Interventions aimed at increasing syphilis screening among non-pregnant individuals in healthcare settings: a systematic review and meta-analysis
Leah Moncrieff A * , Morgan O’Reilly A , Leanne Hall A and Clare Heal A
A * , Morgan O’Reilly A , Leanne Hall A and Clare Heal A
A
Abstract
Syphilis remains a pressing public health concern with potential severe morbidity if left untreated. To improve syphilis screening, targeted interventions are crucial, especially in at-risk populations. This systematic review synthesises studies that compare syphilis screening in the presence and absence of an intervention. A systematic search of four databases was conducted (Medline, Embase, Cinahl and Scopus). The primary outcomes evaluated included syphilis screening, re-screening and detection rates. Findings were synthesised narratively. Where multiple studies were clinically heterogenous, a pooled odds ratio was calculated. Twenty-four studies were included. A variety of interventions showed promise including clinician alerts, which increased syphilis screening rate (OR range, 1.25–1.45) and patient SMS reminders that mostly improved re-screening/re-attendance rates (OR range, 0.93–4.4). Coupling syphilis serology with routine HIV monitoring increased the proportion of HIV-positive individuals undergoing both tests. However, pooling three studies with this intervention using the outcome of syphilis detection rate yielded inconclusive results (pooled OR 1.722 [95% CI 0.721–2.723], I2 = 24.8%, P = 0.264). The introduction of hospital-based packaged testing for screening high-risk individuals is unique given hospitals are not typical locations for public health initiatives. Nurse-led clinics and clinician incentives were successful strategies. Including syphilis screening with other existing programs has potential to increase screening rates (OR range, 1.06–2.08), but requires further investigation. Technology-driven interventions produced cost-effective, feasible and positive outcomes. Challenges were evident in achieving guideline-recommended screening frequencies for men who have sex with men, indicating the need for multifaceted approaches. Wider application of these interventions may improve syphilis screening and detection rates.
Keywords: health facilities and services, HIV, men who have sex with men, screening, sexual health, sexually transmitted infections, syphilis, systematic review.
Introduction
Syphilis poses a significant public health problem. Despite being potentially curable, it often remains asymptomatic and if left undetected, can result in severe morbidity manifesting as cardiovascular syphilis (aortic aneurysm, aortic valvulopathy), neurosyphilis (meningitis, stroke, seizures) or gummatous syphilis (infiltration of any organ and its subsequent destruction).1
Syphilis infection increases the risk of human immunodeficiency virus (HIV) acquisition.2 Moreover, syphilis can increase HIV viral load in individuals already infected, facilitating HIV transmission.3 Syphilis prevention is therefore of greatest concern in HIV-positive individuals and those with high HIV risk, including men who have sex with men (MSM), transgender people and injecting drug users.4
Controlling syphilis outbreaks relies on timely diagnosis and treatment of those infected. Modelling studies indicate more frequent screening of key populations has the potential to improve detection rates.5–9 This approach would enable earlier treatment and contact tracing, and facilitate health promotion initiatives, thereby reducing community transmission and preventing long-term sequelae associated with untreated syphilis.
Several countries have established guidelines promoting regular syphilis screening for MSM. Guidelines in the United States and Australia recommend screening for syphilis in MSM up to 3 monthly and at least annually for those with fewer risk factors (e.g. not sexually active, in a monogamous relationship).10,11 However, available data from the United States12 and Australia13 indicates the rate of screening for syphilis among MSM does not meet these guidelines.
To address this disparity, research has been conducted into targeted interventions to increase screening of all sexually transmitted infections (STIs) among high-risk populations. Systematic reviews have identified methods to increase STI screening including clinician reminders and patient recall systems that have shown promising results in enhancing overall STI screening.14–17
The existing literature lacks comprehensive analysis of interventions specifically targeting syphilis screening, making it challenging to determine optimal strategies and future research directions. This gap is significant given the unique characteristics of syphilis having a primarily asymptomatic course and serious complications. The aim of this review is to evaluate interventions implemented in healthcare settings with the purpose of increasing syphilis screening rates and detection.
Materials and methods
Search strategy
A systematic review of the literature was conducted according to the preferred reporting of items for systematic reviews and meta-analysis (PRISMA) guidelines.18 The review protocol was registered in PROSPERO, the international prospective register of systematic reviews (CRD42023445995). MEDLINE, Embase, CINAHL and Scopus databases were searched, spanning from their respective creation dates to final searches on 8 July 2023, limited to human studies and those published in the English language. The following keywords, along with synonyms, were used: ‘syphilis’, ‘screening’, ‘healthcare facilities’ (Table 1). Reference lists of included articles were also checked for relevant studies.
| Syphilis OR ‘treponema pallidum’ OR ‘treponema pallidum infection’ OR lues OR ‘syphilitic disorder’ OR ‘latent syphilis’ OR ‘latent state syphilis’ OR treponematosis OR ‘great pox’ OR ‘txid160’ | |
| AND | |
| Screen* OR ‘mass screening’ OR ‘health screening’ OR ‘early diagnosis’ OR ‘early detection’ OR ‘secondary prevention’ | |
| AND | |
| Hospital* OR clinic* OR ‘health services’ OR ‘health service’ OR ‘health facilities’ OR ‘health facility’ OR ‘health care services’ OR ‘healthcare services’ OR ‘health care service’ OR ‘healthcare service’ OR ‘health care facilities’ OR ‘healthcare facilities’ OR ‘health care facility’ OR ‘healthcare facility’ |
Titles and abstracts of all publications from the search were uploaded into Covidence.19 Two reviewers (LM, MO) independently screened each abstract for inclusion, with a third reviewer acting as a tiebreaker if there was a discrepancy (LH). Full text screening was individually conducted by two reviewers (LM, MO), guided by the inclusion and exclusion criteria.
Eligibility criteria
The PICO (population, intervention, comparison, outcome) framework was used to guide eligibility criteria.20 Studies of participants who were non-pregnant and asymptomatic for syphilis were included. Screening facilities included sexual health clinics, general practice (GP) and hospitals.
Studies were required to evaluate a clinic- or hospital-based intervention aimed at increasing one or more of the following syphilis-based outcomes: screening rate (proportion of individuals screened); re-screening rate (proportion of individuals who were screening again); or detection rate (proportion of individuals diagnosed with syphilis). A control group or period was required, to ensure comparison to pre-intervention clinical practice. Secondary outcomes, if available, included feasibility (staff burden, resource use, cost analysis) and possible harms of the intervention.
Studies were excluded if they did not include a comparator group or period; reported screening rates in the absence of an intervention; involved STI screening or promotional activities outside of healthcare settings; or were designed to compare sensitivities of different laboratory methods for screening.
Quantitative studies, including randomised controlled trials (RCTs), quasi-experimental, cohort and case-control studies, were eligible for inclusion. Qualitative only studies were excluded from this review as were mathematical modelling studies, review articles, commentaries, editorials, guidelines, and case reports.
Data analysis
Two reviewers (LM, MO) individually extracted data from included articles into a pre-defined template. When completed, data was compared to identify variations in the collected information. Data included study design, study setting, target population, description of the intervention, control groups or periods, outcomes and statistical methods used.
For each study, crude odds ratios (OR), 95% confidence intervals (95% CI) and P-values were calculated based on the data available in the published article. Where multiple studies were considered to be clinically heterogenous with the same outcome, a pooled estimate of the odds ratio was generated. STATA ver. 18,21 was used to conduct a meta-analysis using a random effects model.
Quality assessment
Risk of bias was assessed using the JBI critical appraisal tools.22 Four instruments were used: checklist for RCTs, checklist for quasi-experimental studies, checklist for cohort studies and checklist for analytical cross-sectional studies. Non-randomised pre-post studies were assessed using the checklist for quasi-experimental studies. These instruments allow for calculation of a bias score ranging from 0 to 100% based on 8–13 questions, which varied by study design. A score of 71% was classified as low risk of bias, 51–70% was moderate risk and 50% was high risk.23 Two reviewers (LM, MO) individually assessed for bias and in instances of disagreement, a third reviewer acted as a tiebreaker (LH).
Results
Search results
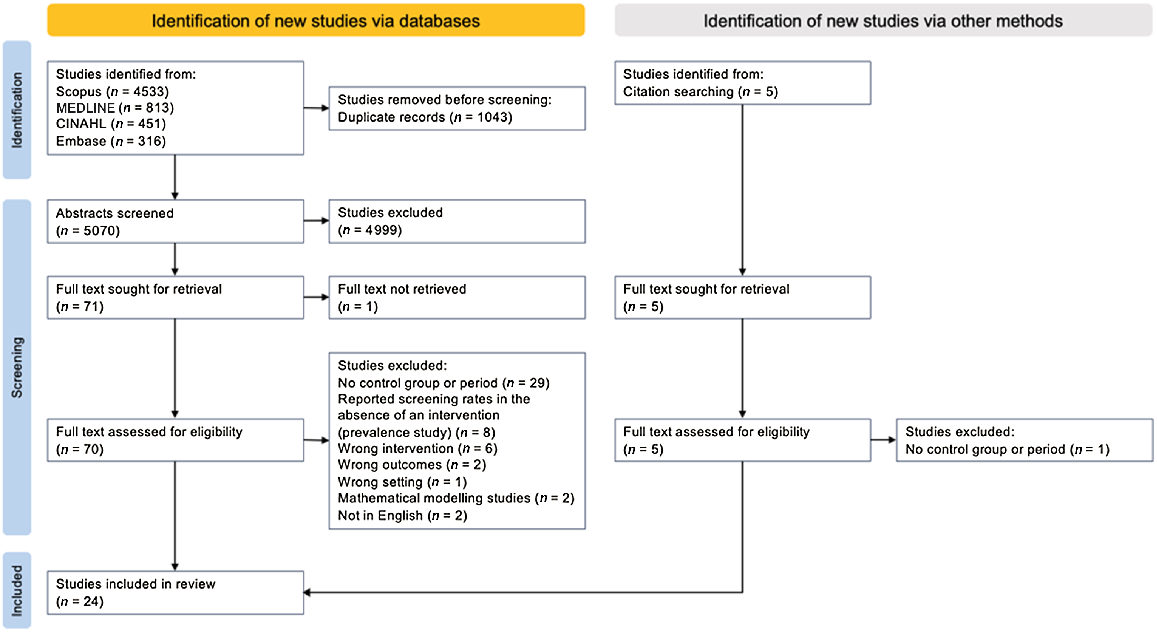
After removing duplicates, 5075 articles were identified, with 24 meeting inclusion criteria (Fig. 1).24–47 Studies and intervention results are summarised in Table 2. Included studies were conducted in Australia (n = 9), the United States (n = 7), the United Kingdom (n = 6), Canada (n = 1) and China (n = 1). All studies, except one randomised control trial,31 used an observational design with a pre-intervention comparator period, concurrent control group or both.
Flowchart for systematic inclusion of studies according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.18
| Study | Study design | Setting | Study population | Intervention | Time after intervention | Control | Outcome(s) | Control group | Intervention group | Statistical findings calculated by reviewers | Risk of bias | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | Crude OR (95% CI) | P-value | ||||||||||
| Reminder systems | |||||||||||||||
| Bissessor et al. 24 | Pre-post | STI clinic, Australia | MSM | Pre-appointment computer-assisted self-interview risk assessment. System-generated clinician alert to test high-risk MSM (>10 sexual partners in previous 12 months) for syphilis. | 12 months. | Before intervention: 12 months. | Screening rate (any risk) | 2787/3902 | 71.42 | 2949/3893 | 75.75 | 1.25 (1.13–1.38) | <0.001 | Low | |
| Screening rate (high-risk) | 1559/2017 | 77.29 | 1282/1445 | 88.72 | 2.31 (1.9–2.8) | <0.001 | |||||||||
| Detection rate of early syphilis in high-risk MSM | 31/2017 | 1.54 | 58/1445 | 4.01 | 2.67 (1.72–4.17) | <0.001 | |||||||||
| Proportion of early syphilis diagnoses that were asymptomatic | 5/31 | 16.13 | 31/58 | 53.45 | 5.97 (2.01–17.71) | 0.0013 | |||||||||
| Scarborough et al. 25 | Quasi-experimental | STI clinic, US | HIV-positive MSM | Self-completed pre-appointment risk assessment provided to doctor during consultation. | 3 months. | Before intervention: 3 months. | Screening rate | 213/437 | 48.74 | 211/364 | 57.97 | 1.45 (1.1–1.92) | 0.0093 | Low | |
| Bourne et al. 26 | Cohort | STI clinic, Australia | HIV-negative MSM | HIV/STI screening SMS reminder: 3–6 months post-appointment. | 9 months. | (1) Before intervention: 9 months. | HIV/STI re-screening rate within 9 months | 544/1753 | 31.03 | 460/714 | 64.43 | 3.1 (2.5–3.8) A | <0.001 | Low | |
| (2) Concurrent control. | 322/1084 | 29.70 | 460/714 | 64.43 | 4.4 (3.5–5.5) A | <0.001 | |||||||||
| (2) Concurrent control. | Detection rate | 8/1084 | 0.74 | 36/714 | 5.04 | 7.14 (3.3–15.46) | <0.001 | ||||||||
| Burton et al. 27 | Pre-post | STI clinic, UK | Patients at risk for STIs (current acute STI, attending for emergency contraception, sex workers, MSM and those in the window period for HIV) | STI screening SMS reminder: 2–12 weeks post-appointment. | 8 months. | Before intervention: 8 months. Risk factors matched. | Re-attendance rate for STI screening within 4 months | 92/266 | 34.59 | 90/273 | 32.97 | 0.93 (0.65–1.33) | 0.691 | Moderate | |
| Nyatsanza et al. 28 | Pre-post | Same as above (Burton et al. 24). More personalised text message including patient’s first name and additional clinic contact details. | 8 months. | (1) Before both interventions: 8 months. Risk factors matched. | Re-attendance rate for STI screening within 4 months | 92/266 | 34.59 | 149/266 | 56.02 | 2.41 (1.7–3.42) | <0.001 | Moderate | |||
| (2) Before intervention: 8 months. Patients who received generic text message. | Re-attendance rate for STI screening within 4 months | 90/273 | 32.97 | 149/266 | 56.02 | 2.59 (1.83–3.67) | <0.001 | ||||||||
| Zou et al. 29 | Cohort | STI clinic, Australia | MSM | SMS reminders 3-, 6- or 12-monthly, depending on patient preference. | 19 months. | Concurrent control. | Re-attendance rate for STI screening within 12 months | 978/1382 | 70.77 | 3-monthly: 587/656 | 89.48 | 3.51 (2.67–4.63) | <0.001 | Low | |
| 6-monthly: 264/301 | 87.71 | 2.95 (2.05–4.24) | <0.001 | ||||||||||||
| Any: 885/997 | 88.77 | 3.26 (2.6–4.1) | <0.001 | ||||||||||||
| Median number of subsequent clinic visits (range) | 1 (1–16) | n/a | 3-monthly: 3 (1–36) | n/a | n/a | <0.001 B | |||||||||
| 6-monthly: 2 (1–14) | n/a | n/a | 0.001 B | ||||||||||||
| Any: 3 (1–36) | n/a | n/a | <0.001 B | ||||||||||||
| Re-screening rate for syphilis | 384/978 | 39.26 | 3-monthly: 393/587 | 66.95 | 3.13 (2.53–3.88) | <0.001 | |||||||||
| 6-monthly: 137/264 | 51.89 | 1.67 (1.27–2.19) | <0.001 | ||||||||||||
| Any: 545/885 | 61.58 | 2.48 (2.06–2.99) | <0.001 | ||||||||||||
| Detection rate of early syphilis at subsequent visits | 15/978 | 1.53 | 3-monthly: 19/587 | 3.24 | 2.15 (1.08–4.26) | 0.0287 | |||||||||
| 6-monthly: 5/264 | 1.89 | 1.24 (0.45–3.44) | 0.6805 | ||||||||||||
| Any: 25/885 | 2.82 | 1.87 (0.98–3.56) | 0.0586 | ||||||||||||
| Detection rate of early latent syphilis at subsequent visits | 4/978 | 0.40 | 3-monthly: 10/587 | 1.70 | 4.22 (1.32–13.52) | 0.0153 | |||||||||
| 6-monthly: 2/264 | 0.76 | 1.86 (0.34–10.2) | 0.4755 | ||||||||||||
| Any: 12/885 | 1.36 | 3.35 (1.08–10.42) | 0.037 | ||||||||||||
| Change in clinic screening guidelines | |||||||||||||||
| Bissessor et al. 30 | Pre-post | STI clinic, Australia | HIV-positive MSM | Syphilis serology included with routine HIV monitoring: 3–6-monthly. | 18 months. | Before intervention: 18 months. | Median number of syphilis tests per man per year | 1 | n/a | 2 | n/a | n/a | n/a | Low | |
| Detection rate of early syphilis | 14/444 | 3.15 | 48/587 | 8.18 | 2.74 (1.49–5.03) | 0.0012 | |||||||||
| Proportion of early syphilis diagnoses that were asymptomatic | 3/14 | 21.43 | 41/48 | 85.42 | 21.48 (4.76–96.96) | <0.001 | |||||||||
| Burchell et al. 31 | Randomised control trial | Four HIV clinics, Canada | HIV-positive males | Syphilis serology included with routine HIV monitoring: 3–6-monthly Randomised stepwise introduction at different clinics. | 6 months. Data given for step 5 when all clinics had the intervention. | Before intervention: 6 months. Data given for step 1 when all clinics were control. | Mean syphilis tests per man per year | 0.53 | n/a | 2.02 | n/a | 2.03 (1.85–2.22) C | Low | ||
| Detection rate of early syphilis | 0.90 | 3.20 | 1.25 (0.71–2.20) C | >0.05 B | |||||||||||
| Proportion screened at least once per year | 36.40 | 79.40 | 3.73 (3.21–4.32) C | ||||||||||||
| Callander et al. 32 | Cohort | GP clinic, Australia | HIV-positive MSM | Syphilis serology included with routine HIV monitoring: 3–6-monthly Implemented in late 2006. | 1 year. Data given for 2007, remains consistent over next 3 years. | Before intervention: 1 year. Data given for 2005. | Mean syphilis tests per man per year | 1.14 | n/a | 2.32 | n/a | n/a | <0.001 B | Moderate | |
| Proportion screened ≥3 times per year | 87/877 | 9.92 | 281/691 | 40.67 | 6.22 (4.76–8.14) | <0.001 | |||||||||
| Proportion with no syphilis tests per year | 240/877 | 27.37 | 20/691 | 2.89 | 0.079 (0.05–0.13) | <0.001 | |||||||||
| Proportion of HIV viral load tests accompanied by syphilis serology | 50.00 | 88.00 | <0.001 B | ||||||||||||
| Cheeks et al. 33 | Cohort | STI clinic, US | HIV-positive MSM | Syphilis serology included with routine HIV monitoring: 3–6-monthly | 15 months. | Before intervention: 15 months. | Detection rate of early syphilis | 4/58 | 6.90 | 29/187 | 15.51 | 2.48 (0.83–7.37) | 0.1028 | Moderate | |
| Winston et al. 34 | Cohort | Hospital out-patient HIV clinic, UK | HIV-positive individuals | Syphilis serology included with routine HIV monitoring: 3–6-monthly | 12 months. | Before intervention: 12 months. | Proportion with CD4 count and syphilis serology performed | 3.00 | 2266/2670 | 84.87 | n/a | n/a | Moderate | ||
| Cohen et al. 35 | Cohort | 12 months (2nd year of intervention). | Before intervention: 12 months. | Proportion with CD4 count and syphilis serology performed | 3.00 | 2389/2655 | 89.98 | n/a | n/a | Moderate | |||||
| 12 months (2nd year of intervention). | 1st year of intervention: 12 months (Winston et al. 31). | Proportion with CD4 count and syphilis serology performed | 2266/2670 | 84.87 | 2389/2655 | 89.98 | 1.6 (1.36–1.89) | <0.001 | |||||||
| Detection rate of asymptomatic syphilis | 26/2670 | 0.97 | 40/2655 | 1.51 | 1.55 (0.95–2.56) | 0.0813 | |||||||||
| Trubiano et al. 36 | Cohort | Hospital out-patient HIV clinic, Australia | HIV-positive individuals | Syphilis serology included with routine HIV monitoring: 3–6-monthly | 4 months. | Before intervention. 4 months. | Proportion with HIV viral load and syphilis serology performed | 136/574 | 23.69 | 317/574 | 55.23 | 3.97 (3.08–5.12) | <0.001 | Moderate | |
| Proportion of HIV viral load tests accompanied by syphilis serology | 175/762 | 22.97 | 417/743 | 56.12 | 4.29 (3.43–5.36) | <0.001 | |||||||||
| Detection rate of syphilis (any stage) | 4/574 | 0.70 | 18/574 | 3.14 | 4.61 (1.55–13.72) | 0.006 | |||||||||
| Guy et al. 37 | Cohort | Three GPs, two STI clinics, two hospital outpatient HIV clinics, Australia | HIV-positive MSM | Opt-out: Four clinics where syphilis serology included with routine HIV monitoring: 3–6-monthly Opt-in: One clinic where clinicians ordered syphilis serology when patients agree (perceived self-risk). Risk-based: Two clinics where clinicians offer syphilis serology only to patients they deemed high-risk. Timing: three clinics introduced opt-out interventions in April 2006, September 2006 and January 2008. The rest had the same policy throughout study period. | 1 year. Data given for 2007, remains consistent over next 3 years. | Before intervention: 1 year. Data given for 2006. | Mean number of syphilis tests per man per year | 1.3 | n/a | 2.2 | n/a | n/a | <0.01 B | Moderate | |
| Proportion screened ≥3 times per year | 15 | 36.00 | <0.01 B | ||||||||||||
| Proportion of HIV viral load tests accompanied by syphilis serology | 37 | 63.00 | <0.01 B | ||||||||||||
| Opt-out interventions: 1 year. Data given for 2010. | Concurrent control groups (opt-in and risk-based clinics). Data given for 2010. | Proportion screened ≥3 times per year in 2010 | Opt-in: 39 | Opt-out: 48 | 0.12 B | ||||||||||
| Risk-based: 8.4 | <0.01 B | ||||||||||||||
| Proportion screened ≥3 times per year in 2010 | Opt-in: 74 | Opt-out: 87 | <0.01 B | ||||||||||||
| Risk-based: 22 | <0.01 B | ||||||||||||||
| Rieg et al. 38 | Cohort | Two HIV clinics, US | HIV-positive MSM | Patients enrolled into the study to have syphilis screening 6-monthly (total of three visits). | 18 months. Data given for 0, 6 and 12 months. | Concurrent control (same population). Data given for 0 and 12 months. | Proportion of syphilis infections diagnosed | 11/16 | 68.75 | 16/16 | 100.00 | 8.5 (0.9–80.03) D | 0.0614 | Moderate | |
| Tang et al. 39 | Cohort | Two STI clinics and one GP, US | HIV-negative MSM (and transgender women) on PrEP | Patients were enrolled to be tested for syphilis 3-monthly (total of four visits). Offered free PrEP as incentive. | 16 months. Data given for 3, 6, 9 and 12 months. | Concurrent control (same population). Data given for 6 and 12 months. | Proportion of syphilis infections diagnosed | 43/54 | 79.63 | 54/54 | 100.00 | 15 (1.88–119.85) D | 0.0106 | Moderate | |
| Hospital-based packaged testing | |||||||||||||||
| Lipps et al. 40 | Cohort | Emergency department, US | ED patients who required STI testing | Educational materials provided to emergency physicians, automated daily reports with results of all syphilis tests and a dedicated STI ‘order set’ that emergency physicians could use to order all STI-related tests when diagnosis of one/more was suspected. | 12 months. | Before intervention: 12 months | Average number of syphilis tests ordered per month in ED | 4 | n/a | 108 | n/a | IRR 30.7 (26.8–35.2) E | <0.001 B | Moderate | |
| Average number of positive syphilis tests per month in ED | 0.63 | n/a | 4.4 | n/a | IRR 7.02 (4.66–10.61) E | <0.001 B | |||||||||
| Marks et al. 41 | Cohort | Three hospitals, US | Individuals hospitalised with serious injection-related infections | Checklist of recommendations to add to patient’s chart for screening patients with invasive infections secondary to injection-related infections. | 13 months. | Before intervention: 6 months. | Screening rate | 48/123 | 39 | 163/271 | 60.15 | 2.36 (1.52–3.65) | <0.001 | Moderate | |
| Enhancing existing health infrastructure | |||||||||||||||
| Snow et al. 42 | Cohort | GP clinic, Australia | MSM | Introduction of a sexual health nurse for STI screening. | 1 year. | (1) Before intervention: 1 year. | Screening rate | 837/1385 | 60.43 | 951/1460 | 65.14 | 1.22 (1.05–1.42) | 0.0095 | Low | |
| (2) Concurrent control. A different GP practice. | Screening rate | 2260/4728 | 47.80 | 951/1460 | 65.14 | 2.04 (1.8–2.3) | <0.001 | ||||||||
| Hamlyn et al. 43 | Cross-sectional | STI clinic, UK | HIV-positive individuals | Introduction of nurse-led clinic for HIV-positive patients within a larger STI clinic. | Audit of 100 consecutive patients. Retrospective data from 18 months (from clinic opening). | Before intervention: audit of 100 consecutive patients. Time period of retrospective data collection not stated. | Proportion of individuals undergoing STI screening at HIV diagnosis | 39/100 | 39.00 | 52/100 | 52.00 | 1.69 (0.97–2.97) | 0.0657 | Moderate | |
| STI screening rate within 12 months preceding the audit | 26/100 | 26.00 | 46/100 | 46.00 | 2.42 (1.34–4.4) | 0.0035 | |||||||||
| Kelly et al. 44 | Cohort | Twelve GP clinics, UK | Heterosexual patients (asymptomatic, >18 year olds) who accessed this service | Training program for GPs and nurses to deliver sexual health care. Previously, done by genitourinary medicine clinics. | Data given for May (start) and October (6 months in). | Before intervention: 1 month. Data given for January. | Screening rate for all four STIs (gonorrhoea, chlamydia, HIV, syphilis) | January: 0/131 | 0 | May: 21/121 | May: 17.36 | 28.75 (3.81–216.9) D | 0.0011 | Moderate | |
| October: 48/144 | October: 33.33 | 66.68 (9.05–491.32) | <0.001 | ||||||||||||
| Zhang et al. 45 | Cohort | Multiple clinics or hospitals, China | Individuals who were drugs users or at risk for syphilis for other reasons (determined by healthcare provider) | Monetary incentive for healthcare providers to screen and treat syphilis. Introduced in 2011. | 1 year. Data given for 2015. | Before intervention: 1 year. Data given for 2010. | Screening rate at ‘voluntary counselling and testing centres’ | 32,877/71,162 | 46.20 | 68,012/69,259 | 98.20 | 63.51 (59.94–67.3) | <0.001 | Moderate | |
| Screening rate at ‘methadone maintenance treatment clinics’ | 9836/18,419 | 53.40 | 17,921/19,737 | 90.80 | 8.61 (8.14–9.1) | <0.001 | |||||||||
| Utilising other screening programs to promote syphilis screening | |||||||||||||||
| Barbee et al. 46 | Cohort | STI clinic, US | MSM | STI self-testing program for chlamydia and gonorrhoea. Then, patient directed to laboratory for syphilis serology, ordered through standing order forms. | 1 year. | Before intervention: 1 year. | Screening rate | 962/1520 | 63.29 | 976/1510 | 64.64 | 1.06 (0.91–1.23) | 0.4403 | Moderate | |
| Botes et al. 47 | Cohort | STI clinic, Australia | HIV-positive MSM | Anal cytology screening program for anal cancer. Also offering STI screening. | 3 months. Includes those who opt-out. | Before intervention: 3 months. | Screening rate of STIs | 67/328 | 20.43 | 123/353 | 34.84 | 2.08 (1.47–2.95) | <0.001 | Moderate | |
| Number syphilis diagnoses | 0 | n/a | 4 | n/a | n/a | n/a | |||||||||
Quality assessment
A majority (n = 14) of the 17 cohort studies exhibited a moderate potential for bias. Since these studies were not randomised, they were unable to minimise allocation or selection bias, so did not adjust for potential confounders in analysis. This resulted in systematic differences in baseline characteristics and risk profiles between the intervention and control groups. Similar limitations were observed in the cross-sectional study43 and two pre-post studies.27,28 The three cohort studies26,29,42 with low bias risk all identified and attempted to overcome potential biases through meticulous study design or by employing multivariate analysis.
Three cohort studies27,28,47 and the cross-sectional study43 grouped all types of STIs together in their results. For the purpose of this review, it was assumed that the individuals were therefore screened for all STIs, including syphilis. However, due to uncertainty in outcome measurements these studies were deemed moderate risk of bias.
The RCT31 and three remaining quasi-experimental studies24,25,30 were of low risk of bias. None of the included studies had high potential for bias.
Interventions and outcomes
The primary outcome of 11 studies was screening rate,22,26,34,37,41–47 and two others emphasised detection rate.27,30 Other included studies reported both screening and detection rates, (n = 4),24,31,35,36 re-screening rates (n = 2)26,29 and re-attendance rate (n = 2).27,28 Studies measuring re-attendance rate were included because their goal was for patients to re-attend specifically for STI screening, including syphilis.
Two cohort studies38,39 reported detection rate at different monthly intervals, with participants serving as their own controls. One cohort study34 reported average number of syphilis tests conducted per month in an emergency department. As this outcome is comparable to screening rate, the study was included.
The studies covered various interventions to increase syphilis screening, such as reminder systems for clinicians (n = 2)24,25 and patients (n = 4),26–29 changes in clinic guidelines for combined HIV and syphilis screening (n = 8)30–37 or increased screening frequency (n = 2),38,39 syphilis serology inclusion in hospital-based packaged testing (n = 2),40,41 improving health infrastructure (n = 4),42–45 and utilising other screening programs for syphilis screening promotion (n = 2).46,47
The implementation of self-reported risk assessments for MSM was conducted by Bissessor et al.24 with a computer-assisted self-interview and by Scarborough et al.25 with paper forms, to be completed prior to their appointment at STI clinics. Clinicians were notified of high-risk MSM either through a computer alert (Bissessor et al.) or by reading the risk assessment form (Scarborough et al.). Both interventions increased the screening rate, compared to a pre-intervention control period (Bissessor: 2787/3902 (71.42%) vs 2949/3893 (75.75%), OR 1.25 [95% CI 1.13–1.38], P < 0.001) (Scarborough: 213/437 (48.74%) vs 211/364 (57.97%), OR 1.45 [95% CI 1.1–1.92], P = 0.0093). Compared to the control period, Bissessor et al. also demonstrated an increased proportion of MSM diagnosed with early syphilis (31/2017 (1.54%) vs 58/1445 (4.01%), OR 2.67 [95% CI 1.72–4.17], P < 0.001) of which a higher proportion were asymptomatic (5/31 (16.13%) vs 31/58 (53.45%), OR 5.97 [95% CI 2.01–17.71], P = 0.0013).
Four studies introduced SMS reminders for patients to return to the clinic for STI re-screening at varying time intervals ranging from 2 weeks to 12 months.26–29 Burton et al.27 and Nyatsanza et al.28 describe this intervention at the same clinic over consecutive years. Only Nyatsanza et al. reported an increase in the proportion of patients re-attending the clinic compared to the pre-intervention period (OR 2.41 [95% CI 1.7–3.42], P < 0.001). The distinguishing factor between these studies was that Nyatsanza et al. used personalised text messages, whereas Burton et al. employed generic texts.
Bourne et al.26 demonstrated an increased re-screening rate of patients for STIs, including syphilis, compared to both a pre-intervention (544/1753 (31.03%) vs 460/714 (64.43%), OR 3.1 [95% CI 2.5–3.8], P < 0.001) and concurrent control group (322/1084 (29.7%) vs 460/714 (64.43%), OR 4.4 [95% CI 3.5–5.5], P < 0.001). Detection rate also increased compared to the concurrent control group (8/1084 (0.74%) vs 36/714 (5.04%), OR 7.14 [95% CI 3.3–15.46], P < 0.001).
Zou et al.29 showed an increased re-screening rate among men receiving 3- and 6-monthly reminders compared to men in the concurrent control group, with the highest re-screening rate (393/587 (66.95%)) in those receiving 3-monthly reminders. Compared to men in the concurrent control group, men receiving the 3-monthly reminders had a significantly higher detection rate of early syphilis (15/978 (1.53%) vs 19/587 (3.24%), OR 2.15 [95% CI 1.08–4.26], P = 0.0287).
The inclusion of syphilis serology with routine blood tests performed for HIV-positive patient monitoring (3–6-monthly) was demonstrated by multiple studies to varying effects. The mean or median number of syphilis tests per individual per year increased in all studies that measured this outcome.30–32,37
Three studies assessed the effectiveness of this intervention by measuring the proportion of HIV viral load tests that were accompanied by syphilis serology. All of these favoured the intervention, demonstrating an increase in this proportion during the post-intervention period compared to the pre-intervention period.32,36,37 Guy et al.37 implemented this intervention at seven clinics using different strategies: four used an opt-out strategy where syphilis serology was automatically added to laboratory requests, one clinic relied on clinicians to order syphilis serology (opt-in) and at two clinics, clinicians offered syphilis serology only to patients they deemed high-risk (risk-based). In the final year of the study period, the proportion of HIV viral load tests accompanied by syphilis serology was highest in clinics with opt-out strategies (87%) compared with opt-in (74%) and risk-based (22%). Of note, all other studies with this intervention used an opt-out method, except for Trubiano et al.36 which employed an opt-in method.
Screening rate was determined as the proportion of individuals who underwent both a HIV viral load or CD4 test and syphilis serology during the study period. Trubiano et al.36 demonstrated that compared to the pre-intervention period, there was an increased proportion of individuals having both tests (136/574 (23.69%) vs 317/574 (55.23%), OR 3.97 [95% CI 3.08–5.12], P < 0.001). Winston et al.34 showed a more substantial difference (3% vs 2266/2670 (84.87%), all raw numbers not provided). Compared to Winston et al., Cohen et al.,35 which reports the second year of the same intervention shows a further increase in this value (2266/2670 (84.87%) vs 2389/2655 (89.98%), OR 1.6 [95% CI 1.36–1.89], P < 0.001), demonstrating continued success of the intervention over time.
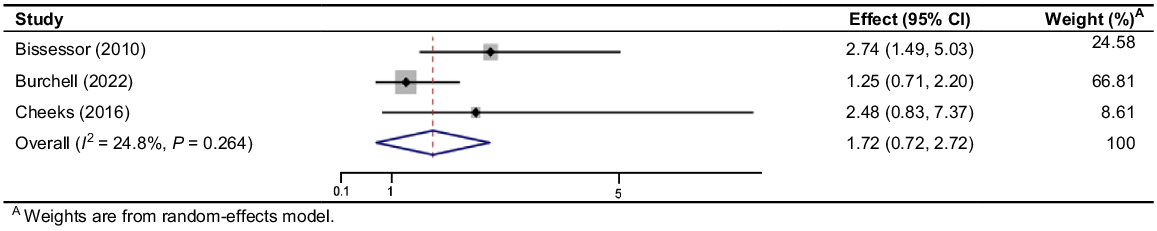
Burchell et al.31 conducted a RCT over 3 years implementing linked syphilis screening with HIV monitoring and reported an increase in the proportion of men screened at least once per year (36.4% vs 79.4%, OR 3.73 [95% CI 3.21–4.32]) compared to a pre-intervention control period. Results regarding the detection rate of early syphilis compared to the pre-intervention period were inconclusive (0.9% vs 3.2%, OR 1.25 [95% CI 0.71–2.20]). The other studies that reported early syphilis detection rate were non-randomised studies and could be impacted by bias. Bissessor et al.30 showed a significant increase in the detection of early syphilis (14/444 (3.15%) vs 48/587 (8.18%), OR 2.74 [95% CI 1.49–5.03], P = 0.0012), whereas Cheeks et al.’s33 results favoured the intervention but were inconclusive (4/58 (6.9%) vs 29/187 (15.51%), OR 2.48 [95% CI 0.83–7.37], P = 0.1028). The pooled OR for this outcome was 1.722 [95% CI 0.721–2.723] and had low heterogeneity (I2 = 24.8%, P = 0.264) (Fig. 2).
Forrest plot of odds ratios of early syphilis detection rate in studies which combine syphilis screening with regular HIV monitoring.
Cohort studies by Rieg et al.38 and Tang et al.39 both enrolled MSM for more frequent syphilis screening. Rieg et al. compared 12-monthly screening (serving as the control) to 6-monthly screening, revealing that 18 individuals with early syphilis infections at 6 months would have potentially remained infectious for an additional 6 months. Tang et al. compared 6-monthly to 3-monthly screening and showed that diagnosis of 11 early syphilis infections would have been delayed. Only Tang et al. showed significant increase in the proportion of syphilis infections diagnosed at 6-monthly versus 3-monthly intervals (43/54 vs 54/54, OR 15 [95% CI 1.88–119.85], P = 0.0106). Both studies had a small sample size.
Lipps et al.40 introduced a dedicated STI order set which included syphilis serology for emergency physicians for use in patients being tested for other STIs, resulting in an increase in the average number of syphilis tests ordered per month (4 vs 108, IRR 30.7 [95% CI 26.8–35.2], P < 0.001) and average number of positive syphilis tests per month (0.63 vs 4.4, IRR 7.02 [95% CI 4.66–10.61], P < 0.001) compared to a pre-intervention period.
Marks et al.41 targeted individuals hospitalised with serious injection-related infections, implementing a standardised checklist of screening recommendations that could be inserted into a patient’s electronic medical record by their treating infectious diseases physician. This resulted in an increase in syphilis screening rate (48/123 (39%) vs 163/271 (60.15%), OR 2.36 [95% CI 1.52–3.65], P < 0.001).
The studies by Snow et al.42 and Hamlyn et al.43 both implemented nurse-led STI clinics for at-risk populations (MSM and HIV-positive individuals, respectively) in different settings (GP practice and STI clinic, respectively). Snow et al. demonstrated a rise in syphilis screening rates compared to both a pre-intervention period (837/1385 (60.43%) vs 951/1460 (65.14%), OR 1.22 [95% CI 1.05–1.42], P = 0.0095) and a similar GP practice without a sexual health nurse (2260/4728 (47.8%) vs 951/1460 (65.14%), OR 2.04 [95% CI 1.8–2.3], P < 0.001). Hamlyn et al.’s audit reported an increase in STI screening rate (26/100 (26%) vs 46/100 (46%), OR 2.42 [95% CI 1.34–4.4], P = 0.0035).
Kelly et al.44 trained general practitioners and practice nurses in Ireland to screen for STIs, traditionally done by genitourinary clinics. Monthly asymptomatic heterosexual patient screenings for four STIs (gonorrhoea, chlamydia, HIV, syphilis) increased (0/131 vs 21/121, OR 28.75 [95% CI 3.81–216.9], P = 0.0011), with sustained increase over the 6-month study period.
Zhang et al.45 introduced a pay-for-performance scheme to incentivise healthcare providers to screen and treat syphilis. Compared to a pre-intervention period, screening rates increased at testing centres (32,877/71,162 (46.2%) vs 68,012/69,259 (98.2%), OR 63.51 [95% CI 59.94–67.3], P < 0.001) and methadone maintenance treatment clinics (9836/18,419 (53.4%) vs 17,921/19,737 (90.8%), OR 8.61 [95% CI 8.14–9.1], P < 0.001).
Barbee et al.46 introduced a self-testing program for chlamydia and gonorrhoea for MSM, and Botes et al.47 introduced an anal cytology screening program for anal cancer for HIV-positive MSM. While not the primary focus of the study, both offered the participating clients to be screened for syphilis using a blood test. There was no change in syphilis screening rate in Barbee et al. (962/1520 (63.39%) vs 976/1510 (64.64%), OR 1.06 [95% CI 0.91–1.23], P = 0.4403), however Botes et al. showed an increased syphilis screening rate after introduction of their anal cytology screening program (67/328 (20.43%) vs 123/353 (34.84%), OR 2.08 [95% CI 1.47–2.95], P < 0.001).
Few studies reported the feasibility of their interventions. Scarborough et al.25 found that a paper-based risk assessment was low-cost but time-consuming for clinic staff, leading to discontinuation of this intervention to adopt an electronic sexual history instrument such as Bissessor et al.24 SMS reminders were low-cost, automatic and required minimal labour,26,29 although no formal cost-benefit analysis was reported.
Adding syphilis serology to HIV monitoring was practical using automatic opt-out methods, avoiding additional staff time or handling.30,35 Trubiano et al.36 faced challenges with their opt-in strategy, struggling to motivate clinicians to screen all MSM attending the clinic for routine review. Overall, this strategy was reported as low cost.30,32 Rieg et al.38 supported this with a cost analysis, demonstrating the annual costs of screening every 6 versus 12 months did not differ substantially (USD10,640 vs USD10,681 per asymptomatic STI detected).
The use of packaged testing in hospital was acceptable to medical providers,40,41 simple and inexpensive.41
Kelly et al.44 reported that providing STI screening in primary care is approximately 1.5-times less expensive than if the same case mix of patients had been seen in secondary care services. The pay-for-performance scheme described by Zhang et al.45 had a mean cost of USD39,000 annually.
No other studies reported feasibility or costs. No studies reported harms of screening.
Discussion
The studies included in this review provide evidence supporting a diverse array of interventions aimed at increasing syphilis screening, with most focusing on tailored approaches for at-risk populations. Technology played a significant role in the reviewed interventions, including clinician alerts, SMS reminders and packaged testing with HIV monitoring or in hospital with other investigations. Computer-assisted self-interviews proved useful for collection of sexual histories and consenting for SMS reminders.
Electronic clinician alerts were deemed more feasible than paper-based methods.24,25 These alerts could also be applied in hospital settings, as shown in a study outside the scope of this review that alerted emergency physicians to screen for syphilis in patients living in high-prevalence areas or with a history of drug use.48 SMS reminders for re-screening are known to be accepted in a sexual health context,49,50 with personalised messages more effective than generic ones.27,28 Once established, these technology-based approaches required minimal staffing and ongoing costs, making them efficient and sustainable solutions for increasing syphilis screening rates.
Incorporating syphilis serology with regular HIV monitoring proved an effective strategy to increase the number of syphilis tests per year and screening rate among HIV-positive individuals. An opt-out method is particularly successful.37
The degree of benefit of linked screening with HIV monitoring in increasing detection rate may be influenced by changes in syphilis incidence and study location. Despite inconclusive meta-analysis results with a pooled OR of 1.722 (0.721–2.723), the clinical significance of the intervention remains notable. Cheeks et al.33 found 3–6-monthly screening identified 27 additional infections that would have otherwise remained undetected until annual syphilis screening. Identifying syphilis infections in the early phase allows for more timely treatment, reducing the period of infectiousness and preventing potential sequelae.
Syphilis screening guidelines suggest 3-monthly screening for MSM,10,11 including those who are HIV-positive,51 which results in four screening episodes annually. Australian HIV monitoring guidelines advise HIV-positive individuals undergo viral load and CD4 count tests 3–6-monthly, potentially extending to annually if virally suppressed.52 Although coupling syphilis screening with HIV monitoring for MSM is convenient and cost effective, it is unlikely to achieve the recommended 3-monthly syphilis screening frequency as per guidelines. This is supported by the included studies, which reveal the mean or median number of screening episodes annually for HIV-positive MSM ranging between 2 and 2.32.30,32,37 Therefore, multiple methods of increasing syphilis screening may be required, such as pairing this strategy with reminder systems, incentives or employing a dedicated sexual health nurse. Zou et al.29 reports that 3-monthly SMS reminders for MSM correspond with a median of three screening episodes annually, although still falling short of guideline recommendations.
HIV-negative MSM may also require further targeted interventions to achieve 3-monthly screening. A current method used in Australia involves screening this population for syphilis on provision of 3-monthly HIV pre-exposure prophylaxis (PrEP) prescriptions.53 This has proven effective, as a 2017 Australian study reported 99% of HIV-negative individuals were screened for syphilis 3-monthly when provided with PrEP.54 A systematic review revealed that globally, the majority (70%) of PrEP programs offer 3-monthly syphilis screening, with lower availability of testing observed primarily in low-income countries.55
Increased syphilis surveillance in hospitals is noteworthy, given their non-traditional role in public health initiatives. This approach becomes especially important for at-risk populations (illicit drug users, cultural subpopulations) who may not access regular healthcare services elsewhere. Recent cross-sectional studies have detailed the implementation of routine syphilis screening in emergency departments, leading to new syphilis diagnoses.56–58
Methods to encourage clinicians to screen for syphilis may be beneficial, such as introduction of a sexual health nurse,42 incentives45 and education of best practice screening for syphilis.40,41,44 As described by Snow et al.,42 general practitioners may have been more inclined to initiate screening, knowing a nurse was available to conduct the tests and spend additional time with these patients, facilitating better adherence to screening guidelines.
The inclusion of syphilis screening in other screening programs or interventions has potential to enhance their public health benefit and merits further investigation, such as integration with cervical cancer screening or HPV vaccine campaigns for at-risk individuals.
Increasing syphilis screening involves two key aspects: initiatives directed at healthcare facilities, as discussed in this review, and efforts targeted at patients themselves to promote attendance to these additional services or to secure consent for reminders and opt-out testing. While not the focus of this review, these interventions hold equal importance. Kelly et al.44 and Barbee et al.46 describe using posters and pamphlets within clinics to advertise their new services. Zou et al.29 employs the use of computer-assisted self-interview to acquire consent for SMS reminders, taking the opportunity to advise MSM of the current syphilis epidemic and its often asymptomatic nature to encourage uptake. Promoting syphilis screening to at-risk populations has been extended through innovative methods such as advertising through mobile dating applications59–61 and social media marketing campaigns.62–66 During an epidemic, patient incentives, such as offering free PrEP in exchange for syphilis screening as demonstrated by Tang et al.,39 could prove valuable. To address the challenge of increasing syphilis screening, a synergistic approach that combines both healthcare driven, and patient-centred strategies is essential.
Strengths and limitations
This review used robust and systematic methodology, minimising bias through meticulous study selection. Data extraction and quality assessment were independently conducted by two researchers, adding further rigour. It addresses a gap in the existing literature by focusing on enhancing syphilis screening in non-pregnant individuals, offering valuable insights for future intervention development and implementation.
There are limitations to this review. Although syphilis testing has been referred to as ‘screening,’ in many cases, it is unclear whether the individuals were indeed asymptomatic or had symptoms and were receiving diagnostic testing rather than screening. Even in studies where the individuals were described as asymptomatic, complete examinations to document symptoms were often not performed. Reasons for non-compliance with interventions in individual studies were often not recorded. For instance, there was no information on why some individuals declined to receive SMS reminders or why some HIV monitoring tests were not accompanied by syphilis serology.
Most studies were observational, so causality between the interventions and outcomes cannot be definitively established. The effectiveness of the interventions across different populations remains uncertain. The review’s lack of representation of low-income countries due, in part, to language restrictions hinders generalisability to setting with potential implementation barriers including access to technology, staff availability and costs of universal screening.
Owing to the clinical heterogeneity of the interventions and various outcomes, pooled outcomes could not be determined for all results to provide a summary effect. Our meta-analysis was limited by the inclusion of different study designs (non-randomised and RCT).
Conclusion
The studies included in this review offer valuable insights into the diverse approaches for increasing syphilis screening. It is important that the benefits of early detection and averting potential morbidity is balanced by the cost of routine screening strategies. Notably, interventions involving reminder systems or syphilis grouped with HIV monitoring should undergo cost-effective analysis to fully assess their impact as they appear to have only modest operating costs. Future research and wider adoption of these interventions in at-risk populations could mitigate the burden of syphilis.
Data availability
All data generated or analysed during this study are included in this published article.
References
1 Tudor ME, Al Aboud AM, Lesie SW, et al. Syphilis. StatPearls. 2023. Available at https://www.ncbi.nlm.nih.gov/books/NBK534780/#:~:text=The%20classic%20primary%20syphilis%20presentation,%2C%20tonsils%2C%20and%20oral%20mucosa [accessed 26 July 2023]
2 Wu MY, Gong HZ, Hu KR, Zheng H, Wan X, Li J. Effect of syphilis infection on HIV acquisition: a systematic review and meta-analysis. Sex Transm Infect 2021; 97: 525-533.
| Crossref | Google Scholar |
3 Buchacz K, Patel P, Taylor M, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS 2004; 18(15): 2075-2079.
| Crossref | Google Scholar | PubMed |
4 World Health Organization. Vulnerable groups and key populations at increased risk of HIV. World Health Organization; 2023. Available at https://www.emro.who.int/asd/health-topics/vulnerable-groups-and-key-populations-at-increased-risk-of-hiv.html [accessed 26 July 2023]
5 Tuite AR, Fisman DN, Mishra S. Screen more or screen more often? Using mathematical models to inform syphilis control strategies. BMC Public Health 2013; 13: 606.
| Crossref | Google Scholar | PubMed |
6 Tuite AR, Shaw S, Reimer JN, Ross CP, Fisman DN, Mishra S. Can enhanced screening of men with a history of prior syphilis infection stem the epidemic in men who have sex with men? A mathematical modelling study. Sex Transm Infect 2018; 94(2): 105-110.
| Crossref | Google Scholar | PubMed |
7 Gray RT, Hoare A, Prestage GP, Donovan B, Kaldor JM, Wilson DP. Frequent testing of highly sexually active gay men is required to control syphilis. Sex Transm Dis 2010; 37(5): 298-305.
| Crossref | Google Scholar | PubMed |
8 Hui BB, Ward JS, Guy R, Law MG, Gray RT, Regan DG. Impact of testing strategies to combat a major syphilis outbreak among Australian Aboriginal and Torres Strait Islander peoples: a mathematical modeling study. Open Forum Infect Dis 2022; 9(5): 119.
| Crossref | Google Scholar |
9 Mitchell KM, Cox AP, Mabey D, Tucker JD, Peeling RW, Vickerman P. The impact of syphilis screening among female sex workers in China: a modelling study. PLoS ONE 2013; 8: e55622.
| Crossref | Google Scholar | PubMed |
10 U.S Preventive Services Task Force. Syphilis infection in nonpregnant adolescents and adults: screening. U.S Preventive Services Task Force; 2022. Available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening [accessed 27 July 2023]
11 Australian STI Management Guidelines. Men who have sex with men. Australian STI Management Guidelines for use in Primary Care; 2021. Available at https://sti.guidelines.org.au/populations-and-situations/men-who-have-sex-with-men/ [accessed 27 July 2023]
12 An Q, Wejnert C, Bernstein K, Paz-Bailey G, for the NHBS Study Group. Syphilis screening and diagnosis among men who have sex with men, 2008–2014, 20 U.S. Cities. J Acquir Immune Defic Syndr 2017; 75(3): S363-S369.
| Crossref | Google Scholar |
13 Guy R, Wand H, Holt M, et al. High annual syphilis testing rates among gay men in Australia, but insufficient retesting. Sex Transm Dis 2012; 39(4): 268-275.
| Crossref | Google Scholar | PubMed |
14 Footman A, Dagama D, Smith CH, Van Der Pol B. A systematic review of new approaches to sexually transmitted infection screening framed in the capability, opportunity, motivation, and behavior model of implementation science. Sex Transm Dis 2021; 48(8): S58-S65.
| Crossref | Google Scholar |
15 Taylor MM, Frasure-Williams J, Burnett P, Park IU. Interventions to improve sexually transmitted disease screening in clinic-based settings. Sex Transm Dis 2016; 43: S28-S41.
| Crossref | Google Scholar | PubMed |
16 Desai M, Woodhall SC, Nardone A, Burns F, Mercey D, Gilson R. Active recall to increase HIV and STI testing: a systematic review. Sex Transm Infect 2015; 91(5): 314-323.
| Crossref | Google Scholar | PubMed |
17 Zou H, Fairley CK, Guy R, Chen MY. The efficacy of clinic-based interventions aimed at increasing screening for bacterial sexually transmitted infections among men who have sex with men: a systematic review. Sex Transm Dis 2012; 39(5): 382-387.
| Crossref | Google Scholar | PubMed |
18 Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009; 6(7): e1000097.
| Crossref | Google Scholar | PubMed |
19 Veritas Health Innovation. Covidence systematic review software. Version 2. Veritas Health Innovation, Melbourne, Australia; 2014. Available at www.covidence.org [accessed 8 July 2023]
20 Richardson WS, Wilson MC, Nishikawa J, Hayward RSA. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995; 123(3): A12-A13.
| Crossref | Google Scholar | PubMed |
21 StataCorp LLC. STATA. Version 18. StataCorp LLC, College Station, Texas; 2023. Available at https://www.stata.com/ [accessed 17 August 2023]
22 Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. Joanna Briggs Institute; 2020. https://doi.org/10.46658/JBIMES-20-01 [accessed August 2023]
23 Turpin R, Rosario AD, Dyer T. Barriers to syphilis testing among men who have sex with men: a systematic review of the literature. Sex Health 2020; 17(3): 201-213.
| Crossref | Google Scholar | PubMed |
24 Bissessor M, Fairley CK, Leslie D, Chen MY. Use of a computer alert increases detection of early, asymptomatic syphilis among higher-risk men who have sex with men. Clin Infect Dis 2011; 53: 57-58.
| Crossref | Google Scholar | PubMed |
25 Scarborough AP, Slome S, Hurley LB, Park IU. Improvement of sexually transmitted disease screening among HIV-infected men who have sex with men through implementation of a standardized sexual risk assessment tool. Sex Transm Dis 2015; 42(10): 595-598.
| Crossref | Google Scholar | PubMed |
26 Bourne C, Knight V, Guy R, Wand H, Lu H, McNulty A. Short message service reminder intervention doubles sexually transmitted infection/HIV re-testing rates among men who have sex with men. Sex Transm Infect 2011; 87(3): 229-231.
| Crossref | Google Scholar | PubMed |
27 Burton J, Brook G, McSorley J, Murphy S. The utility of short message service (SMS) texts to remind patients at higher risk of STIs and HIV to reattend for testing: a controlled before and after study. Sex Transm Infect 2014; 90: 11-13.
| Crossref | Google Scholar | PubMed |
28 Nyatsanza F, McSorley J, Murphy S, Brook G. ‘It’s all in the message’: the utility of personalised short message service (SMS) texts to remind patients at higher risk of STIs and HIV to reattend for testing – a repeat before and after study. Sex Transm Infect 2016; 92(5): 393-395.
| Crossref | Google Scholar | PubMed |
29 Zou H, Fairley CK, Guy R, et al. Automated, computer generated reminders and increased detection of gonorrhoea, chlamydia and syphilis in men who have sex with men. PLoS ONE 2013; 8(4): e61972.
| Crossref | Google Scholar | PubMed |
30 Bissessor M, Fairley CK, Leslie D, Howley K, Chen MY. Frequent screening for syphilis as part of HIV monitoring increases the detection of early asymptomatic syphilis among HIV-positive homosexual men. J Acquir Immune Defic Syndr 2010; 55(2): 211-216.
| Crossref | Google Scholar | PubMed |
31 Burchell AN, Tan DHS, Grewal R, et al. Routinized syphilis screening among men living with human immunodeficiency virus: a stepped wedge cluster randomized controlled trial. Clin Infect Dis 2022; 74(5): 846-853.
| Crossref | Google Scholar | PubMed |
32 Callander D, Baker D, Chen M, Guy R. Including syphilis testing as part of standard HIV management checks and improved syphilis screening in primary care. Sex Transm Dis 2013; 40(4): 338-340.
| Crossref | Google Scholar | PubMed |
33 Cheeks MA, Fransua M, Stringer HG, Jr, Silva S, Relf M. A quality improvement project to increase early detection of syphilis infection or re-infection in HIV-infected men who have sex with men. J Assoc Nurses AIDS Care 2016; 27(2): 143-152.
| Crossref | Google Scholar | PubMed |
34 Winston A, Hawkins D, Mandalia S, Boag F, Azadian B, Asboe D. Is increased surveillance for asymptomatic syphilis in an HIV outpatient department worthwhile? Sex Transm Infect 2003; 79(3): 257-259.
| Crossref | Google Scholar | PubMed |
35 Cohen CE, Winston A, Asboe D, et al. Increasing detection of asymptomatic syphilis in HIV patients. Sex Transm Infect 2005; 81(3): 217-219.
| Crossref | Google Scholar | PubMed |
36 Trubiano JA, Hoy JF. Taming the great: enhanced syphilis screening in HIV-positive men who have sex with men in a hospital clinic setting. Sex Health 2015; 12(2): 176-178.
| Crossref | Google Scholar | PubMed |
37 Guy R, El-Hayek C, Fairley CK, et al. Opt-out and opt-in testing increases syphilis screening of HIV-positive men who have sex with men in Australia. PLoS ONE 2013; 8(8): e71436.
| Crossref | Google Scholar | PubMed |
38 Rieg G, Lewis RJ, Miller LG, Witt MD, Guerrero M, Daar ES. Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDS 2008; 22(12): 947-954.
| Crossref | Google Scholar | PubMed |
39 Tang EC, Vittinghoff E, Philip SS, et al. Quarterly screening optimizes detection of sexually transmitted infections when prescribing HIV preexposure prophylaxis. AIDS 2020; 34(8): 1181-1186.
| Crossref | Google Scholar | PubMed |
40 Lipps AA, Bazan JA, Lustberg ME, et al. A collaborative intervention between emergency medicine and infectious diseases to increase syphilis and HIV screening in the emergency department. Sex Transm Dis 2022; 49(1): 50-54.
| Crossref | Google Scholar | PubMed |
41 Marks LR, Reno H, Liang SY, et al. Value of packaged testing for sexually transmitted infections for persons who inject drugs hospitalized with serious injection-related infections. Open Forum Infect Dis 2021; 8(11): ofab489.
| Crossref | Google Scholar |
42 Snow AF, Vodstrcil LA, Fairley CK, et al. Introduction of a sexual health practice nurse is associated with increased STI testing of men who have sex with men in primary care. BMC Infect Dis 2013; 13: 298.
| Crossref | Google Scholar | PubMed |
43 Hamlyn E, Barrett S, Kelsey J, Lockyer S, Welz T, Poulton M. Improvement in screening for sexually transmitted infections in HIV-positive patients following implementation of a nurse-led clinic. Int J STD AIDS 2007; 18(6): 424-426.
| Crossref | Google Scholar | PubMed |
44 Kelly C, Johnston J, Carey F. Evaluation of a partnership between primary and secondary care providing an accessible Level 1 sexual health service in the community. Int J STD AIDS 2014; 25(10): 751-757.
| Crossref | Google Scholar | PubMed |
45 Zhang W, Luo H, Ma Y, et al. Monetary incentives for provision of syphilis screening, Yunnan, China. Bull World Health Organ 2017; 95(9): 657-662.
| Crossref | Google Scholar | PubMed |
46 Barbee LA, Tat S, Dhanireddy S, Marrazzo JM. Implementation and operational research: effectiveness and patient acceptability of a sexually transmitted infection self-testing program in an HIV care setting. J Acquir Immune Defic Syndr 2016; 72(2): e26-e31.
| Crossref | Google Scholar | PubMed |
47 Botes LP, McAllister J, Ribbons E, Jin F, Hillman RJ. Significant increase in testing rates for sexually transmissible infections following the introduction of an anal cytological screening program, targeting HIV-positive men who have sex with men. Sex Health 2011; 8: 76-78.
| Crossref | Google Scholar | PubMed |
48 Rosenman M, Wang J, Dexter P, Overhage JM. Computerized reminders for syphilis screening in an urban emergency department. AMIA Annu Symp Proc 2003; 2003: 987.
| Google Scholar | PubMed |
49 Lim MSC, Hocking JS, Hellard ME, Aitken CK. SMS STI: a review of the uses of mobile phone text messaging in sexual health. Int J STD AIDS 2008; 19(5): 287-290.
| Crossref | Google Scholar | PubMed |
50 Lim MSC, Sacks-Davis R, Aitken CK, Hocking JS, Hellard ME. Randomised controlled trial of paper, online and SMS diaries for collecting sexual behaviour information from young people. J Epidemiol Community Health 2010; 64(10): 885-889.
| Crossref | Google Scholar | PubMed |
51 Australian STI Management Guidelines for use in Primary Care. People living with HIV. Australian STI Management Guidelines for use in Primary Care; 2021. Available at https://sti.guidelines.org.au/populations-and-situations/people-living-with-hiv/ [accessed 3 August 2023]
52 ASHM. HIV monitoring tool: new patient. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine; 2021. Available at https://ashm.org.au/wp-content/uploads/2022/04/ASHM-HIV-Monitoring-Tool_FA_web-1.pdf [accessed 3 August 2023]
53 ASHM. Clinical follow-up and monitoring of patients on PrEP. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine; 2019. Available at https://prepguidelines.com.au/clinical-follow-up-and-monitoring-of-patients-on-prep/ [accessed 3 August 2023]
54 Lal L, Audsley J, Murphy DA, et al. Medication adherence, condom use and sexually transmitted infections in Australian preexposure prophylaxis users. AIDS 2017; 31(12): 1709-1714.
| Crossref | Google Scholar | PubMed |
55 Ong JJ, Fu H, Baggaley RC, et al. Missed opportunities for sexually transmitted infections testing for HIV pre-exposure prophylaxis users: a systematic review. J Int AIDS Soc 2021; 24(2): e25673.
| Crossref | Google Scholar | PubMed |
56 Larios Venegas A, Melbourne HM, Castillo IA, et al. Enhancing the routine screening infrastructure to address a syphilis epidemic in Miami-Dade County. Sex Transm Dis 2020; 47(5): S61-S65.
| Crossref | Google Scholar |
57 Stanford KA, Hazra A, Friedman E, et al. Opt-out, routine emergency department syphilis screening as a novel intervention in at-risk populations. Sex Transm Dis 2021; 48(5): 347-352.
| Crossref | Google Scholar | PubMed |
58 Yax JA, Niforatos JD, Summers DL, et al. A model for syphilis screening in the emergency department. Public Health Rep 2021; 136(2): 136-142.
| Crossref | Google Scholar | PubMed |
59 Alarcón Gutiérrez M, Fernández Quevedo M, Martín Valle S, et al. Acceptability and effectiveness of using mobile applications to promote HIV and other STI testing among men who have sex with men in Barcelona, Spain. Sex Transm Infect 2018; 94(6): 443-448.
| Crossref | Google Scholar | PubMed |
60 Lampkin D, Crawley A, Lopez TP, Mejia CM, Yuen W, Levy V. Reaching suburban men who have sex with men for STD and HIV services through online social networking outreach: a public health approach. J Acquir Immune Defic Syndr 2016; 72(1): 73-78.
| Crossref | Google Scholar | PubMed |
61 Su J-Y, Holt J, Payne R, Gates K, Ewing A, Ryder N. Effectiveness of using Grindr to increase syphilis testing among men who have sex with men in Darwin, Australia. Aust N Z J Public Health 2015; 39(3): 293-294.
| Crossref | Google Scholar | PubMed |
62 Dowshen N, Lee S, Matty Lehman B, Castillo M, Mollen C. IknowUshould2: feasibility of a youth-driven social media campaign to promote STI and HIV testing among adolescents in Philadelphia. AIDS Behav 2015; 19(S2): 106-111.
| Crossref | Google Scholar |
63 Montoya JA, Kent CK, Rotblatt H, McCright J, Kerndt PR, Klausner JD. Social marketing campaign significantly associated with increases in syphilis testing among gay and bisexual men in San Francisco. Sex Transm Dis 2005; 32(7): 395-399.
| Crossref | Google Scholar | PubMed |
64 Plant A, Javanbakht M, Montoya JA, Rotblatt H, O’Leary C, Kerndt PR. Check Yourself: a social marketing campaign to increase syphilis screening in Los Angeles County. Sex Transm Dis 2014; 41: 50-57.
| Crossref | Google Scholar | PubMed |
65 Wilkinson AL, Pedrana AE, El-Hayek C, et al. The impact of a social marketing campaign on HIV and sexually transmissible infection testing among men who have sex with men in Australia. Sex Transm Dis 2016; 43: 49-56.
| Crossref | Google Scholar | PubMed |
66 Plant A, Montoya JA, Rotblatt H, et al. Stop the sores: the making and evaluation of a successful social marketing campaign. Health Promot Pract 2010; 11: 23-33.
| Crossref | Google Scholar | PubMed |


