The role of decomposer communities in managing surface fuels: a neglected ecosystem service
H. Gibb A * , J. J. Grubb A , O. Decker A , N. Murphy
A , N. Murphy  A , A. E. Franks A and J. L. Wood A
A , A. E. Franks A and J. L. Wood A
A Research Centre for Future Landscapes, School of Life Sciences, La Trobe University, Bundoora, Melbourne, Vic. 3086, Australia.
International Journal of Wildland Fire 31(4) 350-368 https://doi.org/10.1071/WF21112
Submitted: 14 September 2020 Accepted: 18 February 2022 Published: 7 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of IAWF. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY).
Abstract
Surface fuel loads are a key driver of forest fires and the target of hazard reduction burns to reduce fire risk. However, the role of biota in decomposition, or feedbacks between fire and decomposer communities are rarely considered. We review the evidence that decomposer organisms play an important role in surface fuel regulation and how this role is affected by fire. First, we outline the contribution of decomposer organisms to the breakdown of surface fuels. Next, we consider the three distinct phases through which fire regulates decomposer communities and how this may affect decomposition and future fire regimes. Finally, we consider interactions between global change and decomposer–fire feedbacks and the implications for fire management. Evidence indicates that decomposer organisms are important in regulating surface fuels and we propose that the biological basis and dynamic nature of fuel load control require greater attention. This includes better understanding of functional redundancy among decomposer organisms, the impacts of global change on the biota that drive decomposition and the factors that limit decomposer persistence and recolonisation following fires. By filling these knowledge gaps, we will be better armed to conserve and manage these functionally critical taxa in fire-prone ecosystems in a changing world.
Keywords: biodiversity, climate change, dead wood, decomposition, ecosystems, fuel, invertebrates, leaf litter.
Introduction
Ninety gigatons of terrestrial plant biomass, amounting to 90% of annual production, enters the global pool of dead organic matter each year (Cebrian 1999). The biomass is transformed into inorganic forms via complex decomposition steps or exits the pool through consumption by fire. While biological decomposition transforms organic material into microbial mass or stable humic substances, dead organic material consumed by fire is mostly lost to the air as carbon dioxide and methane (Schimmel and Granstrom 1996; Aerts 1997; Miyanishi and Johnson 2002; Van Wagtendonk 2006). Fire is a necessary process in many ecosystems, shaping the distribution of biomes and maintaining the structure and function of fire-prone communities (Bradstock et al. 2002; Bond and Keeley 2005; Bowman et al. 2009). Fires generally start with the ignition of dead organic matter in the surface layer. These surface fuels include fine fuels such as leaves, twigs and bark lying on the ground (Hines et al. 2010), as well as fallen branches and shorter vegetation (Scott and Reinhardt 2001). Across many ecosystems, the spread of fire is driven primarily by the fine fuel component of surface fuels (as well as weather and topography) (Raison et al. 1983; Catchpole 2002).
While the role of biodiversity in fire regimes is increasingly acknowledged, recent work focuses on vegetation (Duff et al. 2017), largely ignoring the role of other biota. Fire not only consumes surface fuels, but also disrupts communities of organisms that decompose those fuels. Invertebrates and microbes are the key players in the decomposition of surface fuels in many ecosystems, yet their role in decomposition is not well studied in the context of fire (Brennan et al. 2009; Buckingham et al. 2015) and invertebrate decomposers were considered only briefly in a recent review of animals as agents of fire (Foster et al. 2020). We propose that decomposer-driven changes in the fire-available fuel have the potential to alter fire frequency, extent and severity, thus creating a feedback cycle among decomposers, surface fuels and fire (Fig. 1). For example, increased fire frequency creates hotter and drier climates, which inhibits litter consumption by decomposer organisms, therefore increasing fuel loads (Hernández and Hobbie 2008; Brennan et al. 2009; Toberman et al. 2014). Further, changes in fire regimes due to global change are likely to have significant impacts on the decomposer species that evolved with natural fire regimes. Despite the potential importance of these feedbacks and the significant economic cost of attempts to control fire, little is understood of the ability of ecosystems to regulate litter and dead wood build-up naturally through the biodiversity of decomposer organisms.
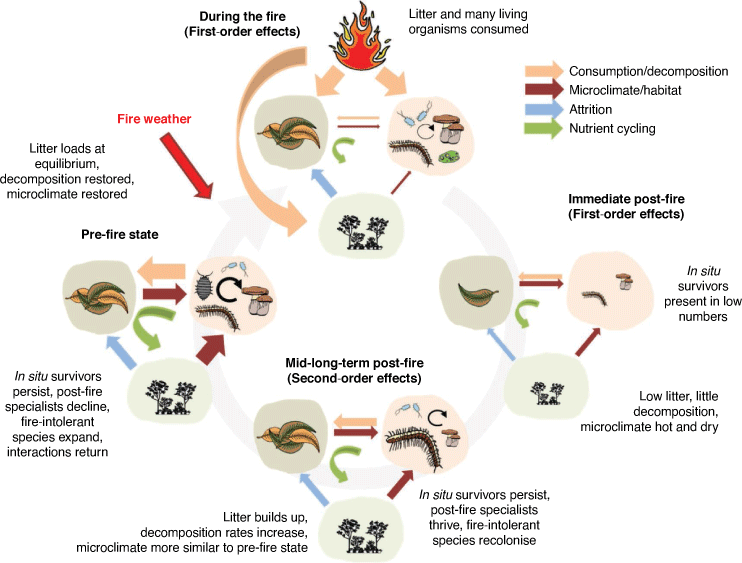
Decomposition is primarily a biological process, driven by decomposer organisms, which are affected by the same anthropogenic perturbations (e.g. climate change and habitat fragmentation) that control the persistence of any species. Accurate predictions of fuel loads and their trajectories through time must therefore incorporate an understanding of the biological nature of decomposition, particularly in wetter ecosystems, where biological decomposition is more important (García-Palacios et al. 2013). Here, we review the contribution of decomposer biodiversity to regulating surface fuel dynamics across biomes and consider how decomposer biodiversity and the function of decomposition are influenced in three distinct temporal phases: (1) during the fire; (2) the immediate post-fire environment (both considered first-order effects); and (3) the long-term regenerated environment (second-order effects). We argue that feedbacks between decomposer biodiversity and fuel loads have the potential to affect the flammability of environments, but that gaps in knowledge prevent us from determining the importance of this effect. Further, we consider the potential impacts of anthropogenic change on the complex relationship between fire, fuels and decomposer organisms. We conclude by considering how fire management programs might better conserve the function of decomposition, thereby allowing biodiversity to better regulate the accumulation of fuels.
Decomposer organisms and their ecosystem service
At a global scale, the decomposition of surface fuels is driven by a range of factors, with climate, litter quality and the soil and litter biota critical in regulating decomposition (Coûteaux et al. 1995; Zhang et al. 2008; García-Palacios et al. 2013). The impacts of soil biota on decomposition are regulated by climate and litter quality and therefore differ substantially among biomes (García-Palacios et al. 2013). The effect of soil fauna exclusion is most pronounced in wetter climates (González and Seastedt 2001) and a meta-analysis suggested that unexplained variation in large-scale decomposition models could be reduced by including biome-specific fauna effects on litter decomposition (García-Palacios et al. 2013).
Both invertebrates and microbes are important in litter decomposition. Invertebrates are directly responsible for an average of 37% of litter loss and up to 20% of wood loss on the forest floor annually across the globe (García-Palacios et al. 2013; Ulyshen 2016). Leaf litter decomposes considerably faster than woody debris (Gessner et al. 2010) and most studies examining the role of decomposers have focused on litter, with few examining woody debris (see Kampichler and Bruckner 2009; García-Palacios et al. 2013; Ulyshen and Wagner 2013). However, the breakdown of wood often requires specialised invertebrate species, which are linked to specific tree traits (Cornwell et al. 2009; Weedon et al. 2009; Zuo et al. 2016; Dossa et al. 2018). Functionally, although there is overlap in roles, invertebrate detritivores can be classified in three general ways: bioturbators that ingest and mix organic matter in the soil (e.g. earthworms and termites), shredders that physically fragment organic matter including transforming it into faeces (e.g. macroinvertebrates, including amphipods, woodlice, millipedes, dipteran larvae and saproxylic beetles) and grazers that consume microbes on detritus (e.g. microinvertebrates, including nematodes, mites and springtails) (Anderson 1988; David 2014; Hoang et al. 2017; Joly et al. 2018; McCary and Schmitz 2021). Bioturbation accelerates decomposition by transporting organic matter to a moister environment and bringing it into closer contact with other decomposers (Ashton and Bassett 1997; Lavelle et al. 2006; Coulis et al. 2016). In addition, it affects soil structure in numerous ways, including creating and degrading soil aggregates and pore formation, which increases decomposition by improving soil hydraulic properties and creating habitat space for smaller decomposers, especially microarthropods (Lavelle 1997; Lavelle et al. 2006; Hoang et al. 2017). Recent work shows that millipede, isopod and snail faeces improve decomposition rates relative to intact litter (Joly et al. 2020). This is likely driven by increasing organic matter lability and consequent leaching, rather than enhanced microbial activity, which is often lower in faeces (after an initial increase) (Joly et al. 2020). In Mediterranean shrubland, burial of millipede faeces led to further improvements of decomposition rates, suggesting possible synergistic effects with bioturbation (Coulis et al. 2016). Assimilation of organic matter consumed by invertebrates ranges from <10% (e.g. some millipedes) to >50% (e.g. some isopods, dipteran larvae), indicating that some taxa are capable of direct litter mineralisation (Dangerfield 1998; David 2014; Abd El-Wakeil 2015; Frouz 2018). Microarthropods, dominated by Collembola and Acari (springtails and mites), contribute to decomposition by moving organic material, which impacts soil aggregation and creates microbial hotspots (Chamberlain et al. 2006; Maaß et al. 2015; Soong and Nielsen 2016).
Although decomposition is often facilitated by invertebrates, the remaining 70–80% of annual decomposition is generally performed by the soil microbiota, which break down recalcitrant compounds such as cellulose and lignin. For example, fungi from the Agaricomycetes are the only organisms that produce enzymes capable of substantial lignin degradation, making them keystone taxa in the decomposition of woody debris (Floudas et al. 2012). Bacteria also contribute to wood and leaf litter decomposition and are capable of producing enzymes for cellobiose, pectin and even lignin degradation (Schink et al. 1981; Brown and Chang 2014; Lladó et al. 2016). While bacteria may not be as proficient at wood decay as fungi, anaerobic taxa such as Acetivibrio, Clostridium and Ruminococcus are primarily responsible for cellulose decomposition in anaerobic ecosystems such as peatlands (Boer et al. 2005). Bacterial nitrogen fixation (N-fixation) is known to occur in decaying wood and may assist in stimulating the decomposition process by replenishing nitrogen in a system that naturally has a high C:N ratio (Johnston et al. 2016; Bani et al. 2018). N-fixing bacteria from the order Rhizobiales, Rhodocyclales, Pseudomonadales, Rhodospirillales, Sphingomonadales and Burkholderiales have all been associated with decaying wood. Litter decomposition studies focusing on bacterial communities have revealed clear successional patterns. In general, leaf surface (phyllosphere) bacteria are present in the first stage of the decomposition but are rapidly replaced with taxa producing proteolytic and cellulolytic enzymes. In the later phase, the most common bacterial taxa are Bradyzhorium, Streptomyces and Burkholderia, the latter of which is strongly associated with the presence of fungi and can utilise fungal hyphae as migratory routes (Johnston et al. 2016; Purahong et al. 2016; Tláskal et al. 2016; Bani et al. 2018).
Interactions among species affect decomposition rates (Gessner et al. 2010). For example, the joint effects of woodlice (Isopoda) and earthworms (Annelida) are stronger than the sum of single-species effects on alder litter, but weaker on oak litter (Zimmer et al. 2005). Decomposition can also be enhanced by multi-trophic interactions (Hedlund and Öhrn 2000) and altered by litter disturbance or predation by vertebrates (Wyman 1998; Nugent et al. 2014) or herbivory by invertebrates prior to leaf fall (Kay et al. 2008). Although microbes are responsible for the majority of decomposition (by mass), their function is mediated by invertebrate decomposers (Lavelle et al. 1995): microbial breakdown is governed by accessibility, which is often enhanced by the action of litter-dwelling invertebrates, which mix the litter and shred and bore into plant matter, increasing the surface area available to bacteria and fungi (Beare et al. 1995; Wolters 2000; Dungait et al. 2012). Invertebrate detritivores may directly consume microbes and commonly have a symbiotic microbe community to assist in digestion of dead plant material (Zimmer and Topp 1998; Ulyshen 2016). The preferential grazing of soil fungi by litter-dwelling insects has been shown to alter fungal community competition dynamics, increasing fungal diversity (Crowther et al. 2013). Further, invertebrates are likely to be vectors for bacterial and fungal detritivores, but these phoretic relationships are poorly understood (but see Persson et al. 2011; Aylward et al. 2015; Jacobsen et al. 2017). This is particularly relevant in the context of recolonisation following disturbances, where insect-mediated transport has the potential to accelerate the recovery of microbe-driven decomposition.
Interactions among microbes may also be critical in the decomposition process: fungi facilitate bacterial penetration into leaves and wood (Boer et al. 2005), while wood decay proceeds at a greater rate when fungi are associated with N-fixing bacteria than when they are not (Blanchette and Shaw 1978; Hoppe et al. 2014). Bacteria enhance decomposition rates by consuming fungal decomposition products, thus stimulating fungi to upregulate their own production of degradative enzymes (Boer et al. 2005; Christofides et al. 2019). Alternatively, bacteria capable of lysing fungal hyphae, either to consume as a food source (mycophagy) or to avoid direct competition for cellulose, may reduce rates of wood decay (Tolonen et al. 2015). The variety of potential interactions among decomposer organisms serves to highlight the complexity of the community that drives decomposition.
Given their significant diversity, the most feasible approach to understanding decomposer communities and variation in their rates of decomposition may be trait-based because this allows us to understand the broader patterns despite limited knowledge of the biology of individual species (McCary and Schmitz 2021). This may be a particularly pertinent approach for microbial decomposers, for which the ability to link genes with function is progressing rapidly (e.g. Langille et al. 2013). For example, genes encoding degradative enzymes and bacterial N-fixation are potential targets for studying decomposer–fire–climate interactions. For invertebrates, dispersal traits have been linked with recolonisation following fire (Langlands et al. 2011; Buckingham et al. 2019). Fewer studies have attempted to link invertebrate decomposer traits with decomposition, but the feeding traits of detritivores covary with the palatability traits of leaf litter (Brousseau et al. 2019; Raymond-Léonard et al. 2019) and determine their impact on decomposition (e.g. detritus shredders, bioturbators and detritus grazers increase decomposition by 28, 22 and 15%, respectively, McCary and Schmitz 2021). Further, trait dissimilarity within detritivore assemblages is positively correlated with decomposition rate as more dissimilar species are more likely to have complementary (rather than redundant) effects on decomposition (Heemsbergen et al. 2004).
Effects of fire on decomposer composition across ecosystems
Fire-prone ecosystems cover a spectrum from fuel-limited to climate-limited ecosystems (Agee 1993; Noss et al. 2006; Pausas and Paula 2012; Steel et al. 2015). In fuel-limited ecosystems, frequent fires consume mostly live and recently dead herbaceous vegetation and litter, where flaming and residence times are short, there is little organic soil and minimal soil heating and most regrowth occurs through resprouting. In contrast, in climate-limited ecosystems, fire-return intervals and succession periods are longer, with greater fuel loads (including duff) accumulating. Across this spectrum, the characteristics of fire range from high frequency and low severity to low frequency and high severity, resulting in different interactions between fire and the decomposer community, depending on the ecosystem. Many species are adapted to natural fire regimes (Gill 1975), resulting in a cyclical change in species communities. Fire impacts decomposer communities through both physical and chemical changes to habitats and substrates, in three distinct temporal phases: (1) during the fire (first-order); (2) the immediate post-fire environment (also first-order); and (3) the longer-term regenerated environment (second-order) (Fig. 1). The extent of the changes to biodiversity wrought by a single fire and length of each of these phases vary among biomes.
First-order effects – during the fire
The immediate effect of fire on decomposer communities depends mainly on severity and duration (DeBano et al. 1998). Heating during fire causes the loss of nutrients and carbon via volatilisation and combustion of litter and soil organic matter, which may have long-term impacts on the ecosystem. The direct and immediate impacts of fire on soil- and ground-dwelling organisms vary among ecosystems. Radiation provides enough heat to combust surface fuels and often kills organisms in the upper soil layer (Wikars and Schimmel 2001). Soil temperature during a fire depends on soil moisture (lower in moister soils; Burrows 1999; Busse et al. 2005), fuel load and soil composition, including rock and moisture content (e.g. Bradstock and Auld 1995; Stoof et al. 2011). High soil temperatures may be short-lived and heat intensity declines with depth, but heat penetration varies among ecosystems (Humphreys and Lambert 1965; Raison et al. 1986; Miranda et al. 1993; Schimmel and Granstrom 1996; Yoshikawa et al. 2002; Carrington 2010; Wanthongchai et al. 2011; Cawson et al. 2016). For example, soil temperatures in cerrado fires in central Brazil do not exceed 38°C below 2-cm depth (Miranda et al. 1993), whereas they may reach up to 80°C at 15-cm depth in jarrah forests in western Australia (Burrows 1999). Depths of 2–10 cm are generally sufficient to avoid lethal temperatures (of 30–60°C) for most invertebrates (Hoffmann et al. 2013; Grubb 2019), suggesting in situ survival is possible. Individuals buried deeper in the soil may stand a very good chance of survival: for example, Thom et al. (2015) showed that butterfly pupae buried deeper than 1.75 cm, where soil temperatures remained ~40°C or lower, had a 50% chance of survival, even where surface temperatures reach 500°C. Further, short heating residence times in fuel-limited savannas and woodlands may have little direct effect on microbes (Hansen et al. 2019) or the abundance and diversity of soil biota (Oliver et al. 2015; Semenova‐Nelsen et al. 2019; Hopkins et al. 2020).
The quality of surface fuels also impacts the survivorship of soil organisms: for example, coarser fuels (e.g. charcoal) can increase the duration for which peak temperatures are held within the soil by up to 10 min, which is long enough to sterilise spores even of pyrophilous fungi (Bruns et al. 2020). In shrubland, fire experiments show that soil temperatures can be lowest where fuel loads are highest, probably owing to greater upwards heat transfer and decreased flame residence time (Stoof et al. 2013). While communities of soil biota may thus suffer a decrease in diversity and biomass during the fire, the impact of fire is spatially heterogeneous (Tangney et al. 2018), and variable among ecosystems (DeBano et al. 1998; Neary et al. 1999; Certini 2005; Pressler et al. 2019).
The mechanisms through which decomposer organisms survive fire in situ have received little attention. For invertebrates and microbes, avoidance usually involves escape to or existence deep in the soil or in unburnt refuges in less severe fires (e.g. in Xanthorrhoea, Brennan et al. 2011; under soil, Dell et al. 2017; or up trees, Sensenig et al. 2017). Survival may be enhanced by extreme temperature tolerance (Kassen 2002) and morphological and behavioural adaptations that allow escape underground. Further, plant endophytic microbial communities can be aerosolised during the combustion and burning of vegetation, which may assist in their survival, dispersal and reestablishment (Moore et al. 2021).
The presence of invertebrate species with limited dispersal capacities after severe fire likely results from in situ survival in burrows or other refuges (e.g. ground-nesting bees, Love and Cane 2016; amphipods, Menz et al. 2016). Low-severity fires can even increase microbial activity in the top 0–10 cm of soil in the short term, owing to warming effects (Bogorodskaya et al. 2010). In Mediterranean ecosystems of south-eastern Australia, amphipods burrow deeper in the soil to avoid desiccation in hotter months (Friend and Richardson 1977; Menz et al. 2016), potentially enhancing survival at the time of year that wildfires occur, but not necessarily protecting against hazard reduction burns in cooler times of year. The recovery of the detritivore community from fire is critical for the resumption of their important role in reducing subsequent fuel loads, so we would benefit from a better understanding of the potential for populations to survive fires in situ and how it varies with ecosystem, fire severity and season.
First-order effects – immediate post-fire environment
Most studies of the impacts of fire on biodiversity in general focus on the period between the fire event and re-establishment or resprouting of the vegetation, which can occur within a fraction of the burning season (e.g. Eva and Lambin 1998; Trigg and Flasse 2000) or take decades (Haslem et al. 2011; Dafni et al. 2012; Bright et al. 2019), depending on the ecosystem. Burning causes distinct shifts in community composition for both microbes and invertebrates (Vilariño and Arines 1991; Baar et al. 1999; York 2000; Simard et al. 2001; New et al. 2010). A recent meta-analysis of 131 studies across a range of biomes found that, while fire decreases diversity of key taxa, impacts on abundance may differ among organisms: fire reduced microorganism abundance by up to 96% (bacteria were more resistant than fungi) and nematode abundance by 88%, but had no effect on soil mesofauna abundance (Pressler et al. 2019). Interestingly, prescribed fires had a more significant impact in the subsoil than wildfires, but the opposite was true for the surface soil fauna. Further, prescribed fire impacts depended on ecosystem types: for example, fire reduced arthropod richness more in grasslands than in forests, probably because vertical movement in the soil is more limited in grasslands (Pressler et al. 2019). Surprisingly, Pressler et al. (2019) were unable to detect a signal of time since fire, in contrast to studies that focussed on invertebrates (e.g. Barratt et al. 2006; Huebner et al. 2012; Oliver et al. 2012).
The community of decomposers present in the immediate post-fire environment includes species that survived the fire in situ and those that dispersed from outside or from refuges inside the fire perimeter following the fire (Robinson et al. 2013), including some fire-favoured or even pyrophilic species (Schütz et al. 1999; Gibb et al. 2006; Johansson et al. 2011; Egidi et al. 2016; Eliott et al. 2019). In climate-limited ecosystems (those where fires are severe and infrequent), these organisms face a very different environment from that experienced prior to the fire. Following fire, the soil biogeochemistry and organic habitat may be severely altered (Baird et al. 1999; González-Pérez et al. 2004; Dove et al. 2020; Pellegrini et al. 2020). Leaf fall may initially increase as scorched foliage falls, rapidly renewing habitat structure (although resource quality may differ) (Springett 1979); alternatively, all foliage may be consumed by canopy fires (Cruz et al. 2012). In high-severity fires in forests, the destruction of the canopy results in microclimate changes such as increased soil temperatures and decreased soil moisture and humidity (Neary et al. 1999). In such ecosystems, species persisting after the fire are expected to be more generalised and tolerant of a broader range of temperatures and soil moistures than those that existed prior to the fire (Kassen 2002). However, there is little evidence that a single fire event causes strong selection for greater physiological tolerance of variable temperatures or low humidity within species in fire-prone forests (Grubb 2019), and fire regime may be more critical than time since fire in selection for fire-tolerant phenotypes.
In addition to changes in microclimate, severe fire also causes far-reaching changes in the nutrient balance, marked changes in hydrothermal conditions and a loss of microhabitat heterogeneity (Butler et al. 2019). These factors act as filters that determine the development of post-fire soil communities of microorganisms and invertebrates, which, in climate-limited ecosystems, can be drastically different from the pre-fire community (Certini 2005). Reestablishment of the microbial community after fire may be hampered in such ecosystems by the reduction of labile forms of organic matter, and changes to soil carbon and soil chemical properties. For example, the combustion of wood and litter has been shown to form recalcitrant carbon molecules enriched in aromatic structures that inhibit microbial saprotrophic growth (Kim et al. 2003). Soil N can also be sequestered into recalcitrant carbon compounds, following burning, depleting the soil of readily accessible N and C reserves, which may contribute to reduced decomposition rates (Mastrolonardo et al. 2014). Understanding soil community recovery post burning may assist in understanding decomposition rates in other habit strata during later stages of succession. For example, while woody debris has a community distinct from that of soils, the soil community is highly predictive of the community in a given piece of woody detritus, suggesting decomposer communities are recruited from the surrounding soil (Sun et al. 2014).
Fire-driven shifts in species functional traits also have the potential to alter decomposition rates. For example, fire severity changes in the post-fire size distribution of invertebrate detritivores (Buckingham et al. 2015; Podgaiski et al. 2018) might affect microbial decomposition by altering the size of litter fragments; and, in grassy ecosystems, fire decreases the prevalence of microbial functional traits related to carbon degradation, which may lead to increased stabilisation of recalcitrant C during the post-fire recovery of the community (Yang et al. 2020).
Second-order fire effects – mid-long-term habitat regeneration
The post-fire habitat proceeds through a succession, as the biota recolonise and re-establish and species are filtered by the changing environment (Sasal et al. 2010; Huebner et al. 2012; Gongalsky and Persson 2013; Haslem et al. 2016; Auclerc et al. 2019). In many fire-prone ecosystems, this return to a pre-fire-like state may be rapid, but in others, habitats may continue to regenerate for decades or even centuries (Haslem et al. 2011; Dafni et al. 2012; Bright et al. 2019). Further, depending on the potential for multiple stable states and the fire interval, the ecosystem may not converge on the pre-fire state (Beckage and Ellingwood 2008; Wood and Bowman 2012; Auclerc et al. 2019).
Different inter-fire intervals (or fire frequencies) are associated with differences in the way in which species are filtered through their physiological tolerance, behaviour and dispersal ability: ecosystems with rapid fire cycles support species that survive the fire or are able to rapidly disperse and re-establish, while those with slower fire cycles support an ongoing succession that may eventually include poor dispersers and fire-intolerant decomposer species (Force 1981; Holliday 1991; Siemann et al. 1997; Malmström 2012). For species unable to survive the fire in situ, dispersal ability is critical to persistence at a landscape scale (Andersen and Müller 2000; Thomas 2000; Whelan et al. 2002; Schmuki et al. 2006). The presence of a mosaic of patches of differing time since fire (pyrodiversity, Parr and Andersen 2006) may provide a colonisation source for species unable to persist during the fire. The interaction between dispersal ability, distance from source populations and the inter-fire interval is thus critical in determining persistence in the landscape (Arnold et al. 2017): low dispersal ability can increase the time for a species to recolonise when inter-fire intervals are long or prevent recolonisation altogether when inter-fire intervals are short (Pippin and Nichols 1996; Panzer 2002; Whelan et al. 2002; Schmuki et al. 2006). While decreased inter-fire intervals might be expected to select for a spectrum of traits that protect species from fire (avoidance behaviours, tolerance and dispersal), fires of increased severity and extent are likely to favour an assemblage dominated by high-dispersal generalist species, which could lead to functional homogenisation of the decomposer community at larger scales (e.g. Clavel et al. 2011; Malmström 2012). Species with traits similar to those of the pre-fire ecosystem are expected to thrive as time since fire increases, but the actual species composition may differ from the pre-fire composition, depending on species’ ability to recolonise or survive the fire. For example, Korobushkin et al. (2017) and Moretti et al. (2006) showed that less-mobile invertebrates suffered the greatest declines and recovered poorly 5 years after fire.
Fire-induced changes in soils may be long-lived in some ecosystems. For example, in coniferous forest, reductions in microbial N/organic N ratios remained 12 years after controlled burns, particularly in the surface layer (0–5 cm) (Fritze et al. 1993; Prieto-Fernández et al. 1998). However, organic carbon has been shown to increase or remain unchanged in most pine-dominated communities, where fire intervals are short (McKee 1982; Godwin et al. 2017; Pellegrini et al. 2018). Shorter inter-fire intervals may also increase the temperature and reduce the moisture of soils, which can reduce decomposition rates (Silveira et al. 2009). The result is that decreased inter-fire intervals are associated with distinct decomposer communities (e.g. fungi, Oliver et al. 2015) and declines in decomposition rates across a range of ecosystems (e.g. temperate forests, Brennan et al. 2009; subtropical sclerophyll forests, Butler et al. 2019; pine savannas, Semenova‐Nelsen et al. 2019; Hopkins et al. 2020). However, the impacts on one taxon may be compensated for by another (e.g. invertebrates may compensate for reduced microbial litter decomposition, Butler et al. 2019).
Most studies of fire effects on invertebrate and microbe assemblages in ecosystems with long fire cycles focus on the period prior to vegetation recovery, but it is critical that we understand the full cycle of decomposer communities between fires. Where inter-fire intervals are shortened by management or anthropogenic global change, impacts on decomposer communities and decomposition are expected to be greatest in ecosystems where fire cycles were historically longest.
How do fire and decomposition interact?
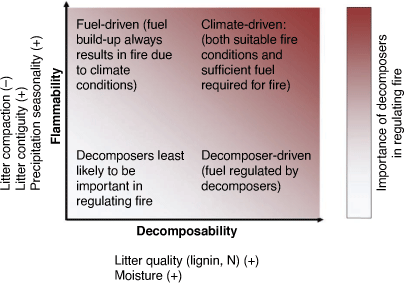
Whether dead plant material follows the pathway of biological decomposition or consumption by fire depends on the traits of leaves, the spatial distribution of vegetation, and climate, all of which vary among biomes (Cornelissen et al. 2017; Pausas and Bond 2020). Leaf biochemical traits determine the decomposability of leaves: leaves that are high in nitrogen but low in lignin and with a low leaf mass per unit area are more decomposable (Cadisch and Giller 1997, p. 409; Cornwell et al. 2008) (Fig. 2). Further, plant traits (such as leaf and stem size) and eventually their litter particle size and shape (particularly curliness) are critical in the ignitability and flammability of litter because larger leaves produce a less compact and more ventilated litter bed that dries more rapidly and is more flammable (Scarff and Westoby 2006; Cornwell et al. 2015; Zylstra et al. 2016) (Fig. 2). At a larger scale, the continuity of vegetation is important in the spread of fire: discontinuous surface fuels often cannot support surface fires (Cornelissen et al. 2017). Finally, climate affects flammability and decomposition through drying – biological decomposition is much slower in drier climates, while flammability is higher. The chance of ignition is also climate-dependent, although human population pressure plays a major role (Ganteaume et al. 2013). Decomposition has the greatest potential to limit fire where both flammability and decomposability are high and that includes biomes such as savannahs and broad-leaved forests (Cornelissen et al. 2017) (Fig. 2).
|
In the absence of fire, more than half of net primary production returns to the soil through decomposition (Wardle et al. 2004). The importance of decomposition in regulating fire is likely to be most important in ecosystems with highly flammable surface fuels, where those fuels are also highly decomposable (Cornelissen et al. 2017) (Fig. 2). Globally, approximately 60–70% of litter decomposition rates are explained by climate and litter quality (Parton et al. 2007). However, soil fauna enhance litter decomposition by 35% (globally, García-Palacios et al. 2013), but their importance varies with biome. Understanding variation in decomposer community contributions and its regulation by climate may therefore be critical in reducing unexplained variation in large-scale decomposition models (García-Palacios et al. 2013). We suggest that understanding how fire alters decomposition across different ecosystems may similarly provide an important contribution to our ability to predict fuel accumulation and manage fire.
The decomposition of dead plant matter is not well studied in the context of fire. Most litterbag studies suggest that prescribed fire reduces decomposition rates relative to unburned controls, although the timeframe differs among climates and vegetation types (Springett 1976; Raison et al. 1983, 1986; Monleon and Cromack 1996; Brennan et al. 2009; Toberman et al. 2014; Köster et al. 2016). However, studies have also found no impacts of time since fire or fire severity on decomposition (Grigal and McColl 1977; Raison et al. 1986; Buckingham et al. 2015; De Long et al. 2016) and even increased decomposition following fire (Throop et al. 2017; Bryanin et al. 2018), highlighting the context dependency of fire impacts on decomposition. Repeated fires create different biodiversity dynamics from single-fire events, and changes in fire frequency (or inter-fire interval) may change the microhabitat and resources for decomposer organisms. More frequent fires decrease decomposition rates and create hotter and drier microclimates, lower litter quality and C:N ratios, and reduce soil C and N, pointing to mechanisms through which litter consumption by decomposer organisms is affected (Hernández and Hobbie 2008; Brennan et al. 2009; Toberman et al. 2014; Cornelissen et al. 2017; Pellegrini et al. 2018). However, detritivore assemblages may change following fire without altering decomposition rates (Vasconcelos and Laurance 2005; Buckingham et al. 2015). Although this suggests that functional redundancy could be high in some ecosystems, most studies indicate a strong link between decomposer assemblages and decomposition (reviewed in García-Palacios et al. 2013).
Functional diversity among detritivores, rather than species richness, drives decomposition rates in laboratory experiments (Heemsbergen et al. 2004). Dynamics of decomposer recolonisation thus do not necessarily indicate rates of functional recovery, particularly if a function is largely driven by a small number of functionally important species. The relationship between decomposition by invertebrates and the traits involved in recolonising an area following fire remains an important knowledge gap. Function is commonly strongly dictated by phylogeny (e.g. Martiny et al. 2013), as are traits associated with recolonisation (e.g. thermal tolerance, Sunday et al. 2012), so it is likely that different functional groups return to the system at different rates. This might reflect availability of their resources, for example, scavengers often increase in abundance with time since fire (e.g. Driscoll et al. 2020). Where inter-fire intervals vary substantially in a landscape or change over time, the period for recolonisation of populations may exceed the inter-fire interval. The result could be that functional groups within the decomposer community are lost, altering rates of decomposition and potentially leading to increased fuel accumulation.
Surface fuel loads are expected to reach an asymptote (equilibrium) over time since fire, with a steady state achieved where litter fall is offset by decomposition (Olson 1963; Fensham 1992). However, litter and woody surface fuel loads show a range of responses to time since fire, remaining constant, peaking, or continuing to increase over time depending on the ecosystem (Schimmel and Granström 1997; Moritz et al. 2004; Gosper et al. 2013; Eskelson and Monleon 2018). Surface fuel loads are often predicted using coarse-scale vegetation maps and time-since-fire relationships (Tolhurst et al. 2008; Price and Gordon 2016). Most models of surface fuel accumulation assume constant decomposition rates throughout post-fire succession (Olson 1963; Raison et al. 1983; McCarthy et al. 2001), although the influence of climatic factors on decomposition is sometimes considered (e.g. Miller and Urban 1999). Inclusion of decomposition as a constant rate in models has long been considered a gross simplification, with early studies acknowledging the importance of season and recent fire history in determining decomposition rate (e.g. Fox et al. 1979; Birk and Simpson 1980), but none to date tackling the complex issue of decomposer assemblages.
The assumption of functional redundancy among decomposer organisms is not supported by laboratory studies (e.g. Heemsbergen et al. 2004; Zimmer et al. 2005) and has not been addressed in the field. This limits our ability to understand spatial and temporal variation in fuel–fire relationships. Oversimplification of the biology and function of decomposer communities reduces the likelihood that we are accurately modelling decomposition (Prescott 2005). It is particularly important to get this right if there is a risk that some approaches to fire management may threaten the very organisms that control fuel loads.
Feedbacks
Surface fuel loads depend on decomposition rates, coming to an equilibrium when decomposition equals input (Minderman 1968). Biological decomposition is slower following fire, explaining why litter build-up following fires is accentuated, particularly where fires are frequent (Raison et al. 1983; Fernandes and Botelho 2003; Litton et al. 2003; Yang et al. 2020). This is likely a direct result of the documented negative impacts of fire on the decomposer community (Pressler et al. 2019) (Fig. 1). Most fire dampening provided by hazard-reduction burns lasts no more than 10 years (Eucalyptus forest and heathland, south-eastern Australia, Sackett 1975; Pinus ponderosa, USA, Wagle and Eakle 1979; Eucalyptus forest, south-eastern Australia, Rawson et al. 1985; shrublands in Western Australia, McCaw et al. 1992; heath and open woodland (mallee) in southern Australia, Grant and Wouters 1993; Pinus pinaster, Spain, Moreira da Silva 1997; shrublands in France, Lambert et al. 1999; Pinus ponderosa, Pollet and Omi 2002), and effectiveness is highly weather-dependent (Fernandes and Botelho 2003). This rapid return to initial fuel loads in some ecosystems with historically long inter-fire intervals encourages forest managers to reduce the inter-fire interval for prescribed burning. However, increased fire frequency (or fire interval squeeze) decreases decomposition rates (Brennan et al. 2009; Butler et al. 2019; Hopkins et al. 2020), thus leading to a greater perceived need for prescribed burning.
The impact of prescribed burning on soil communities may be very different from the impact of the cyclical natural fire regimes to which the biota are adapted (Knapp et al. 2009). In addition to the impacts of increased frequency described above, this may be due to differences in intensity or the timing of fires relative to species’ phenology. For example, in south-eastern Australia, prescribed burns typically occur in spring or autumn, when soils are moister, which is generally when soil communities are more active and, therefore more prone to perturbation (Menz et al. 2016). Further, although moist soils have a reduced heating capacity, the lethal temperature needed to kill soil microbes is dramatically reduced as soil moisture facilitates more effective heat penetration and faster heat distribution: lethal temperatures for bacteria are in excess of 200°C for dry soils, but considerably lower (50–60°C) in moist soils (DeBano 1991). However, moist soils can also protect soil biota: in grasslands, where fires are short-lived, the high specific heat of water can serve to buffer the effects of heating and reduce the likelihood of ignition of organic matter in the soil (Wells 1979).
If inter-fire intervals are ‘squeezed’ (Enright et al. 2015) such that decomposer assemblages are unable to recover within the inter-fire interval, then litter decomposition rates will decrease (Springett 1976; Raison et al. 1986; Monleon and Cromack 1996; Brennan et al. 2009), which may increase the risk of future fires. The recovery of decomposer assemblages is likely to be dictated not only by fire frequency, but also by seasonality, severity and extent, as these factors interact with recolonisation and persistence traits of species, determining whether an assemblage is dominated by superior dispersers or in situ survivors. More frequent, severe, or larger-extent fires may compound the local loss of decomposer diversity. At a landscape scale, source populations may be eliminated. Even if functional effects are not immediately obvious, species richness is associated with functional diversity in heterogeneous environments (Tylianakis et al. 2008) and over time (Reich et al. 2012). It is thus likely that the maintenance of decomposer assemblages is critical in maintaining function and allowing ecosystems to self-regulate litter loads.
Global change, fire and biological decomposition
Biological decomposition is performed by biota, so prediction of the consequences of global change for decomposition requires a better knowledge of how those organisms respond to threats such as landscape modification, climate change and species invasions. These threats will impact fire regimes, altering fire severity, seasonality, extent and frequency (Fig. 3) (third-order fire effects). At the same time, they will alter species assemblages. Further, changes in fire regimes and assemblages of detritivores may act synergistically to complicate outcomes for decomposition.
Landscape modification
Landscape modification remains the most significant threat to biodiversity (Segan et al. 2016). A range of studies have explored the impact of anthropogenic modifications, such as habitat fragmentation, on biodiversity. Fragmentation of forests results in exposure to warmer and more desiccating microclimates, and stronger winds (Malcolm 1998; Briant et al. 2010). This leads to changes in plant species composition (sometimes leading to N-poor litter in small fragments) (Riutta et al. 2012) and vegetation structure (Didham and Lawton 1999). It also decreases rates of litter decomposition in small, isolated fragments and at patch edges (Didham 1998; Martinson and Fagan 2014). Declines in decomposition rates in habitat fragments have been attributed to microclimate (drier climates at forest edges decrease decomposition rates) and changes the composition of detritivore assemblages (de Souza and Brown 1994; Grove 2002; Vasconcelos and Laurance 2005).
At a landscape-scale, the likelihood of fragments burning may differ substantially from the original continuous habitat. Increased anthropogenic use of fire in the modified matrix acts synergistically with the drier edges and decreased decomposition rates of fragmented tropical forests to increase the frequency of fire (Laurence and Vasconcelos 2004). However, fragmentation of flammable habitats can inhibit fire spread at a landscape scale (Duncan and Schmalzer 2004). Protection of small urban fragments from fire can result in less flammable plant communities and fundamental changes in the species composition of litter invertebrates (Gibb and Hochuli 2002). Where fragments do experience fire, they are less likely to be recolonised by dispersal-poor detritivores (Driscoll and Weir 2005; Schmuki et al. 2006). The impacts of habitat fragmentation on fuel loads may thus be difficult to predict, depending on the balance between reduced fire spread and altered fuel loads, and any fragmentation impacts on the decomposers.
Climate change
Global models show that climate is an important driver of litter decomposition (Liski et al. 2003; García-Palacios et al. 2013), and it is therefore likely that changing climate will change decomposition rates. Climate change encompasses a variety of changes, including increasing carbon dioxide (CO2) and temperature, changes in precipitation, and increases in the frequency of extreme weather events (IPCC 2012; National Academies of Sciences, Engineering, and Medicine 2016), including the extremely hot and dry conditions that commonly precede major fires (Hennessy et al. 2005; Nolan et al. 2020). Climate change is thus likely to alter many elements of fire regimes, including fire extent, severity, frequency and seasonality (Fig. 3). As a result, the interaction between future climate projections, fuel loads and decomposition is likely to be complex.
An accurate prediction of how the decomposition of fuel will respond to changes in climate is dependent not just on changes in physical factors, but also shifts in species distributions and changing plant and decomposer communities (Castro et al. 2010; Harris et al. 2016; Koltz et al. 2018). Whereas warming and increased CO2 may increase decomposition rates, studies also predict that under warming conditions, plant communities will change (e.g. Davidson and Janssens 2006; Scherrer et al. 2017), altering the type of litter available to decomposers. Where plant community composition remains unchanged, the nutrient content of fresh litter may decline (Manea et al. 2015; Kasurinen et al. 2017), thus altering the quality of litter (De Long et al. 2016). Further, interactions between invertebrates and microbes change with temperature and soil moisture (Coulis et al. 2013; A’Bear et al. 2014) and the synergistic impact of these changes on decomposition is likely to differ among ecosystems.
In a rapidly changing climate, establishment conditions are also likely to change with time, such that recovering ecosystems may not converge on a pre-fire state. For example, seasonal increases in soil moisture following fire are hypothesised to be critical in facilitating the recovery of soil microbial community structure and function to a pre-burnt state (D’Ascoli et al. 2005; Prendergast-Miller et al. 2017). This suggests that any changes in fire seasonality may alter the direction of community recovery. The same is likely to be true of climate-limited changes in fire frequency, severity and extent.
Species invasions
Invasive species may enhance or inhibit fire, depending on the species and ecosystem. Exotic plants can alter ecosystem flammability (D’Antonio and Vitousek 1992; Mack and D’Antonio 1998; Brooks et al. 2004; Rothstein et al. 2004; Arthur et al. 2012), for example, buffelgrass (Cenchrus ciliaris) invasions increase fuel loads and burn severity (Miller et al. 2010). Litter of invasive species may also decompose more rapidly than native litter (Jo et al. 2016): many successful invaders possess traits, such as limited investment in permanent structures, that are also associated with rapid decomposition (Van Kleunen et al. 2010). Although decomposers are rarely species-specific, they are often adapted to particular plant traits (Kembel and Mueller 2014), such that plant invasions result in changed decomposer assemblages (Cameron and Spencer 1989). Invasive plant species may also alter decomposition through their impact on microclimates, since decomposition rates depend on temperature and moisture (Vallés et al. 2011; Watling et al. 2011). Alternatively, they may affect decomposition indirectly, by altering habitat suitability for decomposer species (Gratton and Denno 2005; Foster et al. 2021).
Invasive predators may dramatically alter ecosystems through their impacts on detritivores. For example, exotic crazy ants (Anoplolepis gracilipes) on Christmas Island caused dramatic increases in forest floor litter loads through predation on native red crabs, a key litter consumer (O’Dowd et al. 2003). Invasive decomposers also affect fuel loads: invasive earthworms are important litter decomposers in the USA and are therefore likely to inhibit fire. However, they live in the litter layer, so are more negatively affected by fire than are native earthworms, which tend to be soil-living (Callaham et al. 2003; Ikeda et al. 2015). In contrast, high abundances of invasive detritivores, such as bark beetles, can increase tree death following fire (Houston 1981; Goldammer and Penafiel 1990), thus increasing fuel for future fires. The feedbacks among species invasion, decomposition and fire are poorly understood, but are clearly important in understanding fire risk in a changing world.
Management implications
Within those fire-prone forests, fire management occurs largely through hazard reduction burns aimed at reducing fine surface fuels, which are the critical habitat of detritivores that naturally undertake biological fuel reduction. The appropriateness of this approach may depend on where on the spectrum of climate-limited to fuel-limited an ecosystem belongs and a one-size fits all approach may lead to negative outcomes in some ecosystems. Inappropriate fire management, i.e. management that leads to population extinctions (Kelly et al. 2015), may lead to a negative feedback loop (Fig. 1). In that case, fire would cause declines in detritivore assemblages, leading to a reduction in litter decomposition, which would be expected to accelerate the accumulation of flammable fuel. This could lead to a requirement for more frequent managed burns, but increased fire frequency is associated with greater loss of biodiversity and function (Hernández and Hobbie 2008; Brennan et al. 2009; Toberman et al. 2014). It is therefore critical that fire managers recognise that surface fuel loads are controlled by organisms and that these organisms and their interactions must be better understood if we are to rebuild diverse and functionally intact decomposer communities and retain the function of decomposition following fire. Active monitoring of not just surface fuels, but also decomposition rates and litter fall would provide the detailed information required to better model this ecosystem service.
Conservation of functionally intact decomposer communities will be achieved through attention to in situ conditions during and after prescribed burns, burn season and the scale and heterogeneity of those burns, and proximity to refuges or other potential colonisation sources in the landscape. Like most species, detritivores have evolved with a natural fire regime. Conflicts between frequent hazard reduction and conservation will be low if there is a history of frequent low-intensity surface fires, but high if the natural fire regime consists of infrequent high-intensity fires (Fernandes and Botelho 2003). Managers may be able to alter the intensity, frequency, seasonality and extent of prescribed fires to mimic natural fires, but mimicking high-intensity, large-extent fires may not be desirable if the aim of prescribed burning is to minimise damage to human populations and infrastructure.
In addition to difficulties in mimicking ‘natural’ fire regimes, today’s unmanaged fires may differ considerably from those of the recent past. A range of anthropogenic factors functioning at larger scales than most fire management operations conspire to alter fire regimes. These include climate change, anthropogenic alteration of landscape structure and composition and changes in the role of people in starting and managing fires, for example, the loss of indigenous fire management, long-term fire suppression or increases in accidental or intentional arson (Bradstock 2010; Ganteaume et al. 2013). Some of the same factors may also limit the ability of species to respond, e.g. temperature affects invertebrate development rates and willingness to fly (Bale et al. 2002; Abram et al. 2017), while habitat degradation and fragmentation drive species loss and limit sources for recolonisation (Didham et al. 1996). Of these factors, landscape structure offers the greatest opportunity for manipulation at a local scale, for example by regulating burn heterogeneity (Kral et al. 2017). However, management must be based on sound knowledge of the dispersal or in situ survival capacities of decomposers across functional groups. It should also be tailored to the ecosystem, considering landscape structure, the position on the climate- and fuel-limited spectrum and traits of the biota.
Additionally, active management of the decomposer communities could provide an avenue for overcoming some of the negative feedbacks associated with increasing fire size, severity and frequency and its interactions with global change. This could involve preserving small-scale litter refuges during management burns (e.g. Holland et al. 2017), enabling recolonisation to occur over much smaller spatial scales, or addition of cellulose into severely burnt soils to favour the development of fungal mycelium and increase microbial C (Fritze et al. 1993). Alternatively, following extreme fires, active litter transplants or targeted transplants of key decomposers to rapidly restore both the invertebrate and microbial components of decomposer communities and their critical ecosystem processes could be used the way that faecal transplants are used for human health or soil transplants for agricultural health (Contos et al. 2021). Similar treatments following hazard reduction burns might assist in lengthening their effectiveness, but these potential solutions remain untested.
Synthesis
Decomposer organisms are important in regulating surface fuels in many ecosystems and fuel loads drive fire. Feedbacks among fire, fuel and decomposer organisms are likely to be important in determining fire regimes (Fig. 1) and how they will respond to global change and should be incorporated into management. However, key questions regarding the relationship between fire, fuel and decomposers remain unanswered (Table 1). For example, data on the importance of decomposer organisms and changes in decomposition rates following fire are currently insufficient to incorporate into models predicting fire risk or spread. In addition to their important functional roles in nutrient cycling and in terrestrial food webs, decomposer organisms present a significant portion of the biodiversity supporting and supported by the surface fuel layer. Efforts can and should be made to conserve and manage these functionally critical taxa in fire-prone ecosystems, particularly those with historically long inter-fire intervals.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported through an Australian Research Council Future Fellowship to HG (FT130100821) and funding to HG, NM and AF from the La Trobe University Research Focus Area of Securing Food, Water and the Environment. Neither funder played a role in the research direction after granting the funding.
Author contributions
HG, NM, AEF conceived ideas for the paper. HG drafted the first version of the manuscript. All authors contributed critically to subsequent drafts and gave final approval for publication.
Supplementary material
Supplementary material is available online.
Acknowledgements
We thank Lucy Johanson for her illustrations and Andrew Bennett and anonymous reviewers for thoughts on earlier versions of the manuscript.
References
Abd El-Wakeil KF (2015) Effects of terrestrial isopods (Crustacea: Oniscidea) on leaf litter decomposition processes. The Journal of Basic & Applied Zoology 69, 10–16.| Effects of terrestrial isopods (Crustacea: Oniscidea) on leaf litter decomposition processes.Crossref | GoogleScholarGoogle Scholar |
A’Bear AD, Jones TH, Boddy L (2014) Potential impacts of climate change on interactions among saprotrophic cord-forming fungal mycelia and grazing soil invertebrates. Fungal Ecology 10, 34–43.
| Potential impacts of climate change on interactions among saprotrophic cord-forming fungal mycelia and grazing soil invertebrates.Crossref | GoogleScholarGoogle Scholar |
Abram PK, Boivin G, Moiroux J, Brodeur J (2017) Behavioural effects of temperature on ectothermic animals: unifying thermal physiology and behavioural plasticity. Biological Reviews 92, 1859–1876.
| Behavioural effects of temperature on ectothermic animals: unifying thermal physiology and behavioural plasticity.Crossref | GoogleScholarGoogle Scholar | 28980433PubMed |
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79, 439–449.
| Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship.Crossref | GoogleScholarGoogle Scholar |
Agee JK (1993) ‘Fire ecology of Pacific Northwest forests.’ (Island Press: Washington DC, USA)
Andersen AN, Müller WJ (2000) Arthropod responses to experimental fire regimes in an Australian tropical savannah: ordinal‐level analysis. Austral Ecology 25, 199–209.
| Arthropod responses to experimental fire regimes in an Australian tropical savannah: ordinal‐level analysis.Crossref | GoogleScholarGoogle Scholar |
Anderson JM (1988) Invertebrate-mediated transport processes in soils. Agriculture, Ecosystems & Environment 24, 5–19.
| Invertebrate-mediated transport processes in soils.Crossref | GoogleScholarGoogle Scholar |
Arnold KT, Murphy NP, Gibb H (2017) Post-fire recovery of litter detritivores is limited by distance from burn edge. Austral Ecology 42, 94–102.
| Post-fire recovery of litter detritivores is limited by distance from burn edge.Crossref | GoogleScholarGoogle Scholar |
Arthur MA, Bray SR, Kuchle CR, McEwan RW (2012) The influence of the invasive shrub, Lonicera maackii, on leaf decomposition and microbial community dynamics. Plant Ecology 213, 1571–1582.
| The influence of the invasive shrub, Lonicera maackii, on leaf decomposition and microbial community dynamics.Crossref | GoogleScholarGoogle Scholar |
Ashton DH, Bassett OD (1997) The effects of foraging by the superb lyrebird (Menura novae-hollandiae) in Eucalyptus regnans forests at Beenak, Victoria. Austral Ecology 22, 383–394.
| The effects of foraging by the superb lyrebird (Menura novae-hollandiae) in Eucalyptus regnans forests at Beenak, Victoria.Crossref | GoogleScholarGoogle Scholar |
Auclerc A, Le Moine JM, Hatton P-J, Bird JA, Nadelhoffer KJ (2019) Decadal post-fire succession of soil invertebrate communities is dependent on the soil surface properties in a northern temperate forest. Science of the Total Environment 647, 1058–1068.
| Decadal post-fire succession of soil invertebrate communities is dependent on the soil surface properties in a northern temperate forest.Crossref | GoogleScholarGoogle Scholar |
Aylward J, Dreyer LL, Steenkamp ET, Wingfield MJ, Roets F (2015) Long-distance dispersal and recolonization of a fire-destroyed niche by a mite-associated fungus. Fungal Biology 119, 245–256.
| Long-distance dispersal and recolonization of a fire-destroyed niche by a mite-associated fungus.Crossref | GoogleScholarGoogle Scholar | 25813511PubMed |
Baar J, Horton TR, Kretzer AM, Bruns TD (1999) Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire. The New Phytologist 143, 409–418.
| Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire.Crossref | GoogleScholarGoogle Scholar |
Baird M, Zabowski D, Everett RL (1999) Wildfire effects on carbon and nitrogen in inland coniferous forests. Plant and Soil 209, 233–243.
| Wildfire effects on carbon and nitrogen in inland coniferous forests.Crossref | GoogleScholarGoogle Scholar |
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology 8, 1–16.
| Herbivory in global climate change research: direct effects of rising temperature on insect herbivores.Crossref | GoogleScholarGoogle Scholar |
Bani A, Pioli S, Ventura M, Panzacchi P, Borruso L, Tognetti R, Tonon G, Brusetti L (2018) The role of microbial community in the decomposition of leaf litter and deadwood. Applied Soil Ecology 126, 75–84.
| The role of microbial community in the decomposition of leaf litter and deadwood.Crossref | GoogleScholarGoogle Scholar |
Barratt BIP, Tozer PA, Wiedemer RL, Ferguson CM, Johnstone PD (2006) Effect of fire on microarthropods in New Zealand indigenous grassland. Rangeland Ecology & Management 59, 383–391.
| Effect of fire on microarthropods in New Zealand indigenous grassland.Crossref | GoogleScholarGoogle Scholar |
Beare MH, Coleman DC, Crossley DA, Hendrix PF, Odum EP (1995) A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant and Soil 170, 5–22. http://www.jstor.org/stable/42947356
Beckage B, Ellingwood C (2008) Fire feedbacks with vegetation and alternative stable states. Complex Systems 18, 159
| Fire feedbacks with vegetation and alternative stable states.Crossref | GoogleScholarGoogle Scholar |
Birk EM, Simpson R (1980) Steady state and the continuous input model of litter accumulation and decomposition in Australian eucalypt forests. Ecology 61, 481–485.
| Steady state and the continuous input model of litter accumulation and decomposition in Australian eucalypt forests.Crossref | GoogleScholarGoogle Scholar |
Blanchette RA, Shaw CG (1978) Associations among bacteria, yeasts and basidiomycetes during wood decay. Phytopathology 68, 631–637.
| Associations among bacteria, yeasts and basidiomycetes during wood decay.Crossref | GoogleScholarGoogle Scholar |
Boer Wd, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiology Reviews 29, 795–811.
| Living in a fungal world: impact of fungi on soil bacterial niche development.Crossref | GoogleScholarGoogle Scholar | 16102603PubMed |
Bogorodskaya AV, Krasnoshchekova EN, Bezkorovainaya IN, Ivanova GA (2010) Post-fire transformation of microbial communities and invertebrate complexes in the pine forest soils, Central Siberia. Contemporary Problems of Ecology 3, 653–659.
| Post-fire transformation of microbial communities and invertebrate complexes in the pine forest soils, Central Siberia.Crossref | GoogleScholarGoogle Scholar |
Bond WJ, Keeley JE (2005) Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends in Ecology & Evolution 20, 387–394.
| Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems.Crossref | GoogleScholarGoogle Scholar |
Bowman DMJS, Balch JK, Artaxo P, et al. (2009) Fire in the Earth system. Science 324, 481–484.
| Fire in the Earth system.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA (2010) A biogeographic model of fire regimes in Australia: current and future implications. Global Ecology and Biogeography 19, 145–158.
| A biogeographic model of fire regimes in Australia: current and future implications.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Auld TD (1995) Soil temperatures during experimental bushfires in relation to fire intensity – consequences for legume germination and fire management in south-eastern Australia. Journal of Applied Ecology 32, 76–84.
| Soil temperatures during experimental bushfires in relation to fire intensity – consequences for legume germination and fire management in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Bradstock RA, Williams JE, Gill MA (2002) ‘Flammable Australia: the fire regimes and biodiversity of a continent.’ (Cambridge University Press: Melbourne, Australia)
Brennan KEC, Christie FJ, York A (2009) Global climate change and litter decomposition: more frequent fire slows decomposition and increases the functional importance of invertebrates. Global Change Biology 15, 2958–2971.
| Global climate change and litter decomposition: more frequent fire slows decomposition and increases the functional importance of invertebrates.Crossref | GoogleScholarGoogle Scholar |
Brennan KEC, Moir ML, Wittkuhn RS (2011) Fire refugia: The mechanism governing animal survivorship within a highly flammable plant. Austral Ecology 36, 131–141.
| Fire refugia: The mechanism governing animal survivorship within a highly flammable plant.Crossref | GoogleScholarGoogle Scholar |
Briant G, Gond V, Laurance SGW (2010) Habitat fragmentation and the desiccation of forest canopies: a case study from eastern Amazonia. Biological Conservation 143, 2763–2769.
| Habitat fragmentation and the desiccation of forest canopies: a case study from eastern Amazonia.Crossref | GoogleScholarGoogle Scholar |
Bright BC, Hudak AT, Kennedy RE, Braaten JD, Henareh Khalyani A (2019) Examining post-fire vegetation recovery with Landsat time series analysis in three western North American forest types. Fire Ecology 15, 8
| Examining post-fire vegetation recovery with Landsat time series analysis in three western North American forest types.Crossref | GoogleScholarGoogle Scholar |
Brooks ML, D’antonio CM, Richardson DM, Grace JB, Keeley JE, DiTomaso JM, Hobbs RJ, Pellant M, Pyke D (2004) Effects of invasive alien plants on fire regimes. Bioscience 54, 677–688.
| Effects of invasive alien plants on fire regimes.Crossref | GoogleScholarGoogle Scholar |
Brousseau PM, Gravel D, Handa IT (2019) Traits of litter‐dwelling forest arthropod predators and detritivores covary spatially with traits of their resources. Ecology 100, e02815
| Traits of litter‐dwelling forest arthropod predators and detritivores covary spatially with traits of their resources.Crossref | GoogleScholarGoogle Scholar | 31287928PubMed |
Brown ME, Chang MCY (2014) Exploring bacterial lignin degradation. Current Opinion in Chemical Biology 19, 1–7.
| Exploring bacterial lignin degradation.Crossref | GoogleScholarGoogle Scholar | 24780273PubMed |
Bruns TD, Chung JA, Carver AA, Glassman SI (2020) A simple pyrocosm for studying soil microbial response to fire reveals a rapid, massive response by Pyronema species. PLoS One 15, e0222691
| A simple pyrocosm for studying soil microbial response to fire reveals a rapid, massive response by Pyronema species.Crossref | GoogleScholarGoogle Scholar | 32130222PubMed |
Bryanin S, Abramova E, Makoto K (2018) Fire-derived charcoal might promote fine root decomposition in boreal forests. Soil Biology and Biochemistry 116, 1–3.
| Fire-derived charcoal might promote fine root decomposition in boreal forests.Crossref | GoogleScholarGoogle Scholar |
Buckingham S, Murphy N, Gibb H (2015) The effects of fire severity on macroinvertebrate detritivores and leaf litter decomposition. PLoS One 10, e0124556
| The effects of fire severity on macroinvertebrate detritivores and leaf litter decomposition.Crossref | GoogleScholarGoogle Scholar | 25880062PubMed |
Buckingham S, Murphy N, Gibb H (2019) Effects of fire severity on the composition and functional traits of litter-dwelling macroinvertebrates in a temperate forest. Forest Ecology and Management 434, 279–288.
| Effects of fire severity on the composition and functional traits of litter-dwelling macroinvertebrates in a temperate forest.Crossref | GoogleScholarGoogle Scholar |
Burrows ND (1999) A soil heating index for interpreting ecological impacts of jarrah forest fires. Australian Forestry 62, 320–329.
| A soil heating index for interpreting ecological impacts of jarrah forest fires.Crossref | GoogleScholarGoogle Scholar |
Busse MD, Hubbert KR, Fiddler GO, Shestak CJ, Powers RF (2005) Lethal soil temperatures during burning of masticated forest residues. International Journal of Wildland Fire 14, 267–276.
| Lethal soil temperatures during burning of masticated forest residues.Crossref | GoogleScholarGoogle Scholar |
Butler OM, Lewis T, Rezaei Rashti M, Maunsell SC, Elser JJ, Chen C (2019) The stoichiometric legacy of fire regime regulates the roles of micro‐organisms and invertebrates in decomposition. Ecology 100, e02732
| The stoichiometric legacy of fire regime regulates the roles of micro‐organisms and invertebrates in decomposition.Crossref | GoogleScholarGoogle Scholar | 30993678PubMed |
Cadisch G, Giller K (1997) ‘Driven by nature: plant residue quality and decomposition’. (CAB International, Wallingford, UK)
Callaham MA, Hendrix PF, Phillips RJ (2003) Occurrence of an exotic earthworm (Amynthas agrestis) in undisturbed soils of the southern Appalachian Mountains, USA. Pedobiologia 47, 466–470.
| Occurrence of an exotic earthworm (Amynthas agrestis) in undisturbed soils of the southern Appalachian Mountains, USA.Crossref | GoogleScholarGoogle Scholar |
Cameron GN, Spencer SR (1989) Rapid leaf decay and nutrient release in a Chinese tallow forest. Oecologia 80, 222–228.
| Rapid leaf decay and nutrient release in a Chinese tallow forest.Crossref | GoogleScholarGoogle Scholar | 28313111PubMed |
Carrington ME (2010) Effects of soil temperature during fire on seed survival in Florida sand pine scrub. International Journal of Forestry Research 2010, 1–10.
| Effects of soil temperature during fire on seed survival in Florida sand pine scrub.Crossref | GoogleScholarGoogle Scholar |
Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW (2010) Soil microbial community responses to multiple experimental climate change drivers. Applied and Environmental Microbiology 76, 999–1007.
| Soil microbial community responses to multiple experimental climate change drivers.Crossref | GoogleScholarGoogle Scholar | 20023089PubMed |
Catchpole W (2002) Fire properties and burn patterns in heterogeneous landscapes. In ‘Flammable Australia: the fire regimes and biodiversity of a continent’. (Eds RA Bradstock, JE Williams, AM Gill) pp. 50–75. (Cambridge University Press: Cambridge, UK)
Cawson JG, Nyman P, Smith HG, Lane PNJ, Sheridan GJ (2016) How soil temperatures during prescribed burning affect soil water repellency, infiltration and erosion. Geoderma 278, 12–22.
| How soil temperatures during prescribed burning affect soil water repellency, infiltration and erosion.Crossref | GoogleScholarGoogle Scholar |
Cebrian J (1999) Patterns in the fate of production in plant communities. The American Naturalist 154, 449–468.
| Patterns in the fate of production in plant communities.Crossref | GoogleScholarGoogle Scholar | 10523491PubMed |
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143, 1–10.
| Effects of fire on properties of forest soils: a review.Crossref | GoogleScholarGoogle Scholar | 15688212PubMed |
Chamberlain P, Mcnamara N, Chaplow J, Stott A, Black H (2006) Translocation of surface litter carbon into soil by Collembola. Soil Biology and Biochemistry 38, 2655–2664.
| Translocation of surface litter carbon into soil by Collembola.Crossref | GoogleScholarGoogle Scholar |
Christofides SR, Hiscox J, Savoury M, Boddy L, Weightman AJ (2019) Fungal control of early-stage bacterial community development in decomposing wood. Fungal Ecology 42, 100868
| Fungal control of early-stage bacterial community development in decomposing wood.Crossref | GoogleScholarGoogle Scholar |
Clavel J, Julliard R, Devictor V (2011) Worldwide decline of specialist species: toward a global functional homogenization? Frontiers in Ecology and the Environment 9, 222–228.
| Worldwide decline of specialist species: toward a global functional homogenization?Crossref | GoogleScholarGoogle Scholar |
Contos P, Wood JL, Murphy NP, Gibb H (2021) Rewilding with invertebrates and microbes to restore ecosystems: Present trends and future directions. Ecology and Evolution 11, 7187–7200.
| Rewilding with invertebrates and microbes to restore ecosystems: Present trends and future directions.Crossref | GoogleScholarGoogle Scholar | 34188805PubMed |
Cornelissen JHC, Grootemaat S, Verheijen LM, Cornwell WK, van Bodegom PM, van der Wal R, Aerts R (2017) Are litter decomposition and fire linked through plant species traits? New Phytologist 216, 653–669.
| Are litter decomposition and fire linked through plant species traits?Crossref | GoogleScholarGoogle Scholar |
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy PN, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters 11, 1065–1071.
| Plant species traits are the predominant control on litter decomposition rates within biomes worldwide.Crossref | GoogleScholarGoogle Scholar | 18627410PubMed |
Cornwell WK, Cornelissen JHC, Allison SD, Bauhus J, Eggleton P, Preston CM, Scarff F, Weedon JT, Wirth C, Zanne AE (2009) Plant traits and wood fates across the globe: rotted, burned, or consumed? Global Change Biology 15, 2431–2449.
| Plant traits and wood fates across the globe: rotted, burned, or consumed?Crossref | GoogleScholarGoogle Scholar |
Cornwell WK, Elvira A, van Kempen L, van Logtestijn RSP, Aptroot A, Cornelissen JHC (2015) Flammability across the gymnosperm phylogeny: the importance of litter particle size. New Phytologist 206, 672–681.
| Flammability across the gymnosperm phylogeny: the importance of litter particle size.Crossref | GoogleScholarGoogle Scholar |
Coulis M, Hättenschwiler S, Fromin N, David JF (2013) Macroarthropod-microorganism interactions during the decomposition of Mediterranean shrub litter at different moisture levels. Soil Biology and Biochemistry 64, 114–121.
| Macroarthropod-microorganism interactions during the decomposition of Mediterranean shrub litter at different moisture levels.Crossref | GoogleScholarGoogle Scholar |
Coulis M, Hättenschwiler S, Coq S, David J-F (2016) Leaf litter consumption by macroarthropods and burial of their faeces enhance decomposition in a Mediterranean ecosystem. Ecosystems 19, 1104–1115.
| Leaf litter consumption by macroarthropods and burial of their faeces enhance decomposition in a Mediterranean ecosystem.Crossref | GoogleScholarGoogle Scholar |
Coûteaux M-M, Bottner P, Berg B (1995) Litter decomposition, climate and liter quality. Trends in Ecology & Evolution 10, 63–66.
| Litter decomposition, climate and liter quality.Crossref | GoogleScholarGoogle Scholar |
Crowther TW, Stanton DWG, Thomas SM, A’Bear AD, Hiscox J, Jones TH, Voříšková J, Baldrian P, Boddy L (2013) Top-down control of soil fungal community composition by a globally distributed keystone consumer. Ecology 94, 2518–2528.
| Top-down control of soil fungal community composition by a globally distributed keystone consumer.Crossref | GoogleScholarGoogle Scholar | 24400503PubMed |
Cruz MG, Sullivan AL, Gould JS, Sims NC, Bannister AJ, Hollis JJ, Hurley RJ (2012) Anatomy of a catastrophic wildfire: the Black Saturday Kilmore East fire in Victoria, Australia. Forest Ecology and Management 284, 269–285.
| Anatomy of a catastrophic wildfire: the Black Saturday Kilmore East fire in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Dafni A, Izhaki I, Ne’eman G (2012) The effect of fire on biotic interactions in Mediterranean Basin ecosystems: pollination and seed dispersal. Israel Journal of Ecology & Evolution 58, 235–250.
| The effect of fire on biotic interactions in Mediterranean Basin ecosystems: pollination and seed dispersal.Crossref | GoogleScholarGoogle Scholar |
Dangerfield JM (1998) Biology and ecology of millipedes in the Kalahari. Transactions of the Royal Society of South Africa 53, 183–194.
| Biology and ecology of millipedes in the Kalahari.Crossref | GoogleScholarGoogle Scholar |
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annual Review of Ecology and Systematics 23, 63–87.
| Biological invasions by exotic grasses, the grass/fire cycle, and global change.Crossref | GoogleScholarGoogle Scholar |
D’Ascoli R, Rutigliano FA, De Pascale RA, Gentile A, De Santo AV (2005) Functional diversity of the microbial community in Mediterranean maquis soils as affected by fires. International Journal of Wildland Fire 14, 355–363.
| Functional diversity of the microbial community in Mediterranean maquis soils as affected by fires.Crossref | GoogleScholarGoogle Scholar |
David JF (2014) The role of litter-feeding macroarthropods in decomposition processes: a reappraisal of common views. Soil Biology and Biochemistry 76, 109–118.
| The role of litter-feeding macroarthropods in decomposition processes: a reappraisal of common views.Crossref | GoogleScholarGoogle Scholar |
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173.
| Temperature sensitivity of soil carbon decomposition and feedbacks to climate change.Crossref | GoogleScholarGoogle Scholar | 16525463PubMed |
De Long JR, Dorrepaal E, Kardol P, Nilsson M-C, Teuber LM, Wardle DA (2016) Understory plant functional groups and litter species identity are stronger drivers of litter decomposition than warming along a boreal forest post-fire successional gradient. Soil Biology and Biochemistry 98, 159–170.
| Understory plant functional groups and litter species identity are stronger drivers of litter decomposition than warming along a boreal forest post-fire successional gradient.Crossref | GoogleScholarGoogle Scholar |
de Souza OFF, Brown VK (1994) Effects of habitat fragmentation on Amazonian termite communities. Journal of Tropical Ecology 10, 197–206.
| Effects of habitat fragmentation on Amazonian termite communities.Crossref | GoogleScholarGoogle Scholar |
DeBano LF (1991) The effect of fire on soil properties. In ‘Proceedings management and productivity of western-Montane forest soils’ 10–12 April 1990, Boise, ID. Gen. Tech. Rep. INT-280. (Compilers AE Harvey, LF Neuenschwander) pp. 151–156. (USDA Forest Service, Intermountain Research Station: Ogden, UT).
DeBano L, Neary D, Folliott P (1998) ‘Fire effects on ecosystems.’ (Wiley: New York)
Dell J, O’Brien J, Doan L, Richards L, Dyer L (2017) An arthropod survival strategy in a frequently burned forest. Ecology 98, 2972–2974.
| An arthropod survival strategy in a frequently burned forest.Crossref | GoogleScholarGoogle Scholar | 28837744PubMed |
Didham RK (1998) Altered leaf-litter decomposition rates in tropical forest fragments. Oecologia 116, 397–406.
| Altered leaf-litter decomposition rates in tropical forest fragments.Crossref | GoogleScholarGoogle Scholar | 28308072PubMed |
Didham RK, Lawton JH (1999) Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments. Biotropica 31, 17–30.
| Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments.Crossref | GoogleScholarGoogle Scholar |
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends in Ecology and Evolution 11, 255–260.
| Insects in fragmented forests: a functional approach.Crossref | GoogleScholarGoogle Scholar | 21237834PubMed |
Dossa GGO, Schaefer D, Zhang J-L, Tao J-P, Cao K-F, Corlett RT, Cunningham AB, Xu J-C, Cornelissen JHC, Harrison RD (2018) The cover uncovered: Bark control over wood decomposition. Journal of Ecology 106, 2147–2160.
| The cover uncovered: Bark control over wood decomposition.Crossref | GoogleScholarGoogle Scholar |
Dove NC, Safford HD, Bohlman GN, Estes BL, Hart SC (2020) High-severity wildfire leads to multi-decadal impacts on soil biogeochemistry in mixed-conifer forests. Ecological Applications 30, e02072
| High-severity wildfire leads to multi-decadal impacts on soil biogeochemistry in mixed-conifer forests.Crossref | GoogleScholarGoogle Scholar | 31925848PubMed |
Driscoll DA, Weir T (2005) Beetle responses to habitat fragmentation depend on ecological traits, habitat condition, and remnant size. Conservation Biology 19, 182–194.
| Beetle responses to habitat fragmentation depend on ecological traits, habitat condition, and remnant size.Crossref | GoogleScholarGoogle Scholar |
Driscoll DA, Smith AL, Blight S, Sellar I (2020) Interactions among body size, trophic level, and dispersal traits predict beetle detectability and occurrence responses to fire. Ecological Entomology 45, 300–310.
| Interactions among body size, trophic level, and dispersal traits predict beetle detectability and occurrence responses to fire.Crossref | GoogleScholarGoogle Scholar |
Duff TJ, Keane RE, Penman TD, Tolhurst KG (2017) Revisiting wildland fire fuel quantification methods: the challenge of understanding a dynamic, biotic entity. Forests 8, 351
| Revisiting wildland fire fuel quantification methods: the challenge of understanding a dynamic, biotic entity.Crossref | GoogleScholarGoogle Scholar |
Duncan BW, Schmalzer PA (2004) Anthropogenic influences on potential fire spread in a pyrogenic ecosystem of Florida, USA. Landscape Ecology 19, 153–165.
| Anthropogenic influences on potential fire spread in a pyrogenic ecosystem of Florida, USA.Crossref | GoogleScholarGoogle Scholar |
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biology 18, 1781–1796.
| Soil organic matter turnover is governed by accessibility not recalcitrance.Crossref | GoogleScholarGoogle Scholar |
Egidi E, McMullan-Fisher S, Morgan JW, May T, Zeeman B, Franks AE (2016) Fire regime, not time-since-fire, affects soil fungal community diversity and composition in temperate grasslands. FEMS Microbiology Letters 363, fnw196
| Fire regime, not time-since-fire, affects soil fungal community diversity and composition in temperate grasslands.Crossref | GoogleScholarGoogle Scholar | 27528692PubMed |
Eliott M, Lawson S, Hayes A, Debuse V, York A, Lewis T (2019) The response of cerambycid beetles (Coleoptera: Cerambycidae) to long‐term fire frequency regimes in subtropical eucalypt forest. Austral Ecology 44, 609–620.
| The response of cerambycid beetles (Coleoptera: Cerambycidae) to long‐term fire frequency regimes in subtropical eucalypt forest.Crossref | GoogleScholarGoogle Scholar |
Enright NJ, Fontaine JB, Bowman DM, Bradstock RA, Williams RJ (2015) Interval squeeze: altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Frontiers in Ecology and the Environment 13, 265–272.
| Interval squeeze: altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes.Crossref | GoogleScholarGoogle Scholar |
Eskelson BN, Monleon VJ (2018) Post-fire surface fuel dynamics in California forests across three burn severity classes. International Journal of Wildland Fire 27, 114–124.
| Post-fire surface fuel dynamics in California forests across three burn severity classes.Crossref | GoogleScholarGoogle Scholar |
Eva H, Lambin EF (1998) Remote sensing of biomass burning in tropical regions: Sampling issues and multisensor approach. Remote Sensing of Environment 64, 292–315.
| Remote sensing of biomass burning in tropical regions: Sampling issues and multisensor approach.Crossref | GoogleScholarGoogle Scholar |
Fensham R (1992) The management implications of fine fuel dynamics in bushlands surrounding Hobart, Tasmania. Journal of Environmental Management 36, 301–320.
| The management implications of fine fuel dynamics in bushlands surrounding Hobart, Tasmania.Crossref | GoogleScholarGoogle Scholar |
Fernandes PM, Botelho HS (2003) A review of prescribed burning effectiveness in fire hazard reduction. International Journal of Wildland Fire 12, 117–128.
| A review of prescribed burning effectiveness in fire hazard reduction.Crossref | GoogleScholarGoogle Scholar |
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336, 1715–1719.
| The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes.Crossref | GoogleScholarGoogle Scholar | 22745431PubMed |
Force DC (1981) Postfire insect succession in southern California chaparral. The American Naturalist 117, 575–582.
| Postfire insect succession in southern California chaparral.Crossref | GoogleScholarGoogle Scholar |
Foster CN, Banks SC, Cary GJ, Johnson CN, Lindenmayer DB, Valentine LE (2020) Animals as agents in fire regimes. Trends in Ecology & Evolution 35, 346–356.
| Animals as agents in fire regimes.Crossref | GoogleScholarGoogle Scholar |
Foster JG, Gervan CA, Coghill MG, Fraser LH (2021) Are arthropod communities in grassland ecosystems affected by the abundance of an invasive plant? Oecologia 196, 1–12.
| Are arthropod communities in grassland ecosystems affected by the abundance of an invasive plant?Crossref | GoogleScholarGoogle Scholar | 33507399PubMed |
Fox BJ, Fox MD, Mckay GM (1979) Litter accumulation after fire in a eucalypt forest. Australian Journal of Botany 27, 157–165.
| Litter accumulation after fire in a eucalypt forest.Crossref | GoogleScholarGoogle Scholar |
Friend J, Richardson A (1977) A preliminary study of niche partition in two Tasmanian terrestrial amphipod species. Ecological Bulletins 25, 24–35. Available at https://www.jstor.org/stable/20112562
Fritze H, Pennanen T, Pietikäinen J (1993) Recovery of soil microbial biomass and activity from prescribed burning. Canadian Journal of Forest Research 23, 1286–1290.
| Recovery of soil microbial biomass and activity from prescribed burning.Crossref | GoogleScholarGoogle Scholar |
Frouz J (2018) Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 332, 161–172.
| Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization.Crossref | GoogleScholarGoogle Scholar |
Ganteaume A, Camia A, Jappiot M, San-Miguel-Ayanz J, Long-Fournel M, Lampin C (2013) A review of the main driving factors of forest fire ignition over Europe. Environmental Management 51, 651–662.
| A review of the main driving factors of forest fire ignition over Europe.Crossref | GoogleScholarGoogle Scholar | 23086400PubMed |
García-Palacios P, Maestre FT, Kattge J, Wall DH (2013) Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecology Letters 16, 1045–1053.
| Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes.Crossref | GoogleScholarGoogle Scholar | 23763716PubMed |
Gessner MO, Swan CM, Dang CK, McKie BG, Bardgett RD, Wall DH, Hättenschwiler S (2010) Diversity meets decomposition. Trends in Ecology & Evolution 25, 372–380.
| Diversity meets decomposition.Crossref | GoogleScholarGoogle Scholar |
Gibb H, Hochuli DF (2002) Habitat fragmentation in an urban environment: large and small fragments support different arthropod assemblages. Biological Conservation 106, 91–100.
| Habitat fragmentation in an urban environment: large and small fragments support different arthropod assemblages.Crossref | GoogleScholarGoogle Scholar |
Gibb H, Pettersson RB, Hjältén J, Hilszczański J, Ball JP, Johansson T, Atlegrim O, Danell K (2006) Conservation-oriented forestry and early successional saproxylic beetles: Responses of functional groups to manipulated dead wood substrates. Biological Conservation 129, 437–450.
| Conservation-oriented forestry and early successional saproxylic beetles: Responses of functional groups to manipulated dead wood substrates.Crossref | GoogleScholarGoogle Scholar |
Gill AM (1975) Fire and the Australian flora: a review. Australian Forestry 38, 4–25.
| Fire and the Australian flora: a review.Crossref | GoogleScholarGoogle Scholar |
Goldammer JG, Penafiel SR (1990) Fire in the pine-grassland biomes of tropical and subtropical Asia. In ‘Fire in the Tropical Biota’. (Ed. JG Goldammer) pp. 45–62. (Springer)
Godwin DR, Kobziar LN, Robertson KM (2017) Effects of fire frequency and soil temperature on soil CO2 efflux rates in old‐field pine‐grassland forests. 8, 274
| Effects of fire frequency and soil temperature on soil CO2 efflux rates in old‐field pine‐grassland forests.Crossref | GoogleScholarGoogle Scholar |
Gongalsky KB, Persson T (2013) Recovery of soil macrofauna after wildfires in boreal forests. Soil Biology and Biochemistry 57, 182–191.
| Recovery of soil macrofauna after wildfires in boreal forests.Crossref | GoogleScholarGoogle Scholar |
González G, Seastedt TR (2001) Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 82, 955–964.
| Soil fauna and plant litter decomposition in tropical and subalpine forests.Crossref | GoogleScholarGoogle Scholar |
González-Pérez JA, González-Vila FJ, Almendros G, Knicker H (2004) The effect of fire on soil organic matter—a review. Environment International 30, 855–70.
| The effect of fire on soil organic matter—a review.Crossref | GoogleScholarGoogle Scholar | 15120204PubMed |
Gosper CR, Prober SM, Yates CJ (2013) Multi-century changes in vegetation structure and fuel availability in fire-sensitive eucalypt woodlands. Forest Ecology and Management 310, 102–109.
| Multi-century changes in vegetation structure and fuel availability in fire-sensitive eucalypt woodlands.Crossref | GoogleScholarGoogle Scholar |
Grant SR, Wouters MA (1993) ‘The effect of fuel reduction burning on the suppression of four wildfires in Western Victoria.’ (Department of Conservation and Natural Resources: Melbourne)
Gratton C, Denno RF (2005) Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis. Restoration Ecology 13, 358–372.
| Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis.Crossref | GoogleScholarGoogle Scholar |
Grigal DF, McColl JG (1977) Litter decomposition following forest fire in north-eastern Minnesota. Journal of Applied Ecology 14, 531–538.
| Litter decomposition following forest fire in north-eastern Minnesota.Crossref | GoogleScholarGoogle Scholar |
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annual Review of Ecology and Systematics 33, 1–23.
| Saproxylic insect ecology and the sustainable management of forests.Crossref | GoogleScholarGoogle Scholar |
Grubb JJ (2019) Going Through Hell: Drivers of invertebrate detritivore recovery after fire. PhD thesis, La Trobe University: Melbourne, Australia.
Hansen PM, Semenova-Nelsen TA, Platt WJ, Sikes BA (2019) Recurrent fires do not affect the abundance of soil fungi in a frequently burned pine savanna. Fungal Ecology 42, 100852
| Recurrent fires do not affect the abundance of soil fungi in a frequently burned pine savanna.Crossref | GoogleScholarGoogle Scholar | 32863864PubMed |
Harris RMB, Remenyi TA, Williamson GJ, Bindoff NL, Bowman DMJS (2016) Climate–vegetation–fire interactions and feedbacks: trivial detail or major barrier to projecting the future of the Earth system? Wiley Interdisciplinary Reviews: Climate Change 7, 910–931.
| Climate–vegetation–fire interactions and feedbacks: trivial detail or major barrier to projecting the future of the Earth system?Crossref | GoogleScholarGoogle Scholar |
Haslem A, Kelly LT, Nimmo DG, Watson SJ, Kenny SA, Taylor RS, Avitabile SC, Callister KE, Spence-Bailey LM, Clarke MF, Bennett AF (2011) Habitat or fuel? Implications of long-term, post-fire dynamics for the development of key resources for fauna and fire. Journal of Applied Ecology 48, 247–256.
| Habitat or fuel? Implications of long-term, post-fire dynamics for the development of key resources for fauna and fire.Crossref | GoogleScholarGoogle Scholar |
Haslem A, Leonard SWJ, Bruce MJ, Christie F, Holland GJ, Kelly LT, MacHunter J, Bennett AF, Clarke MF, York A (2016) Do multiple fires interact to affect vegetation structure in temperate eucalypt forests? Ecological Applications 26, 2414–2423.
| Do multiple fires interact to affect vegetation structure in temperate eucalypt forests?Crossref | GoogleScholarGoogle Scholar |
Hedlund K, Öhrn MS (2000) Tritrophic interactions in a soil community enhance decomposition rates. Oikos 88, 585–591.
| Tritrophic interactions in a soil community enhance decomposition rates.Crossref | GoogleScholarGoogle Scholar |
Heemsbergen DA, Berg MP, Loreau M, van Hal JR, Faber JH, Verhoef HA (2004) Biodiversity effects on soil processes explained by interspecific functional dissimilarity. Science 306, 1019–1020.
| Biodiversity effects on soil processes explained by interspecific functional dissimilarity.Crossref | GoogleScholarGoogle Scholar | 15528441PubMed |
Hennessy K, Lucas C, Nicholls N, Bathols J, Suppiah R, Ricketts J (2005) ‘Climate change impacts on fire-weather in south-east Australia.’ (Climate Impacts Group, CSIRO Atmospheric Research and the Australian Government Bureau of Meteorology: Melbourne, Vic.)
Hernández DL, Hobbie SE (2008) Effects of fire frequency on oak litter decomposition and nitrogen dynamics. Oecologia 158, 535–543.
| Effects of fire frequency on oak litter decomposition and nitrogen dynamics.Crossref | GoogleScholarGoogle Scholar | 18850116PubMed |
Hines F, Hines F, Tolhurst KG, Wilson AA, McCarthy GJ (2010) ‘Overall fuel hazard assessment guide.’ (Victorian Government, Department of Sustainability and Environment: Melbourne)
Hoang DTT, Bauke SL, Kuzyakov Y, Pausch J (2017) Rolling in the deep: priming effects in earthworm biopores in topsoil and subsoil. Soil Biology and Biochemistry 114, 59–71.
| Rolling in the deep: priming effects in earthworm biopores in topsoil and subsoil.Crossref | GoogleScholarGoogle Scholar |
Hoffmann AA, Chown SL, Clusella-Trullas S (2013) Upper thermal limits in terrestrial ectotherms: how constrained are they? Functional Ecology 27, 934–949.
| Upper thermal limits in terrestrial ectotherms: how constrained are they?Crossref | GoogleScholarGoogle Scholar |
Holland GJ, Clarke MF, Bennett AF (2017) Prescribed burning consumes key forest structural components: implications for landscape heterogeneity. Ecological Applications 27, 845–858.
| Prescribed burning consumes key forest structural components: implications for landscape heterogeneity.Crossref | GoogleScholarGoogle Scholar | 27992957PubMed |
Holliday NJ (1991) Species responses of carabid beetles (Coleoptera: Carabidae) during post-fire regeneration of boreal forest. The Canadian Entomologist 123, 1369–1389.
| Species responses of carabid beetles (Coleoptera: Carabidae) during post-fire regeneration of boreal forest.Crossref | GoogleScholarGoogle Scholar |
Hopkins JR, Huffman JM, Platt WJ, Sikes BA (2020) Frequent fire slows microbial decomposition of newly deposited fine fuels in a pyrophilic ecosystem. Oecologia 193, 631–643.
| Frequent fire slows microbial decomposition of newly deposited fine fuels in a pyrophilic ecosystem.Crossref | GoogleScholarGoogle Scholar | 32699992PubMed |
Hoppe B, Kahl T, Karasch P, Wubet T, Bauhus J, Buscot F, Krüger D (2014) Network analysis reveals ecological links between N-fixing bacteria and wood-decaying fungi. PLoS One 9, e88141
| Network analysis reveals ecological links between N-fixing bacteria and wood-decaying fungi.Crossref | GoogleScholarGoogle Scholar | 24505405PubMed |
Houston DR (1981) ‘Stress triggered tree diseases: the diebacks and declines.’ NE-INF-41-81. (USDA Forest Service, Northeastern Forest Experiment Station: Broomall, PA)
Huebner K, Lindo Z, Lechowicz MJ (2012) Post-fire succession of collembolan communities in a northern hardwood forest. European Journal of Soil Biology 48, 59–65.
| Post-fire succession of collembolan communities in a northern hardwood forest.Crossref | GoogleScholarGoogle Scholar |
Humphreys F, Lambert MJ (1965) An examination of a forest site which has exhibited the ash-bed effect. Soil Research 3, 81–94.
| An examination of a forest site which has exhibited the ash-bed effect.Crossref | GoogleScholarGoogle Scholar |
Ikeda H, Callaham Jr MA, O’Brien JJ, Hornsby BS, Wenk ES (2015) Can the invasive earthworm, Amynthas agrestis, be controlled with prescribed fire? Soil Biology and Biochemistry 82, 21–27.
| Can the invasive earthworm, Amynthas agrestis, be controlled with prescribed fire?Crossref | GoogleScholarGoogle Scholar |
IPCC (2012) Managing the risks of extreme events and disasters to advance climate change adaptation. In ‘A special report of working groups I and II of the Intergovernmental Panel on Climate Change’. (Eds CB Field, V Barros, TF Stocker, D Qin, DJ Dokken, KL Ebi, MD Mastrandrea, KJ Mach, G-K Plattner, SK Allen, M Tignor, PM Midgley) (Cambridge University Press: Cambridge, UK)
Jacobsen RM, Kauserud H, Sverdrup-Thygeson A, Bjorbækmo MM, Birkemoe T (2017) Wood-inhabiting insects can function as targeted vectors for decomposer fungi. Fungal Ecology 29, 76–84.
| Wood-inhabiting insects can function as targeted vectors for decomposer fungi.Crossref | GoogleScholarGoogle Scholar |
Jo I, Fridley JD, Frank DA (2016) More of the same? In situ leaf and root decomposition rates do not vary between 80 native and nonnative deciduous forest species. New Phytologist 209, 115–122.
| More of the same? In situ leaf and root decomposition rates do not vary between 80 native and nonnative deciduous forest species.Crossref | GoogleScholarGoogle Scholar |
Johansson T, Andersson J, Hjältén J, Dynesius M, Ecke F (2011) Short‐term responses of beetle assemblages to wildfire in a region with more than 100 years of fire suppression. Insect Conservation and Diversity 4, 142–151.
| Short‐term responses of beetle assemblages to wildfire in a region with more than 100 years of fire suppression.Crossref | GoogleScholarGoogle Scholar |
Johnston SR, Boddy L, Weightman AJ (2016) Bacteria in decomposing wood and their interactions with wood-decay fungi. FEMS Microbiology Ecology 92, fiw179
| Bacteria in decomposing wood and their interactions with wood-decay fungi.Crossref | GoogleScholarGoogle Scholar | 27559028PubMed |
Joly FX, Coq S, Coulis M, Nahmani J, Hättenschwiler S (2018) Litter conversion into detritivore faeces reshuffles the quality control over C and N dynamics during decomposition. Functional Ecology 32, 2605–2614.
| Litter conversion into detritivore faeces reshuffles the quality control over C and N dynamics during decomposition.Crossref | GoogleScholarGoogle Scholar |
Joly F-X, Coq S, Coulis M, David J-F, Hättenschwiler S, Mueller CW, Prater I, Subke J-A (2020) Detritivore conversion of litter into faeces accelerates organic matter turnover. Communications Biology 3, 1–9.
| Detritivore conversion of litter into faeces accelerates organic matter turnover.Crossref | GoogleScholarGoogle Scholar |
Kampichler C, Bruckner A (2009) The role of microarthropods in terrestrial decomposition: a meta‐analysis of 40 years of litterbag studies. Biological Reviews 84, 375–389.
| The role of microarthropods in terrestrial decomposition: a meta‐analysis of 40 years of litterbag studies.Crossref | GoogleScholarGoogle Scholar | 19485987PubMed |
Kassen R (2002) The experimental evolution of specialists, generalists, and the maintenance of diversity. Journal of Evolutionary Biology 15, 173–190.
| The experimental evolution of specialists, generalists, and the maintenance of diversity.Crossref | GoogleScholarGoogle Scholar |
Kasurinen A, Silfver T, Rousi M, Mikola J (2017) Warming and ozone exposure effects on silver birch (Betula pendula Roth) leaf litter quality, microbial growth and decomposition. Plant and Soil 414, 127–142.
| Warming and ozone exposure effects on silver birch (Betula pendula Roth) leaf litter quality, microbial growth and decomposition.Crossref | GoogleScholarGoogle Scholar |
Kay AD, Mankowski J, Hobbie SE (2008) Long‐term burning interacts with herbivory to slow decomposition. Ecology 89, 1188–1194.
| Long‐term burning interacts with herbivory to slow decomposition.Crossref | GoogleScholarGoogle Scholar | 18543612PubMed |
Kelly LT, Bennett AF, Clarke MF, McCarthy MA (2015) Optimal fire histories for biodiversity conservation. Conservation Biology 29, 473–481.
| Optimal fire histories for biodiversity conservation.Crossref | GoogleScholarGoogle Scholar | 25163611PubMed |
Kembel SW, Mueller RC (2014) Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany-Botanique 92, 303–311.
| Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities.Crossref | GoogleScholarGoogle Scholar |
Kim E-J, Oh J-E, Chang Y-S (2003) Effects of forest fire on the level and distribution of PCDD/Fs and PAHs in soil. Science of the Total Environment 311, 177–189.
| Effects of forest fire on the level and distribution of PCDD/Fs and PAHs in soil.Crossref | GoogleScholarGoogle Scholar |
Knapp EE, Estes BL, Skinner CN (2009) Ecological effects of prescribed fire season: a literature review and synthesis for managers. Gen. Tech. Rep. PSW-GTR-224. (USDA Forest Service, Pacific Southwest Research Station: Albany, CA)
Koltz AM, Burkle LA, Pressler Y, Dell JE, Vidal MC, Richards LA, Murphy SM (2018) Global change and the importance of fire for the ecology and evolution of insects. Current Opinion in Insect Science 29, 110–116.
| Global change and the importance of fire for the ecology and evolution of insects.Crossref | GoogleScholarGoogle Scholar | 30551816PubMed |
Korobushkin DI, Gorbunova AY, Zaitsev AS, Gongalsky KB (2017) Trait-specific response of soil macrofauna to forest burning along a macrogeographic gradient. Applied Soil Ecology 112, 97–100.
| Trait-specific response of soil macrofauna to forest burning along a macrogeographic gradient.Crossref | GoogleScholarGoogle Scholar |
Köster K, Berninger F, Heinonsalo J, Lindén A, Köster E, Ilvesniemi H, Pumpanen J (2016) The long-term impact of low-intensity surface fires on litter decomposition and enzyme activities in boreal coniferous forests. International Journal of Wildland Fire 25, 213–223.
| The long-term impact of low-intensity surface fires on litter decomposition and enzyme activities in boreal coniferous forests.Crossref | GoogleScholarGoogle Scholar |
Kral KC, Limb RF, Harmon JP, Hovick TJ (2017) Arthropods and fire: previous research shaping future conservation. Rangeland Ecology & Management 70, 589–598.
| Arthropods and fire: previous research shaping future conservation.Crossref | GoogleScholarGoogle Scholar |
Lambert B, Casteignau D, Costa M, Etienne M, Guiton J, Rigolot E (1999) ‘Analyse après incendie de six coupures de combustible.’ (La Cardère)
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology 31, 814
| Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences.Crossref | GoogleScholarGoogle Scholar | 23975157PubMed |
Langlands PR, Brennan KEC, Framenau VW, Main BY (2011) predicting the post-fire responses of animal assemblages: testing a trait-based approach using spiders. Journal of Animal Ecology 80, 558–568.
| predicting the post-fire responses of animal assemblages: testing a trait-based approach using spiders.Crossref | GoogleScholarGoogle Scholar |
Lavelle P (1997) Faunal activities and soil processes: adaptive strategies that determine ecosystem function Advances in Ecological Research 27, 93–132.
| Faunal activities and soil processes: adaptive strategies that determine ecosystem functionCrossref | GoogleScholarGoogle Scholar |
Lavelle P, Lattaud C, Trigo D, Barois I (1995) Mutualism and biodiversity in soils. Plant and Soil 170, 23–33.
| Mutualism and biodiversity in soils.Crossref | GoogleScholarGoogle Scholar |
Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, Bureau F, Margerie P, Mora P, Rossi J-P (2006) Soil invertebrates and ecosystem services. European Journal of Soil Biology 42, S3–S15.
| Soil invertebrates and ecosystem services.Crossref | GoogleScholarGoogle Scholar |
Liski J, Nissinen A, Erhard M, Taskinen O (2003) Climatic effects on litter decomposition from Arctic tundra to tropical rainforest. Global Change Biology 9, 575–584.
| Climatic effects on litter decomposition from Arctic tundra to tropical rainforest.Crossref | GoogleScholarGoogle Scholar |
Litton CM, Ryan MG, Knight DH, Stahl PD (2003) Soil‐surface carbon dioxide efflux and microbial biomass in relation to tree density 13 years after a stand replacing fire in a lodgepole pine ecosystem. Global Change Biology 9, 680–696.
| Soil‐surface carbon dioxide efflux and microbial biomass in relation to tree density 13 years after a stand replacing fire in a lodgepole pine ecosystem.Crossref | GoogleScholarGoogle Scholar |
Lladó S, Žifčáková L, Větrovský T, Eichlerová I, Baldrian P (2016) Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biology and Fertility of Soils 52, 251–260.
| Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition.Crossref | GoogleScholarGoogle Scholar |
Love BG, Cane JH (2016) Limited direct effects of a massive wildfire on its sagebrush steppe bee community. Ecological Entomology 41, 317–326.
| Limited direct effects of a massive wildfire on its sagebrush steppe bee community.Crossref | GoogleScholarGoogle Scholar |
Maaß S, Caruso T, Rillig MC (2015) Functional role of microarthropods in soil aggregation. Pedobiologia 58, 59–63.
| Functional role of microarthropods in soil aggregation.Crossref | GoogleScholarGoogle Scholar |
Mack MC, D’Antonio CM (1998) Impacts of biological invasions on disturbance regimes. Trends in Ecology & Evolution 13, 195–198.
| Impacts of biological invasions on disturbance regimes.Crossref | GoogleScholarGoogle Scholar |
Malcolm JR (1998) A Model of conductive heat flow in forest edges and fragmented landscapes. Climatic Change 39, 487–502.
| A Model of conductive heat flow in forest edges and fragmented landscapes.Crossref | GoogleScholarGoogle Scholar |
Malmström A (2012) Life-history traits predict recovery patterns in Collembola species after fire: a 10 year study. Applied Soil Ecology 56, 35–42.
| Life-history traits predict recovery patterns in Collembola species after fire: a 10 year study.Crossref | GoogleScholarGoogle Scholar |
Manea A, Grootemaat S, Leishman MR (2015) Leaf flammability and fuel load increase under elevated CO2 levels in a model grassland. International Journal of Wildland Fire 24, 819–827.
| Leaf flammability and fuel load increase under elevated CO2 levels in a model grassland.Crossref | GoogleScholarGoogle Scholar |
Martinson HM, Fagan WF (2014) Trophic disruption: a meta‐analysis of how habitat fragmentation affects resource consumption in terrestrial arthropod systems. Ecology Letters 17, 1178–1189.
| Trophic disruption: a meta‐analysis of how habitat fragmentation affects resource consumption in terrestrial arthropod systems.Crossref | GoogleScholarGoogle Scholar | 24866984PubMed |
Martiny AC, Treseder K, Pusch G (2013) Phylogenetic conservatism of functional traits in microorganisms. The ISME Journal 7, 830–838.
| Phylogenetic conservatism of functional traits in microorganisms.Crossref | GoogleScholarGoogle Scholar | 23235290PubMed |
Mastrolonardo G, Francioso O, Di Foggia M, Bonora S, Rumpel C, Certini G (2014) Application of thermal and spectroscopic techniques to assess fire-induced changes to soil organic matter in a Mediterranean forest. Journal of Geochemical Exploration 143, 174–182.
| Application of thermal and spectroscopic techniques to assess fire-induced changes to soil organic matter in a Mediterranean forest.Crossref | GoogleScholarGoogle Scholar |
McCarthy MA, Gill AM, Bradstock RA (2001) Theoretical fire-interval distributions. International Journal of Wildland Fire 10, 73–77.
| Theoretical fire-interval distributions.Crossref | GoogleScholarGoogle Scholar |
McCary MA, Schmitz OJ (2021) Invertebrate functional traits and terrestrial nutrient cycling: insights from a global meta‐analysis. Journal of Animal Ecology 90, 1714–1726.
| Invertebrate functional traits and terrestrial nutrient cycling: insights from a global meta‐analysis.Crossref | GoogleScholarGoogle Scholar |
McCaw L, Maher T, Gillen K, Lewis MR (1992) Wildfires in the Fitzgerald River National Park, Western Australia, December 1989. Technical report no. 26. (Department of Conservation and Land Management: Western Australia).
McKee WH (1982) Changes in soil fertility following prescribed burning on coastal plain pine sites. Research Paper‐RE‐234. (Southern Forest Experiment Station: Asheville, NC). Available at https://doi.org/10.2737/SE‐RP‐234
Menz L, Gibb H, Murphy N (2016) Dispersal-limited detritivores in fire-prone environments: persistence and population structure of terrestrial amphipods (Talitridae). International Journal of Wildland Fire 25, 753–761.
| Dispersal-limited detritivores in fire-prone environments: persistence and population structure of terrestrial amphipods (Talitridae).Crossref | GoogleScholarGoogle Scholar |
Miller C, Urban DL (1999) A model of surface fire, climate and forest pattern in the Sierra Nevada, California. Ecological Modelling 114, 113–135.
| A model of surface fire, climate and forest pattern in the Sierra Nevada, California.Crossref | GoogleScholarGoogle Scholar |
Miller G, Friedel M, Adam P, Chewings V (2010) Ecological impacts of buffel grass (Cenchrus ciliaris L.) invasion in central Australia – does field evidence support a fire–invasion feedback? The Rangeland Journal 32, 353–365.
| Ecological impacts of buffel grass (Cenchrus ciliaris L.) invasion in central Australia – does field evidence support a fire–invasion feedback?Crossref | GoogleScholarGoogle Scholar |
Minderman G (1968) Addition, decomposition and accumulation of organic matter in forests. The Journal of Ecology 56, 355–362.
| Addition, decomposition and accumulation of organic matter in forests.Crossref | GoogleScholarGoogle Scholar |
Miranda AC, Miranda HS, de Fátima Oliveira Dias I, de Souza Dias BF (1993) Soil and air temperatures during prescribed cerrado fires in Central Brazil. Journal of Tropical Ecology 9, 313–320.
| Soil and air temperatures during prescribed cerrado fires in Central Brazil.Crossref | GoogleScholarGoogle Scholar |
Miyanishi K, Johnson EA (2002) Process and patterns of duff consumption in the mixedwood boreal forest. Canadian Journal of Forest Research 32, 1285–1295.
| Process and patterns of duff consumption in the mixedwood boreal forest.Crossref | GoogleScholarGoogle Scholar |
Monleon VJ, Cromack K (1996) Long-term effects of prescribed underburning on litter decomposition and nutrient release in ponderosa pine stands in central Oregon. Forest Ecology and Management 81, 143–152.
| Long-term effects of prescribed underburning on litter decomposition and nutrient release in ponderosa pine stands in central Oregon.Crossref | GoogleScholarGoogle Scholar |
Moore RA, Bomar C, Kobziar LN, Christner BC (2021) Wildland fire as an atmospheric source of viable microbial aerosols and biological ice nucleating particles. The ISME Journal 15, 461–472.
| Wildland fire as an atmospheric source of viable microbial aerosols and biological ice nucleating particles.Crossref | GoogleScholarGoogle Scholar | 33009511PubMed |
Moreira da Silva J (1997) Historique des feux contrôlés au Portugal: brûlage dirigé. Forêt Méditerranéenne 18, 299–310.
Moretti M, Duelli P, Obrist MK (2006) Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 149, 312–327.
| Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests.Crossref | GoogleScholarGoogle Scholar | 16804704PubMed |
Moritz MA, Keeley JE, Johnson EA, Schaffner AA (2004) Testing a basic assumption of shrubland fire management: how important is fuel age? Frontiers in Ecology and the Environment 2, 67–72.
| Testing a basic assumption of shrubland fire management: how important is fuel age?Crossref | GoogleScholarGoogle Scholar |
National Academies of Sciences, Engineering, and Medicine (2016) ‘Attribution of extreme weather events in the context of climate change.’ (The National Academies Press: Washington, DC). Available at
| Crossref |
Neary DG, Klopatek CC, DeBano LF, Ffolliott PF (1999) Fire effects on belowground sustainability: a review and synthesis. Forest Ecology and Management 122, 51–71.
| Fire effects on belowground sustainability: a review and synthesis.Crossref | GoogleScholarGoogle Scholar |
New TR, Yen AL, Sands DPA, Greenslade P, Neville PJ, York A, Collett NG (2010) Planned fires and invertebrate conservation in south-east Australia. Journal of Insect Conservation 14, 567–574.
| Planned fires and invertebrate conservation in south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Nolan RH, Boer MM, Collins L, Resco de Dios V, Clarke HG, Jenkins M, Kenny B, Bradstock RA (2020) Causes and consequences of eastern Australia’s 2019–20 season of mega-fires. Global Change Biology 26, 1039–1041.
| Causes and consequences of eastern Australia’s 2019–20 season of mega-fires.Crossref | GoogleScholarGoogle Scholar | 31916352PubMed |
Noss RF, Franklin JF, Baker WL, Schoennagel T, Moyle PB (2006) Managing fire‐prone forests in the western United States. Frontiers in Ecology and the Environment 4, 481–487.
| Managing fire‐prone forests in the western United States.Crossref | GoogleScholarGoogle Scholar |
Nugent DT, Leonard SWJ, Clarke MF (2014) Interactions between the superb lyrebird (Menura novaehollandiae) and fire in south-eastern Australia. Wildlife Research 41, 203–211.
| Interactions between the superb lyrebird (Menura novaehollandiae) and fire in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
O’Dowd DJ, Green PT, Lake PS (2003) Invasional ‘meltdown’ on an oceanic island. Ecology Letters 6, 812–817.
| Invasional ‘meltdown’ on an oceanic island.Crossref | GoogleScholarGoogle Scholar |
Oliver AA, Bogan MT, Herbst DB, Dahlgren RA (2012) Short-term changes in-stream macroinvertebrate communities following a severe fire in the Lake Tahoe basin, California. Hydrobiologia 694, 117–130.
| Short-term changes in-stream macroinvertebrate communities following a severe fire in the Lake Tahoe basin, California.Crossref | GoogleScholarGoogle Scholar |
Oliver AK, Callaham Jr MA, Jumpponen A (2015) Soil fungal communities respond compositionally to recurring frequent prescribed burning in a managed south-eastern US forest ecosystem. Forest Ecology and Management 345, 1–9.
| Soil fungal communities respond compositionally to recurring frequent prescribed burning in a managed south-eastern US forest ecosystem.Crossref | GoogleScholarGoogle Scholar |
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44, 322–331.
| Energy storage and the balance of producers and decomposers in ecological systems.Crossref | GoogleScholarGoogle Scholar |
Panzer R (2002) Compatibility of prescribed burning with the conservation of insects in small, isolated prairie reserves. Conservation Biology 16, 1296–1307.
| Compatibility of prescribed burning with the conservation of insects in small, isolated prairie reserves.Crossref | GoogleScholarGoogle Scholar |
Parr CL, Andersen AN (2006) Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm. Conservation Biology 20, 1610–1619.
| Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm.Crossref | GoogleScholarGoogle Scholar | 17181796PubMed |
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315, 361–364.
| Global-scale similarities in nitrogen release patterns during long-term decomposition.Crossref | GoogleScholarGoogle Scholar | 17234944PubMed |
Pausas JG, Bond WJ (2020) On the three major recycling pathways in terrestrial ecosystems. Trends in Ecology & Evolution 35, 767–775.
| On the three major recycling pathways in terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pausas JG, Paula S (2012) Fuel shapes the fire–climate relationship: evidence from Mediterranean ecosystems. Global Ecology and Biogeography 21, 1074–1082.
| Fuel shapes the fire–climate relationship: evidence from Mediterranean ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pellegrini AFA, Ahlström A, Hobbie SE, et al. (2018) Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 553, 194–198.
| Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity.Crossref | GoogleScholarGoogle Scholar | 29227988PubMed |
Pellegrini AFA, Hobbie SE, Reich PB, Jumpponen A, Brookshire ENJ, Caprio AC, Coetsee C, Jackson RB (2020) Repeated fire shifts carbon and nitrogen cycling by changing plant inputs and soil decomposition across ecosystems. Ecological Monographs 90, e01409
| Repeated fire shifts carbon and nitrogen cycling by changing plant inputs and soil decomposition across ecosystems.Crossref | GoogleScholarGoogle Scholar |
Persson Y, Ihrmark K, Stenlid J (2011) Do bark beetles facilitate the establishment of rot fungi in Norway spruce? Fungal Ecology 4, 262–269.
| Do bark beetles facilitate the establishment of rot fungi in Norway spruce?Crossref | GoogleScholarGoogle Scholar |
Pippin WF, Nichols B (1996) Observations of arthropod populations following the La Mesa Fire of 1977. USDA Forest Service General Technical Report RM161‐165, No. 0277-5786. (USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO)
Podgaiski LR, Cavalleri A, Ferrando CPR, Pillar VD, Mendonça Jr MdS (2018) Prescribed patch burnings increase thrips species richness and body size in grassland communities. Insect Conservation and Diversity 11, 204–212.
| Prescribed patch burnings increase thrips species richness and body size in grassland communities.Crossref | GoogleScholarGoogle Scholar |
Pollet J, Omi PN (2002) Effect of thinning and prescribed burning on crown fire severity in ponderosa pine forests. International Journal of Wildland Fire 11, 1–10.
| Effect of thinning and prescribed burning on crown fire severity in ponderosa pine forests.Crossref | GoogleScholarGoogle Scholar |
Prendergast-Miller MT, De Menezes AB, Macdonald LM, Toscas P, Bissett A, Baker G, Farrell M, Richardson AE, Wark T, Thrall PH (2017) Wildfire impact: natural experiment reveals differential short-term changes in soil microbial communities. Soil Biology and Biochemistry 109, 1–13.
| Wildfire impact: natural experiment reveals differential short-term changes in soil microbial communities.Crossref | GoogleScholarGoogle Scholar |
Prescott CE (2005) Do rates of litter decomposition tell us anything we really need to know? Forest Ecology and Management 220, 66–74.
| Do rates of litter decomposition tell us anything we really need to know?Crossref | GoogleScholarGoogle Scholar |
Pressler Y, Moore JC, Cotrufo MF (2019) Belowground community responses to fire: meta‐analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 128, 309–327.
| Belowground community responses to fire: meta‐analysis reveals contrasting responses of soil microorganisms and mesofauna.Crossref | GoogleScholarGoogle Scholar |
Price OF, Gordon CE (2016) The potential for LiDAR technology to map fire fuel hazard over large areas of Australian forest. Journal of Environmental Management 181, 663–673.
| The potential for LiDAR technology to map fire fuel hazard over large areas of Australian forest.Crossref | GoogleScholarGoogle Scholar | 27558828PubMed |
Prieto-Fernández A, Acea MJ, Carballas T (1998) Soil microbial and extractable C and N after wildfire. Biology and Fertility of Soils 27, 132–142.
| Soil microbial and extractable C and N after wildfire.Crossref | GoogleScholarGoogle Scholar |
Purahong W, Wubet T, Lentendu G, Schloter M, Pecyna MJ, Kapturska D, Hofrichter M, Krüger D, Buscot F (2016) Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Molecular Ecology 25, 4059–4074.
| Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition.Crossref | GoogleScholarGoogle Scholar | 27357176PubMed |
Raison RJ, Woods PV, Khanna PK (1983) Dynamics of fine fuels in recurrently burnt eucalypt forests. Australian Forestry 46, 294–302.
| Dynamics of fine fuels in recurrently burnt eucalypt forests.Crossref | GoogleScholarGoogle Scholar |
Raison RJ, Woods PV, Khanna PK (1986) Decomposition and accumulation of litter after fire in sub‐alpine eucalypt forests. Austral Ecology 11, 9–19.
| Decomposition and accumulation of litter after fire in sub‐alpine eucalypt forests.Crossref | GoogleScholarGoogle Scholar |
Rawson R, Rees B, Billing P (1985) ‘Effectiveness of fuel-reduction burning.’ (Department of Conservation, Forests and Lands, Fire Protection Branch: Victoria, Australia)
Raymond-Léonard LJ, Gravel D, Handa IT (2019) A novel set of traits to describe Collembola mouthparts: taking a bite out of the broad chewing mandible classification. Soil Biology and Biochemistry 138, 107608
| A novel set of traits to describe Collembola mouthparts: taking a bite out of the broad chewing mandible classification.Crossref | GoogleScholarGoogle Scholar |
Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DFB, Eisenhauer N (2012) Impacts of biodiversity loss escalate through time as redundancy fades. Science 336, 589–592.
| Impacts of biodiversity loss escalate through time as redundancy fades.Crossref | GoogleScholarGoogle Scholar | 22556253PubMed |
Riutta T, Slade EM, Bebber DP, Taylor ME, Malhi Y, Riordan P, Macdonald DW, Morecroft MD (2012) Experimental evidence for the interacting effects of forest edge, moisture and soil macrofauna on leaf litter decomposition. Soil Biology and Biochemistry 49, 124–131.
| Experimental evidence for the interacting effects of forest edge, moisture and soil macrofauna on leaf litter decomposition.Crossref | GoogleScholarGoogle Scholar |
Robinson NM, Leonard SWJ, Ritchie EG, Bassett M, Chia EK, Buckingham S, Gibb H, Bennett AF, Clarke MF (2013) Refuges for fauna in fire‐prone landscapes: their ecological function and importance. Journal of Applied Ecology 50, 1321–1329.
| Refuges for fauna in fire‐prone landscapes: their ecological function and importance.Crossref | GoogleScholarGoogle Scholar |
Rothstein DE, Vitousek PM, Simmons BL (2004) An exotic tree alters decomposition and nutrient cycling in a Hawaiian montane forest. Ecosystems 7, 805–814.
| An exotic tree alters decomposition and nutrient cycling in a Hawaiian montane forest.Crossref | GoogleScholarGoogle Scholar |
Sackett SS (1975) Scheduling prescribed burns for hazard reduction in the Southeast. Journal of Forestry 73, 143–147.
| Scheduling prescribed burns for hazard reduction in the Southeast.Crossref | GoogleScholarGoogle Scholar |
Sasal Y, Raffaele E, Farji-Brener AG (2010) Succession of ground-dwelling beetle assemblages after fire in three habitat types in the Andean forest of NW Patagonia, Argentina. Journal of Insect Science 10, 1–17.
| Succession of ground-dwelling beetle assemblages after fire in three habitat types in the Andean forest of NW Patagonia, Argentina.Crossref | GoogleScholarGoogle Scholar |
Scarff FR, Westoby M (2006) Leaf litter flammability in some semi‐arid Australian woodlands. Functional Ecology 20, 745–752.
| Leaf litter flammability in some semi‐arid Australian woodlands.Crossref | GoogleScholarGoogle Scholar |
Scherrer D, Massy S, Meier S, Vittoz P, Guisan A (2017) Assessing and predicting shifts in mountain forest composition across 25 years of climate change. Diversity and Distributions 23, 517–528.
| Assessing and predicting shifts in mountain forest composition across 25 years of climate change.Crossref | GoogleScholarGoogle Scholar |
Schimmel J, Granstrom A (1996) Fire severity and vegetation response in the boreal Swedish forest. Ecology 77, 1436–1450.
| Fire severity and vegetation response in the boreal Swedish forest.Crossref | GoogleScholarGoogle Scholar |
Schimmel J, Granström A (1997) Fuel succession and fire behaviour in the Swedish boreal forest. Canadian Journal of Forest Research 27, 1207–1216.
| Fuel succession and fire behaviour in the Swedish boreal forest.Crossref | GoogleScholarGoogle Scholar |
Schink B, Ward JC, Zeikus JG (1981) Microbiology of wetwood: importance of pectin degradation and clostridium species in living trees. Applied and Environmental Microbiology 42, 526–32.
| Microbiology of wetwood: importance of pectin degradation and clostridium species in living trees.Crossref | GoogleScholarGoogle Scholar | 16345848PubMed |
Schmuki C, Vorburger C, Runciman D, Maceachern S, Sunnucks P (2006) When log‐dwellers meet loggers: impacts of forest fragmentation on two endemic log‐dwelling beetles in south-eastern Australia. Molecular Ecology 15, 1481–1492.
| When log‐dwellers meet loggers: impacts of forest fragmentation on two endemic log‐dwelling beetles in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 16629805PubMed |
Schütz S, Weissbecker B, Hummel HE, Apel K-H, Schmitz H, Bleckmann H (1999) Insect antenna as a smoke detector. Nature 398, 298–299.
| Insect antenna as a smoke detector.Crossref | GoogleScholarGoogle Scholar |
Scott J, Reinhardt E (2001) Assessing crown fire potential by linking models of surface and crown fire behavior. USDA Forest Service Res. Pap. RMRS-RP-29. (U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO)
Segan DB, Murray KA, Watson JEM (2016) A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Global Ecology and Conservation 5, 12–21.
| A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions.Crossref | GoogleScholarGoogle Scholar |
Semenova‐Nelsen TA, Platt WJ, Patterson TR, Huffman J, Sikes BA (2019) Frequent fire reorganizes fungal communities and slows decomposition across a heterogeneous pine savanna landscape. New Phytologist 224, 916–927.
| Frequent fire reorganizes fungal communities and slows decomposition across a heterogeneous pine savanna landscape.Crossref | GoogleScholarGoogle Scholar |
Sensenig RL, Kimuyu DK, Ruiz Guajardo JC, Veblen KE, Riginos C, Young TP (2017) Fire disturbance disrupts an acacia ant–plant mutualism in favor of a subordinate ant species. Ecology 98, 1455–1464.
| Fire disturbance disrupts an acacia ant–plant mutualism in favor of a subordinate ant species.Crossref | GoogleScholarGoogle Scholar | 28273343PubMed |
Siemann E, Haarstad J, Tilman D (1997) Short-term and long-term effects of burning on oak savanna arthropods. The American Midland Naturalist 137, 349–361.
| Short-term and long-term effects of burning on oak savanna arthropods.Crossref | GoogleScholarGoogle Scholar |
Silveira JM, Barlow J, Krusche AV, Orwin KH, Balch JK, Moutinho P (2009) Effects of experimental fires on litter decomposition in a seasonally dry Amazonian forest. Journal of Tropical Ecology 25, 657–663.
| Effects of experimental fires on litter decomposition in a seasonally dry Amazonian forest.Crossref | GoogleScholarGoogle Scholar |
Simard DG, Fyles JW, Paré D, Nguyen T (2001) Impacts of clearcut harvesting and wildfire on soil nutrient status in the Quebec boreal forest. Canadian Journal of Soil Science 81, 229–237.
| Impacts of clearcut harvesting and wildfire on soil nutrient status in the Quebec boreal forest.Crossref | GoogleScholarGoogle Scholar |
Soong JL, Nielsen UN (2016) The role of microarthropods in emerging models of soil organic matter. Soil Biology and Biochemistry 102, 37–39.
| The role of microarthropods in emerging models of soil organic matter.Crossref | GoogleScholarGoogle Scholar |
Springett JA (1976) The effect of prescribed burning on the soil fauna and on litter decomposition in Western Australian forests. Austral Ecology 1, 77–82.
| The effect of prescribed burning on the soil fauna and on litter decomposition in Western Australian forests.Crossref | GoogleScholarGoogle Scholar |
Springett JA (1979) The effects of a single hot summer fire on soil fauna and on litter decomposition in jarrah (Eucalyptus marginata) forest in Western Australia. Australian Journal of Ecology 4, 279–291.
| The effects of a single hot summer fire on soil fauna and on litter decomposition in jarrah (Eucalyptus marginata) forest in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Steel ZL, Safford HD, Viers JH (2015) The fire frequency–severity relationship and the legacy of fire suppression in California forests. Ecosphere 6, 1–23.
| The fire frequency–severity relationship and the legacy of fire suppression in California forests.Crossref | GoogleScholarGoogle Scholar |
Stoof CR, De Kort A, Bishop TFA, Moore D, Wesseling JG, Ritsema CJ (2011) How Rock fragments and moisture affect soil temperatures during fire. Soil Science Society of America Journal 75, 1133–1143.
| How Rock fragments and moisture affect soil temperatures during fire.Crossref | GoogleScholarGoogle Scholar |
Stoof CR, Moore D, Fernandes PM, Stoorvogel JJ, Fernandes RES, Ferreira AJD, Ritsema CJ (2013) Hot fire, cool soil. Geophysical Research Letters 40, 1534–1539.
| Hot fire, cool soil.Crossref | GoogleScholarGoogle Scholar |
Sun H, Terhonen E, Kasanen R, Asiegbu FO (2014) Diversity and community structure of primary wood-inhabiting bacteria in boreal forest. Geomicrobiology Journal 31, 315–324.
| Diversity and community structure of primary wood-inhabiting bacteria in boreal forest.Crossref | GoogleScholarGoogle Scholar |
Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nature Climate Change 2, 686–690.
| Thermal tolerance and the global redistribution of animals.Crossref | GoogleScholarGoogle Scholar |
Tangney R, Issa NA, Merritt DJ, Callow JN, Miller BP (2018) A method for extensive spatiotemporal assessment of soil temperatures during an experimental fire using distributed temperature sensing in optical fibre. International Journal of Wildland Fire 27, 135–140.
| A method for extensive spatiotemporal assessment of soil temperatures during an experimental fire using distributed temperature sensing in optical fibre.Crossref | GoogleScholarGoogle Scholar |
Thom MD, Daniels JC, Kobziar LN, Colburn JR (2015) Can butterflies evade fire? Pupa location and heat tolerance in fire prone habitats of Florida. PLoS One 10, e0126755
| Can butterflies evade fire? Pupa location and heat tolerance in fire prone habitats of Florida.Crossref | GoogleScholarGoogle Scholar | 26016779PubMed |
Thomas CD (2000) Dispersal and extinction in fragmented landscapes. Proceedings of the Royal Society of London, B 267, 139–145.
| Dispersal and extinction in fragmented landscapes.Crossref | GoogleScholarGoogle Scholar |
Throop HL, Abu Salem M, Whitford WG (2017) Fire enhances litter decomposition and reduces vegetation cover influences on decomposition in a dry woodland. Plant Ecology 218, 799–811.
| Fire enhances litter decomposition and reduces vegetation cover influences on decomposition in a dry woodland.Crossref | GoogleScholarGoogle Scholar |
Tláskal V, Voříšková J, Baldrian P (2016) Bacterial succession on decomposing leaf litter exhibits a specific occurrence pattern of cellulolytic taxa and potential decomposers of fungal mycelia. FEMS Microbiology Ecology 92, fiw177
| Bacterial succession on decomposing leaf litter exhibits a specific occurrence pattern of cellulolytic taxa and potential decomposers of fungal mycelia.Crossref | GoogleScholarGoogle Scholar | 27543318PubMed |
Toberman H, Chen C, Lewis T, Elser JJ (2014) High‐frequency fire alters C:N:P stoichiometry in forest litter. Global Change Biology 20, 2321–2331.
| High‐frequency fire alters C:N:P stoichiometry in forest litter.Crossref | GoogleScholarGoogle Scholar | 24132817PubMed |
Tolhurst K, Shields B, Chong D (2008) Phoenix: development and application of a bushfire risk management tool. Australian Journal of Emergency Management 23, 47–54.
Tolonen AC, Cerisy T, El-Sayyed H, Boutard M, Salanoubat M, Church GM (2015) Fungal lysis by a soil bacterium fermenting cellulose. Environmental Microbiology 17, 2618–2627.
| Fungal lysis by a soil bacterium fermenting cellulose.Crossref | GoogleScholarGoogle Scholar | 24798076PubMed |
Trigg S, Flasse S (2000) Characterizing the spectral-temporal response of burned savannah using in situ spectroradiometry and infrared thermometry. International Journal of Remote Sensing 21, 3161–3168.
| Characterizing the spectral-temporal response of burned savannah using in situ spectroradiometry and infrared thermometry.Crossref | GoogleScholarGoogle Scholar |
Tylianakis JM, Rand TA, Kahmen A, Klein A-M, Buchmann N, Perner J, Tscharntke T (2008) Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems. PLoS Biology 6, e122
| Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems.Crossref | GoogleScholarGoogle Scholar |
Ulyshen MD (2016) Wood decomposition as influenced by invertebrates. Biological Reviews 91, 70–85.
| Wood decomposition as influenced by invertebrates.Crossref | GoogleScholarGoogle Scholar | 25424353PubMed |
Ulyshen MD, Wagner TL (2013) Quantifying arthropod contributions to wood decay. Methods in Ecology and Evolution 4, 345–352.
| Quantifying arthropod contributions to wood decay.Crossref | GoogleScholarGoogle Scholar |
Vallés SM, Fernández JBG, Dellafiore C, Cambrollé J (2011) Effects on soil, microclimate and vegetation of the native-invasive Retama monosperma (L.) in coastal dunes. Plant Ecology 212, 169–179.
| Effects on soil, microclimate and vegetation of the native-invasive Retama monosperma (L.) in coastal dunes.Crossref | GoogleScholarGoogle Scholar |
Van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters 13, 235–245.
| A meta-analysis of trait differences between invasive and non-invasive plant species.Crossref | GoogleScholarGoogle Scholar | 20002494PubMed |
Van Wagtendonk JW (2006) Fire as a physical process. In ‘Fire in California’s Ecosystems’. (Eds NG Sugihara, JW van Wagtendonk, KE Shaffer, J FitesKaufman, AE Thode) pp. 38–57. (University of California Press)
Vasconcelos HL, Laurance WF (2005) Influence of habitat, litter type, and soil invertebrates on leaf-litter decomposition in a fragmented Amazonian landscape. Oecologia 144, 456–462.
| Influence of habitat, litter type, and soil invertebrates on leaf-litter decomposition in a fragmented Amazonian landscape.Crossref | GoogleScholarGoogle Scholar | 15942762PubMed |
Vilariño A, Arines J (1991) Numbers and viability of vesicular-arbuscular fungal propagules in field soil samples after wildfire. Soil Biology and Biochemistry 23, 1083–1087.
| Numbers and viability of vesicular-arbuscular fungal propagules in field soil samples after wildfire.Crossref | GoogleScholarGoogle Scholar |
Wagle R, Eakle TW (1979) A controlled burn reduces the impact of a subsequent wildfire in a ponderosa pine vegetation type. Forest Science 25, 123–129.
| A controlled burn reduces the impact of a subsequent wildfire in a ponderosa pine vegetation type.Crossref | GoogleScholarGoogle Scholar |
Wanthongchai K, Goldammer JG, Bauhus J (2011) Effects of fire frequency on prescribed fire behaviour and soil temperatures in dry dipterocarp forests. International Journal of Wildland Fire 20, 35–45.
| Effects of fire frequency on prescribed fire behaviour and soil temperatures in dry dipterocarp forests.Crossref | GoogleScholarGoogle Scholar |
Wardle DA, Walker LR, Bardgett RD (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305, 509–513.
| Ecosystem properties and forest decline in contrasting long-term chronosequences.Crossref | GoogleScholarGoogle Scholar | 15205475PubMed |
Watling JI, Hickman CR, Orrock JL (2011) Invasive shrub alters native forest amphibian communities. Biological Conservation 144, 2597–2601.
| Invasive shrub alters native forest amphibian communities.Crossref | GoogleScholarGoogle Scholar |
Weedon JT, Cornwell WK, Cornelissen JHC, Zanne AE, Wirth C, Coomes DA (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species? Ecology Letters 12, 45–56.
| Global meta-analysis of wood decomposition rates: a role for trait variation among tree species?Crossref | GoogleScholarGoogle Scholar | 19016827PubMed |
Wells CG (1979) ‘Effects of fire on soil: a state-of-knowledge review.’ (Department of Agriculture, Forest Service)
Whelan RJ, Rodgerson L, Dickman CR, Sutherland EF (2002) Critical life cycles of plants and animals: developing a process-based understanding of population changes in fire-prone landscapes. In ‘Flammable Australia: the fire regimes and biodiversity of a continent’. (Eds RA Bradstock, JE Williams, AM Gill) pp. 94–124. (Cambridge University Press: Cambridge)
Wikars L-O, Schimmel J (2001) Immediate effects of fire-severity on soil invertebrates in cut and uncut pine forests. Forest Ecology and Management 141, 189–200.
| Immediate effects of fire-severity on soil invertebrates in cut and uncut pine forests.Crossref | GoogleScholarGoogle Scholar |
Wolters V (2000) Invertebrate control of soil organic matter stability. Biology and Fertility of Soils 31, 1–19.
| Invertebrate control of soil organic matter stability.Crossref | GoogleScholarGoogle Scholar |
Wood SW, Bowman DMJS (2012) Alternative stable states and the role of fire–vegetation–soil feedbacks in the temperate wilderness of southwest Tasmania. Landscape Ecology 27, 13–28.
| Alternative stable states and the role of fire–vegetation–soil feedbacks in the temperate wilderness of southwest Tasmania.Crossref | GoogleScholarGoogle Scholar |
Wyman RL (1998) Experimental assessment of salamanders as predators of detrital food webs: effects on invertebrates, decomposition and the carbon cycle. Biodiversity & Conservation 7, 641–650.
| Experimental assessment of salamanders as predators of detrital food webs: effects on invertebrates, decomposition and the carbon cycle.Crossref | GoogleScholarGoogle Scholar |
Yang S, Zheng Q, Yang Y, Yuan M, Ma X, Chiariello NR, Docherty KM, Field CB, Gutknecht JLM, Hungate BA, et al. (2020) Fire affects the taxonomic and functional composition of soil microbial communities, with cascading effects on grassland ecosystem functioning. Global Change Biology 26, 431–442.
| Fire affects the taxonomic and functional composition of soil microbial communities, with cascading effects on grassland ecosystem functioning.Crossref | GoogleScholarGoogle Scholar | 31562826PubMed |
York A (2000) Long-term effects of frequent low-intensity burning on ant communities in coastal blackbutt forests of south-eastern Australia. Austral Ecology 25, 83–98.
| Long-term effects of frequent low-intensity burning on ant communities in coastal blackbutt forests of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Yoshikawa K, Bolton WR, Romanovsky VE, Fukuda M, Hinzman LD (2002) Impacts of wildfire on the permafrost in the boreal forests of Interior Alaska. Journal of Geophysical Research 108, FFR 4-1–FFR 4-14.
| Impacts of wildfire on the permafrost in the boreal forests of Interior Alaska.Crossref | GoogleScholarGoogle Scholar |
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. Journal of Plant Ecology 1, 85–93.
| Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors.Crossref | GoogleScholarGoogle Scholar |
Zimmer M, Topp W (1998) Microorganisms and cellulose digestion in the gut of the woodlouse Porcellio scaber. Journal of Chemical Ecology 24, 1397–1408.
| Microorganisms and cellulose digestion in the gut of the woodlouse Porcellio scaber.Crossref | GoogleScholarGoogle Scholar |
Zimmer M, Kautz G, Topp W (2005) Do woodlice and earthworms interact synergistically in leaf litter decomposition? Functional Ecology 19, 7–16.
| Do woodlice and earthworms interact synergistically in leaf litter decomposition?Crossref | GoogleScholarGoogle Scholar |
Zuo J, Berg MP, Klein R, Nusselder J, Neurink G, Decker O, Hefting MM, Sass-Klaassen U, van Logtestijn RSP, Goudzwaard L, van Hal J, Sterck FJ, Poorter L, Cornelissen JHC (2016) Faunal community consequence of interspecific bark trait dissimilarity in early-stage decomposing logs. Functional Ecology 30, 1957–1966.
| Faunal community consequence of interspecific bark trait dissimilarity in early-stage decomposing logs.Crossref | GoogleScholarGoogle Scholar |
Zylstra P, Bradstock RA, Bedward M, Penman TD, Doherty MD, Weber RO, Gill AM, Cary GJ (2016) Biophysical mechanistic modelling quantifies the effects of plant traits on fire severity: species, not surface fuel loads, determine flame dimensions in eucalypt forests. PLoS One 11, e0160715
| Biophysical mechanistic modelling quantifies the effects of plant traits on fire severity: species, not surface fuel loads, determine flame dimensions in eucalypt forests.Crossref | GoogleScholarGoogle Scholar | 27529789PubMed |