Chemical composition of wildfire ash produced in contrasting ecosystems and its toxicity to Daphnia magna
Ashleigh R. Harper A E , Cristina Santin A B , Stefan H. Doerr A , Cynthia A. Froyd B , Dania Albini B , Xose Luis Otero C , Lucia Viñas D and Begoña Pérez-Fernández D
A E , Cristina Santin A B , Stefan H. Doerr A , Cynthia A. Froyd B , Dania Albini B , Xose Luis Otero C , Lucia Viñas D and Begoña Pérez-Fernández D
A Department of Geography, Swansea University, Singleton Park, Swansea, SA2 8PP, UK.
B Department of Biosciences, Swansea University, Singleton Park, Swansea, SA2 8PP, UK.
C Department of Edaphology and Agricultural Chemistry, Faculty of Biology, University of Santiago de Compostela, 15782 Santiago de Compostela, Spain.
D Instituto Español de Oceanografía (IEO), Centro Oceanográfico de Vigo, Subida a Radio Faro 50, 36390 Vigo, Spain.
E Corresponding author. Email: ashleigh.r.harper@hotmail.co.uk
International Journal of Wildland Fire 28(10) 726-737 https://doi.org/10.1071/WF18200
Submitted: 15 November 2018 Accepted: 20 June 2019 Published: 6 August 2019
Journal Compilation © IAWF 2019 Open Access CC BY-NC-ND
Abstract
It is well established in the world’s fire-prone regions that wildfires can considerably change the hydrological dynamics of freshwater catchments. Limited research, however, has focused on the potential impacts of wildfire ash toxicity on aquatic biota. Here, we assess the chemical composition and toxicity of ash generated from wildfires in six contrasting vegetation types distributed globally (UK grassland, Spanish pine forest, Spanish heathland, USA chaparral, Australian eucalypt forest and Canadian spruce forest). Acute (48 h) immobilisation tests were conducted on the extensively studied aquatic macroinvertebrate Daphnia magna, a sensitive indicator of aquatic contaminants. We found significant differences between the chemical composition and toxicity of these ash types. The UK and Spanish ash had no detectable toxicity to Daphnia magna, whereas the Australian eucalypt, USA chaparral and Canadian spruce ash all caused significant toxicity (immobilisation). The principal characteristics of the latter ash types were their high pH, and NO3−, Cl− and conductivity levels. Elevated water-soluble and total concentrations of metals (e.g. Mn, Fe, Zn, Pb, Cu and As) and total polycyclic aromatic hydrocarbons (PAHs) were not linked to toxicity.
Additional keywords: bioassays, ecotoxicology, polycyclic aromatic hydrocarbons (PAH), wildfire impacts.
Introduction
Fires are a natural process in many habitat types worldwide (Bixby et al. 2015), but they can be a social and environmental concern, potentially impacting public health, safety, infrastructure, biodiversity, land-use, water and air pollution (Bladon et al. 2014; Brito et al. 2017). Fire activity is projected to increase in many locations and ecotypes as a result of climate and societal changes, making the full understanding of their impacts crucial (Scholze et al. 2006; Chen et al. 2018).
During wildland fires, combustion of fuels releases a wide range of organic and inorganic components into the atmosphere but also concentrates some of them into wildfire ash left on the ground (Bodí et al. 2014). Fresh wildfire ash typically consists of mineral materials and charred organic components, is non-cohesive, has a low density, and is not attached to the soil, which facilitates its mobilisation and transportation by post-fire water and wind erosion (Bodí et al. 2014; Abraham et al. 2017). The release of soluble elements and particulate matter from eroded ash and underlying soil into aquatic systems following fires can cause increases in water turbidity, pH, organic matter, suspended sediment and conductivity and depletion of dissolved oxygen, among other effects (Smith et al. 2011; Tsai et al. 2017). Ash is however not usually examined as a distinct part of the post-fire sediment and few laboratory studies have characterised the composition of wildfire ash (Bodí et al. 2014).
The majority of the general studies into the effects of wildfire on water quality have focused on runoff amounts and nutrient levels and only more recently has increased research attention been given to pyrolytic substances, chemical elements and biological reactivity (Shakesby and Doerr 2006; Campos et al. 2012; Silva et al. 2015). Key areas receiving particular attention as a result of concern about their environmental effects are the production and mobilisation of polycyclic aromatic hydrocarbons (PAHs) and heavy metals (e.g. Vila-Escalé et al. 2007; Campos et al. 2012; Oliveira-Filho et al. 2018). Both present major biological concern owing to their carcinogenic potential, persistence within ecosystems and tendency to bio-accumulate (Smith et al. 2011; Chen et al. 2018). These contaminants are thought to have complex impacts on water quality and the biological effects of these in aquatic systems have been observed to persist across long spatial and temporal scales (Earl and Blinn 2003; Costa et al. 2014).
Ash has also begun to receive increasing recognition as a source of diffuse contamination in freshwater systems and detrimental impacts on both lake and stream biota, including fish (e.g. Nunes et al. 2017; Oliveira-Filho et al. 2018; Gonino et al. 2019a), amphibians (Pilliod et al. 2003), macroinvertebrates (Brito et al. 2017) and algae (Campos et al. 2012) have all been observed. Highly variable impacts of ash contamination on freshwater biota have been reported between different ecosystems, types of ash, fires and species (Smith et al. 2011; Silva et al. 2015; Oliveira-Filho et al. 2018). Campos et al. (2012) and Silva et al. (2015), for example, found no significant impact of eucalypt ash on the planktonic crustacean Daphnia magna reproduction or immobilisation rates over a chronic (21 day) and acute (48 h) exposures respectively. Toxicity was, however, observed on several lower trophic level species in these studies, the bacteria Vibrio fischeri, algae Pseudokirchneriella subcapitata and the macrophyte Lemna minor. A similar study by Brito et al. (2017) tested toxicity over acute exposures (48 h) of three types of ash from the Brazilian Cerrado ecoregion on the planktonic crustacean Ceriodaphnia dubia, the fish Danio rerio and the mollusc Biomphalaria glabrata and found that all ash types caused toxicity to C. dubia, none affected B. glabrata and only one type was toxic for D. rerio. At higher trophic levels, negative impacts of Brazilian sugarcane ash have also been observed on several native fish species (Astyanax lacustris, Moenkhausia bonita and M. forestii) over 24-h acute exposures but not for two non-native fish species (Oreochromis niloticus and Poecilia reticulata) (Gonino et al. 2019b). These studies demonstrate the variability and complexity of influencing factors in relation to the impacts of ash contamination on aquatic biota, highlighting the limited breadth of available research in this area (Hallema et al. 2018).
To enhance our understanding of the impacts of ash contamination on aquatic biota, the present study aimed to (1) determine the chemical composition of wildfire ash produced in six contrasting ecosystems; (2) examine the ecotoxicological effect of these ash types on the freshwater indicator species Daphnia magna; and (3) evaluate the relationship between chemical composition and observed toxicity and its implications for the relative water contamination potential of ash produced in these differing ecosystems. To the best of our knowledge, this constitutes the first ecotoxicology assessment allowing the direct comparison of the composition and toxicity of ash from several globally distributed contrasting ecosystems.
Materials and methods
Ash samples
Six composite ash samples were collected after wildland fires, before any rainfall, in each of the selected ecosystems types (Table 1): Australian eucalypt forest (AUS), USA chaparral (USA), Canadian spruce forest (CAN), Spanish heathland (URIA), Spanish pine forest (SPA), UK grassland (UK). Fire and vegetation characteristics are summarised in Table 1. Each composite ash sample was sieved through a 1-mm mesh before chemical characterisation or use in the bioassays.

|
Chemical characterisation
Chemical characterisation of the six ash types collected was undertaken to determine the total and water-soluble concentrations of major (Ca, Cl−, Mg, Na, Si, SO42−, NO3−) and trace elements and compounds (Al, B, Cu, F−, Fe, Ni, NH4+, As, Cd, Hg, Pb and PO43−), in addition to pH, dissolved organic carbon (DOC) and electric conductivity. This characterisation was undertaken using established methods (Plumlee et al. 2007; Santín et al. 2015, 2018; see supplementary material for full details).
The concentrations of 35 PAHs were also determined according to Pérez-Fernández et al. (2015) and Viñas et al. (2009) with gas chromatography-mass spectrometry (GC/MS) (Thermo mod DSQ II, Thermo Electron Corporation). The first step consisted of a 10-h Soxhlet extraction with a 1 : 3 acetone :hexane mixture. The extract was then treated overnight with activated copper for elemental sulfur removal and then cleaned using column chromatography with deactivated alumina (see supplementary material for full details).
Daphnia toxicity testing
Ecotoxicological assays consisting of acute ash exposures (48 h) were conducted using the planktonic crustacean Daphnia magna. This species is extensively used in ecological and toxicological studies as a sensitive indicator of the effects of contaminants on aquatic biota (OECD 2004; USEPA 2016). Daphnia spp. are also particularly relevant to freshwater lentic ecosystems (lakes, reservoirs and ponds) and ideal for investigating contamination potential in downstream waterbodies (Robinson and Thorn 2005; Nikinmaa 2014).
A monoclonal starter culture of D. magna was obtained from a long-term (2-year) rearing program. The new culture was reared and maintained according to recommended guidelines (OECD 2004; USEPA 2016), under controlled temperature (20 ± 2°C) and light conditions (uniform illumination of cool-white type, approximately 5000 lx; photoperiod 16 h light : 8 h dark) and fed every 2 days with a distilled suspension of Pseudokirchneriella subcapitata at ~0.1–0.2 mL per Daphnid per day.
To produce the test solutions, each ash sample was combined with a culture medium (synthetic hardwater medium – (ASTM 1996)) at the ratio 1 : 10 (mass : volume) (e.g. 100 g of ash in 1 L of medium). The samples were then homogenised in an orbital shaker for 4 h and stored at 4°C (maximum 24 h) before using in the ecotoxicological assays.
The acute toxicity tests were conducted according to the OECD 202 (OECD 2004) guidelines, with the exception of full pH adjustment. pH was not adjusted to control levels (pH 7.2 ± 0.2) in the bioassays to reproduce as close to natural conditions as possible, given pH is one of the most important factors affecting the toxicity and bioavailability of elements to freshwater species (Franklin et al. 2000). OECD 202 guidelines acknowledge that tests should be carried out without the adjustment of pH where values are within pH 6–9 at the highest test concentration (OECD 2004). It is crucially important that pH adjustment does not cause significant changes to the test substances and owing to the complex and varying compositions and reactivity of wildfire ash, potential interactions are unclear. Little is known to date on wildfire ash concentrations in water bodies; therefore, a wide range of ash concentrations was tested, trying to represent the potential variability of different natural scenarios. Six different concentrations of the ash–medium solutions were used during testing (3.12, 6.25, 12.5, 25.0, 50.0, 75.0 g L−1), plus four controls per concentration.
Tests were initiated using new-borns less than 24 h old, originating from the third to fifth brood of the culture. For each ash type, 150 daphnids were used. This sample size was divided into five individuals per test vessel for each concentration with four replicates and one control per concentration. The test was conducted for 48 h and the immobilisation of neonates was documented at 24 and 48 h. Immobilisation of neonates is defined here as individuals not able to swim within 15 s of gentle agitation of the test vessel. During this period, the same temperature (20 ± 2°C) and photoperiod (photoperiod 16 h light : 8 h dark) conditions as during rearing were maintained. D. magna were not fed during the acute exposure (USEPA 2016).
Statistical analysis
The water-soluble (leachates) chemical composition results were subjected to principal component analysis (PCA) (RStudio version 5.4.1) to identify constituents most strongly correlated with the different ash types. This approach to assessing the characteristic components in a given sample is widely used in environmental research when dealing with complex datasets (Brito et al. 2017). The leachates data were chosen for this analysis, as opposed to the total elements data, because this is likely the most bioavailable fraction and, therefore, the most likely to have affected the daphnia over an acute exposure.
To identify thresholds in the D. magna toxicity results and in agreement with standard procedures (Musset 2006), the data were subjected to single-factor analysis of variance tests (RStudio version 5.4.1). Where significant results were identified, post-hoc Dunnett’s analysis was used to test if the response at each concentration was significantly different to the control groups and, therefore, identify critical thresholds (lethal concentrations) in the response relationships. This enables the effect concentrations (EC10 = concentration at which 10% of individuals are immobilised and EC50 = concentration at which 50% of individuals are immobilised) for each ash to be interpolated, along with the lowest observed effect concentration (LOEC) (Musset 2006). A significance level of 5% (0.05) was used in all statistical tests.
Results
Ash chemistry
The total elemental composition of the six ash types overall contained several potential contaminants, but in highly variable concentrations (Supplementary material Table S1). The most abundant element in all samples was Ca (range 11 800–177 000 mg kg−1) with Al (range 1320–22 600 mg kg−1) and Fe (range 979–30 600 mg kg−1) both present in high concentrations throughout. The elements found in the lowest total concentrations were: As (range 0.46–9.67 mg kg−1), Cd (range 0.17–1.13 mg kg−1) and Hg (range 0–0.05 mg kg−1) (supplementary material Table S1).
pH and electrical conductivity (EC) measured in the leachates notably varied across ash samples, with pH levels ranging from moderately alkaline in the UK ash (7.9), to strongly alkaline in the USA ash (11.2). Equally, EC levels varied greatly from 233 µS cm−1 in the SPA ash to 3880 µS cm−1 in the AUS ash. High pH and EC values were both characteristic features of the ash types producing immobilisation of Daphnia magna tested (see Acute toxicity test section). pH within the bioassays themselves, however, was notably less variable (7.31–9.08), likely due to differences in dilution between leachates and the bioassay testing, and the addition of the culture medium in the latter.
The water-soluble (leachate) composition of the ash types was also highly variable (Table 2), with the most abundant components being SO42− (range 1203–10 180 mg kg−1), Cl− (range 228–1509 mg kg−1) and Na (range 17–3893 mg kg−1). The minor metal and metalloids elements were similarly the components found in the lowest concentration in the leachates: Cd (range 0–7 µg kg−1), Ni (range 60–844 µg kg−1), Zn (range 0–140 µg kg−) and Hg (range 1–2 µg kg−1) (Table 2).
Some soluble elements occurred in particularly high levels, highlighting the variation in element content within the ash (Table 2). For example, in the UK sample, PO43− (620 mg kg−1) and metals such as Fe (4378 µg kg−1) and Mn (9292 µg kg−1) were notably high in comparison with the other ash types. There were also notably high levels of, for example, Ca (5864 mg kg−1) and SO42− (32 289 mg kg−1) in the CAN sample; B (85 mg kg−1) and Na (3893 mg kg−1) in the AUS sample; and Cu (5158 µg kg−1) and As (329 µg kg−1) in the URIA sample (Table 2).
The water-soluble concentrations of each element were relatively low when compared with the total dry concentration within each ash type (Table 2). On average, the proportions of water-soluble Al, Pb, Mn, Fe, Zn were <1% total dry weight; As, Si, Ca, P, Ni, Cu, Cd were <5% and Mg was <10%. The levels of Na (2–77%) and Hg (5–57%) solubility were highly variable and they are clearly the most soluble of the components analysed.
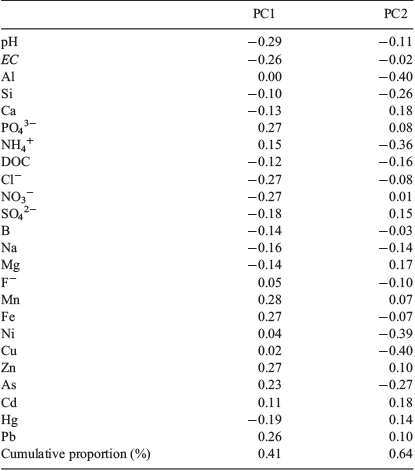
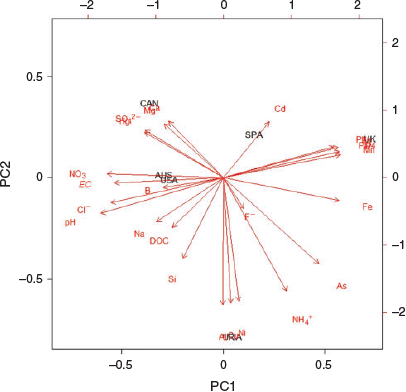
PCA identified three primary components explaining 79% of the total leachates dataset variance (PC1 = 41%; PC2 = 23%; PC3 = 15%) (Table 3). PC1 is most strongly positively correlated with Mn, Fe, Zn, As, Pb and PO43− levels and most strongly negatively correlated with pH, EC, NO3−, Cl−, Hg and SO42− (Table 3 and Fig. 1). A biplot of the standardised PC1 and PC2 values (Fig. 1) shows which components best characterised each ash type and pH, EC, NO3−, Cl−, Hg and SO42− were most closely correlated with the three ash types producing significant immobilisation of D. magna, whereas Al, Cu, Ni, NH4+, As, Fe, Mn, PO43−, Pb, Cd were more closely correlated with the three non-toxic ash types (Fig. 1).
|
|
Thirty-five PAHs were analysed across the ash types including the 16 USEPA priority PAHs, which provide the focus of the following discussion (Table 4). The total concentration of these priority contaminants ranged from 1155 to 14 078 ng g−1 ash, the highest total being found in the UK ash originating from an upland grassland ecosystem in south Wales (∑16 EPA PAHs: 12 336 ng g−1 ash) (Table 4). Notably high PAHs concentrations were also found in the CAN (∑16 EPA PAHs: 7486 ng g−1) and the SPA ash (∑16 EPA PAHs: 4393 ng g−1 ash) (Table 4).

|
The proportion of the methylated and non-methylated PAHs was very similar in all the samples with approximately three times more non-methylated PAHs in each ash type, except the USA ash, which contained over 15 times the amount of non-methylated PAHs (Table 4). There was also a predominance of two-ring PAHs in all the samples. Generally, the quantity of each ring type decreases sequentially with the number of rings, 2 > 3 > 4 > 5 and 6 with the exception of the USA sample, which had a relatively similar quantity of three-, four-, five- and six-ring PAHs. The predominant two-ring PAH in all samples was naphthalene. Phenanthrene was the most common three-ring PAH, except in the UK sample where it was acenaphthylene. All three of these abundant PAHs (naphthalene, phenanthrene and acenaphthylene) are classified as EPA priority contaminants (Table 4).
Acute toxicity test
High levels of D. magna immobilisation were recorded at both 24- and 48-h exposure for three of the six ash types tested: AUS, USA and CAN (P < 0.001 for all three ash types) (Fig. 2; Tables 5 and 6). The response relationships identify the AUS ash as the most toxic, with a 100% immobilisation of D. magna individuals at less than 25 g ash L−1 within the first 24 h of exposure (Table 5; Fig. 2). The immobilisation effect of both the North American ash samples (USA and CAN) were relatively similar, with 48-h EC50 being achieved at 20 and 26 g ash L−1 respectively, despite the notably different source vegetation (Table 6; Fig. 2). In contrast, no significant immobilisation occurred in response to the remaining three ash types (URIA, SPA and UK) (Tables 5 and 6). The UK ash did not produce any observable immobilisation across any of the test concentrations after 48 h of exposure. The Spanish samples (URIA, SPA) only produced low rates of immobilisation at the highest concentrations (Tables 5 and 6).
Discussion
Overall ash chemical properties
The total concentration of each element within the six ash types showed a wide variability (Supplementary material Table S1). These variations may be explained by the accumulative capacity of the different vegetation types, taking up different levels of elements from the soil and surrounding environment (Peralta-Videa et al. 2009; Brito et al. 2017). Fire dynamics (e.g. burn temperature) and soil properties are also important features in the composition of elements within ash (Pitman 2006; Bodí et al. 2014; Chen et al. 2018). In general, oxides and hydroxides of Ca, Mg, Si and P particularly tend to be abundant in wildfire ash (Pereira and Úbeda 2010; Silva et al. 2015) as found in the ash tested here (Supplementary material Table S1).
Overall, the water solubility of the studied elements in all ash types was low (<20% except for Na and Hg). This agrees with previous findings (Khanna et al. 1994; Santín et al. 2015; Silva et al. 2015; Brito et al. 2017). The most abundant ions in all leachates were SO42−, Cl− and Na+ (Table 2), likely owing to them forming very soluble salts (i.e. sulfates or chlorides). These components are thus commonly found in high concentrations in the dissolved residue of ash (Freitas and Rocha 2011; Santín et al. 2015) (Table 2). In contrast, heavy metals such as Cd, Ni and Zn showed the lowest concentration in the leachates owing to being relatively insoluble in alkaline (pH 8–10) conditions, precipitating mainly as hydroxides (Weiner 2012). These results are similar to those found in other studies assessing post-fire runoff and ash leachates in a range of ecosystem types (Jung et al. 2009; Pereira et al. 2011) and in agreement with the general concentration trend of alkali (Na, K) > alkaline (Ca, Mg) >> heavy metals (Pb, Cd and Hg) found by Santín et al. (2015) in eucalypt forest ash.
Ash types and element solubility
Despite the overall similarities in ash solubility in the ash leachates, there are also substantial variations among the ash types, making their chemical profiles notably different. Brito et al. (2017), assessing Brazilian Cerrado ash types, also found there were little qualitative differences in the overall composition of the different ash tested, but large variations in the concentration of the chemical elements between sampling areas.
The PCA analysis allowed detection of key differences in the composition of the ash types studied here. The UK ash leachate has a distinctly soluble profile in comparison with the others. PCA analysis shows several heavy metals (Mn, Fe, Zn and Pb) and PO43− to be characteristic elements of the UK ash leachate (Fig. 1). This leachate shows high concentrations of soluble Fe, Mn and PO43− in comparison with the other ash types (Table 2). The pH (7.9) of the UK leachate was 1 to 3 units lower than the extracts from the other samples (Table 2). These less alkaline conditions favour the solubility of metals and P compared with the other samples where the metals tend to precipitate as hydroxides for pH values above 8–9 and the phosphate as hydroxyapatite for pH values >8.5 (for example see: Diaz et al. 1994; Stumm and Morgan 1995).
A characteristic component of the CAN sample (identified by PCA, Fig. 1) was the high levels of soluble Ca, despite the total concentration in dry ash being relatively similar to that of the AUS, SPA and USA ash (Table 2). It is unclear why the solubility of Ca is notably higher in the CAN ash in comparison with the other ash types (Jung et al. 2009; Brito et al. 2017), but it may be responsible for the reduced PO43− levels (1.2 mg kg−1) in the CAN leachate as P has a tendency to precipitate in the presence of Ca (Diaz et al. 1994). This P–Ca interaction may influence algal and cyanobacterial growth (and thus, eutrophication) by regulating P levels in freshwater systems (Bladon et al. 2008; Blake et al. 2009). In the broader context, Ca is not normally considered hazardous, but can significantly influence the overall toxicity of ash eluates (e.g. its strong relationship with SO42− leaching) (Mount et al. 1997; Tian et al. 2018). Stiernström et al. (2013) even propose that Ca might be one of the key elements responsible for the ecotoxicity of ash eluates on the crustacean Nitocra spinipes, despite Ca not being classified as individually ecotoxic. The CAN ash tested here produced significant immobilisation of Daphnia magna over the 48-h exposure also potentially influenced by its high Ca concentration.
For the AUS ash sample, the levels of soluble B and Na are higher than in the other ash types (Fig. 1, Table 2). These elements are often found in high concentrations in ash leachates (Jung et al. 2009; Pereira et al. 2011), particularly B in other eucalyptus ash tested (Freitas and Rocha 2011). High Na+ levels in freshwater systems can present an issue for water purification processes as they cannot be removed using conventional methods (Smith et al. 2011). Unlike reported by Silva et al. (2015), where the principal potential toxic components of their eucalypt ash were Mn and Zn, neither of these elements were found in the eucalypt (AUS) ash analysed here. This further highlights the differences in ash composition comparing individual fire events and ecosystem types (Bodí et al. 2014).
In the URIA ash, the most defining components were Cu, Al, Ni, NH4+ and As (Fig. 1). This ash contained comparatively high concentrations of soluble Cu (5158 µg kg−1) and the carcinogen As (329 µg kg−1). Similar elevated soluble levels of Cu have, however, been found in mixed eucalyptus ash (Cu 5100–6200 µg kg−1) by Santín et al. (2015). The reason for the significantly higher solubility rate of Cu in this heathland ash (URIA 12.9%, range excluding URIA 0.49–2.01%) is worth further consideration to identify areas or components likely to increase the risk of Cu contamination. The concentration of As, although elevated in the URIA (and UK) sample here, has been reported as higher in several other wildfire ash samples (e.g. 4000–7300 µg kg−1 in Santín et al. (2015); 42 000 µg kg−1 in Silva et al. (2015)) and despite being above the 0.01-mg L−1 World Health Organizations drinking-water guideline (World Health Organization 2011), it did not appear to cause significant immobilisation of D. magna in the URIA or UK ash.
The SPA ash has a relatively insoluble overall profile with notably high concentrations of the metals Al, Fe, Zn, Cb, Pb and the metalloid As in the dry ash (Supplementary material S1) but limited to no leaching of Al, Fe, Zn and Pb into the water-soluble extract (Table 2). Despite this, Cd presented as a distinct principal component of the SPA ash with a comparatively high soluble concentration (7 µg kg−1) and as the only sample to register a solubility percentage greater than 1% (2.85%). Similar dry quantities of Cd were recorded by Brito et al. (2017) assessing Brazilian Cerrado ash types (0.1–0.3 mg kg−1) but Cd solubility was lower in these ash types (<0.01%).
PAHs composition
The organic fraction of ash may also contain organic contaminants of biological concern (Vila-Escalé et al. 2007; Chen et al. 2018). The data available on PAHs release following a fire, however, are quite limited (Vila-Escalé et al. 2007; Kim et al. 2011; Campos et al. 2012; Rey-Salgueiro et al. 2018).
The concentrations of PAHs found in the ash analysed here are also widely variable, with a range of 1155 ng g−1 in the AUS ash to 14 078 ng g−1 in the UK ash (16 USEPA priority PAHs) (Table 4). The values contained within the ash tested here are substantially higher than those presented by Olivella et al. (2006) testing wildfire ash from pine and oak forests (∑12 PAHs: 1–19 ng g−1 ash). The lowest concentration, found in the AUS ash type (∑16 EPA PAHs: 1155 ng g−1 ash), was of a comparable level with those found by Silva et al. (2015) assessing dry wildfire ash in a predominantly eucalypt ecosystem in Portugal (∑16 EPA PAHs: 1100 ng g−1 ash). The full range of PAH concentrations found here are within the range 1000–50 000 ng g1 (∑16 EPA PAHs) found by Santín et al. (2017) analysing PAHs in pine forest floor and wood under wildfire charring and slow pyrolysis.
The UK ash shows a much higher PAHs concentration than the other types (Table 4). It is unclear why this is the case as no other research has been conducted on the PAHs composition of wildfire ash originating from comparable grassland ecosystems. The type of fuel and variations in combustion temperatures and oxygen availability are thought to strongly affect the concentration and type of PAHs in ash (Enell et al. 2008; Rey-Salgueiro et al. 2018). Chen et al. (2018) found that PAHs concentrations were significantly higher in black wildfire ash (moderate burn severity) in comparison with white wildfire ash (high burn severity). This was also true of the ash types tested here with the darker (dark grey-black) ash samples (UK, URIA, CAN) containing a much higher concentration of PAHs than the lighter (light grey-white) samples (AUS, USA, SPA) (Table 4). Although variations in combustion completeness could be related to PAHs content here, the proportion of methylated PAHs in the UK ash is similar to that of the other samples tested (Table 4). The proportion of methylated/total PAHs is usually considered an indicator of combustion completeness as during combustion, the methylated component of the compound is lost first (Keiluweit et al. 2012) (Table 4).
The high presence of low-molecular-weight and therefore, greater-volatility PAHs (i.e. Naphthalene (Naph) and Phenanthrene (Phe)) in the ash tested here may seem contradictory as it can be expected that these compounds would be lost during a fire. It is, however, likely that these PAHs preferentially recondense in the ash layer and are retained in microporous structures of pyrogenic material (Santín et al. 2017). Other studies support this idea, reporting high concentrations of Naph and Phe (Kim et al. 2011) or Naph, Chrysene (Chry), Benz(a)anthracene and Acenaphthylene (Campos et al. 2012) from wood burning. Ash studies of beech and similar species (Bundt et al. 2001) were dominated by Naph and by Benzo(ghi)perylen, Benzo(b)fluoranthene, Benzo(k)fluoranthene, Chry, Triphenylene and Phe but at lower concentrations.
Caution is required when making comparisons between the PAHs values across studies as there are important variations in the methodologies employed. Some studies examine PAHs in ash (Enell et al. 2008; Silva et al. 2015) or sediment (Olivella et al. 2006; Kim et al. 2011) and others in stream water (Olivella et al. 2006), pond water (Vila-Escalé et al. 2007), runoff water (Campos et al. 2012) or aqueous extracts (Enell et al. 2008; Silva et al. 2015), meaning concentration and compositional differences are to be expected. It is likely the high to very high PAHs concentrations recorded in the ash studied here would be dramatically reduced if the leachable fraction of the samples were tested, as opposed to total concentrations, therefore making the portion more accessible to interact with aquatic fauna lower (Frišták et al. 2019).
Implications for toxicology
The wildfire ash analysis conducted here not only demonstrates the high variability in the concentration of chemical components of ash produced in contrasting ecosystems (Table 2), but also the differences in their potential toxic effects in aquatic systems (Table 5 and 6). Significant toxicity was observed to D. magna over the acute exposures for three of the six ash types tested: AUS, USA and CAN (Fig. 2; Table 5 and 6). Ash type and composition, therefore, seem crucial to the level of toxicity on cladoceran species, as also demonstrated previously (Campos et al. 2012; Silva et al. 2015; Brito et al. 2017).
The combination of the chemical data with the D. magna immobilisation results highlights several possible relationships (Fig. 1). The PCA identified pH and EC as two of the parameters strongly characteristic of the three ash types causing significant D. magna immobilisation (AUS, USA, CAN) (Table 3, Fig. 1). It is well established that extreme values of pH and EC have a detrimental impact on zooplankton species (Mount et al. 1997; Franklin et al. 2000; Silva et al. 2015). The pH values in the bioassays themselves, however, were notably lower and less variable than in the leachate results used during the PCA analysis and within a range thought acceptable for the survival of D. magna and similar cladoceran species (OECD 2004) (Table 5 and 6). Crucially, however, the relationship between pH and immobilisation is very similar between the leachates and bioassays pH results, with higher pH values characteristic of the ash types producing immobilisation in D. magna. This perhaps suggests that pH has an indirect effect on D. magna immobilisation as pH can also influence the dissolution of elements from ash into water and therefore the relative toxic potential of other ash components (Fedje et al. 2010). Low pH values, for example, encourage the leaching of oxyanion-forming (As, B, Cr, Sb and V) and cation-forming elements (Ca), and neutral pH greatly reduces the leaching of amphoteric elements (e.g. Al, Cd, Cu, Pb and Zn) (Fedje et al. 2010). The more neutral pH of the UK sample, however, does not seem to have reduced the leaching of Al, Cd, Cu and Pb. pH has an inconsistent relationship with toxicity, and, often, results are difficult to interpret (Wilde et al. 2006; Silva et al. 2015).
The influence of key nutrients on D. magna immobilisation is perhaps less well established (Smith et al. 2011) (Fig. 1), as ions such as Cl− and NO3− are required at minimum levels to support aquatic life. However, the PCA also identified high concentrations of Cl− and NO3− as being key characteristic components of the three toxic ash types, particularly the more toxic AUS and USA ash (Table 3 and Fig. 1). Many anthropogenic (e.g. oil and gas production, irrigation methods and industrial and agricultural processes) and natural (e.g. sediment pore waters and burning) conditions have been shown to increase nutrient concentrations to toxic levels (e.g. Hoke et al. 1993; Ferreira et al. 2005; Mast and Clow 2008). Scott and Crunkilton (2000) demonstrated NO3− produces immobilisation of D. magna at 462 mg L−1 with no observable effect concentration at 358 mg L−1. Similarly, Mount et al. (1997) estimated a concentration of 1000–2000 mg L−1 as the concentration of Cl− required to produce EC50 in Ceriodaphnia dubia. This suggests despite the correlations between NO3− and Cl− with the toxic ash types found here, the relatively low quantities of these components alone are not likely capable of causing the observed toxicity (Table 2). The limited number of studies focusing on Na+, Cl−, SO42− and NO3− exports after a fire have found maximum levels sampled in ash fall well below recommended safe limits (Smith et al. 2011).
The relatively high PAHs concentrations found in the ash tested here appear to produce no observable toxicity on D. magna and, furthermore, higher PAHs concentrations seem to be associated with reduced toxicity. It has to be noted that PAHs concentrations were only determined in bulk ash samples. PAHs have limited solubility in water, particularly of the larger-ring-size PAHs (>3 rings) (Chen et al. 2018). The lack of relationship between high levels of PAHs and toxicity found here and in other studies (Campos et al. 2012; Silva et al. 2015) raises questions about the bioavailability of PAHs in this context. In an assessment of the methylated PAHs composition of sludge-derived pyrogenic material, Frišták et al. (2019) found during pyrolysis methylated aromatics mainly bind to insoluble carbon fractions or get trapped in microporous structures of pyrogenic material and, therefore, are unlikely to be bioavailable and hazardous to freshwater systems. This may be one reason why the PAHs concentrations are not associated with toxicity in D. magna here. The potential more subtle and longer-term impacts of PAHs on aquatic biota, such as reductions in the rate of growth, metabolic activity and reproduction or increased mutation and cancer risk (Hellou et al. 2006; Campos et al. 2012), were beyond the scope of the present study. Potential synergistic, antagonistic and additive effects of the complex and variable PAHs composition of the ash types tested could also not be ruled out as a source of toxicity. Further research should be conducted investigating if these levels of total PAHs pose a water contamination risk from a wider ecological or drinking water perspective.
Despite the variations in ash composition and the subsequent significant differences in D. magna immobilisation, it is difficult to isolate the primary causes of toxicity. In addition to the most likely, if indirect, influential parameters pH and EC, there are also likely components that are not necessarily toxic by themselves but could be variables influencing toxicity at certain concentrations (e.g. DOC, Na, Ca and Mg) (Freitas and Rocha 2011; Simplício et al. 2016). Physical characteristics of the ash types may also be a possible cause of immobilisation as variations in particle size and distribution of the suspended particulate matter in the unfiltered samples used could have compromised the food intake and locomotive ability of D. magna, leading to immobilisation or death (Brito et al. 2017). Even when using a standardised laboratory approach, as employed here, it remains difficult to untangle the effects of such components from those caused by other variables in such complex samples (Wilde et al. 2006; Silva et al. 2015; Brito et al. 2017).
Earlier work has suggested macroinvertebrate species such as D. magna are less sensitive to contamination than lower trophic species (Campos et al. 2012), and thus the effects of ash contamination on these higher trophic organisms are expected to be primarily indirect through the propagation of toxicity across the food chain via bottom-up bioaccumulation processes (Abrantes et al. 2008). A few notable studies have demonstrated this premise with no observable effect of ash toxicity on daphnid survival or reproduction rates over both acute (48 h) and chronic (21 day) exposures, but significant impacts have been observed on lower trophic species (bacteria, algae and macrophytes) (Campos et al. 2012; Silva et al. 2015). Understanding the mechanisms influencing the bioaccumulation and availability of ash contaminants in freshwater systems should thus be a focus of future research. The results presented here, along with other studies, appear to justify the concerns around the impacts of wildfire ash on aquatic biota and water quality even without the assessment of bioaccumulation processes (Campos et al. 2012; Brito et al. 2017).
Conclusion
The chemical characterisation of the six wildfire ash types shows an overall similar composition of elements, but significant variations in the concentration, reactivity and solubility of these elements. The solubility of all elements was low for all ash types comparing the total and leachate characterisation data.
Our results demonstrate significant immobilisation of Daphnia magna over acute exposure (48 h) to three of the six ash types (AUS, USA and CAN). The main characteristics of these ash types producing immobilisation, derived from PCA, were high PC values of pH, EC, NO3−, Cl−, Hg and SO42−. None of these components, however, appear likely to have directly caused the immobilisation response (ecotoxicity) observed. It is perhaps more likely that these components, and possible others (e.g. Ca), have contributed indirectly to the observed toxicity. Elevated water-soluble concentrations of metal and metalloid contaminants (Mn, Fe, Zn, Pb, Cu and As) did not produce any significant inhibition and tended to be characteristic of the non-toxic ash types. The total PAHs concentrations were also not linked to significant inhibition. It continues to prove difficult to identify specific causes of toxicity in aquatic biota using test substances as complex and variable as wildfire ash.
Combining the detailed chemical characterisation of the ash types with the ecotoxicology results helps to provide further insight into the composition and variations in ash produced in different ecosystem types and potential implications of wildfire ash contamination on the environment. A detailed understanding of the interactions and impacts of metals, nutrients and PAHs in different ecosystem types is essential for evaluating the pollution risk of fires and for informing management. The results presented here justify the concerns around the downstream contamination potential of ash in certain ecosystems on aquatic biota and highlight the need for a greater understanding of possible direct and indirect chemical causalities. Further research is therefore required in order to (i) identify and predict conditions creating certain chemical signatures in ash, and (ii) to investigate the specific direct (or indirect) causality of toxicity in key groups of aquatic species.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by a Swansea University College of Science PhD studentship (Harper), a Leverhulme Trust Research Fellowship REF-2016–456\2 (Doerr) and a Ser Cymru Fellowship supported by European Union’s 2020 research and innovation program under the Marie Sklodowska–Curie grant agreement no. 663830 (Santin) and Natural Environment Research Council (NERC) grant NE/R011125/1. Xose Luis Otero was supported by Cross-Research in Environmental Technologies (CRETUS) strategic group (Conselleria de Educacion REF. 2018-PG100). We would also like to acknowledge and thank A. Cerdà and M. Bodi for kindly providing the Spanish pine forest (SPA) ash sample.
References
Abraham J, Dowling K, Florentine S (2017) Risk of post-fire metal mobilization into surface water resources: a review. The Science of the Total Environment 599, 1740–1755.| Risk of post-fire metal mobilization into surface water resources: a review.Crossref | GoogleScholarGoogle Scholar | 28535601PubMed |
Abrantes N, Pereira R, Soares AMVM, Gonçalves F (2008) Evaluation of the ecotoxicological impact of the pesticide Lasso® on non-target freshwater species, through leaching from nearby agricultural fields, using terrestrial model ecosystems. Water, Air, and Soil Pollution 192, 211–220.
| Evaluation of the ecotoxicological impact of the pesticide Lasso® on non-target freshwater species, through leaching from nearby agricultural fields, using terrestrial model ecosystems.Crossref | GoogleScholarGoogle Scholar |
ASTM (American Society for Testing and Materials) (1996) Standard guide for conducting acute toxicity test on test materials with fishes, macroinvertebrates and hydrobiologia. Annual Book of ASTM Standards E 729-796 (Philadelphia, PA, USA).
Bixby RJ, Cooper SD, Gresswell RE, Brown LE, Dahm CN, Dwire KA (2015) Fire effects on aquatic ecosystems: an assessment of the current state of the science. Freshwater Science 34, 1340–1350.
| Fire effects on aquatic ecosystems: an assessment of the current state of the science.Crossref | GoogleScholarGoogle Scholar |
Bladon KD, Silins U, Wagner MJ, Stone M, Emelko MB, Mendoza CA, Devito KJ, Boon S (2008) Wildfire impacts on nitrogen concentration and production from headwater streams in southern Alberta’s Rocky Mountains. Canadian Journal of Forest Research 38, 2359–2371.
| Wildfire impacts on nitrogen concentration and production from headwater streams in southern Alberta’s Rocky Mountains.Crossref | GoogleScholarGoogle Scholar |
Bladon KD, Emelko MB, Silins U, Stone M (2014) Wildfire and the future of water supply. Environmental Science & Technology 48, 8936–8943.
| Wildfire and the future of water supply.Crossref | GoogleScholarGoogle Scholar |
Blake WH, Wallbrink PJ, Droppo IG (2009) Sediment aggregation and water quality in wildfire-affected river basins. Marine and Freshwater Research 60, 653–659.
| Sediment aggregation and water quality in wildfire-affected river basins.Crossref | GoogleScholarGoogle Scholar |
Bodí MB, Martin DA, Balfour VN, Santín C, Doerr SH, Pereira P, Cerdà A, Mataix-Solera J (2014) Wildland fire ash: production, composition and eco-hydro-geomorphic effects. Earth-Science Reviews 130, 103–127
| Wildland fire ash: production, composition and eco-hydro-geomorphic effects.Crossref | GoogleScholarGoogle Scholar |
Brito DQ, Passos CJS, Muniz DHF, Oliveira-Filho EC (2017) Aquatic ecotoxicity of ashes from Brazilian savanna wildfires. Environmental Science and Pollution Research International 24, 19671–19682.
| Aquatic ecotoxicity of ashes from Brazilian savanna wildfires.Crossref | GoogleScholarGoogle Scholar | 28681306PubMed |
Bundt M, Krauss M, Blaser P, Wilcke W (2001) Forest fertilization with wood ash: effect on the distribution and storage of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Journal of Environmental Quality 30, 1296–1304.
| Forest fertilization with wood ash: effect on the distribution and storage of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs).Crossref | GoogleScholarGoogle Scholar | 11476508PubMed |
Campos I, Abrantes N, Vidal T, Bastos AC, Gonçalves F, Keizer JJ (2012) Assessment of the toxicity of ash-loaded runoff from a recently burnt eucalypt plantation. European Journal of Forest Research 131, 1889–1903.
| Assessment of the toxicity of ash-loaded runoff from a recently burnt eucalypt plantation.Crossref | GoogleScholarGoogle Scholar |
Chen H, Chow AT, Li XW, Ni HG, Dahlgren RA, Zeng H, Wang JJ (2018) Wildfire burn intensity affects the quantity and speciation of polycyclic aromatic hydrocarbons in soils. ACS Earth & Space Chemistry 2, 1262–1270.
| Wildfire burn intensity affects the quantity and speciation of polycyclic aromatic hydrocarbons in soils.Crossref | GoogleScholarGoogle Scholar |
Costa MR, Calvão AR, Aranha J (2014) Linking wildfire effects on soil and water chemistry of the Marão River watershed, Portugal, and biomass changes detected from Landsat imagery. Applied Geochemistry 44, 93–102.
| Linking wildfire effects on soil and water chemistry of the Marão River watershed, Portugal, and biomass changes detected from Landsat imagery.Crossref | GoogleScholarGoogle Scholar |
Diaz OA, Reddy KR, Moore PA (1994) Solubility of inorganic phosphorus in stream water as influenced by pH and calcium concentration. Water Research 28, 1755–1763.
| Solubility of inorganic phosphorus in stream water as influenced by pH and calcium concentration.Crossref | GoogleScholarGoogle Scholar |
Earl SR, Blinn DW (2003) Effects of wildfire ash on water chemistry and biota. Freshwater Biology 48, 1015–1030.
| Effects of wildfire ash on water chemistry and biota.Crossref | GoogleScholarGoogle Scholar |
Enell A, Fuhrman F, Lundin L, Warfvinge P, Thelin G (2008) Polycyclic aromatic hydrocarbons in ash: determination of total and leachable concentrations. Environmental Pollution 152, 285–292.
| Polycyclic aromatic hydrocarbons in ash: determination of total and leachable concentrations.Crossref | GoogleScholarGoogle Scholar | 17719155PubMed |
Ferreira AJD, Coelho COA, Boulet AK, Lopes FP (2005) Temporal patterns of solute loss following wildfires in central Portugal. International Journal of Wildland Fire 14, 401–412.
| Temporal patterns of solute loss following wildfires in central Portugal.Crossref | GoogleScholarGoogle Scholar |
Franklin NM, Stauber JL, Markich SJ, Lim RP (2000) pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.). Aquatic Toxicology 48, 275–289.
| pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.).Crossref | GoogleScholarGoogle Scholar | 10686332PubMed |
Freitas EC, Rocha O (2011) Acute and chronic effects of sodium and potassium on the tropical freshwater cladoceran Pseudosida ramosa. Ecotoxicology 20, 88–96.
| Acute and chronic effects of sodium and potassium on the tropical freshwater cladoceran Pseudosida ramosa.Crossref | GoogleScholarGoogle Scholar | 20978846PubMed |
Frišták V, Laughinghouse HD, Packová A, Graser M, Soja G (2019) Monitoring of methylated naphthalenes in sludge-derived pyrogenic carbonaceous materials. Chemosphere 217, 456–462.
| Monitoring of methylated naphthalenes in sludge-derived pyrogenic carbonaceous materials.Crossref | GoogleScholarGoogle Scholar | 30439658PubMed |
Gonino G, Benedito E, Branco P, Ferreira MT, Santos JM (2019a) Short-term effects of wildfire ash exposure on behaviour and hepatosomatic condition of a potamodromous cyprinid fish, the Iberian barbel Luciobarbus bocagei (Steindachner, 1864). The Science of the Total Environment 665, 226–234.
| Short-term effects of wildfire ash exposure on behaviour and hepatosomatic condition of a potamodromous cyprinid fish, the Iberian barbel Luciobarbus bocagei (Steindachner, 1864).Crossref | GoogleScholarGoogle Scholar | 30772552PubMed |
Gonino GMR, Figueiredo BRS, Manetta GI, Zaia Alves GH, Benedito E (2019b) Fire increases the productivity of sugarcane, but it also generates ashes that negatively affect native fish species in aquatic systems. The Science of the Total Environment 664, 215–221.
| Fire increases the productivity of sugarcane, but it also generates ashes that negatively affect native fish species in aquatic systems.Crossref | GoogleScholarGoogle Scholar | 30743114PubMed |
Hallema DW, Sun G, Caldwell PV, Norman SP, Cohen EC, Liu Y, Bladon KD, McNulty SG (2018) Burned forests impact water supplies. Nature Communications 9, 1307
| Burned forests impact water supplies.Crossref | GoogleScholarGoogle Scholar | 29636465PubMed |
Hellou J, Leonard J, Collier TK, Ariese F (2006) Assessing PAH exposure in feral finfish from the north-west Atlantic. Marine Pollution Bulletin 52, 433–441.
| Assessing PAH exposure in feral finfish from the north-west Atlantic.Crossref | GoogleScholarGoogle Scholar | 16364371PubMed |
Hoke RA, Giesy JP, Zabik M, Unger M (1993) Toxicity of sediments and sediment pore waters from the Grand Calumet River–Indiana Harbor, Indiana area of concern. Ecotoxicology and Environmental Safety 26, 86–112.
| Toxicity of sediments and sediment pore waters from the Grand Calumet River–Indiana Harbor, Indiana area of concern.Crossref | GoogleScholarGoogle Scholar | 7691537PubMed |
Jung HY, Hogue TS, Rademacher LK, Meixner T (2009) Impact of wildfire on source water contributions in Devil Creek, CA: evidence from end-member mixing analysis. Hydrological Processes 23, 183–200.
| Impact of wildfire on source water contributions in Devil Creek, CA: evidence from end-member mixing analysis.Crossref | GoogleScholarGoogle Scholar |
Fedje KK, Ekberg C, Skarnemark G, Steenari BM (2010) Removal of hazardous metals from MSW fly ash – an evaluation of ash-leaching methods. Journal of Hazardous Materials 173, 310–317.
| Removal of hazardous metals from MSW fly ash – an evaluation of ash-leaching methods.Crossref | GoogleScholarGoogle Scholar | 19744790PubMed |
Keiluweit M, Kleber M, Sparrow MA, Simoneit BRT, Prahl FG (2012) Solvent-extractable polycyclic aromatic hydrocarbons in biochar: influence of pyrolysis temperature and feedstock. Environmental Science & Technology 46, 9333–9341.
| Solvent-extractable polycyclic aromatic hydrocarbons in biochar: influence of pyrolysis temperature and feedstock.Crossref | GoogleScholarGoogle Scholar |
Keith LH (2015) The source of US EPA’s sixteen PAH priority pollutants. Polycyclic Aromatic Compounds 35, 147–160.
| The source of US EPA’s sixteen PAH priority pollutants.Crossref | GoogleScholarGoogle Scholar |
Khanna PK, Raison RJ, Falkiner RA (1994) Chemical properties of ash derived from eucalyptus litter and its effects on forest soils. Forest Ecology and Management 66, 107–125.
| Chemical properties of ash derived from eucalyptus litter and its effects on forest soils.Crossref | GoogleScholarGoogle Scholar |
Kim EJ, Choi SD, Chang YS (2011) Levels and patterns of polycyclic aromatic hydrocarbons (PAHs) in soils after forest fires in South Korea. Environmental Science and Pollution Research International 18, 1508–1517.
| Levels and patterns of polycyclic aromatic hydrocarbons (PAHs) in soils after forest fires in South Korea.Crossref | GoogleScholarGoogle Scholar | 21553034PubMed |
Mast MA, Clow DW (2008) Effects of 2003 wildfires on stream chemistry in Glacier National Park, Montana. Hydrological Processes 22, 5013–5023.
| Effects of 2003 wildfires on stream chemistry in Glacier National Park, Montana.Crossref | GoogleScholarGoogle Scholar |
Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM (1997) Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environmental Toxicology and Chemistry 16, 2009–2019.
| Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows).Crossref | GoogleScholarGoogle Scholar |
Musset ML (2006) ‘Current approaches in the statistical analysis of ecotoxicity data: a guidance to application’ OECD Environment Health and Safety Publications - Series on Testing and Assessment. 54
Nikinmaa M (2014) An introduction to aquatic toxicology. (Academic Press – Elsevier: Oxford)
Nunes B, Silva V, Campos I, Pereira JL, Pereira P, Keizer JJ, Gonçalves F, Abrantes N (2017) Off-site impacts of wildfires on aquatic systems – biomarker responses of the mosquitofish Gambusia holbrooki. The Science of the Total Environment 581–582, 305–313.
| Off-site impacts of wildfires on aquatic systems – biomarker responses of the mosquitofish Gambusia holbrooki.Crossref | GoogleScholarGoogle Scholar | 28088544PubMed |
OECD (2004) OECD Guideline for testing of chemicals: OECD 202. Daphnia sp., acute immobilisation test. (Organisation for Economic Cooperation and Development: Paris)
Oliveira-Filho EC, Brito DQ, Dias ZMB, Guarieiro MS, Carvalho EL, Fascineli ML, Niva CC, Grisolia CK (2018) Effects of ashes from a Brazilian savanna wildfire on water, soil and biota: an ecotoxicological approach. The Science of the Total Environment 618, 101–111.
| Effects of ashes from a Brazilian savanna wildfire on water, soil and biota: an ecotoxicological approach.Crossref | GoogleScholarGoogle Scholar | 29127867PubMed |
Olivella MA, Ribalta TG, De Febrer AR, Mollet JM, De Las Heras FXC (2006) Distribution of polycyclic aromatic hydrocarbons in riverine waters after Mediterranean forest fires. The Science of the Total Environment 355, 156–166.
| Distribution of polycyclic aromatic hydrocarbons in riverine waters after Mediterranean forest fires.Crossref | GoogleScholarGoogle Scholar | 15885751PubMed |
Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J (2009) The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. The International Journal of Biochemistry & Cell Biology 41, 1665–1677.
| The biochemistry of environmental heavy metal uptake by plants: implications for the food chain.Crossref | GoogleScholarGoogle Scholar |
Pereira P, Úbeda X (2010) Spatial distribution of heavy metals released from ashes after a wildfire. Journal of Environmental Engineering and Landscape Management 18, 13–22.
| Spatial distribution of heavy metals released from ashes after a wildfire.Crossref | GoogleScholarGoogle Scholar |
Pereira P, Beda X, Martin D, Mataix-Solera J, Guerrero C (2011) Effects of a low-severity prescribed fire on water-soluble elements in ash from a cork oak (Quercus suber) forest located in the north-east of the Iberian Peninsula. Environmental Research 111, 237–247.
| Effects of a low-severity prescribed fire on water-soluble elements in ash from a cork oak (Quercus suber) forest located in the north-east of the Iberian Peninsula.Crossref | GoogleScholarGoogle Scholar | 20869047PubMed |
Pérez-Fernández B, Viñas L, Franco MÁ, Bargiela J (2015) PAHs in the Ría de Arousa (NW Spain): a consideration of PAHs sources and abundance. Marine Pollution Bulletin 95, 155–165.
| PAHs in the Ría de Arousa (NW Spain): a consideration of PAHs sources and abundance.Crossref | GoogleScholarGoogle Scholar | 25960270PubMed |
Pilliod DS, Bury RB, Hyde EJ, Pearl CA, Corn PS (2003) Fire and amphibians in North America. Forest Ecology and Management 178, 163–181.
| Fire and amphibians in North America.Crossref | GoogleScholarGoogle Scholar |
Pitman RM (2006) Wood ash use in forestry – a review of the environmental impacts. Forestry 79, 563–588.
| Wood ash use in forestry – a review of the environmental impacts.Crossref | GoogleScholarGoogle Scholar |
Plumlee GS, Martin DA, Hoefen T, Kokaly R, Eckberg A, Meeker GP, Adams M, Anthony M, Lamothe PJ (2007) Preliminary analytical results for ash and burned soils from the October 2007 southern California wildfires. U.S. Geological Survey. Open-File Report 2007-1407
Rey-Salgueiro L, Martínez-Carballo E, Merino A, Vega JA, Fonturbel MT, Simal-Gandara J (2018) Polycyclic aromatic hydrocarbons in soil organic horizons depending on the soil burn severity and type of ecosystem. Land Degradation & Development 29, 2112–2123.
| Polycyclic aromatic hydrocarbons in soil organic horizons depending on the soil burn severity and type of ecosystem.Crossref | GoogleScholarGoogle Scholar |
Robinson L, Thorn I (2005) Toxicology and ecotoxicology in chemical safety assessment. (Blackwell Publishing. Oxford)
Santín C, Doerr SH, Otero XL, Chafer CJ (2015) Quantity, composition and water contamination potential of ash produced under different wildfire severities. Environmental Research 142, 297–308.
| Quantity, composition and water contamination potential of ash produced under different wildfire severities.Crossref | GoogleScholarGoogle Scholar | 26186138PubMed |
Santín C, Doerr SH, Merino A, Bucheli TD, Bryant R, Ascough P, Gao X, Masiello CA (2017) Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Scientific Reports 7, 11233
| Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars.Crossref | GoogleScholarGoogle Scholar | 28894167PubMed |
Santín C, Otero XL, Doerr SH, Chafer CJ (2018) Impact of a moderate/high-severity prescribed eucalypt forest fire on soil phosphorous stocks and partitioning. The Science of the Total Environment 621, 1103–1114.
| Impact of a moderate/high-severity prescribed eucalypt forest fire on soil phosphorous stocks and partitioning.Crossref | GoogleScholarGoogle Scholar | 29103642PubMed |
Scholze M, Knorr W, Arnell NW, Prentice IC (2006) A climate-change risk analysis for world ecosystems. Proceedings of the National Academy of Sciences of the United States of America 103, 13116–13120.
| A climate-change risk analysis for world ecosystems.Crossref | GoogleScholarGoogle Scholar | 16924112PubMed |
Scott G, Crunkilton RL (2000) Acute and chronic toxicity of nitrate to fathead minnows (Pimephales promelas), Ceriodephnia dubia and Daphnia magna. Environmental Toxicology and Chemistry 19, 2918–2922
| Acute and chronic toxicity of nitrate to fathead minnows (Pimephales promelas), Ceriodephnia dubia and Daphnia magna.Crossref | GoogleScholarGoogle Scholar |
Shakesby RA, Doerr SH (2006) Wildfire as a hydrological and geomorphological agent. Earth-Science Reviews 74, 269–307.
| Wildfire as a hydrological and geomorphological agent.Crossref | GoogleScholarGoogle Scholar |
Silva V, Pereira JL, Campos I, Keizer JJ, Gonçalves F, Abrantes N (2015) Toxicity assessment of aqueous extracts of ash from forest fires. Catena 135, 401–408.
| Toxicity assessment of aqueous extracts of ash from forest fires.Crossref | GoogleScholarGoogle Scholar |
Simplício N, Muniz D, Rocha F, Martins D, Dias Z, Farias B, Oliveira-Filho E (2016) Comparative analysis between ecotoxicity of nitrogen-, phosphorus-, and potassium-based fertilizers and their active ingredients. Toxics 5,
| Comparative analysis between ecotoxicity of nitrogen-, phosphorus-, and potassium-based fertilizers and their active ingredients.Crossref | GoogleScholarGoogle Scholar | 29051434PubMed |
Smith HG, Sheridan GJ, Lane PNJ, Nyman P, Haydon S (2011) Wildfire effects on water quality in forest catchments: a review with implications for water supply. Journal of Hydrology 396, 170–192.
| Wildfire effects on water quality in forest catchments: a review with implications for water supply.Crossref | GoogleScholarGoogle Scholar |
Stiernström S, Lindé M, Hemström K, Wik O, Ytreberg E, Bengtsson BE, Breitholtz M (2013) Improved understanding of key elements governing the toxicity of energy ash eluates. Waste Management 33, 842–849.
| Improved understanding of key elements governing the toxicity of energy ash eluates.Crossref | GoogleScholarGoogle Scholar | 23312131PubMed |
Stumm W, Morgan J (1995) Aquatic chemistry: chemical equilibria and rates in natural waters. (Wiley-Interscience, New York, NY, USA).
Tian Q, Guo B, Nakama S, Sasaki K (2018) Distributions and leaching behaviors of toxic elements in fly ash. ACS Omega 3, 13055–13064.
| Distributions and leaching behaviors of toxic elements in fly ash.Crossref | GoogleScholarGoogle Scholar |
Tsai KP, Uzun H, Karanfil T, Chow AT (2017) Dynamic changes of disinfection byproduct precursors following exposures of Microcystis aeruginosa to wildfire ash solutions. Environmental Science & Technology 51, 8272–8282.
| Dynamic changes of disinfection byproduct precursors following exposures of Microcystis aeruginosa to wildfire ash solutions.Crossref | GoogleScholarGoogle Scholar |
USEPA (United States Environmental Protection Agency) (2016) Aquatic invertebrate acute toxicity test, freshwater daphnids. EPA-OCSPP-850-1010. (US Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention: Washington, DC, USA)
Vila-Escalé M, Vegas-Vilarrúbia T, Prat N (2007) Release of polycyclic aromatic compounds into a Mediterranean creek (Catalonia, NE Spain) after a forest fire. Water Research 41, 2171–2179.
| Release of polycyclic aromatic compounds into a Mediterranean creek (Catalonia, NE Spain) after a forest fire.Crossref | GoogleScholarGoogle Scholar | 17397897PubMed |
Viñas L, Franco MA, González JJ (2009) Polycyclic aromatic hydrocarbon composition of sediments in the Ría de Vigo (NW Spain). Archives of Environmental Contamination and Toxicology 57, 42–49.
| Polycyclic aromatic hydrocarbon composition of sediments in the Ría de Vigo (NW Spain).Crossref | GoogleScholarGoogle Scholar | 18825447PubMed |
Weiner ER (2012) Applications of environmental aquatic chemistry: a practical guide. 3rd edn. (CRC Press: Boca Raton, FL, USA)
Wilde KL, Stauber JL, Markich SJ, Franklin NM, Brown PL (2006) The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.). Archives of Environmental Contamination and Toxicology 51, 174–185.
| The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.).Crossref | GoogleScholarGoogle Scholar | 16583260PubMed |
World Health Organization (2011) Guidelines for drinking-water quality – 4th edn. (World Health Organization: Geneva)






