prescribed fire restoration strategies in a mesquite savanna: vegetation responses. Rangeland Ecology and Management 63, 275–285.
| Integrated grazing and prescribed fire restoration strategies in a mesquite savanna: vegetation responses.Crossref | GoogleScholarGoogle Scholar |
Tonn JR, Jurgensen MF, Mroz GD, Page-Dumroese DS (2000) Miller Creek: ecosystem recovery in a western Montana forest 30 years after prescribed burning and wildfire. In ‘Fire and Forest Ecology: Innovative Silviculture and Vegetation Management: Proceedings of the 21st Tall Timbers Fire Ecology Conference: an International Symposium’, 14–16 April 1998, Tallahassee, FL. (Eds, WK Moser, CF Moser) Tall Timbers Research Station, number 21, pp. 67–73. (Tallahassee, FL)
Vaitkus M, Eddleman LE (1987) Composition and productivity of a western juniper understory and its response to canopy removal. In ‘Proceedings: Pinyon–Juniper Conference’, 13–16 January 1986, Reno, NV. (Ed RL Everett) USDA Forest Service, Intermountain Research Station, General Technical Report INT-215, pp. 456–460. (Ogden, UT)
Van Auken OW (2000) Shrub invasions of North American semiarid grasslands. Annual Review of Ecology and Systematics 31, 197–215.
| Shrub invasions of North American semiarid grasslands.Crossref | GoogleScholarGoogle Scholar |
Westoby M, Walker B, Noy-Meir I (1989) Opportunistic management for rangelands not at equilibrium. Journal of Range Management 42, 266–274.
| Opportunistic management for rangelands not at equilibrium.Crossref | GoogleScholarGoogle Scholar |
Whisenant SG (1990) Changing fire frequencies on Idaho’s Snake River Plains: ecological and management implication. In ‘Proceedings: Symposium on Cheatgrass Invasion, Shrub Die-off, and Other Aspects of Shrub Biology and Management’, 5–7 April 1989, Las Vegas, NV. USDA Forest Service, Intermountain Research Station General Technical Report INT-276, pp. 4–10. (Ogden, UT)
Wisdom MJ, Rowland MM, Suring LH, Schueck L, Meinke CW, Knick ST (2005) Evaluating species of conservation concern at regional scales. In ‘Habitat Threats in the Sagebrush Ecosystem’. (Eds MJ Wisdom, MM Rowland, LH Suring) pp. 5–74. (Alliance Communications Group: Lawrence, KS)
Wright HA, Neuenschwander LF, Britton CM (1979) The role and use of fire in sagebrush-grass and pinyon–juniper plant communities: a state-of-the-art review. USDA Forest Service, Intermountain Forest and Range Experiment Station, General Technical Report INT-58. (Ogden, UT)
Yates CJ, Hobbs RJ (1997) Temperate eucalypt woodlands: a review of their status, processes threatening their persistence and techniques for restoration. Australian Journal of Botany 45, 949–973.
| Temperate eucalypt woodlands: a review of their status, processes threatening their persistence and techniques for restoration.Crossref | GoogleScholarGoogle Scholar |
Young RP 1983. Fire as a vegetation management tool in rangelands of the Intermountain Region. In ‘Proceedings; Managing Intermountain Rangelands – Improvement of Range and Wildlife Habitats’. 15–17 September 1981, Twin Falls, ID and 22–24 June 1982, Elko, NV. (Eds SB Monsen, N Shaw) USDA Forest Service, Intermountain Forest and Range Experiment Station, General Technical Report INT-157, pp. 18–31. (Ogden, UT)
Zerihun W, Backleus I (1991) The shrub land vegetation in Western Shewa, Ethiopia and its possible recovery. Journal of Vegetation Science 2, 173–180.
| The shrub land vegetation in Western Shewa, Ethiopia and its possible recovery.Crossref | GoogleScholarGoogle Scholar |
Ziegenhagen LL, Miller RF (2009) Postfire recovery of two shrubs in the interiors of large burns in the Intermountain West. Western North American Naturalist 69, 195–205.
| Postfire recovery of two shrubs in the interiors of large burns in the Intermountain West.Crossref | GoogleScholarGoogle Scholar |


