A comparative study of brain activation patterns associated with sexual arousal between males and females using 3.0-T functional magnetic resonance imaging
Gwang-Won Kim A and Gwang-Woo Jeong A B CA Research Institute of Medical Imaging, Chonnam National University Medical School, Gwangju 501-757, Korea.
B Department of Radiology, Chonnam National University Medical School, Chonnam National University Hospital, Gwangju 501-757, Korea.
C Corresponding author. Email: gwjeong@jnu.ac.kr
Sexual Health 11(1) 11-16 https://doi.org/10.1071/SH13127
Submitted: 15 May 2013 Accepted: 1 October 2013 Published: 13 December 2013
Abstract
Background: In contrast to the previous studies using a 1.5-T magnetic resonance imaging system, our study was performed on a higher magnetic field strength, 3.0 T, to gain more valuable information on the functional brain anatomy associated with visual sexual arousal for discriminating the gender difference by increasing the detection power of brain activation. Methods: Twenty-four healthy subjects consisting of 12 males and 12 females underwent functional magnetic resonance imaging examination for this study. Brain activity was measured while viewing erotic videos. Results: The predominant activation areas observed in males as compared with females included the hypothalamus, the globus pallidus, the head of the caudate nucleus, the parahippocampal gyrus, the amygdala and the septal area, whereas the predominant activation in females was observed in the anterior cingulate gyrus and the putamen. Conclusion: Our findings suggest that the brain activation patterns associated with visual sexual arousal are specific to gender. This gender difference in brain activation patterns is more remarkable at higher magnet field (3.0 T) than at 1.5 T.
Additional keywords: gender difference, high field.
Introduction
Several lines of evidence have provided insight into the gender differences in the neural mechanisms responsible for the organisation and function in various species.1,2 From invertebrates to humans, both males and females of a given species display identifiable differences in behaviour that mostly pertain to sexual and social behaviour. This phenomenon has been explained by biological, social and psychological differences between men and women.3
Research papers4–6 on sexual arousal in humans have been published to assess the functional anatomical differences and physiological changes in males and females through physiological methodologies and neuroimaging techniques in combination with statistical verification. A functional near-infrared spectroscopy study4 reported on gender differences in the timing and intensity of dorsolateral prefrontal cortex activation in response to a sexually explicit visual stimulus. Moreover, an eye-tracking study5 reported that both men and women subjected to different aspects of the same visual sexual stimuli could reflect pre-existing cognitive biases that possibly contribute to sex differences for neural, subjective and physiological arousal. These studies mentioned above suggest that there is a considerable interest in the gender difference of brain activation in response to erotic visual stimuli.
The blood oxygenation level-dependent (BOLD) technique is a commonly used functional magnetic resonance imaging (fMRI) method, which has advantages over other neuroimaging techniques such as positron emission tomography because of its intrinsic merits of noninvasiveness and high spatiotemporal resolution. Therefore, the use of the fMRI provides valuable information on the neural mechanism(s) associated with sexual arousal. Moreover, with the advent of 3.0-T magnetic resonance imaging (MRI) systems, performance and image quality are even further enhanced compared with the 1.5-T systems. With an increase in the magnetic field strength, the functional signal-to-noise ratio (SNR) also increases, which leads to enhanced detection power and spatial specificity. The functional SNR is the ratio between the intensity of a signal associated with changes in brain function and the variability in the data due to all sources of noise. Increased functional SNR can provide more information on brain activation in two ways. First, it is possible to identify more voxels as active at the same statistical threshold. Second, by increasing the statistical threshold, estimates can be improved for active voxels without changing the spatial extent of activation.7
Several studies8–10 concerned with sexual arousal while viewing erotic videos have been carried out using 1.5-T MRI units. These studies have demonstrated that the brain regions specifically associated with sexual arousal included the amygdala, the anterior cingulate gyrus, the ventral striatum, the thalamus, the hypothalamus and the insula. It is interesting to note that other studies11–13 have identified differences in brain activation patterns associated with sexual arousal between males and females using 1.5-T fMRI: the brain areas commonly activated in males more than in females included the amygdala and hypothalamus. They revealed that men become more aroused compared with women when watching sexually explicit films. In the commonly used 1.5-T MRI unit, it is much more difficult to demonstrate significant activation compared with the 3.0-T system, especially in the smaller anatomical structures, such as the septal area and hypothalamus, due to the low SNR of the BOLD signal. In other words, the higher field strength allows the use of smaller voxels, improving spatial resolution. For this reason, a combination of 3-T fMRI and a reduction in voxel size results in more informative results than does the 1.5-T MRI system.
The purpose of this study was to discriminate the differential brain areas in response to visual sexual stimulation between males and females using BOLD-based fMRI at 3.0 T, which is capable of providing a superior SNR and spatiotemporal as compared with 1.5-T systems.
Material and methods
Subjects and activation paradigm
Twenty-four right-handed subjects consisting of 12 males (age range: 23.5 ± 2.5 years) and 12 females (age range: 22.7 ± 2.9 years), who were all college students majoring in biological science and technology, participated in this study. None of the subjects had received any hormones. After a complete description of the study, informed consent was obtained from all subjects following the guidelines of our institutional review board. None of the participants displayed any symptoms of psychological disease or a personality disorder.
Sexual stimuli were presented in the form of a video clip. The erotic video was not disclosed in advance in order to increase sexual arousal in subjects. As for the activation paradigm, a traditional block analysis using contrast between the condition during the sexually arousing video and a resting condition was performed. The visual sexual stimulation began with a 1-min rest with a black screen, 3 min of stimulation by viewing of an erotic video and a 1-min rest. The erotic videos, which were edited and approved by a urologist and a psychologist with a major in sexuality, showed consensual sexual interactions between one man and one woman. However, the erotic video did not include an audio component. In our study, a thin white cross mark on black background screen was used as the rest condition so that the subjects could hardly feel any emotional or physical attraction, or cognition. Our study aimed only to identify the brain centres associated with gender differences in response to visually evoked sexual stimulation. The videos for visual stimulation were edited on a personal computer and were projected via a liquid crystal display projector (MP-3220, Hewlett-Packard Development Company, Palo Alto, CA, USA) onto a custom-built white screen in the MR room. Subjects viewed the screen through a mirror attached to the head coil in front of the forehead of the subject.The volunteers were asked to answer a questionnaire concerning the subjects’ preconceptions of sexual behaviour using a quartile scale: 0 (very conservative), 25 (conservative), 50 (moderate), 75 (liberal) and 100 (very liberal). After completion of the fMRI exams, the volunteers answered another questionnaire on their subjectively perceived sexual arousal (0, no change; 25, minimal increase; 50, moderate increase; 75, large increase; 100, maximal increase) in response to the question ‘To what degree were you sexually aroused?’
Acquisition of raw data of fMRI
Functional MRI was performed on a 3.0-T Forte MR Scanner (Isol Technology, Seoul, Korea) with a birdcage head coil. Functional images were acquired using a gradient-echo echo planar pulse sequence with the following parameters: repetition time ÷ echo time = 3000 ms 35 ms–1; flip angle = 70°; field of view = 22 cm × 22 cm; matrix size = 64 × 64; number of excitations = 1; slice thickness = 5 mm. The number of slices acquired was 20. The first two phases of dummy scans were supplemented to circumvent unstable fMRI signals. In addition, high-resolution anatomical images of the whole brain were acquired with T1-weighted images (repetition time ÷ echo time = 600 ms 14 ms–1; field of view = 22 cm × 22 cm; matrix size = 256 × 192; number of excitations = 2; slice thickness = 5 mm).
Data analysis
The fMRI data were analysed by postprocessing and data analysis using Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, University College London, London, UK) and our homemade software (functional and anatomical labelling of brain activation (FALBA)).10,14 Prior to statistical analysis, images were realigned utilising sinc interpolation to match each functional volume to the reference volume, and were spatially normalised to the standard stereotactic space corresponding to the template from the Montreal Neurological Institute space, which is a template created from 152 brain datasets. Bilinear interpolation was applied for normalisation. The images were then smoothed with an 8 mm full-width half-maximum Gaussian filter. No global scaling was used and the resulting time series across each voxel were high-pass filtered with a cut-off of 120 s. The individual data were analysed using a single-subject fixed effect model which was built by convolution of boxcar functions for the two conditions: sexually arousing video clips and the rest condition.
After specification of the appropriate design matrix, signal changes in the haemodynamic response function produced by the different experimental conditions were assessed at each voxel using a general linear model with a boxcar method. Statistical activation maps were obtained for the contrast between activation and resting. This analysis was performed in order to identify brain areas with an increased BOLD signal while viewing the erotic videos compared with the rest periods. Significant signal changes for each contrast were assessed by means of t-statistics on a voxel-by-voxel basis. The threshold at the voxel level value was set at uncorrected P < 0.05 with a spatial extent of at least 20 adjacent voxels.
For the group analysis of male and female subjects, the following regions of interest (ROIs), which are involved in sexual arousal in males and females,9–13 were created using Wake Forest University Pick Atlas (Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, NC, USA):15 the hippocampus, the parahippocampal gyrus, the amygdala, the anterior cingulate gyrus, the septal area, the putamen, the globus pallidus, the caudate nucleus, the thalamus and the hypothalamus. The ROI mask was applied to the evaluation of the contrasting areas between males and females using two-sample t-test.
Results
Subjective response to visual sexual stimulation
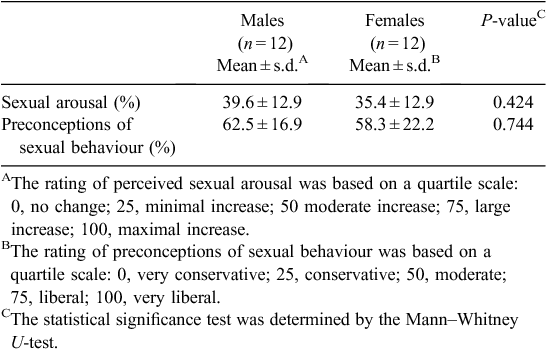
After completion of the fMRI study, the subjects were asked to provide their perceived sexual arousal and preconceptions of sexual behaviour using a quartile scale (Table 1). The reported scores of perceived sexual arousal were 39.6 ± 12.9 and 35.4 ± 12.9 in males and females, respectively, and the scores of preconceptions of sexual behaviour were 62.5 ± 16.9 and 58.3 ± 22.2, respectively. In the questionnaire for rating perceived sexual arousal, both males and females reported that the erotic videos were not disgusting but favourable to them.
|
Differential activation patterns between males and females
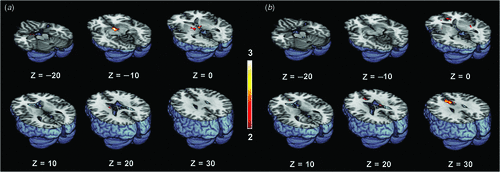
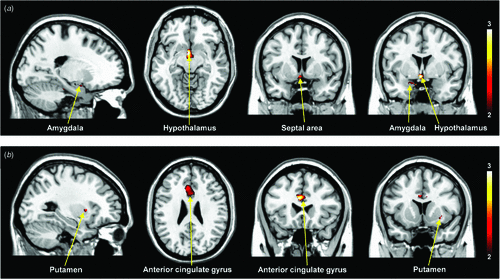
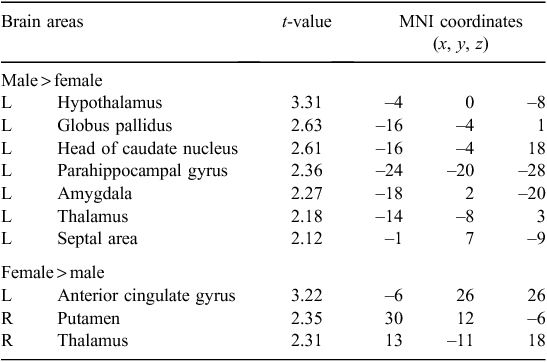
Figure 1 shows the differential activation patterns between males and females, which was analysed by a two-sample t-test for the ROI mask; Fig. 2 demonstrates the neuroanatomical details. Differential brain activities between two groups are summarised in Table 2. The predominant activation areas observed in males more than in females included the hypothalamus, the globus pallidus, the head of the caudate nucleus, the parahippocampal gyrus, the amygdala, the septal area and the thalamus, whereas the predominant activation in females was observed in the anterior cingulate gyrus, the putamen and the thalamus. These brain areas are supposed to be involved in a specific role for the gender difference related to sexual arousal.
|
|
|
Discussion
Several studies7,16 have been published in the literature describing the advantage of 3.0 T over 1.5 T in fMRI. Kranow et al.16 examined the effects of magnetic field strength on several different tasks, including perceptual, memory and emotional processing paradigms, comparing 1.5 T and 3.0 T. They found that across the brain regions, there were substantial increases in the number of activated voxels with increasing field strength in a cognitive task, suggesting that detection power throughout the brain improved with field strength.7,16 The use of an MRI system with a higher magnetic field provides higher signal intensity in fMRI because of its stronger magnetic susceptibility effects.17
All of the fMRI studies11–13 concerning gender differences of sexual arousal have been performed on a 1.5-T MRI system and these studies were not able to investigate the smaller brain centres, such as the septal area, because of the dominant effect of large vessels at such a low magnet field (1.5 T). As shown in Table 2, our study demonstrated the predominant activation areas observed in males rather than females: the hypothalamus, the globus pallidus, the head of the caudate nucleus, the parahippocampal gyrus, the amygdala and the septal area. Activation of the hypothalamus and amygdala in males was also reported in the previous 1.5-T fMRI studies.11–13 However, it should be noted that activation of the septal area was observed in male subjects with the help of a higher magnetic field in the 3-T MRI system. This area is extensively interconnected with the amygdala and hypothalamus, and is related to hypothalamic activity and sexual arousal.18 Numerous animal studies19,20 have considerably clarified the role of the septal area associated with sexual function and behaviour. Also, the septal area has been implicated in the control of sexual arousal in human males.21,22 Although extensive animal studies have found activation in the septal area with sexual stimuli in males, most neuroimaging studies21,22 concerned with correlations between sexual response and activation of the septal area in humans have not yet been discussed. Activation of the septal area noted in our study using a 3-T MRI system would be helpful to assess the neural mechanisms of sexual arousal in males.
The other brain structure with predominant activation in males rather than females included the hypothalamus (P < 0.005). Activation of the hypothalamus in male subjects suggests that males became physiologically more aroused to the erotic visual stimulus compared with females. In animal studies, the paraventricular nucleus of the hypothalamus, lateral hypothalamus and preoptic area appeared to be involved in erectile functions.23 Redoute et al.20 found a significant correlation between activation in the hypothalamus and measures of penile tumescence in human males using positron emission tomography. One possible explanation for this gender difference is that the hypothalamus may be involved in the physiological reaction to sexual stimuli, such as erection, or that sexual arousal activates the hypothalamic gonadal axis, resulting in increased steroid secretion, as seen in men following sexual activation.24 Moreover, Temel et al.25 suggested that the most important structures involved in penile erection are the frontal lobe, the amygdala, the thalamus and the hypothalamus in males. Especially, increased tumescence was associated with activation of the globus pallidus and the caudate nucleus.18 Activation of the amygdala, the thalamus and hypothalamus, the globus pallidus and the caudate nucleus in our study may reflect recognition of penile erection.
In addition to activation of the hypothalamus, the amygdala was predominantly activated in male subjects. Several fMRI studies12,13 have also demonstrated greater activation of the amygdala in males than in females in response to sexual stimuli. The amygdala has multiple functions and although processes related to emotional arousal are clearly of prime importance, in a specific context, other roles may take precedence to determine amygdala activity. Hamann et al.13 suggested that amygdala activation based on sex differences was independent of the degree of subjective sexual arousal for both sexes and purely reflected the sex differences. In particular, a larger amygdala size is related to higher sexual drive in humans, further supporting the role of the human amygdala in sexual motivation.26 Moreover, it has been suggested that the amygdala mediates sex differences in memory for emotional visual stimuli.27 Thus the hypothalamus, the globus pallidus, the head of the caudate nucleus, the parahippocampal gyrus, the amygdala and the septal area observed in our study may reflect the higher level of sexual arousal in males than in females.
The thalamus, another interesting area in our study, showed significant activation in both males and females. The males, in contrast with the females showed higher activity in the left thalamus, whereas the females showed higher activities in the right thalamus. Several fMRI studies10,28 have demonstrated thalamus activation during sexual arousal in males and females. The thalamus can transmit input information of sense and consciousness to the cerebral cortices including the frontal, temporal and occipital lobes. As the extensive interconnectivity of the thalamus has been supported by a previous neurological study,29 we expected to identify a connection between the cerebral cortices and the thalamus for sensory information. The thalamus may be a hub of activation areas related to sexual arousal and could be implicated as a cognitive factor of sexual arousal both in males and females.
Most studies11–13 have identified the brain areas that are predominantly activated in males more than in females; however, the fMRI studies for the areas predominantly activated in females more than in males have not yet been completely specified. Our study demonstrates the brain areas predominantly activated in females more than in males include the anterior cingulate gyrus and putamen (Table 2). These results are partly similar to the brain areas commonly activated in women during exposure to sexual arousal stimuli.10,11 The anterior cingulate gyrus of the internal components of the limbic system is related to the emotional processing of sexual arousal, and this area has been implicated in the induction and control of affective behaviour for sexual arousal.30 Beauregard et al.8 reported that attempted inhibition of the sexual arousal generated by viewing the erotic stimuli was associated with activation of the anterior cingulate gyrus. Thus this area associated with the inhibition of sexual arousal showed higher brain activity in females than in males. However, these results in females cannot be clearly explained by a correlation between activation areas and sexual stimulation because of the lack of physiological measures of sexual arousal. The brain areas predominantly activated in females need to be elucidated with further investigations.
There are some limitations in this study. First, the participants included females without consideration of the menstrual cycle. The phases of the menstrual cycle include the follicular phase and luteal phase, which bring about fluctuations in hormone levels at different times of the month. The peak levels of luteinising hormone and follicle stimulating hormone fall in the middle of the menstrual cycle when females of various species become sexually more responsive.31 However, evidence for an association between the modulation of sexual receptivity or arousal, and phases of the menstrual cycle in females appears inconsistent and even contradictory.11 In addition, both the brain areas predominantly activated in males more than in females in the luteal phase and the menstrual phase were the same.12 Second, we used a thin white cross mark on a black background screen as the resting condition, towards which the subjects hardly felt any emotional or physical attraction, or cognition. We assumed that the emotional feelings of males and females were different from each other when viewing neutral stimuli. Therefore the resting stimulus was selected to be a proper control condition to minimise gender differences between the responses to neutral stimuli. Third, the participants rated their sexual arousal as being below the average level, but this level is similar to that in the previous study11 at 1.5 T. Although the reported sexual arousal was between a minimal and moderate levels, the perceived sexual arousal score range in females was about the same as in males. The fourth limitation of this study is concerned with the lack of objective measurements of sexual arousal such as penile tumescence in males and vaginal blood volume in females. However, the visual sexual stimuli had been assessed for their erotic effect as shown in Table 1.
This study was conducted to discriminate the activated brain areas in response to visual sexual stimulation between males and females using an fMRI with higher magnetic field strength (3T) for the first time. We validated the clinical usefulness of 3-T fMRI in our findings that the differential neural activation pattern associated with sexual arousal is specific to gender. This finding will be useful in understanding gender differences in neural mechanisms on sexual arousal.
Conflicts of interest
None declared.
Acknowledgement
This research was supported by the National Research Foundation of Korea (NRF) grants funded by the Ministry of Science, ICT & Future Planning (no. 2011–0016291), and the Basic Science Research Program through the NRF funded by the Ministry of Education (no. 2009–0077677).
References
[1] Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol 2007; 17 675–83.| Neural mechanisms underlying sex-specific behaviors in vertebrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjs1Kmsrg%3D&md5=138e99e353798e3e2861244be5625075CAS | 18343651PubMed |
[2] Kelly SJ, Ostrowski NL, Wilson MA. Gender differences in brain and behavior: hormonal and neural bases. Pharmacol Biochem Behav 1999; 64 655–64.
| Gender differences in brain and behavior: hormonal and neural bases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnsVKksL0%3D&md5=8028cecd543ed32705fcdab54d0255ffCAS | 10593187PubMed |
[3] Baldwin JD, Baldwin JI. Gender differences in sexual interest. Arch Sex Behav 1997; 26 181–210.
| Gender differences in sexual interest.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s3lsFKrtA%3D%3D&md5=3759ce69bf6d58d886943001bd863f3eCAS | 9101033PubMed |
[4] Leon-Carrion J, Martin-Rodriguez JF, Damas-Lopez J, Pourrezai K, Izzetoglu K, Barroso Y, et al Does dorsolateral prefrontal cortex (DLPFC) activation return to baseline when sexual stimuli cease? The role of DLPFC in visual sexual stimulation. Neurosci Lett 2007; 416 55–60.
| Does dorsolateral prefrontal cortex (DLPFC) activation return to baseline when sexual stimuli cease? The role of DLPFC in visual sexual stimulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjsVeku7s%3D&md5=051b063741ec84853b5076771634c6f8CAS | 17316990PubMed |
[5] Rupp HA, Wallen K. Sex differences in viewing sexual stimuli: an eye-tracking study in men and women. Horm Behav 2007; 51 524–33.
| Sex differences in viewing sexual stimuli: an eye-tracking study in men and women.Crossref | GoogleScholarGoogle Scholar | 17362952PubMed |
[6] Canli T, Gabrieli JD. Imaging gender differences in sexual arousal. Nat Neurosci 2004; 7 325–6.
| Imaging gender differences in sexual arousal.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisFCisrk%3D&md5=403704cfa40514f487af3e94a52992cfCAS | 15048119PubMed |
[7] Huettel SA, Son AW, Mccarthy G. Functional magnetic resonance imaging. Sunderland: Sinauer Associates; 2004.
[8] Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci 2001; 21 RC165
| 1:STN:280:DC%2BD3Mrgt1Ohuw%3D%3D&md5=36a66738f948d939e2207228395ed151CAS | 11549754PubMed |
[9] Park K, Kang HK, Seo JJ, Kim HJ, Ryu SB, Jeong GW. Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology 2001; 57 1189–94.
| Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD387hslOrtA%3D%3D&md5=57099a50b00558fe0be903c7db4829f7CAS | 11377345PubMed |
[10] Jeong GW, Park K, Youn G, Kang HK, Kim HJ, Seo JJ, et al Assessment of cerebrocortical regions associated with sexual arousal in premenopausal and menopausal women by using BOLD-based functional MRI. J Sex Med 2005; 2 645–51.
| Assessment of cerebrocortical regions associated with sexual arousal in premenopausal and menopausal women by using BOLD-based functional MRI.Crossref | GoogleScholarGoogle Scholar | 16422822PubMed |
[11] Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, et al Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp 2002; 16 1–13.
| Areas of brain activation in males and females during viewing of erotic film excerpts.Crossref | GoogleScholarGoogle Scholar | 11870922PubMed |
[12] Gizewski ER, Krause E, Karama S, Baars A, Senf W, Forsting M. There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli: a fMRI study. Exp Brain Res 2006; 174 101–8.
| There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli: a fMRI study.Crossref | GoogleScholarGoogle Scholar | 16604320PubMed |
[13] Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci 2004; 7 411–6.
| Men and women differ in amygdala response to visual sexual stimuli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisFCis7k%3D&md5=d0960dfaf0f5dfb9afd488612024f34cCAS | 15004563PubMed |
[14] Yang JC, Park K, Eun SJ, Lee MS, Yoon JS, Shin IS, et al Assessment of cerebrocortical areas associated with sexual arousal in depressive women using functional MR imaging. J Sex Med 2008; 5 602–9.
| Assessment of cerebrocortical areas associated with sexual arousal in depressive women using functional MR imaging.Crossref | GoogleScholarGoogle Scholar | 18194182PubMed |
[15] Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19 1233–9.
| An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets.Crossref | GoogleScholarGoogle Scholar | 12880848PubMed |
[16] Krasnow B, Tamm L, Greicius MD, Yang TT, Glover GH, Reiss AL, et al Comparison of fMRI activation at 3 and 1.5 T during perceptual, cognitive, and affective processing. Neuroimage 2003; 18 813–26.
| Comparison of fMRI activation at 3 and 1.5 T during perceptual, cognitive, and affective processing.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s3gs12ksQ%3D%3D&md5=a221084752b824d5581872cf6fdfbebfCAS | 12725758PubMed |
[17] Scarabino T, Giannatempo GM, Popolizio T, Tosetti M, d’Alesio V, Esposito F, et al 3.0-T functional brain imaging: a 5-year experience. Radiol Med (Torino) 2007; 112 97–112.
| 3.0-T functional brain imaging: a 5-year experience.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s7lsVequg%3D%3D&md5=50aa7719fbcbf556cd318d6266c75da2CAS |
[18] Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al Brain activation and sexual arousal in healthy, heterosexual males. Brain 2002; 125 1014–23.
| Brain activation and sexual arousal in healthy, heterosexual males.Crossref | GoogleScholarGoogle Scholar | 11960892PubMed |
[19] Sachs BD, Meisel RL. The physiology of male sexual behavior. In Knobil E, Neill JD, editors. Physiology of reproduction. New York: Raven Press; 1994.pp. 3–105
[20] Redouté J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, et al Brain processing of visual sexual stimuli in human males. Hum Brain Mapp 2000; 11 162–77.
| Brain processing of visual sexual stimuli in human males.Crossref | GoogleScholarGoogle Scholar | 11098795PubMed |
[21] Gorman DG, Cummings JL. Hypersexuality following septal injury. Arch Neurol 1992; 49 308–10.
| Hypersexuality following septal injury.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK387mtF2ksg%3D%3D&md5=dedb50dce76466d9df1052909a55cd39CAS | 1536635PubMed |
[22] Stoléru S, Grégoire MC, Gérard D, Decety J, Lafarge E, Cinotti L, et al Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav 1999; 28 1–21.
| Neuroanatomical correlates of visually evoked sexual arousal in human males.Crossref | GoogleScholarGoogle Scholar | 10097801PubMed |
[23] Giuliano F, Bernabe J, Brown K, Droupy S, Benoit G, Rampin O. Erectile response to hypothalamic stimulation in rats: role of peripheral nerves. Am J Physiol 1997; 273 R1990–7.
| 1:CAS:528:DyaK1cXjsVOhug%3D%3D&md5=9eec63fa0d1e173243eed67a981a2391CAS | 9435653PubMed |
[24] Stoleru SG, Ennaji A, Cournot A, Spira A. LH pulsatile secretion and testosterone blood levels are influenced by sexual arousal in human males. Psychoneuroendocrinology 1993; 18 205–18.
| LH pulsatile secretion and testosterone blood levels are influenced by sexual arousal in human males.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVWhtLs%3D&md5=36c53b51ad1800774d767fb2d942fa96CAS | 8516424PubMed |
[25] Temel Y, Hafizi S, Tan S, Visser-Vandewalle V. Role of the brain in the control of erection. Asian J Androl 2006; 8 259–64.
| Role of the brain in the control of erection.Crossref | GoogleScholarGoogle Scholar | 16625274PubMed |
[26] Baird AD, Wilson SJ, Bladin PF, Saling MM, Reutens DC. The amygdala and sexual drive: insights from temporal lobe epilepsy surgery. Ann Neurol 2004; 55 87–96.
| The amygdala and sexual drive: insights from temporal lobe epilepsy surgery.Crossref | GoogleScholarGoogle Scholar | 14705116PubMed |
[27] Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, et al Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem 2001; 75 1–9.
| Sex-related difference in amygdala activity during emotionally influenced memory storage.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7htFWkug%3D%3D&md5=e02340fb3ca3ed45a520c64d9c35d26bCAS | 11124043PubMed |
[28] Ponseti J, Bosinski HA, Wolff S, Peller M, Jansen O, Medhorn HM, et al A functional endophenotype for sexual orientation in humans. Neuroimage 2006; 33 825–33.
| A functional endophenotype for sexual orientation in humans.Crossref | GoogleScholarGoogle Scholar | 16979350PubMed |
[29] Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 1997; 154 918–25.
| 1:STN:280:DyaK2szlsVCluw%3D%3D&md5=82dd381a5edf7d7c27ff7174cc9fde13CAS | 9210741PubMed |
[30] Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci 1998; 353 1841–9.
| The neuronal basis for consciousness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FntFChsg%3D%3D&md5=925983c5930201b0f663e1752cdc4cc2CAS | 9854256PubMed |
[31] Harvey SM. Female sexual behavior: fluctuations during the menstrual cycle. J Psychosom Res 1987; 31 101–10.
| Female sexual behavior: fluctuations during the menstrual cycle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s7ktVyqtA%3D%3D&md5=2765932ee1be24eb7eff5de41bd3939eCAS | 3820137PubMed |


