Podzolisation affects the spatial allocation and chemical composition of soil organic matter fractions
Agnes Krettek A B , Ludger Herrmann A and Thilo Rennert
A B , Ludger Herrmann A and Thilo Rennert  A
A
A Department of Soil Chemistry and Pedology, Institute of Soil Science and Land Evaluation, University of Hohenheim, D-70593 Stuttgart.
B Corresponding author. Email: agnes.krettek@uni-hohenheim.de.
Soil Research 58(8) 713-725 https://doi.org/10.1071/SR20164
Submitted: 10 June 2020 Accepted: 11 August 2020 Published: 25 September 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Podzols are soils that display a unique vertical distribution of soil organic matter (SOM). We hypothesise that podzolisation, as a pedogenetic process, influences or even controls content, allocation and quality of SOM. We determined soil organic carbon (SOC) and nitrogen (N) contents in six SOM fractions obtained from mineral horizons of five soils with increasing degree of podzolisation: sand and stable aggregates (S + A), particulate organic matter (POM) > 63 µm and <63 µm, silt and clay (s + c), resistant SOC and dissolved organic matter. We applied infrared spectroscopy to evaluate SOM decomposition state, relative abundance of functional groups and SOM-metal complexation. In topsoil horizons, relative SOC allocation shifted from the larger to the smaller size POM fraction with increasing podzolisation. Accompanied with size reduction, the POM < 63 µm fraction was progressively less decomposed, as derived from infrared spectroscopy and C : N ratios. In illuvial subsoils, the proportion of SOC in the S + A fraction increased with increasing podzolisation, implying SOM accumulation in aggregates and coatings on sand grains. Elevated abundance of carboxylate and aromatic C in the s + c fractions of subsoil horizons indicated their preferred sorption. Additionally, metal-carboxyl complexation increased during podzolisation.
Additional keywords: fractionation, infrared techniques, Podzols.
Introduction
Podzols are soils characterised by transformation, mobilisation, vertical transport and subsequent partial precipitation of soil organic matter (SOM) together with iron (Fe), aluminium (Al) and other elements in subsoil horizons (IUSS Working Group WRB 2015). In the mid- to high-latitudes, Podzols develop on parent materials that provide coarse material during weathering or on highly permeable parent materials (Lundström et al. 2000; Sauer et al. 2007). Podzol sites are characterised by low contents of neutral cations and Fe (or depletion in these elements by intensive chemical weathering), sufficient percolating water and low soil temperatures (Lundström et al. 2000; Schaetzl 2002; Sauer et al. 2007). In addition, a vegetation providing low-nutrient litter favours the formation of poorly decomposed organic surface layers (Lundström et al. 2000; Sauer et al. 2007). Nutrient depletion and acidification aggravate the living conditions for soil organisms and, consequently, lead to the deceleration of litter decomposition and the enhanced formation of water-soluble organic acids. Additionally, acidic conditions facilitate the destruction of primary silicates, clay minerals and metal oxides, releasing Al, Fe and silicon (Si) into the soil solution. The formation of an eluvial horizon (e.g. EA or E) in the topsoil and mostly several illuvial horizons in the subsoil characterised by SOM (Bh) or metal oxides (Bs) or both (Lundström et al. 2000; Buurman and Jongmans 2005; Sauer et al. 2007) is a result of several combined processes, which we hereafter summarise and label as podzolisation. The term ‘metal oxides’ used includes oxide, hydroxides and oxyhydroxides.
As vertical podzolisation proceeds, genetic eluvial and illuvial horizons progressively deepen and differentiate (Cornelis et al. 2014). Several hypotheses have been proposed to explain the entire or aspects of podzolisation (e.g. Browne 1995; Lundström et al. 2000; Buurman and Jongmans 2005; Sauer et al. 2007). These comprised the mobilisation of SOM in topsoil horizons with the subjacent illuvial horizon receiving percolating water containing Al and Fe ions complexed by dissolved organic matter (DOM) or hydrophilic organic colloids. The saturation of organic molecules or colloids in deeper soil horizons by additional metal complexes results in formation of poorly crystalline Fe and Al (hydr-)oxides and accumulation of organo–mineral associations. Microbial decomposition of the organic ligands could also influence the precipitation of metals, which could possibly be remobilised by supply of fresh DOM (Buurman and Jongmans 2005). Further processes suggested to be involved in podzolisation include (i) reductive dissolution of Fe oxides by organic acids and subsequent transport of metal–organic complexes into the subsoil (Bloomfield 1953; Skjemstad et al. 1992), (ii) downward transport of inorganic colloidal sols of Al, Si and Fe and their precipitation in B horizons and (iii) adsorption of DOM on minerals formed in B horizons (e.g. Anderson et al. 1982; Farmer 1982).
Podzolisation continuously removes SOM from the mineral topsoil (Jansen et al. 2004) and modifies the chemical composition of the bulk mineral soil, inducing changes of SOM stabilisation mechanisms and SOM allocation (Rumpel et al. 2002, 2004). Illuvial B horizons in a Podzol chronosequence showed a decreasing proportion of aromatic carbon (C) in the order Bh–Bhs–Bs, and increasing aromaticity with increasing podzolisation – evidence that not only the allocation but also the composition of SOM in B horizons is dynamic in time and space (Skjemstad et al. 1992). By means of 13C-NMR spectroscopy, Rumpel et al. (2004) interpreted SOM in Podzol subsoils as adsorbed, i.e. as species dissolved and transported from the topsoil and deposited on mineral surfaces in the subsoil. This was consistent with a younger 14C age of mineral-associated SOM in Bh and Bs horizons compared with those of the underlying Bw horizons where input of DOM was possibly small (Schulze et al. 2009). Studies in Germany (Spielvogel et al. 2008) and the USA (Rasmussen et al. 2005) have shown the relevance of organic Al and Fe complexes and poorly crystalline mineral phases for SOM stabilisation in the subsoil of temperate forest soils. Next to increasing binding to the mineral phase, the mean residence time of SOM was observed to increase with soil depth (Scharpenseel et al. 1989; Rumpel et al. 2002).
Based on this literature review, we hypothesise that podzolisation influences or even controls the content, allocation and quality of SOM. More specifically, we hypothesise that the effects of podzolisation on SOM dynamics are reflected in quantitative and qualitative shifts of SOM in physical and chemical fractions. We expect a proportion of SOM in mineral association and in aggregates, relative to total SOM. Concomitantly, we expect increasing accumulation of reactive species in these fractions of illuvial B horizons with increasing podzolisation. Apart from mineral-associated SOM, we expect effects of podzolisation on particulate organic matter that reflect deteriorating SOM decomposition in the course of podzolisation. The aim of this study was to test these hypotheses (1) by quantifying the distribution of SOM among six physical and chemical SOM fractions, obtained from eluvial and illuvial horizons of soils along a podzolisation gradient and (2) by characterising SOM in these fractions by diffuse reflectance infrared Fourier-transform spectroscopy and their variability in the course of podzolisation.
Materials and methods
Site, soils, and sampling
We studied five soil profiles (designated P1–P5) with increasing morphological degree of podzolisation – from initial podzolisation (P1) to a fully developed Podzol (P5; IUSS Working Group WRB 2015), located in the Lower Rhine Plain in north-west Germany (51°10′16″N, 6°11′40″E). All profiles (Fig. S1 in the supplementary material) developed from aeolian sand on top of Pleistocene clastic sediments of the main terrace of the river Rhine (Dickhof et al. 2006). The profiles are located within a dune field at 82–93 m above sea level. The climate of the area is temperate-oceanic with mild winters, average annual precipitation of 750 mm and average annual temperature of 9°C (Dickhof et al. 2006). The vegetation consists mainly of Scots pine (Pinus sylvestris).
Bulk samples (~2 kg each) were collected from each mineral horizon of all profiles in excavated pits, air-dried and passed through a 2-mm sieve after manual removal of visible roots and coarser fragments. All samples were homogenised and analysed in duplicate.
Fractionation of SOM
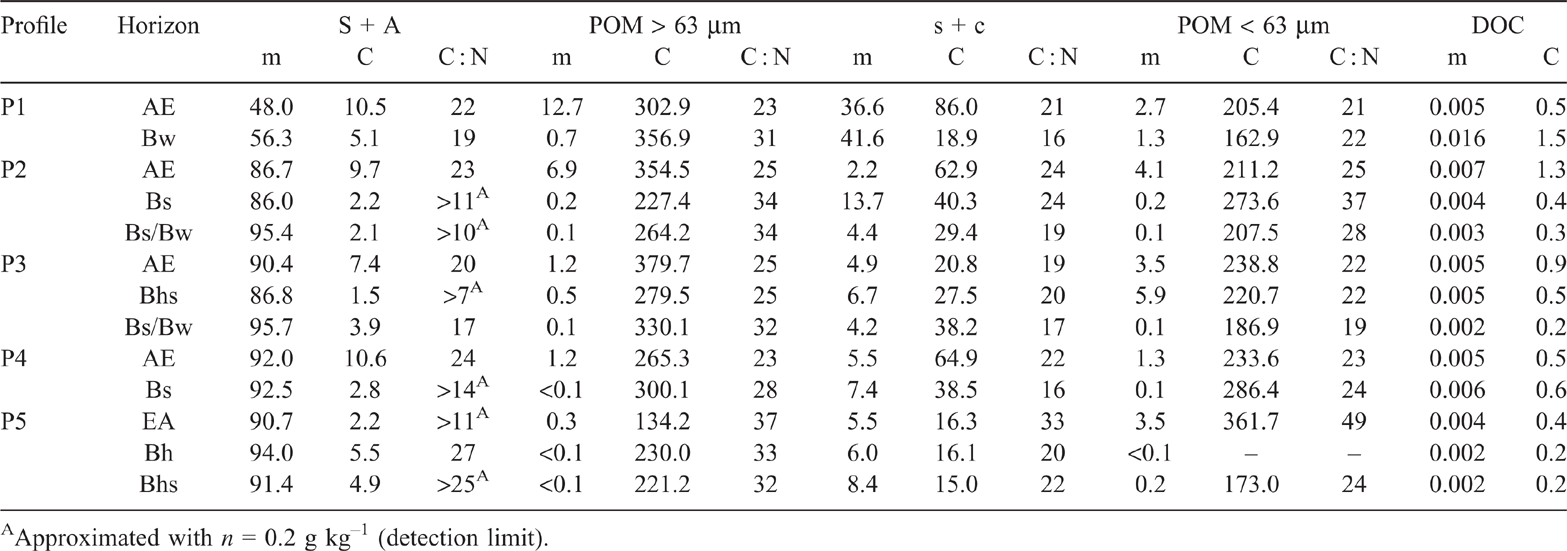
We fractionated all mineral horizons using a modified scheme adapted from Zimmermann et al. (2007). A sketch of the modified fractionation scheme (Fig. S2) and further details can be found in the supplementary material. Briefly, we disrupted the soil material by defined ultrasonication (i.e. 22 J ml–1) and separated fractions <63 µm and >63 µm by wet sieving. By density separation (i.e. at 1.8 g cm–1) of the fraction >63 µm, we obtained a heavy fraction of sand and sand-sized aggregates (S + A) and the floating light particulate organic matter (POM > 63 µm). The suspension <63 µm was pressure filtered to 0.45 µm. The filtrate constituted the fraction of dissolved organic carbon (DOC). Following the original protocol (Zimmermann et al. 2007), the fraction within 0.45–63 µm represents the mineral silt and clay (s + c) fraction. The blackish colour of the s + c fractions indicated the presence of POM. In fact, scanning-electron microscope images revealed plant debris on mineral surfaces (Fig. S3). Therefore, modifying the original method, we carried out a second density fractionation of the s + c fraction at a density of 1.8 g cm–3, as for the fraction >63 µm. The original fraction of 0.45–63 µm was, thus, divided into a heavy fraction <63 µm consisting of silt- and clay-sized particles (s + c) and a light organic fraction (POM < 63 µm). A chemically resistant fraction (rSOC) was obtained from the s + c fraction by treatment with sodium hypochlorite (NaOCl).
Analyses
Soil pH was measured potentiometrically in suspensions of 10 g of soil in 25 mL of 0.01 M CaCl2 solution. After sonication and dispersion with 0.05 M NH4OH solution and H2O2 treatment, soil particle-size analysis was conducted by sieving (sand fractions) and sedimentation (clay (<2 µm), fine, medium, and coarse silt; Table 1). The qualitative mineral composition of bulk samples and clay-size fractions was analysed by X-ray diffractometry (XRD) using a Siemens D500 X-ray diffractometer (Co-Kα radiation, U = 40 kV, I = 40 mA). For further clay-mineral differentiation, a Bruker D2 Phaser was used (Co-Kα radiation, U = 40 kV, I = 5 mA). Bulk samples were milled and measured as topfill powder mounts. Clay fractions were quantitatively obtained by centrifugation and the obtained suspension was divided into two. One part was saturated with Mg2+ and later treated with glycol. The other part was saturated with K+ and underwent additional temperature treatments (400°C and 600°C for 1 h each). Clay suspensions were transferred to ceramic (Al2O3) plates (oriented mounts) for diffraction measurements. Powder XRD measurements were carried out at diffraction angles in the range of 2θ = 3–69° with a step size of 0.05° and a counting time of 12 s per step, and at 3–35° for clay-fraction analysis.

|
We extracted the soils with citrate (Reyes and Torrent 1997), targeting organic–metal (Al and Fe) complexes, with ammonium oxalate–oxalic acid in darkness (Schwertmann 1964), targeting poorly crystalline Fe oxides and aluminosilicates, and partially organic Al and Fe complexes (Rennert 2019) and with dithionite–citrate– bicarbonate (Mehra 1958), targeting all pedogenic Fe oxides. Additionally, we extracted the S + A fractions with ammonium oxalate–oxalic acid. We determined the concentrations of Al, Fe and Si in the extracts by microwave plasma-atomic emission spectrometry (4200 MP-AES, Agilent, Waldbronn, Germany).
All solid fractions were ground (MM 200, Retsch, Reutlingen, Germany) before elemental analysis (C and N) with an elemental analyser (Vario macro EL, Elementar, Hanau, Germany). A subsample of the DOC fraction was analysed for the DOC concentration by thermal oxidation (Multi N/C 2100 S, Analytik Jena, Jena, Germany).
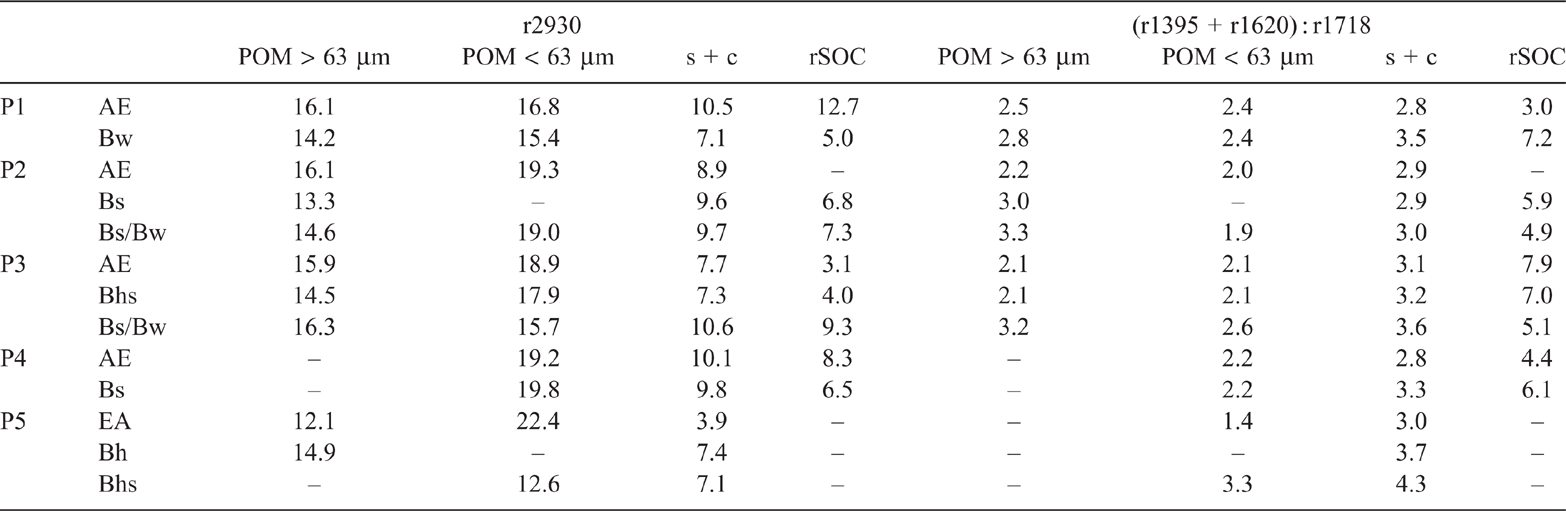
We characterised the SOM fractions by diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, using the external DRIFT accessory of a LUMOS infrared microscope (Bruker, Ettlingen, Germany). We mixed 75 mg of each sample with 75 mg of KBr for dilution and recorded background spectra of pure KBr; 200 spectra per sample were collected at a resolution of 4 cm–1 in the spectral range of 4000–600 cm–1. The spectra were converted to Kubelka-Munk units using the OPUS 7.2 software (Bruker). We calculated the ratio of the intensity at 2930 cm–1 (aliphatic C) and the sum of the intensities at 2930, 1620 (aromatic and carboxyl C) and 1520 cm–1 (aromatic C) as an indicator of oxidation and transformation (Rennert et al. 2017). The smaller the value of this ratio multiplied by 100 (r2930), the more oxidised and transformed the SOM, whereas large values indicate fresh plant-derived OM such as free POM (Rennert et al. 2017; Rennert 2018). Due to the sandy texture of the soils and the associated predominant presence of numerous Si–O groups, the peak at 1159 cm–1 was, unlike in previous studies (Rennert 2018; Rennert et al. 2018), not considered for the calculation of r2930. Additionally, the intensities at 1718 (carboxyl, also in aromatic esters; Veum et al. 2014), 1620, 1520 and 1395 cm–1 (carboxylate; Dick et al. 2003) were normalised with division by the SOC content. These ratios were multiplied by 100 and designated as r1718, r1620, r1520 and r1395 respectively. The ratio r1718 functioned as an indicator of oxidation and r1395 as an indicator of complexation of SOM with metals via the carboxyl group (Rennert 2018). The relative abundance of carboxylic metal bonds was derived from the ratio of deprotonated (or complexed) and protonated carboxyl groups (r1395 + r1620) : r1718 (Kaiser et al. 1997).
Results and discussion
Soil properties and profile characterisation
The stringent interpretation of differences between horizons and profiles is only possible when the parent material is homogenous, i.e. in this case aeolian sand. To test the homogeneity, we evaluated texture and mineral composition.
With respect to granulometric data, the first distinct feature is that the texture of all profiles was pure sand with >97 mass % in the sand fractions (Table 1). From this perspective, we can assume the parent material was uniform and homogeneous. To test the homogeneity among horizons, we evaluated the most prominent fractions: medium (mS) and fine sand (fS). The ratio mS : fS increased with depth in all profiles (Table 1). In most subsoils (except for P1), mS : fS was close to 3 and in the respective topsoils around 1.5. These regular gradients reflect alternating aeolian cycles, with increased transport capacity of strong erosive winds depositing the lower lying sediments and decreasing transport capacity of abating winds depositing finer upper sediments later on. In P3 even one and a half cycles are reflected by mS : fS. Only in the flatter area of P1 were the ratios all below 1.5 but with the same tendency to decrease towards the topsoil. Most probably, the materials here represent re-allocated topsoil material of the dune field that was further fractionated.
According with the texture data, the bulk mineral composition indicated homogeneous parent material, as quartz was the dominant mineral (>90 mass %) in all soil samples. Accessory minerals were feldspars (potassium feldspars as well as plagioclase) and clay minerals (illite, chlorite, interstratified minerals and kaolinite with vertical trends at the expense of chlorite towards topsoil horizons). Potassium feldspars dominated since they tend to be more resistant to weathering. Plagioclase appeared with a clear reflex only in the Bs horizon of P2 and in all horizons of P1.
All profiles (P1–P5) were strongly acidic with pH range of 2.7–3.6 in the topsoil and 2.9–4.1 in the subsoil (Table 2). Thus, pH slightly increased with depth and varied within the expected range for Podzols under coniferous forest (Browne 1995; Sauer et al. 2007). Compared with the other soils, P5 had elevated clay contents in the illuvial B horizons, in accordance with increased silt and clay proportions in subsoils of sandy Podzols (Schmidt et al. 2000; Sauer et al. 2008).

|
The contents of dithionite-extractable Fe (Fed) and Al (Ald) showed a tendency to increase from topsoil to subsoil horizons (in particular Bh, Bhs and Bs; Table 2). Precipitation of Fe oxides in the subsoil could be attributed to increased pH with depth (Browne 1995; Lundström et al. 2000). The Fed contents tended to increase down to the illuvial horizons and to be lower in Bw horizons. The Ald contents were well below those of Fed (except for P5) and tended to increase with depth. With progressive podzolisation, Fed contents tended to decrease in all horizons, with by far the lowest Fed contents (219–490 mg kg–1) in the fully developed Podzol (P5). This is in contrast to Vermeire et al. (2019), who reported increasing accumulation of Fed in subsoils of a Podzol chronosequence. Overall, Fed contents were at least one order of magnitude lower than in other sandy Podzols (Egli et al. 2001; Mokma et al. 2004; Cornelis et al. 2018), indicating that for our case the parent material was generally very low in Fe.
In the topsoil, Ald contents generally decreased with increasing podzolisation but there was no such consistent pattern in the subsoil. However, the subsoil horizons of P5 showed highest Ald contents, which exceeded the Fed contents by a factor of 2.5–4. Fitze (1982) observed that weathering released more Al than Fe with time in a Podzol chronosequence of the Swiss Alps. Potential Al sources at the current study site could include potassium feldspars and other Al-bearing primary minerals (Berggren and Mulder 1995). Moreover, litter leachate of Scots pine promotes Al release from minerals (Hongve et al. 2000). Schaetzl and Rothstein (2016) showed that, annually, considerably more Al than Fe was transported into the subsoil of Podzols by the percolating soil solution.
The illuvial subsoil of P5 also evinced a predominance of oxalate-soluble (Alo : Feo = 10.1 ± 1.8) and citrate-soluble Al over Fe (Alc : Fec = 10.1 ± 1.3). All soils tended to have lower Alo contents in the A horizon (140–340 mg kg–1) and larger Alo contents in the B horizons, which was most prominent in the illuvial horizons of P5 (up to 1550 mg kg–1). This corroborated with findings of Blaser et al. (1997) of a distinct increase of oxalate- and pyrophosphate-extractable Al contents in B horizons of a Haplic Podzol. However, we did not apply pyrophosphate extraction because of its poor selectivity (Rennert 2019) but used neutral citrate for extraction of metals complexed with SOM. Citrate-extractable Fe and Al contents revealed similar trends to those by oxalate throughout all profiles. That was, the contents not only followed a similar trend, with close correlations between Feo and Fec (r2 = 0.93) as well as Alo and Alc (r2 = 0.92), but extraction by both oxalate and citrate yielded nearly the same contents of these elements in all horizons (Table 2), indicating that most of the metals were organically bound. This was contrary to Reyes and Torrent (1997), who reported that Feo and Alo contents in Podzol Bs horizons substantially exceeded those of Fec and Alc, and attributed this to the presence of allophane.
There was overall less Al than Fe extractable with citrate except for the subsoil of P5. Derived from extraction with citrate (Reyes and Torrent 1997) or pyrophosphate (Higashi et al. 1981; Kodama and Wang 1989; Gustafsson et al. 1995; Blaser et al. 1997; Karltun et al. 2000; Tiberg et al. 2018) more Al than Fe associated with SOM was present in B horizons of developed Podzols. In all profiles of the current study, Fec contents were highest in the uppermost illuvial B horizon indicating accumulation of Fe associated with SOM in the horizon directly underlying the eluvial horizon, whereas Alc contents further increased with depth. Similarly, Ferro-Vázquez et al. (2014) detected maximum accumulation of Fe–organic complexes just below the E horizon, using pyrophosphate extraction, while Al–organic complexes accumulated deeper in soil. Lundström (1993) reported that Fe–SOM complexes precipitated more readily from the soil solution than Al–SOM complexes. However, the continuous input of organic acids from the surface horizons may have led to the re-mobilisation of metals from precipitated metal–organic complexes (Buurman et al. 2005; Ferro-Vázquez et al. 2014). Then, dissolved organic–Al complexes would migrate to deeper soil horizons, while organic–Fe complexers are assumed to be immobile in spodic horizons (Nierop et al. 2002; Jansen et al. 2003, 2005).
The Feo : Fed ratios were generally below 0.5 and lower in topsoil than in subsoil horizons, apart from P5 where the EA horizon exhibited the highest Feo : Fed (0.86). Previous studies on acidic soils with strong leaching showed that Feo : Fed > 0.6 indicates preferential formation of poorly crystalline Fe oxides in the presence of SOM (Schwertmann 1964; Eger and Hewitt 2008). In the topsoil horizons, Feo : Fed increased with advancing podzolisation from P2 to P5 (Table 2). However, except for P5, Feo : Fed was exceptionally low (Schwertmann 1964; Eger and Hewitt 2008; Jankowski 2014). We also found unusually low Feo : Fed for the subsoil horizons. Almost identical contents of oxalate- and citrate-soluble Fe suggested that oxalate extracted Fe from organic forms, as similarly detected by do Nascimento et al. (2004), rather than from poorly crystalline Fe oxides.
In the topsoil, SOC contents declined successively with increasing podzolisation (Table 2), complying with the increase in downward translocation of SOM (Lundström et al. 2000; Buurman and Jongmans 2002; Sauer et al. 2007). Consequently, SOC contents of the illuvial B horizons of P5 exceeded that of the fully developed E horizon, characteristic of a spodic horizon (IUSS Working Group WRB 2015).
The distribution pattern of N was generally similar to SOC. The C : N ratio decreased with depth in all profiles (Table 2). Decreasing C : N was ascribed to higher proportions of microbial-derived than plant-derived SOM in subsoil horizons (Rumpel and Kögel-Knabner 2011). Although C : N remained consistent in the corresponding horizons of P1–P4, it increased considerably in all corresponding mineral horizons of P5. Coniferous forest soils have large SOC contents because of their low pH or reduced litter quality with wide C : N (Kaiser et al. 2002; Crow et al. 2007). However, N contents that were throughout close to the detection limit probably contributed to greater variability of C : N.
According to IUSS Working Group WRB (2015), we classified P1 as Dystric Arenosol. Soils P2–P4 were classified as Dystric Brunic Arenosols. However, the Bw horizon of P1 was too shallow (<15 cm) for the ‘brunic’ qualifier. All profiles had an AE or EA horizon, which appeared predominantly light-coloured. Nevertheless, only the Munsell colour in moist state of the E horizon of P5 met the diagnostic criterion (10YR 6/2) to be classified as albic material. All other profiles (P1–P4), reflecting earlier pedogenic development stages, showed only visually an ascending tendency towards albic material without matching the required Munsell colours. With distinct spodic horizons containing illuvial compounds within the upper 200 cm, albic material and its texture coarser than very fine sand, P5 was classified as Albic Podzol (arenic).
Allocation of SOM and its shifts in the course of podzolisation
The SOC and N contents of bulk samples were recovered after fractionation by 97 ± 10% and 101 ± 10% respectively. Mass recovery was 104 ± 6%. The slightly lower recovery of SOC compared with mass could be attributed to the partial mobilisation of SOC by Na6(H2W12O40) and subsequent removal during density fractionation (Crow et al. 2007; Schulze et al. 2009) or by loss of dissolved and colloidal SOM during centrifugation and filtration (Castanha et al. 2008). Mass recoveries exceeding 100% might indicate the residual presence of Na6(H2W12O40) after density fractionation.
For all horizons, the POM fractions had the largest SOC contents, with the >63 µm fraction having a slightly higher SOC content (average 284.7 g kg–1) than the <63 µm fraction (230.1 g kg–1) (Table 3). During the separation of POM and minerals by density fractionation, small mineral particles may be transferred into the POM resulting in decreased SOC contents of the POM fraction, e.g. 162.9 g C kg–1 (POM < 63 µm, Bw horizon of P1) and an underestimation of mineral-associated SOM (Kaiser and Guggenberger 2007). In general, this effect was more pronounced for the POM < 63 µm fraction than the >63 µm fraction. The mass proportion of the POM > 63 µm fraction in the subsoils was generally very low, indicating small input of root-derived SOM or other coarse organic particles (Table 3). This is consistent with Buurman and Jongmans (2002), who reported little root-derived SOM in subsoils of nutrient-poor Podzols.
|
The mass proportion of the s + c fraction in the A horizons was always smaller than in the corresponding directly underlying B horizons (Fig. 1), consistent with elevated silt and clay contents in the subsoil of sandy Podzols (Schmidt et al. 2000; Sauer et al. 2008). The enrichment of SOC in the s + c fraction may be attributed to accumulation of silt- and clay-sized minerals that have interacted with SOM (Guggenberger et al. 1995; Rumpel et al. 2004), or may originate from SOM encapsulated in silt-sized aggregates (Zimmermann et al. 2012). In the topsoil, the SOC contents of the s + c fraction decreased with increasing podzolisation (except for P4; Table 3). In the subsoil, the s + c fractions of P2–P4 had substantially higher SOC contents than correspondingly in P1 and P5. The reversed pattern occurred for the S + A fraction, where the SOC contents in P1 and P5 were notably higher than in P2–P4. Overall, the SOC proportion of the S + A fractions in the illuvial horizons showed an increasing trend with successive podzolisation (Fig. 1). Sand-sized separates of a Haplic Podzol in north-west Germany with similar vegetation were lower in absolute and relative SOC content (Schmidt et al. 2000) than the Podzols of the current study. These disparities might have been caused by differences in the energy applied to disrupt aggregates by Schmidt et al. (2000). They utilised an energy of 440 J ml–1, which may have led to the enhanced release of smaller mineral and organic particles building up sand-sized aggregates, as compared with the applied fractionation (22 J ml–1) in the current study. The SOC contained in these particles would, thus, be detected in other fractions (POM < 63 µm).
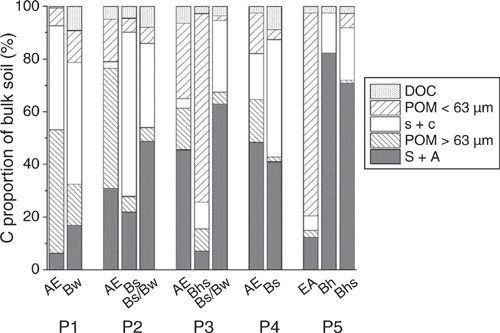
|
During Podzol development, the relative contribution of the POM < 63 µm fraction to total SOC in topsoil mineral horizons increased, while that of the >63 µm fraction strongly decreased (Fig. 1). In illuvial subsoils, the relative proportion of the S + A fractions increased with successive podzolisation. In the most developed Podzol, the S + A fraction represented the major SOC proportion in the B horizons, implying accumulation of SOM stabilised in aggregates, coatings of SOM on sand grains or both. Rumpel et al. (2004) showed that in the subsoil of acidic forest soils, sorption of DOM occurred in all particle fractions. Coarser particles could be coated by Fe oxides, increasing the surface area and providing an effective adsorbent for SOM (Ransom et al. 1998; Kaiser and Zech 2000). Using scanning-electron microscopy with energy dispersive spectroscopy, Jankowski (2014) found well-developed coatings on sand grains in Bhs horizons with micromorphological features typical of spodic horizons containing mainly SOM, Si, Al, Fe and manganese. For a further verification of whether increasing SOC contents in the S + A fraction of subsoils were caused by the formation of sand-sized aggregates or oxide coatings on sand grains, we conducted an oxalate extraction with the S + A fractions. We initially assumed that oxalate dissolved the poorly crystalline Fe and Al minerals that typically form coatings on sand grains and act as cementing agents in aggregates and solubilised Fe and Al associated with SOM. The Feo, Alo and Sio (except for P4) contents of the S + A fractions tended to increase with depth in all profiles (Table 4). Although P1–P3 had hardly any soluble Si in the topsoil, Si was present in P4 and P5 where podzolisation was most intense, thus, the strongest destruction of primary silicates and clay minerals took place. Generally, Sio contents were very low. There was no clear pattern with Podzol development for either Feo or Alo contents. However, as for the bulk soil, P5 showed the lowest contents of Feo (43 and 130 mg kg–1 in topsoil and subsoil respectively) and highest Alo contents in the subsoil (up to 1110 mg kg–1) in the S + A fraction, with the latter indicating the accumulation of organic Al in illuvial horizons.

|
With increasing podzolisation, the proportion of Alo and Feo of the S + A fraction related to the Alo and Feo of the bulk soil increased (largest values for P5, Fig. 2). The proportions of Feo and Alo were strongly positively correlated with the respective proportions of SOC (r2 = 0.92 for Feo, r2 = 0.87 for Alo; Fig. 2). Kaiser et al. (2002) already demonstrated the close correlation between Feo and SOC contents in Podzol and Cambisol horizons. Yuan et al. (1998) showed enrichment in SOC, N and Al on particle surfaces from Podzol subsoil horizons by X-ray photoelectron spectroscopy, which would explain our findings. The relevance of Al–organic associations and poorly crystalline Al mineral phases in SOM stabilisation in subsoil horizons of temperate forest soils was previously evidenced (e.g. Rasmussen et al. 2005). Because the Alo content of the S + A fractions of subsoil horizons increased relatively and absolutely in the course of podzolisation, we can confirm these past findings.

|
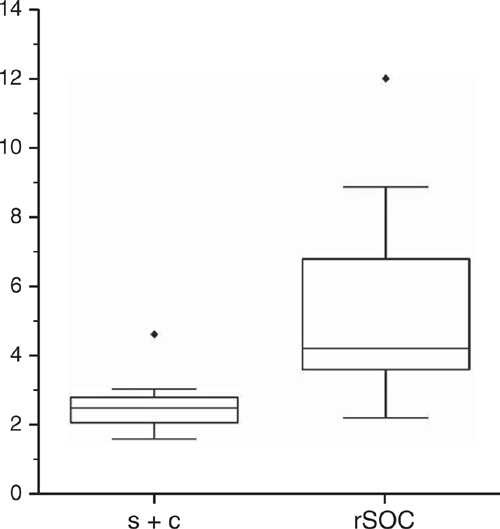
We obtained a chemically resistant rSOC fraction by treating the s + c fractions with NaOCl. Although 88% SOC remained after treatment in the topsoil of P1, it decreased successively until there was no more than 23% left in the topsoil of P5 (Fig. 3). In subsoils, the removal was overall stronger, preserving merely 40–47% SOC in P1–P4 and 17% and 11% in the Bh and Bhs horizon of P5 respectively. Apparently, SOM in the s + c fraction became more oxidisable in the course of podzolisation throughout all mineral horizons, pointing to increased potential degradability of SOM. Accordingly, Eusterhues et al. (2003) observed that SOC removal by Na2S2O8 treatment was ~70% in the subsoil of a Haplic Podzol and less than 20% in corresponding horizons of a Dystric Cambisol. Leifeld and Kögel-Knabner (2001) reported greater resistance of aliphatic C to oxidative treatment with H2O2. However, due to marginal changes in the amounts of aliphatic compounds among horizons of a Dystric Cambisol and a Haplic Podzol, Eusterhues et al. (2003) did not assume the chemical composition to be the only controlling factor for the resistance of SOM towards oxidation. In the current study, NaOCl treatment was conducted at pH 8 with samples having pH < 4. Increasing the pH during the treatment induced at least partial desorption of anionic organic species, e.g. of carboxylic groups, which was more pronounced in more strongly podzolised soils, i.e. with soils that have experienced inputs of organic acids to a larger extent or for a longer time or both. Smaller SOC loads in deeper soil horizons were assumed to be adsorbed with more ligands in direct contact with mineral surfaces (Kaiser and Guggenberger 2003). Consequently, Mikutta et al. (2005) suggested SOM desorption from mineral surfaces by NaOCl or Na2S2O8 treatment stronger in topsoils than in subsoils. Consistently, Eusterhues et al. (2003) observed SOC removal by Na2S2O8 (pH 7–8.5) treatment in a Haplic Podzol decreasing with depth. Considering both oxidation and desorption, we could not confirm that SOM was more strongly bound in the subsoil and therefore more difficult to remove. However, it is rather unlikely that the method used mirrored real conditions in Podzols. If the SOC loss was based on oxidation, the material in the soil would only be potentially labile, as decomposition would not set in under the extremely poor conditions in Podzols. If desorption was decisive for the loss of SOC, a corresponding adjustment to pH 8 in the subsoil of Podzols was very unlikely. Without taking into account real conditions, no conclusive assessments could be made regarding the actual degradability of organo–mineral associations in Podzols.
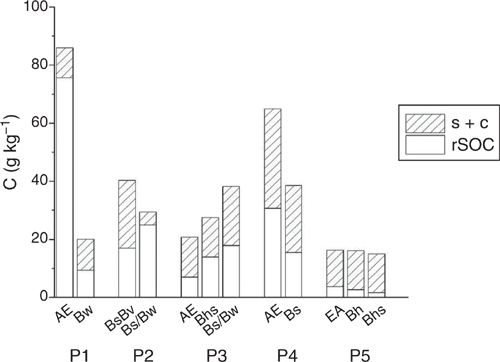
|
The mass proportion of the water-soluble fraction DOC was <0.2% (Table 3). The relative proportion of SOC released as DOC was the smallest among the fractions with 0.6–9.2%. There was a weak tendency towards higher DOC contents in the subsoil (5.5%) than in the topsoil (3.4%). However, SOM released into the aqueous phase during fractionation did not reflect solubility in situ, as easily dissociable compounds might likewise be extracted by water, leading to higher concentrations of aromatic C than in the actual soil solution (Rennert et al. 2007).
Throughout, N allocation and corresponding trends were almost identical with SOC for topsoil and subsoil. Due to very low N contents, several C : N ratios could only be approximated by means of the detection limit, particularly in the S + A and rSOC fraction (Table 3). The POM > 63 µm generally showed considerably higher C : N than the bulk samples, and POM < 63 µm exhibited a distinctly higher C : N mainly in subsoil. Golchin et al. (1994) considered wide C : N of light fractions as an indicator of poorly degraded material. All C : N in the topsoil of P1–P4 differed to a lesser extent than in the subsoil. Similarly, Schrumpf et al. (2013) observed more pronounced differences between the C : N of various density fractions in Podzol subsoil horizons. As in the bulk soil, C : N of the P5 fractions distinctly exceeded those of P1–P4, particularly in the topsoil. By trend, C : N decreased from the topsoil to the adjacent B horizon in mineral fractions (s + c and S + A) and increased in organic fractions (POM > 63 µm and < 63 µm). Similarly, C : N of a heavy fraction decreased with depth, but C : N of the corresponding light fraction increased (Schrumpf et al. 2013). Preferential association, i.e. stabilisation of N-rich organic species with clay-sized minerals (e.g. Knicker 2004), would account for the decrease of C : N in the s + c fraction with depth. Organic coatings with sorbed N-rich compounds could explain the low C : N of S + A fractions (Sollins et al. 2006). Schmidt et al. (2000) reported C : N of 36 and 26 for the clay fractions of an AE and a Bh horizon respectively, which were smaller than those of other particle-size fractions. However, Schmidt et al. (2000) investigated particle-size separates without previous density separation and this might explain greater C : N compared with the s + c fractions of our study.
Spectroscopic characterisation of SOM fractions
Spectral differences within one fraction tended to be smaller than among fractions, both between the topsoil and subsoil of a profile and in the course of podzolisation. Beyond that, the spectra of all POM < 63 µm fractions showed absorption within 3700–3600 cm–1, indicating silicates carried over during density separation in accord with SOC contents in POM fractions (Table 3). In the S + A fraction, absorption bands occurred particularly around 1000 cm–1, reflecting the predominance of quartz and the sandy texture of the profiles (Table 1).
Both POM fractions consistently showed the highest values for the indicator r2930, implying the least transformed SOM in these fractions (Table 5). For the POM > 63 µm fraction, r2930 for P1 and P2 decreased from topsoil to B horizons, whereas the values decreased and then increased along the profile for P3 and increased for P5. The POM < 63 µm fraction showed decreasing r2930 values with depth, indicating increasingly decomposed material from topsoil to subsoil (except for P4). With increasing podzolisation, r2930 of the POM < 63 µm fraction in the topsoil increased, indicating progressively less degraded SOM induced by soil acidification and deteriorating decomposition conditions (Schulze et al. 2009). The C : N of the POM < 63 µm fraction in topsoil increased accordingly. A decreasing degree of decomposition in the topsoil resulted in the POM < 63 µm being more differentiated within P5 than within the other profiles, i.e. with increasing podzolisation. Hence, besides an increase in the relative SOC proportion from the POM > 63 µm to the <63 µm fraction with increasing podzolisation (Table 3), there was also a qualitative shift accompanying the size difference of the organic debris.
Another indication of the qualitative difference of the A horizon of P5 was given by striking absorption at 1718 cm–1 (Fig. S4), indicating the pronounced presence of carboxyl groups in the POM < 63 µm fraction. Similarly, do Nascimento et. al. (2004) found an increase in intensity of the absorption band at 1715 cm–1 in IR spectra of clay fractions from SOM-rich A horizons of a developed Podzol, whereas those at 1620 and 1400 cm–1 decreased compared with less podzolised soils. Kaiser et al. (1997) observed that the band at 1725 cm–1 disappeared almost completely but bands at 1605 and 1400 cm–1 increased when SOM was adsorbed on amorphous Al(OH)3 and interpreted it as an indicator of inner-sphere complexation of carboxyl groups. Although our spectra showed slight shifts of the bands described by Kaiser et al. (1997), bands at 1718, 1620 and 1395 cm–1 could be assigned to the targeted functional groups (Mikutta et al. 2007) for considering the extent of binding of carboxyl groups with the ratio (r1395 + r1620) : r1718 (Table 5). The ratio increased for both POM fractions with depth (except for POM < 63 µm in P2 and P4, where ratios remained constant) pointing to increased metal complexation of carboxyl groups in the subsoil. The previously discussed extraction results (increasing contents of Alo and Alc with depth) revealed high mobility of Al, i.e. translocation into the subsoil in the course of podzolisation. Dissolved Al may have reacted with carboxyl groups of the POM < 63 µm fractions as manifested in the IR spectrum. The (r1395 + r1620) : r1718 of the POM < 63 µm fraction of the fully developed Podzol P5 was highest in the subsoil and lowest in the topsoil compared with all other developmental stages. Consistently, Nuzzo et al. (2020) found carboxyl groups in B horizons mostly dissociated or complexed with Fe and Al ions in contrast to H horizons. Rumpel et al. (2004) reported highly oxidised molecules preferentially advancing to deeper horizons in a Podzol while aliphatic C was shown to be a particularly important SOM constituent of the A horizon of a sandy Haplic Podzol. The highest r2930 in the A horizon of P5 indicated selectively preserved aliphatic plant-litter compounds such as cutin and suberin as possible remaining contributors to the insoluble SOM fraction in Podzol topsoil (Kögel‐Knabner et al. 1989; Nierop and Buurman 1999).
The SOM in mineral fractions is more decomposed than POM (Poirier et al. 2018), which we confirmed with the smaller r2930 values for the s + c fraction than for the POM fractions (Table 5). Rennert (2018) assigned smaller r2930 values in the clay fraction to oxidised species that have interacted with phyllosilicates and Fe oxides. The r2930 values for the rSOC fractions were consistently smaller than those of the s + c fractions (except for the AE horizon of P1), suggesting accumulation of oxidised and reactive species that were neither further oxidised nor desorbed, thus constituting the most stable SOM.
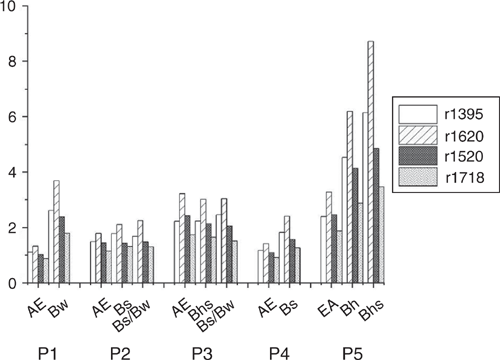
We assessed changes in the composition of SOM by evaluating indicators: r1395 (Fig. 4a) for metal complexation, r1718 (Fig. 4b) for carboxyl groups and (r1395+r1620) : r1718 (Table 5) for the relative extent of carboxylic bonds. The S + A fractions had low SOC contents, implying DRIFT intensities too low for a calculation of ratios. All three indicators were markedly higher in the rSOC fraction, pointing to the highest abundance of carboxyls as well as most SOM associated with metals and most metal complexed carboxylic groups in the rSOC fraction. Differences between the s + c fraction and the POM fractions were mostly marginal for r1395 and (r1395+r1620) : r1718 as numerical values, but were distinct in terms of process-related effects. The SOM can be bound to mineral surfaces by a variety of adsorption mechanisms. The absorption band at 1395 cm–1 did not allow for a distinction between sorption mechanisms such as hydrogen bonding, anion exchange, ligand exchange, cation bridges and co-precipitation. In the present environment at pH < 4, cation bridging was a conceivable mechanism, of both organic solutes to mineral surfaces and Al3+ promoting intramolecular and intermolecular associations of SOM causing the precipitation of organic substances (Römkens and Dolfing 1998) together with oxides and previously immobilised SOM (e.g. Guggenberger and Zech 1993; Jansen et al. 2003). Increasing oxidation and desorption of SOM by NaOCl with progressive podzolisation indicated decreasing adsorption energies (Gu et al. 1994; Mikutta et al. 2007), decreasing amount of reactive functional groups or increasing amount of less aromatic compounds that are more susceptible to desorption (Mikutta et al. 2005). Relating the intensity at 1520 cm–1 of aromatic C and that at 2930 cm–1 of aliphatic C before and after NaOCl treatment indicates preferential removal of aliphatic C relative to aromatic species (Fig. 5).

|
|
The ratio (r1395+1620) : r1718 increased with depth for the s + c fraction of all profiles as well as with successive podzolisation, indicating increasing metal complexation of carboxyl groups with the greatest ratio in the subsoil of P5. Considering a few individual bands that were normalised by the SOC content in the corresponding s + c fraction (Fig. 6), the increase in carboxylate (1395 and 1620 cm–1) and aromatic C (1520 and 1620 cm–1) in the s + c fraction was evident with increasing podzolisation, particularly in the subsoil of P5. Eusterhues et al. (2007) postulated chemical fractionation in Podzol subsoils, i.e. preferred sorption of aromatic and carboxylic groups, which would provide a conclusive explanation for the data presented here. Unlike mineral-associated SOM, aromatic compounds were found to be a minor constituent of HF-resistant, i.e. non-mineral-associated, SOM (Eusterhues et al. 2007). Correspondingly, we observed increasingly less aromatic C in the POM < 63 µm fraction. Thus, SOM in the s + c fraction probably originated from previously nonstabilised DOM from the POM < 63 µm or the >63 µm fraction of the overlying horizon. Eusterhues et al. (2007) did not verify whether these organic compounds originated from litter leachate or from DOM formed in situ, such as microbially altered material or root exudates. The range in C : N of 16–24 in the s + c fraction of the subsoil suggests litter leachate as the probable source of mineral-associated SOM in the Podzol subsoils under study.
|
Podzolisation affected the contents and allocation of SOM as follows:
-
increasing relative contribution of the POM < 63 µm fraction to the total SOC in topsoil mineral horizons, while that of the POM > 63 µm fraction strongly decreased, and
-
accumulation of SOM in aggregates, coatings of SOM on sand grains or both intimately associated with Fe phases and increasing amounts of Al in illuvial subsoil horizons.
Further, podzolisation affected the chemical composition of SOM fractions as follows:
-
progressively less degraded SOM in the POM < 63 µm fraction induced by deteriorating decomposition conditions,
-
leachate DOM as the most likely source of mineral-associated SOM in the subsoil,
-
preferred sorption or residual accumulation of carboxylate and aromatic C in mineral-associated subsoil SOM, and
-
possibly increasingly lower adsorption energies of SOM in the s + c fraction.
Conclusions
This study showed changing vertical quantitative and qualitative distribution patterns of SOC fractions as a function of podzolisation. The more developed the soils in terms of podzolisation, the larger the relative contribution of SOC associated with Fe and Al species in illuvial horizons. Particularly the composition of mineral-associated SOM in subsoil horizons documented a well-known podzolisation process, i.e. DOM leaching and transport. Association with minerals might transfer virtually readily degradable SOM into a more stabilised form. With progressing podzolisation, SOM accumulates in subsoil aggregates by interaction with Fe and Al ions, potentially precipitating rather than by interaction with poorly crystalline oxides. Additional SOM accumulation in the subsoil is given by interactions with dithionite-soluble Fe oxides and clay minerals. Although subsoil SOM accumulates during podzolisation in fractions commonly accepted as stable (aggregates and clay), subsoil SOM is not necessarily intrinsically stable and sequestered in the long term in these fractions. Stabilisation may be restricted to the rather harsh prevailing conditions, e.g. regarding pH and microbial activity, as shown by chemical oxidation and desorption. However, changing soil conditions, for instance increasing subsoil temperature, may destabilise subsoil SOM in Podzols, as it may promote microbial decomposition.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge Dr Carsten Schilli (GD NRW) for site selection as well as valuable information on site characterisation and site data. Further, we thank study site contact Erik Ludwig and the Regionalforstamt Niederrhein for site access. This work was funded by the Deutsche Forschungsgemeinschaft (RE 2251/10–1).
References
Anderson HA, Berrow ML, Farmer VC, Hepburn A, Russell JD, Walker AD (1982) A reassessment of Podzol formation processes. Journal of Soil Science 33, 125–136.| A reassessment of Podzol formation processes.Crossref | GoogleScholarGoogle Scholar |
Berggren D, Mulder J (1995) The role of organic matter in controlling aluminum solubility in acidic mineral soil horizons. Geochimica et Cosmochimica Acta 59, 4167–4180.
| The role of organic matter in controlling aluminum solubility in acidic mineral soil horizons.Crossref | GoogleScholarGoogle Scholar |
Blaser P, Kernbeek P, Tebbens L, Van Breemen N, Luster J (1997) Cryptopodzolic soils in Switzerland. European Journal of Soil Science 48, 411–423.
| Cryptopodzolic soils in Switzerland.Crossref | GoogleScholarGoogle Scholar |
Bloomfield C (1953) A study of podzolization. Part I. The mobilization of iron and aluminium by scots pine needles. Journal of Soil Science 4, 5–16.
| A study of podzolization. Part I. The mobilization of iron and aluminium by scots pine needles.Crossref | GoogleScholarGoogle Scholar |
Browne BA (1995) Toward a new theory of podzolization. In ‘Carbon forms and functions in forest soils’. (Eds WW McFee, JM Kelly) pp. 253–273. (Soil Science Society of America: Madison, WI, USA)
Buurman P, Jongmans AG (2002) Podzolization – an additional paradigm. Edafologia 9, 107–114.
Buurman P, Jongmans AG (2005) Podzolisation and soil organic matter dynamics. Geoderma 125, 71–83.
| Podzolisation and soil organic matter dynamics.Crossref | GoogleScholarGoogle Scholar |
Buurman P, van Bergen PF, Jongmans AG, Meijer EL, Duran B, Van Lagen B (2005) Spatial and temporal variation in Podzol organic matter studied by pyrolysis-gas chromatography/mass spectrometry and micromorphology. European Journal of Soil Science 56, 253–270.
| Spatial and temporal variation in Podzol organic matter studied by pyrolysis-gas chromatography/mass spectrometry and micromorphology.Crossref | GoogleScholarGoogle Scholar |
Castanha C, Tumbore S, Amundson R (2008) Methods of separating soil carbon pools affect the chemistry and turnover time of isolated fractions. Radiocarbon 50, 83–97.
| Methods of separating soil carbon pools affect the chemistry and turnover time of isolated fractions.Crossref | GoogleScholarGoogle Scholar |
Cornelis JT, Weis D, Lavkulich L, Vermeire ML, Delvaux B, Barling J (2014) Silicon isotopes record dissolution and re-precipitation of pedogenic clay minerals in a podzolic soil chronosequence. Geoderma 235–236, 19–29.
| Silicon isotopes record dissolution and re-precipitation of pedogenic clay minerals in a podzolic soil chronosequence.Crossref | GoogleScholarGoogle Scholar |
Cornelis JT, Delvaux B, Van Ranst E, Rouxhet PG (2018) Sub-micrometer distribution of Fe oxides and organic matter in Podzol horizons. Geoderma 323, 126–135.
| Sub-micrometer distribution of Fe oxides and organic matter in Podzol horizons.Crossref | GoogleScholarGoogle Scholar |
Crow SE, Swanston CW, Lajtha K, Brooks JR, Keirstead H (2007) Density fractionation of forest soils: Methodological questions and interpretation of incubation results and turnover time in an ecosystem context. Biogeochemistry 85, 69–90.
| Density fractionation of forest soils: Methodological questions and interpretation of incubation results and turnover time in an ecosystem context.Crossref | GoogleScholarGoogle Scholar |
Dick DP, Santos JHZ, Ferranti EM (2003) Chemical characterization and infrared spectroscopy of soil organic matter from two southern Brazilian soils. Revista Brasileira de Ciência do Solo 27, 29–39.
| Chemical characterization and infrared spectroscopy of soil organic matter from two southern Brazilian soils.Crossref | GoogleScholarGoogle Scholar |
Dickhof A, Hornig G, Hozman P, Milbert G (2006) Bodenkarte zur Standorterkundung: Verfahren Wassenberg / Wegberg (Forst), Erläuterungen, Geologischer Dienst Nordrhein-Westfalen, Krefeld.
do Nascimento NR, Bueno GT, Fritsch E, Herbillon AJ, Allard T, Melfi AJ, Astolfo R, Boucher H, Li Y (2004) Podzolisation as a deferralitization process: a study of Acrisol-Podzol sequence derived from Palaeozoic sandstones in the northern upper Amazon basin. European Journal of Soil Science 55, 523–538.
| Podzolisation as a deferralitization process: a study of Acrisol-Podzol sequence derived from Palaeozoic sandstones in the northern upper Amazon basin.Crossref | GoogleScholarGoogle Scholar |
Eger A, Hewitt A (2008) Soils and their relationship to aspect and vegetation history in the Eastern Southern Alps, Canterbury High Country, South Island, New Zealand. Catena 75, 297–307.
| Soils and their relationship to aspect and vegetation history in the Eastern Southern Alps, Canterbury High Country, South Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Egli M, Fitze P, Mirabella A (2001) Weathering and evolution of soils formed on granitic, glacial deposits: Results from chronosequences of Swiss alpine environments. Catena 45, 19–47.
| Weathering and evolution of soils formed on granitic, glacial deposits: Results from chronosequences of Swiss alpine environments.Crossref | GoogleScholarGoogle Scholar |
Eusterhues K, Rumpel C, Kleber M, Kögel-Knabner I (2003) Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation. Organic Geochemistry 34, 1591–1600.
| Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation.Crossref | GoogleScholarGoogle Scholar |
Eusterhues K, Rumpel C, Kögel-Knabner I (2007) Composition and radiocarbon age of HF-resistant soil organic matter in a Podzol and a Cambisol. Organic Geochemistry 38, 1356–1372.
| Composition and radiocarbon age of HF-resistant soil organic matter in a Podzol and a Cambisol.Crossref | GoogleScholarGoogle Scholar |
FAO (2006) Guidelines for soil description, 4th edition. (FAO: Rome)
Farmer VC (1982) Significance of the presence of allophane and imogolite in Podzol Bs horizons for podzolization mechanisms: a review. Soil Science and Plant Nutrition 28, 571–578.
| Significance of the presence of allophane and imogolite in Podzol Bs horizons for podzolization mechanisms: a review.Crossref | GoogleScholarGoogle Scholar |
Ferro-Vázquez C, Nóvoa-Muñoz JC, Costa-Casais M, Klaminder J, Martínez-Cortizas A (2014) Metal and organic matter immobilization in temperate Podzols: a high resolution study. Geoderma 217–218, 225–234.
| Metal and organic matter immobilization in temperate Podzols: a high resolution study.Crossref | GoogleScholarGoogle Scholar |
Fitze PF (1982) Zur Relativdatierung von Moränen aus der Sicht der Bodenentwicklung in den kristallinen Zentralalpen. Catena 9, 265–306.
| Zur Relativdatierung von Moränen aus der Sicht der Bodenentwicklung in den kristallinen Zentralalpen.Crossref | GoogleScholarGoogle Scholar |
Golchin A, Oades JM, Skjemstad JO, Clarke P (1994) Study of free and occluded particulate organic matter in soils by solid state 13C CP/MAS NMR spectroscopy and scanning electron microscopy. Australian Journal of Soil Research 32, 285–309.
| Study of free and occluded particulate organic matter in soils by solid state 13C CP/MAS NMR spectroscopy and scanning electron microscopy.Crossref | GoogleScholarGoogle Scholar |
Gu B, Schmitt J, Chen Z, Liang L, McCarthy JF (1994) Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environmental Science & Technology 28, 38–46.
| Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models.Crossref | GoogleScholarGoogle Scholar |
Guggenberger G, Zech W (1993) Dissolved organic carbon control in acid forest soils of the Fichtelgebirge (Germany) as revealed by distribution patterns and structural composition analyses. Geoderma 59, 109–129.
| Dissolved organic carbon control in acid forest soils of the Fichtelgebirge (Germany) as revealed by distribution patterns and structural composition analyses.Crossref | GoogleScholarGoogle Scholar |
Guggenberger G, Zech W, Haumaier L, Christensen B (1995) Land-use effects on the composition of organic matter in particle-size fractions of soil: II. CPMAS and solution 13C NMR analysis. European Journal of Soil Science 46, 147–158.
| Land-use effects on the composition of organic matter in particle-size fractions of soil: II. CPMAS and solution 13C NMR analysis.Crossref | GoogleScholarGoogle Scholar |
Gustafsson JP, Bhattacharya P, Bain DC, Fraser AR, McHardy WJ (1995) Podzolisation mechanisms and the synthesis of imogolite in northern Scandinavia. Geoderma 66, 167–184.
| Podzolisation mechanisms and the synthesis of imogolite in northern Scandinavia.Crossref | GoogleScholarGoogle Scholar |
Higashi T, De Coninck F, Gelaude F (1981) Characterization of some spodic horizons of the Campine (Belgium) with dithionite-citrate, pyrophosphate and sodium hydroxide-tetraborate. Geoderma 25, 131–142.
| Characterization of some spodic horizons of the Campine (Belgium) with dithionite-citrate, pyrophosphate and sodium hydroxide-tetraborate.Crossref | GoogleScholarGoogle Scholar |
Hongve D, Van Hees PAW, Lundström US (2000) Dissolved components in precipitation water percolated through forest litter. European Journal of Soil Science 51, 667–677.
| Dissolved components in precipitation water percolated through forest litter.Crossref | GoogleScholarGoogle Scholar |
IUSS Working Group WRB (2015) ‘World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps.’ World Soil Resources Reports No. 106. (FAO: Rome)
Jankowski M (2014) The evidence of lateral podzolization in sandy soils of northern Poland. Catena 112, 139–147.
| The evidence of lateral podzolization in sandy soils of northern Poland.Crossref | GoogleScholarGoogle Scholar |
Jansen B, Nierop KGJ, Verstraten JM (2003) Mobility of Fe(II), Fe(III) and Al in acidic forest soils mediated by dissolved organic matter: influence of solution pH and metal/organic carbon ratios. Geoderma 113, 323–340.
| Mobility of Fe(II), Fe(III) and Al in acidic forest soils mediated by dissolved organic matter: influence of solution pH and metal/organic carbon ratios.Crossref | GoogleScholarGoogle Scholar |
Jansen B, Nierop KGJ, Verstraten JM (2004) Mobilization of dissolved organic matter, aluminium and iron in Podzol eluvial horizons as affected by formation of metal-organic complexes and interactions with solid soil material. European Journal of Soil Science 55, 287–297.
| Mobilization of dissolved organic matter, aluminium and iron in Podzol eluvial horizons as affected by formation of metal-organic complexes and interactions with solid soil material.Crossref | GoogleScholarGoogle Scholar |
Jansen B, Nierop KGJ, Verstraten JM (2005) Mechanisms controlling the mobility of dissolved organic matter, aluminium and iron in Podzol B horizons. European Journal of Soil Science 56, 537–550.
| Mechanisms controlling the mobility of dissolved organic matter, aluminium and iron in Podzol B horizons.Crossref | GoogleScholarGoogle Scholar |
Kaiser K, Guggenberger G (2003) Mineral surfaces and soil organic matter. European Journal of Soil Science 54, 219–236.
| Mineral surfaces and soil organic matter.Crossref | GoogleScholarGoogle Scholar |
Kaiser K, Guggenberger G (2007) Distribution of hydrous aluminium and iron over density fractions depends on organic matter load and ultrasonic dispersion. Geoderma 140, 140–146.
| Distribution of hydrous aluminium and iron over density fractions depends on organic matter load and ultrasonic dispersion.Crossref | GoogleScholarGoogle Scholar |
Kaiser K, Zech W (2000) Dissolved organic matter sorption by mineral constituents of subsoil clay fractions. Journal of Plant Nutrition and Soil Science 163, 531–535.
| Dissolved organic matter sorption by mineral constituents of subsoil clay fractions.Crossref | GoogleScholarGoogle Scholar |
Kaiser K, Guggenberger G, Haumaier L, Zech W (1997) Dissolved organic matter sorption on subsoils and minerals studied by 13C-NMR and DRIFT spectroscopy. European Journal of Soil Science 48, 301–310.
| Dissolved organic matter sorption on subsoils and minerals studied by 13C-NMR and DRIFT spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Kaiser K, Eusterhues K, Rumpel C, Guggenberger G, Kögel-Knabner I (2002) Stabilization of organic matter by soil minerals - Investigations of density and particle-size fractions from two acid forest soils. Journal of Plant Nutrition and Soil Science 165, 451–459.
| Stabilization of organic matter by soil minerals - Investigations of density and particle-size fractions from two acid forest soils.Crossref | GoogleScholarGoogle Scholar |
Karltun E, Bain DC, Gustafsson JP, Mannerkoski H, Murad E, Wagner U, Fraser AR, McHardy WJ, Starr M (2000) Surface reactivity of poorly-ordered minerals in Podzol B horizons. Geoderma 94, 265–288.
| Surface reactivity of poorly-ordered minerals in Podzol B horizons.Crossref | GoogleScholarGoogle Scholar |
Knicker H (2004) Stabilization of N-compounds in soil and organic-matter-rich sediments - What is the difference? Marine Chemistry 92, 167–195.
| Stabilization of N-compounds in soil and organic-matter-rich sediments - What is the difference?Crossref | GoogleScholarGoogle Scholar |
Kodama H, Wang C (1989) Distribution and characterization of noncrystalline inorganic components in Spodosols and Spodosol-like soils. Soil Science Society of America Journal 53, 526–534.
| Distribution and characterization of noncrystalline inorganic components in Spodosols and Spodosol-like soils.Crossref | GoogleScholarGoogle Scholar |
Kögel‐Knabner I, Ziegler F, Riederer M, Zech W (1989) Distribution and decomposition pattern of cutin and suberin in forest soils. Journal of Plant Nutrition and Soil Science 152, 409–413.
| Distribution and decomposition pattern of cutin and suberin in forest soils.Crossref | GoogleScholarGoogle Scholar |
Leifeld J, Kögel-Knabner I (2001) Organic carbon and nitrogen in fine soil fractions after treatment with hydrogen peroxide. Soil Biology & Biochemistry 33, 2155–2158.
| Organic carbon and nitrogen in fine soil fractions after treatment with hydrogen peroxide.Crossref | GoogleScholarGoogle Scholar |
Lundström US (1993) The role of organic acids in the soil solution chemistry of a podzolized soil. Journal of Soil Science 44, 121–133.
| The role of organic acids in the soil solution chemistry of a podzolized soil.Crossref | GoogleScholarGoogle Scholar |
Lundström US, Van Breemen N, Bain D (2000) The podzolization process. A review. Geoderma 94, 91–107.
| The podzolization process. A review.Crossref | GoogleScholarGoogle Scholar |
Mehra OP (1958) Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays and Clay Minerals 7, 317–327.
| Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate.Crossref | GoogleScholarGoogle Scholar |
Mikutta R, Kleber M, Kaiser K, Jahn R (2005) Review: Organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate. Soil Science Society of America Journal 69, 120–135.
| Review: Organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate.Crossref | GoogleScholarGoogle Scholar |
Mikutta R, Mikutta C, Kalbitz K, Scheel T, Kaiser K, Jahn R (2007) Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochimica et Cosmochimica Acta 71, 2569–2590.
| Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms.Crossref | GoogleScholarGoogle Scholar |
Mokma DL, Yli-Halla M, Lindqvist K (2004) Podzol formation in sandy soils of Finland. Geoderma 120, 259–272.
| Podzol formation in sandy soils of Finland.Crossref | GoogleScholarGoogle Scholar |
Nierop KGJ, Buurman P (1999) Insoluble organic matter fractions in incipient Podzol B horizons: preservation of aliphatic biopolymers from roots. Humic Substances in the Environment 1, 29–37.
Nierop KGJ, Jansen B, Verstraten JM (2002) Dissolved organic matter, aluminium and iron interactions: precipitation induced by metal/carbon ratio, pH and competition. The Science of the Total Environment 300, 201–211.
| Dissolved organic matter, aluminium and iron interactions: precipitation induced by metal/carbon ratio, pH and competition.Crossref | GoogleScholarGoogle Scholar |
Nuzzo A, Buurman P, Cozzolino V, Spaccini R, Piccolo A (2020) Infrared spectra of soil organic matter under primary vegetation sequence. Chemical and Biological Technologies in Agriculture 7, 6
| Infrared spectra of soil organic matter under primary vegetation sequence.Crossref | GoogleScholarGoogle Scholar |
Poirier V, Roumet C, Munson AD (2018) The root of the matter: linking root traits and soil organic matter stabilization processes. Soil Biology & Biochemistry 120, 246–259.
| The root of the matter: linking root traits and soil organic matter stabilization processes.Crossref | GoogleScholarGoogle Scholar |
Ransom B, Kim D, Kastner M, Wainwright S (1998) Organic matter preservation on continental slopes: importance of mineralogy and surface area. Geochimica et Cosmochimica Acta 62, 1329–1345.
| Organic matter preservation on continental slopes: importance of mineralogy and surface area.Crossref | GoogleScholarGoogle Scholar |
Rasmussen C, Torn MS, Southard RJ (2005) Mineral assemblage and aggregates control carbon dynamics in a California conifer forest. Soil Science Society of America Journal 69, 1711–1721.
| Mineral assemblage and aggregates control carbon dynamics in a California conifer forest.Crossref | GoogleScholarGoogle Scholar |
Rennert T (2018) Geogenic CO2 affects inorganic soil properties and the composition of soil organic matter in physical fractions. Soil Research 56, 396–403.
| Geogenic CO2 affects inorganic soil properties and the composition of soil organic matter in physical fractions.Crossref | GoogleScholarGoogle Scholar |
Rennert T (2019) Wet-chemical extractions to characterise pedogenic Al and Fe species - A critical review. Soil Research 57, 1–16.
| Wet-chemical extractions to characterise pedogenic Al and Fe species - A critical review.Crossref | GoogleScholarGoogle Scholar |
Rennert T, Gockel KF, Mansfeldt T (2007) Extraction of water-soluble organic matter from mineral horizons of forest soils. Journal of Plant Nutrition and Soil Science 170, 514–521.
| Extraction of water-soluble organic matter from mineral horizons of forest soils.Crossref | GoogleScholarGoogle Scholar |
Rennert T, Ghong NP, Rinklebe J (2017) Permanganate-oxidizable soil organic matter in floodplain soils. Catena 149, 381–384.
| Permanganate-oxidizable soil organic matter in floodplain soils.Crossref | GoogleScholarGoogle Scholar |
Rennert T, Georgiadis A, Ghong NP, Rinklebe J (2018) Compositional variety of soil organic matter in mollic floodplain-soil profiles - Also an indicator of pedogenesis. Geoderma 311, 15–24.
| Compositional variety of soil organic matter in mollic floodplain-soil profiles - Also an indicator of pedogenesis.Crossref | GoogleScholarGoogle Scholar |
Reyes I, Torrent J (1997) Citrate-ascorbate as a highly selective extractant for poorly crystalline iron oxides. Soil Science Society of America Journal 61, 1647–1654.
| Citrate-ascorbate as a highly selective extractant for poorly crystalline iron oxides.Crossref | GoogleScholarGoogle Scholar |
Römkens PFAM, Dolfing J (1998) Effect of Ca on the solubility and molecular size distribution of DOC and Cu binding in soil solution samples. Environmental Science & Technology 32, 363–369.
| Effect of Ca on the solubility and molecular size distribution of DOC and Cu binding in soil solution samples.Crossref | GoogleScholarGoogle Scholar |
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter - A key but poorly understood component of terrestrial C cycle. Plant and Soil 338, 143–158.
| Deep soil organic matter - A key but poorly understood component of terrestrial C cycle.Crossref | GoogleScholarGoogle Scholar |
Rumpel C, Kögel-Knabner I, Bruhn F (2002) Vertical distribution, age, and chemical composition of organic carbon in two forest soils of different pedogenesis. Organic Geochemistry 33, 1131–1142.
| Vertical distribution, age, and chemical composition of organic carbon in two forest soils of different pedogenesis.Crossref | GoogleScholarGoogle Scholar |
Rumpel C, Eusterhues K, Kögel-Knabner I (2004) Location and chemical composition of stabilized organic carbon in topsoil and subsoil horizons of two acid forest soils. Soil Biology & Biochemistry 36, 177–190.
| Location and chemical composition of stabilized organic carbon in topsoil and subsoil horizons of two acid forest soils.Crossref | GoogleScholarGoogle Scholar |
Sauer D, Sponagel H, Sommer M, Giani L, Jahn R, Stahr K (2007) Podzol: soil of the year 2007. A review on its genesis, occurrence, and functions. Journal of Plant Nutrition and Soil Science 170, 581–597.
| Podzol: soil of the year 2007. A review on its genesis, occurrence, and functions.Crossref | GoogleScholarGoogle Scholar |
Sauer D, Schülli-Maurer I, Sperstad R, Sørensen R, Stahr K (2008) Podzol development with time in sandy beach deposits in southern Norway. Journal of Plant Nutrition and Soil Science 171, 483–497.
| Podzol development with time in sandy beach deposits in southern Norway.Crossref | GoogleScholarGoogle Scholar |
Schaetzl RJ (2002) A Spodosol-Entisol transition in Northern Michigan. Soil Science Society of America Journal 66, 1272–1284.
| A Spodosol-Entisol transition in Northern Michigan.Crossref | GoogleScholarGoogle Scholar |
Schaetzl RJ, Rothstein DE (2016) Temporal variation in the strength of podzolization as indicated by lysimeter data. Geoderma 282, 26–36.
| Temporal variation in the strength of podzolization as indicated by lysimeter data.Crossref | GoogleScholarGoogle Scholar |
Scharpenseel HW, Becker-Heidmann P, Neue HU, Tsutsuki K (1989) Bomb-carbon, 14C-dating and 13C-measurements as tracers of organic matter dynamics as well as of morphogenetic and turbation processes. Science of the Total Environment 81–82, 99–110.
| Bomb-carbon, 14C-dating and 13C-measurements as tracers of organic matter dynamics as well as of morphogenetic and turbation processes.Crossref | GoogleScholarGoogle Scholar |
Schmidt MWI, Knicker H, Kögel-Knabner I (2000) Organic matter accumulating in Aeh and Bh horizons of a Podzol - Chemical characterization in primary organo-mineral associations. Organic Geochemistry 31, 727–734.
| Organic matter accumulating in Aeh and Bh horizons of a Podzol - Chemical characterization in primary organo-mineral associations.Crossref | GoogleScholarGoogle Scholar |
Schrumpf M, Kaiser K, Guggenberger G, Persson T, Kögel-Knabner I, Schulze ED (2013) Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 10, 1675–1691.
| Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals.Crossref | GoogleScholarGoogle Scholar |
Schulze K, Borken W, Muhr J, Matzner E (2009) Stock, turnover time and accumulation of organic matter in bulk and density fractions of a Podzol soil. European Journal of Soil Science 60, 567–577.
| Stock, turnover time and accumulation of organic matter in bulk and density fractions of a Podzol soil.Crossref | GoogleScholarGoogle Scholar |
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Zeitschrift für Pflanzenernährung Düngung und Bodenkunde 105, 194–202.
| Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Zeitschrift für PflanzenernährungCrossref | GoogleScholarGoogle Scholar |
Skjemstad JO, Waters AG, Hanna JV, Oades JM (1992) Genesis of Podzols on coastal dunes in Southern Queensland. IV. Nature of the organic fraction as seen by 13C nuclear magnetic resonance spectroscopy. Australian Journal of Soil Research 30, 667–681.
| Genesis of Podzols on coastal dunes in Southern Queensland. IV. Nature of the organic fraction as seen by 13C nuclear magnetic resonance spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Sollins P, Swanston C, Kleber M, Filley T, Kramer M, Crow S, Caldwell BA, Lajtha K, Bowden R (2006) Organic C and N stabilization in a forest soil: Evidence from sequential density fractionation. Soil Biology & Biochemistry 38, 3313–3324.
| Organic C and N stabilization in a forest soil: Evidence from sequential density fractionation.Crossref | GoogleScholarGoogle Scholar |
Spielvogel S, Prietzel J, Kögel-Knabner I (2008) Soil organic matter stabilization in acidic forest soils is preferential and soil type-specific. European Journal of Soil Science 59, 674–692.
| Soil organic matter stabilization in acidic forest soils is preferential and soil type-specific.Crossref | GoogleScholarGoogle Scholar |
Tiberg C, Sjöstedt C, Gustafsson JP (2018) Metal sorption to Spodosol Bs horizons: organic matter complexes predominate. Chemosphere 196, 556–565.
| Metal sorption to Spodosol Bs horizons: organic matter complexes predominate.Crossref | GoogleScholarGoogle Scholar | 29329088PubMed |
Vermeire M, Bonneville S, Stenuit B, Cornélis J, Delvaux B (2019) Is microbial reduction of Fe (III) in podzolic soils influencing C release? Geoderma 340, 1–10.
| Is microbial reduction of Fe (III) in podzolic soils influencing C release?Crossref | GoogleScholarGoogle Scholar |
Veum KS, Goyne KW, Kremer RJ, Miles RJ, Sudduth KA (2014) Biological indicators of soil quality and soil organic matter characteristics in an agricultural management continuum. Biogeochemistry 117, 81–99.
| Biological indicators of soil quality and soil organic matter characteristics in an agricultural management continuum.Crossref | GoogleScholarGoogle Scholar |
Yuan G, Soma M, Seyama H, Theng BK, Lavkulich L, Takamatsu T (1998) Assessing the surface composition of soil particles from some podzolic soils by X-ray photoelectron spectroscopy. Geoderma 86, 169–181.
| Assessing the surface composition of soil particles from some podzolic soils by X-ray photoelectron spectroscopy.Crossref | GoogleScholarGoogle Scholar |
Zimmermann M, Leifeld J, Schmidt MWI, Smith P, Fuhrer J (2007) Measured soil organic matter fractions can be related to pools in the RothC model. European Journal of Soil Science 58, 658–667.
| Measured soil organic matter fractions can be related to pools in the RothC model.Crossref | GoogleScholarGoogle Scholar |
Zimmermann M, Leifeld J, Conen F, Bird MI, Meir P (2012) Can composition and physical protection of soil organic matter explain soil respiration temperature sensitivity? Biogeochemistry 107, 423–436.
| Can composition and physical protection of soil organic matter explain soil respiration temperature sensitivity?Crossref | GoogleScholarGoogle Scholar |