Incident hepatitis B infection subsequent to the diagnosis of HIV infection in a Melbourne cohort: missed opportunities for prevention
Amy Body A , Jennifer F. Hoy A B , Allen C. Cheng B C and Michelle L. Giles A B DA Infectious Diseases Unit, Alfred Hospital, Melbourne, Vic. 3004, Australia.
B Department of Infectious Diseases, Monash University, Melbourne, Vic. 3004, Australia.
C Department of Epidemiology and Preventive Medicine, Monash University and Alfred Hospital, Melbourne, Vic. 3004, Australia.
D Corresponding author. Email: michelle.giles@monash.edu
Sexual Health 11(1) 5-10 https://doi.org/10.1071/SH13019
Submitted: 4 February 2013 Accepted: 22 July 2013 Published: 25 October 2013
Abstract
Background: The characteristics associated with incident hepatitis B (HBV) infection in HIV-positive individuals are not well described in the Australian setting. The aim of this study is to determine the characteristics of and risk factors for HBV infection within HIV-infected individuals in a Melbourne cohort between 1985 and 2011. Methods: Individuals susceptible to HBV at their HIV diagnosis were identified using their HBV serology stored within the Victorian HIV database. Within this group, those who had a subsequent positive test for hepatitis B surface antigen or hepatitis B core antibody were identified as infected with HBV after their HIV diagnosis. Incident cases were matched with controls from the initially susceptible group who did not seroconvert for analysis. An incidence rate was calculated from the number of seroconversions and the cumulative time at risk (in 1000 patient-years of follow-up). Results: Of the 4711 patients with HIV seen more than once, 3223 had HBV testing. Of the 174 with positive HBV test results, 39 individuals met the definition of seroconversion after HIV diagnosis, representing the incident cases. The estimated HBV incidence rate was 1.81 (95% confidence interval: 1.28–2.47) per 1000 patient-years at risk. These individuals form the basis of a detailed case series and case–control study. Data collected include demographic details, immunological and virological characteristics, antiretroviral treatment and vaccination history. Conclusions: HIV-infected individuals should be screened for HBV and monitored for incident infection. Optimal control of HIV and improved vaccination coverage provide the best opportunity for prevention.
Additional keywords: coinfection, incidence, serology, susceptibility.
Introduction
Hepatitis B virus (HBV) and HIV share common modes of transmission and, as such, are common coinfections worldwide. Coinfection with HIV and HBV has potential for greater health implications for the individual than infection with either virus alone, the most striking problem being a significantly higher rate of liver-related mortality in coinfected individuals.1 Therefore, prevention of chronic HBV infection in HIV-infected individuals is vital.
Prevention of HBV infection in HIV-infected individuals is complicated by the suboptimal response to the HBV vaccine in this population, particularly in those with low CD4 cell counts.2 This can be improved, however, with vaccine administration strategies such as using a higher dose, giving an additional dose (four v. three) and intradermal administration compared with intramuscular.3 Poor vaccine response may also be compounded by poor rates of vaccination schedule completion.4,5
Risk factors for HIV–HBV coinfection have varied, depending on the setting in which they have been investigated. The epidemiology of adult-acquired HBV differs from country to country, with the role of various risk factors varying depending on their prevalence within the community under study.
In countries with a low background rate of HBV, HBV is usually contracted during adulthood,6 presenting an opportunity for prevention in the HIV-infected community. Within large HIV-positive cohorts in Europe and the United States, risk factors for HBV coinfection include male gender, recent injecting drug use or alcohol abuse and being a man who has sex with men.5,7 These risk factors were also observed in an East Asian cohort, despite the higher background prevalence of HBV in these countries and therefore higher rates of early life transmission.8 HIV-specific risk factors for incident infection include increasing HIV viral load, prior AIDS-defining illness, and lower current and nadir CD4 counts.7,9,10
The aim of this study was to determine the risk factors for incident cases of HBV infection occurring after HIV infection in Australia and to demonstrate any missed opportunities for HBV prevention within a Melbourne cohort of HIV-infected individuals.
Methods
This study was approved by the Alfred Hospital Human Research Ethics Committee and the Monash University Human Research Ethics Committee in Melbourne, Australia. Cases and controls were identified from the Victorian HIV Service Database (VHIVSD), which contains records of all individuals treated for HIV infection at the Alfred Hospital (Melbourne, Australia) and the former Fairfield Infectious Diseases Hospital (Melbourne, Australia, closed 1996). From 1996, data on current patients of the HIV Service have been entered prospectively and patients active before this date have had data entered retrospectively.
Cases were selected by an initial search of the VHIVSD for individuals with a recorded negative HBV test (hepatitis B surface antigen (HBsAg) or hepatitis B core antibody (HBcAb)) on or after the date of their HIV diagnosis, followed by a positive HBV test (HBsAg or HBcAb) at a later date. Individuals with negative HBsAg and negative HBcAb tests at baseline with a subsequent positive test were included in the study. The date of incident HBV infection was defined as the midpoint between the last negative and the first positive HBV test.
Cases of incident HBV infection were reviewed in detail, with all paper and electronic hospital records reviewed, as well as database records. Demographic details, blood-borne virus risk factors, medical history, HBV vaccination receipt, HBV disease features and HBV outcomes were collected.
Controls were selected from individuals in the VHIVSD who had been tested for HBsAg and HBcAb with no positive test ever being recorded. Controls were matched on the basis of HBV test date, and then matched for date of HIV diagnosis (within two years of case HIV diagnosis). Where possible, cases were matched with three controls. In some cases there were insufficient eligible controls. In cases with less than three eligible controls, the maximum possible number of controls was allocated. One case was excluded from the analysis due to inability to allocate a suitable control.
Univariate and multivariate conditional logistic regression were used to analyse the case-control data. Multivariate analysis was restricted to three variables (intravenous drug use, HBV vaccination and receipt of HBV-active highly active antiretroviral therapy (HAART)). Variables for multivariate analysis were selected based on their clinical relevance and the completeness of data available.
All odds ratios (ORs) are the odds of having an incident HBV infection calculated on univariate conditional logistic regression unless otherwise stated. All ranges reported are the numerical range from the smallest to the largest value.
An incidence rate was calculated from the number of seroconversions and the cumulative time at risk (in 1000 patient-years of follow-up). The time at risk was defined as the time from HIV diagnosis to the last negative test (in patients that did not seroconvert) or the time from the HIV diagnosis to the midpoint of the time between the last negative and the first positive test (in patients who seroconverted). We excluded patients whose first HBV test after HIV diagnosis was positive (that is, who were positive at baseline) or those who only had negative HBV serology before the time of HIV diagnosis. Confidence intervals were calculated assuming the Poisson distribution.
Results
Case series of incident HBV infection
On the VHIVSD, 3223 of 4711 patients (68.4%) had tests recorded for HBsAg or HBcAb. Of these, 174 (5.4%) had a positive test ever recorded and 39 of the 174 (22.4%) met the criteria for inclusion in the study (15 out of 39 were HBsAg-positive and 24 out of 39 were HBcAb-positive; see Fig. 1).
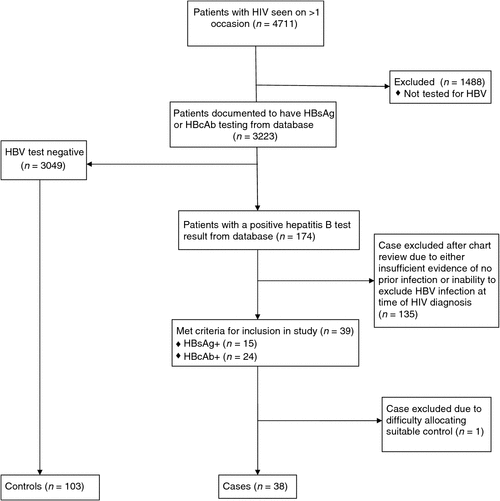
|
Of the 39 individuals with incident HBV infection, 89.7% were male, similar to the gender distribution of the entire VHIVSD cohort (93.3% male) and 66.7% were born in Australia. The median year of HIV diagnosis was 1990 (compared with 1993 for the entire VHIVSD).
The median time from the date of HIV diagnosis to the estimated date of HBV infection was 4.6 years (range: 4 months–17 years). The median time from HIV diagnosis to first positive HBV test was 7.77 years (range: 8 months–20 years). The median date of HBV infection was July 1996 (range: October 1986–October 2007; see Fig. 2).
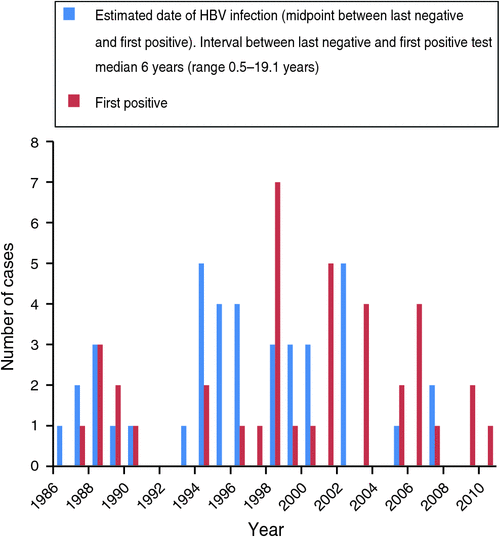
|
An acute illness was documented in 5 of the 39 individuals (12.8%) and an additional 7 (17.9%) had gastrointestinal symptoms that were possibly attributable to acute HBV infection. Nine (23.1%) had documented chronic infection with HBV (defined as two positive tests at least 6 months apart) and 5 (12.8%) did not have a subsequent HBsAg test on record to determine whether chronic infection resulted. Fourteen cases (35.9%) died during follow-up, the majority from AIDS-related conditions but none from liver-related disease.
Of the 39 cases, 10 had documentation of vaccination, although the date and number of administered doses was not consistently recorded. Of the 10 individuals with documentation of some HBV vaccination, six had also undergone post-vaccination serological testing, with only 2 (33.3%) being HBsAb-positive, but they were not simultaneously tested for HBcAb, making it impossible to exclude positive HBsAb as a result of infection. They both subsequently had undetectable HBsAb titres.
Risk factors and potential protective factors for incident HBV infection such as HBV-active antiretroviral therapy (ART) are summarised in Table 1.
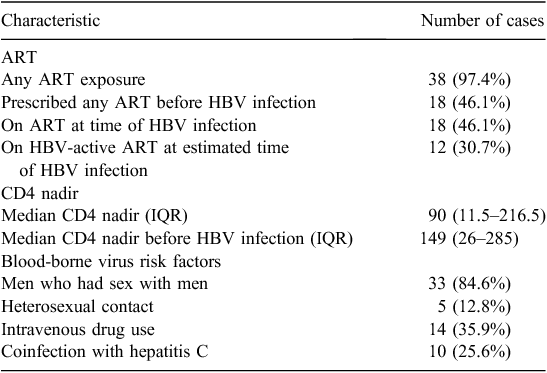
|
Of the 13 individuals naive to ART at their first positive HBV date, five had a first positive HBV test before 1990, before the widespread availability of antiretroviral drugs in Australia.
Twelve individuals (30.7%) were receiving HBV-active ART at the estimated date of infection. Eleven of the 12 had a history of interrupted HBV-active ART during the window between the last negative and first positive HBV tests. The remaining individual was prescribed uninterrupted lamivudine between their last negative and first positive test. This individual had detectable plasma HIV RNA twice during the 7-year window for infection, indicating possible nonadherence to their antiretroviral regimen.
Thirty-eight of 39 (97.4%) had at least one CD4 count recorded before HBV infection. Overall, the median CD4 cell nadir was 90; before HBV infection it was 149. Twenty-five of the 38 cases with a CD4 cell count recorded before HBV infection had their CD4 cell nadir before HBV infection. The remaining 13 individuals experienced a further decline of their CD4 cell count subsequent to their HBV diagnosis.
Thirty-eight of 39 cases had documentation of a hepatitis C (HCV) antibody test. Ten cases (25.6%) had documented HCV antibody positivity. Of these, six had a documented HCV polymerase chain reaction (PCR) result, all of which were positive and all of which indicated a chronic infection (>6 months between two positive PCR tests or a positive antibody test >6 months before positive PCR).
Case–control study
There were 38 cases and 103 controls included in the analysis. Characteristics of the cases and controls are summarised in Table 2. There was no significant difference between any of the parameters collected including risk factors for HBV and potential HBV prevention strategies such as HBV-active ART or HBV vaccination.

|
A significantly higher proportion of cases (13 out of 38, 34%) died than controls (14 out of 103, 14%). This association was retained after adjustment for ART, length of follow-up, CD4 nadir, injecting drug use (IDU) and age (OR: 3.99, P = 0.007). No case died of liver-related illness.
Incidence rate of HBV
The cumulative time at risk of the cohort was calculated at 21 587 patient-years, in which 39 seroconversions were documented. Therefore, the estimated incidence rate of HBV was 1.81 (95% confidence interval: 1.28–2.47) per 1000 patient-years at risk.
Discussion
The main finding from this study is that incident HBV, a preventable infection, continues to occur in the Australian context of HIV-infected individuals at a higher rate than in the general population. This suggests that preventive approaches are suboptimal. In this study, HBV infection in this HIV-infected cohort was likely to become chronic, exposing individuals to the potential sequelae of chronic disease.
A relatively high proportion of cases (10 out of 39, 25.6%) had some record of receipt of HBV vaccination. This is likely to be an underestimate of the total number vaccinated, with many individuals vaccinated at other medical services and inconsistent written communications of the same. For the purpose of this study, an individual was counted as vaccinated if there was any record of vaccination, which may have meant suboptimal dosing or total number of vaccinations, thereby resulting in nonprotective levels of surface antibody. Only two cases in this series had evidence of protective HBsAb titres after vaccination. This confirms others’ findings that HBV vaccination cannot be assumed to be protective in the absence of a positive HBsAb titre.11
The ability of HBV vaccine to induce a protective HBsAb response in HIV-infected individuals has increased as immunological status has improved with HAART and modified vaccine schedules have been employed, with seroconversion rates recently exceeding 80%,3 approaching that observed in HIV-negative individuals of >90%.12 It is a timely reminder that this preventive opportunity be seized and all HIV-infected individuals be offered four intramuscular double-dose (40 µg) vaccinations followed by documentation of HBsAb seroconversion.
Two cases in this series had evidence of protective HBsAb titres after vaccination yet went on to develop HBV infection. As HBcAb was not simultaneously tested, it is not possible to exclude a positive HBsAb being due to infection. A positive HBsAb response has been shown to protect against HBV infection; however, a small percentage of positive responders may go on to contract HBV.11 This emphasises that other modes of prevention may be necessary.
The incidence rate we have estimated suggests that for every 100 susceptible seronegative HIV-positive individuals, ~2 will acquire HBV infection over 10 years. A caveat of this analysis was that the exact timing of seroconversion was not known and we assumed that this occurred between the last negative and the first positive test. This also limited our ability to analyse for some time-varying factors of interest, such as whether the use of hepatitis B active antiretrovirals such as tenofovir or lamivudine might be protective against HBV infection. Given the limited numbers, we also did not estimate incidence in specific patient subgroups.
We were unable to demonstrate a protective role of HBV-active HAART in our study. The ability to assess this was limited due to the small sample size overall, and particularly the small subset who were infected with HBV subsequent to the widespread use of HBV-active HAART. Previous studies that have attempted to answer this question have also suffered from the limitation of small numbers in each treatment subgroup. One study showed a protective effect of HBV-active HAART, however included only very small numbers of individuals not on an HBV-active regimen.9
A high proportion (35.9%) of cases had a history of IDU. A higher percentage of cases than controls had IDU exposure. On multivariate analysis the association between IDU and incident HBV approached significance. Importantly, the prevalence of IDU amongst controls (20.6%) was higher than that observed amongst the entire database (10.6%). This reflects the inherent selection bias in selecting controls from among those with recorded HBV tests where testing has not been universal. It is likely that those who were perceived as being at higher risk of HBV exposure through IDU were tested more often and thus were more likely to be selected as controls. This selection bias reduced the ability of the study to clearly distinguish the role of IDU in incident HBV risk.
Measurement of the effect of IDU on incident HBV risk in various HIV cohorts is difficult due to the varying rates of IDU in different settings and the background rates of HBV in the community. One study found an association between recent IDU and incident HBV in an HIV cohort.13 This study was able to distinguish between recent and any IDU, which was not possible for the controls in our study design. Other studies have not been able to demonstrate a clear role for IDU in incident HBV,7,8 possibly due to exposure status being determined by source of HIV, recorded as a single possible exposure rather than as one of multiple possible exposures.
The individuals who did not use intravenous drugs had no risk factors for exposure other than sexual exposure. Due to the overwhelming predominance of men who had sex with men in our cohort, it was difficult to assess different patterns of sexual behaviour as a risk factor for HBV. However other studies have found that incident HBV was more common among men who had sex with men than in other HIV sexual exposure categories.5,7
The course and outcomes of HBV in this cohort were similar to that experienced in other cohorts. A low percentage had a recorded acute HBV illness, consistent with known patterns in HIV-negative individuals14. A high percentage of cases (23%) went on to have confirmed chronic HBV infection. This is comparable to that observed in other HIV cohorts.15,16 It has been shown that the incidence of chronic HBV infection has decreased since the HAART era.9 Although we were unable to subdivide our group of patients between before and after the HAART era for analysis due to small numbers; it was evident that several cases of chronic HBV arose in the post-HAART era.
The increased mortality findings in this study were unexpected (OR: 3.99). Previous studies have demonstrated a much more modest effect of HBV on all-cause mortality in HIV cohorts (OR 1.537) This finding may have been due to a combination of true factors but also by unavoidable confounding factors unable to be measured in our study. Confounding factors increasing the true mortality rate of the HBV-positive group could include poor engagement with health care services, less antiretroviral administration or adherence, or increased risk-taking behaviour.
Implications for clinical care
HIV-infected individuals should be screened for HBV and monitored for incident infection. Active education and prevention strategies should be pursued to reduce the morbidity associated with coinfection. In particular, rates of vaccine coverage and protection should be optimised by identifying those who are susceptible and administering a complete and adequately dosed vaccine schedule with documentation of protective titres of hepatitis B surface antibody after vaccination.
It should be reinforced to clinicians that incident HBV infection still occurs, even with the widespread use of HBV-active ART, but with optimal control of HIV and a modified vaccination schedule, prevention of HIV–HBV co-infection is achievable.
References
[1] Thio CL, Seaberg EC, Skolasky R, Phair J, Visscher B, Munoz A, et al HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360 1921–6.| HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS).Crossref | GoogleScholarGoogle Scholar | 12493258PubMed |
[2] Fonseca MO, Pang LW. Cavalheiro NdP, Barone AA, Lopes MH. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005; 23 2902–8.
| Cavalheiro NdP, Barone AA, Lopes MH. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisVWktL4%3D&md5=880031fb027104f45931eb5bbc64c0aaCAS | 15780739PubMed |
[3] Launay O, van der Vliet D, Rosenberg AR, Michel M-L, Piroth L, Rey D, et al Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011; 305 1432–40.
| Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvFWls7k%3D&md5=f4ef8ea09f32501ddc231a5876ea1d2cCAS | 21486976PubMed |
[4] Bailey CL, Smith V, Sands M. Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. Int J Infect Dis 2008; 12 e77–83.
| Hepatitis B vaccine: a seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults.Crossref | GoogleScholarGoogle Scholar | 18723381PubMed |
[5] Spradling PR, Richardson JT, Buchacz K, Moorman AC, Brooks JT, HIV Outpatient Study (HOPS) Investigators Prevalence of chronic hepatitis B virus infection among patients in the HIV Outpatient Study, 1996–2007†. J Viral Hepat 2010; 17 879–86.
| Prevalence of chronic hepatitis B virus infection among patients in the HIV Outpatient Study, 1996–2007†.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cbpsFylsg%3D%3D&md5=97d70a90016e26d0f58c8aaca51f44d3CAS | 20158604PubMed |
[6] Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004; 38 S158–68.
| Global epidemiology of hepatitis B virus.Crossref | GoogleScholarGoogle Scholar | 15602165PubMed |
[7] Konopnicki D, Mocroft A, De Wit S, Antunes F, Ledergerber B, Katlama C, et al Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005; 19 593–601.
| Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort.Crossref | GoogleScholarGoogle Scholar | 15802978PubMed |
[8] Zhou J, Dore G, Zhang F, Lim P, Chen Y, TREAT Asia HIV Observational Database Hepatitis B and C virus coinfection in The TREAT Asia HIV Observational Database. Journal of Gastroenterology and Hepatology 2007; 22 1510–8.
| 17645479PubMed |
[9] Chun HM, Fieberg AM, Hullsiek KH, Lifson AR, Crum-Cianflone NF, Weintrob AC, et al Epidemiology of hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis 2010; 50 426–36.
| Epidemiology of hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years.Crossref | GoogleScholarGoogle Scholar | 20047484PubMed |
[10] Tedaldi E, Peters L, Neuhaus J, Puoti M, Rockstroh J, Klein MB, et al Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the Strategic Management of Antiretroviral Therapy (SMART) Study. Clinical Infectious Diseases 2008; 47 1468–75.
| 18959492PubMed |
[11] Landrum ML, Hullsiek KH, Ganesan A, Weintrob AC, Crum-Cianflone NF, Barthel RV, et al Hepatitis B vaccination and risk of hepatitis B infection in HIV-infected individuals. AIDS 2010; 24 545–55.
| Hepatitis B vaccination and risk of hepatitis B infection in HIV-infected individuals.Crossref | GoogleScholarGoogle Scholar | 19487908PubMed |
[12] Dentico P, Buongiorno R, Volpe A, Zavoianni A, Pastore G, Schiraldi O. Long-term immunogenicity safety and efficacy of a recombinant hepatitis B vaccine in healthy adults. Eur J Epidemiol 1992; 8 650–5.
| Long-term immunogenicity safety and efficacy of a recombinant hepatitis B vaccine in healthy adults.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s%2FltVSktg%3D%3D&md5=a1a0a6b34d959ab5cb257c1de787a390CAS | 1426164PubMed |
[13] Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis 2003; 188 571–7.
| Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects.Crossref | GoogleScholarGoogle Scholar | 12898445PubMed |
[14] McMahon BJ, Alward WLM, Hall DB, Heyward WL, Bender TR, Francis DP, et al Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985; 151 599–603.
| Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M7jt12hsg%3D%3D&md5=367925707bd4fea76fd62fe05b622f7bCAS | 3973412PubMed |
[15] Landrum ML, Fieberg AM, Chun HM, Crum-Cianflone NF, Marconi VC, et al The effect of human immunodeficiency virus on hepatitis B virus serologic status in co-infected adults. PLoS ONE 2010; 5 e8687
| The effect of human immunodeficiency virus on hepatitis B virus serologic status in co-infected adults.Crossref | GoogleScholarGoogle Scholar | 20084275PubMed |
[16] Hadler SC, Judson FN, O’Malley PM, Altman NL, Penley K, Buchbinder S, et al Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection. J Infect Dis 1991; 163 454–7.
| Outcome of hepatitis B virus infection in homosexual men and its relation to prior human immunodeficiency virus infection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M7ktVSjtA%3D%3D&md5=fcd5b8c04a6a193bfe3a52de9c7a76aaCAS | 1825315PubMed |


