Simonachne, a new genus for Australia segregated from Ancistrachne s.l. (Poaceae : Panicoideae : Paniceae) and a new subtribe Cleistochloinae
E. J. Thompson A *
A *
A c/o Queensland Herbarium, Department of Environment and Science, Brisbane Botanic Gardens, Mt Coot-tha Road, Toowong, Qld 4066, Australia.
Australian Systematic Botany 35(1) 19-62 https://doi.org/10.1071/SB20024
Submitted: 4 September 2020 Accepted: 18 January 2022 Published: 11 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
A new genus, Simonachne E.J.Thomps. is described and Ancistrachne maidenii (A.A.Ham.) Vickery is transferred to it as Simonachne maidenii (A.A.Ham.) E.J.Thomps. The new subtribe Cleistochloinae E.J.Thomps. is described and is composed of four genera, Calyptochloa, Cleistochloa, Dimorphochloa and Simonachne, united by distinctive morphology that is associated with reproductive dimorphism. Phenetic analyses were used to examine the similarities of taxa and to test the consistency of results with variation in analysis inputs. Input variations included the dataset in terms of composition of the samples and morphological characters, and the cluster analysis algorithms, viz. classification, ordination and association measure. A baseline dataset was used for comparison of results and comprised 24 samples and 161 characters relating to anatomy, micro- and macromorphology of spikelets, leaves and fertile culms. Three major clusters were resolved, Cleistochloinae (‘the cleistogamy group’), Neurachninae in its original sense, and a cluster referred to as the ‘paniculate inflorescence group’ composed of Ancistrachne s.s., Entolasia and Panicum s.s. The results were congruent with a recent phylogenetic study that showed that Ancistrachne s.l., Cleistochloa s.l. and Dimorphochloa s.l. were not monophyletic. The process provided an array of morphological characters for descriptions of species and for distinguishing taxa at multiple ranks in natural groups, components of alpha and beta taxonomy respectively.
Keywords: Ancistrachne, Ancistrachne maidenii, Calyptochloa, Cleistochloa, Cleistochloinae, clusters, Dimorphochloa, morphology, Neurachninae, phenetics, similarity, Simonachne, ‘the cleistogamy group’.
Introduction
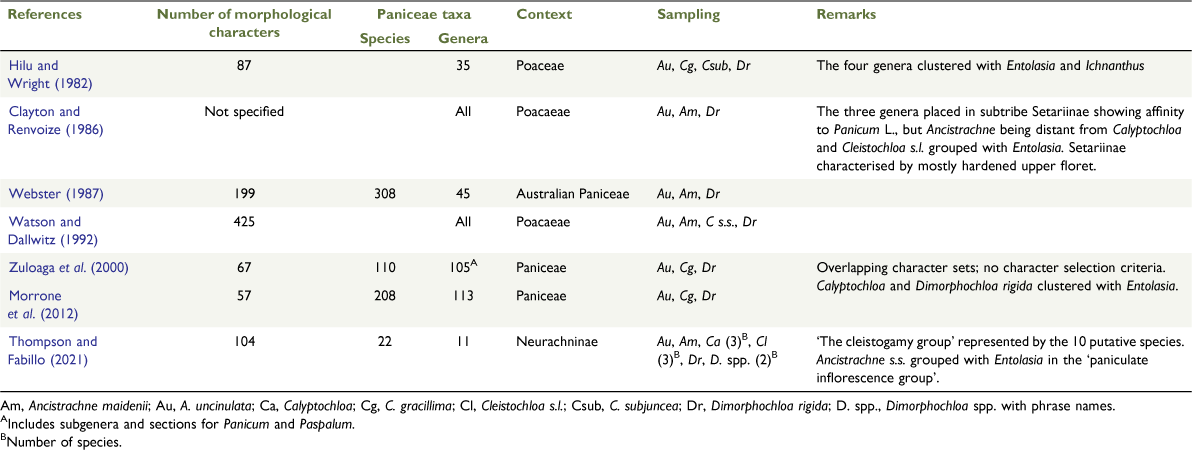
A recent phylogenetic reconstruction of the panicoid grass subtribe Neurachninae sensu Morrone et al. (2012) showed that it consists of three major clades, viz. ‘the cleistogamy group’, the ‘paniculate inflorescence group’ and Neurachninae s.s., and, additionally, that the genus Ancistrachne sensu Vickery (1961b) is not monophyletic (Thompson and Fabillo 2021). ‘The cleistogamy group’ was represented by Ancistrachne maidenii (A.A.Ham.) Vickery and placed sister to Calyptochloa C.E.Hubb., Cleistochloa s.l. and Dimorphochloa s.l., with all taxa united by several morphological characters and, most notably, by a rare dimorphic breeding system (Table 1). Ancistrachne s.s. was sister to Entolasia Stapf. in the ‘paniculate inflorescence group’, defined by the terminal inflorescences in panicles and several characters relating to spikelets. Neurachninae in its original sense as circumscribed by Clayton and Renvoize (1986) comprised Neurachne R.Br. (syn. Paraneurachne (Hack.) S.T.Blake) and Thyridolepis S.T.Blake that share a definitive combination of characters (Clayton and Renvoize 1986; Thompson and Fabillo 2021).

|
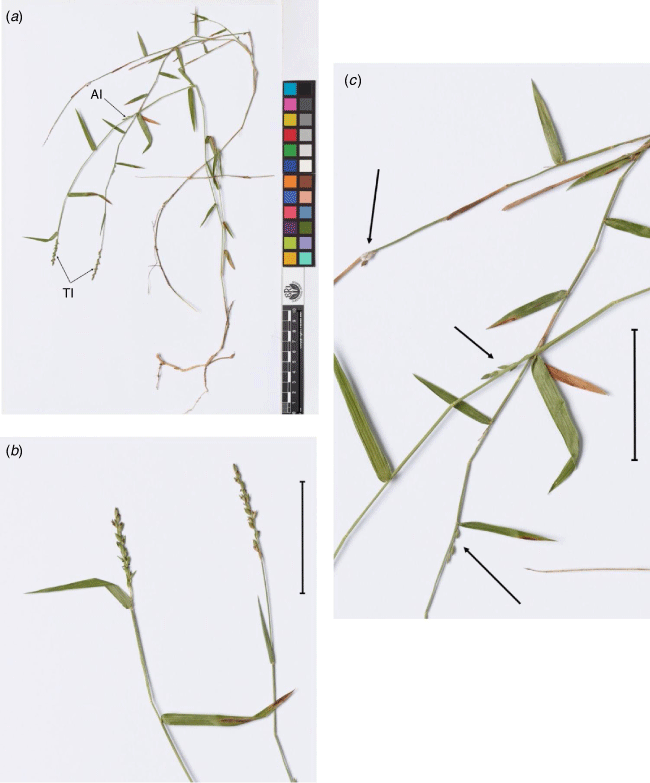
The dimorphic breeding system of the ‘the cleistogamy group’ has a distinctive morphology in tribe Paniceae. It consists of two types of inflorescences, terminal and axillary, in different locations on the same plant (amphigamy), with corresponding spikelet dimorphism (Fig. 1, 2). The spikelet dimorphism across the genera is either of two forms, namely similar or very different (Fig. 2). Ancistrachne maidenii and Dimorphochloa rigida S.T.Blake are similar in that both species have relatively inconspicuous spikelet dimorphism compared with other species in the group (Fig. 2). Conversely, Calyptochloa, Cleistochloa s.l. and Dimorphochloa s.l. have axillary spikelets that are very different from their corresponding terminal spikelets. All species in ‘the cleistogamy group’ share anther dimorphism where anthers in the axillary spikelets are considerably smaller than those in the terminal spikelets. The axillary spikelets, if appraised independently, could be considered to belong to a different taxon in a similar perspective as remarked by Chase (1918, p. 255) in her assessment of several North American grasses with dimorphic breeding systems. The spikelets in the axillary inflorescences are obligately cleistogamous (CL), i.e. pollination takes place within a closed flower that never opens, whereas the spikelets in the terminal inflorescences are usually chasmogamous (CH), as for most grasses, or uncommonly mixed with CL spikelets in some species.
Ancistrachne, in its original sense as circumscribed by Blake (1941), is unique in the Australian Paniceae. The genus is identifiable by a combination of characters, including spikelets that are tilted on the pedicels, adorned with hooked hairs, and exhibit abaxial orientation (Webster 1987; Simon 2002). Ancistrachne sensu Blake (1941) comprises three species, viz. A. uncinulata (R.Br.) S.T.Blake, an Australian endemic occurring along the eastern coast of Australia, and two species from the tropical Asia–Pacific region, A. ancylotricha (Quisumb. & Merr.) S.T.Blake and A. numaeensis (Balansa) S.T.Blake, recorded in the Philippines and New Caledonia respectively. A. ancylotricha was omitted from the study by Thompson and Fabillo (2021) becaue of poor quality of extracted DNA and polymerase chain-reaction failures. However, Ancistrachne maidenii, a rare species from the eastern coast of Australia (Fig. 3) transferred to Ancistrachne from Eriochloa in 1961 (Vickery 1961a), has woolly hairs and lacks the hooked hairs and differs from Ancistrachne s.s. in other morphological characters (Hamilton 1913; Vickery 1961a, 1961b; Simon 2002; Thompson and Fabillo 2021; Table 1, Fig. 4).

|

|
The affinities and placement of Ancistrachne s.l. (i.e. including A. maidenii) have differed in several studies using various methods of analysis of morphological or molecular data (Table 2). Ancistrachne s.l., Calyptochloa, Cleistochloa S.T.Blake and Dimorphochloa rigida have frequently been grouped together and occasionally Entolasia has been allied to these genera (Table 2). In the intuitive diagram of morphological relationships of genera in subtribe Setariinae by Clayton and Renvoize (1986), Ancistrachne s.l. had close affinity to Panicum L., reflecting the basionym for A. uncinulata, Panicum uncinulatum R.Br., and Calyptochloa and Cleistochloa s.s. were grouped with Entolasia. In molecular phylogenetic reconstructions, Ancistrachne s.l. was positioned in subtribe Neurachninae s.l. with Calyptochloa, Cleistochloa s.s. (subsuming D. rigida), Neurachne and Thyridolepis (Christin et al. 2012; Grass Phylogeny Working Group II 2012; Morrone et al. 2012; Soreng et al. 2015, 2017). In these studies, Ancistrachne uncinulata was used as the sole representative of Ancistrachne s.l., usually with one other species from each of the other four genera in Neurachninae s.l. (Table 2). Morrone et al. (2012) considered Neurachninae to have two uniting characters, viz. inflorescences with reduced secondary branching, and abaxial spikelet orientation. However, most of the taxa in the clade have reduced primary branching and lack secondary branches, although Ancistrachne s.s. sometimes bears secondary branching. Ancistrachne s.s., Neurachne and Thyridolepis have abaxial spikelet orientation, whereas members of ‘the cleistogamy group’ have adaxial spikelet orientation (Webster 1987; Thompson and Fabillo 2021; Table 1). All genera in Neurachninae s.l. have C3 photosynthetic pathway, although some species of Neurachne have been recorded as having C3–C4 intermediacy (Hattersley et al. 1982; Christin et al. 2012). On the basis of the phylogenetic reconstructions by Morrone et al. (2012) and Grass Phylogeny Working Group II (2012), Kellogg (2015) synonomised Ancistrachne s.l. and Calyptochloa with Cleistochloa s.s. and listed several uniting characters (Table 1). Byn contrast, Ancistrachne uncinulata, Calyptochloa, Cleistochloa s.l. and Dimorphochloa s.l. have been maintained by Brown and Bostock (http://data.qld.gov.au/dataset/census-of-the-queensland-flora-2020/, accessed 15 May 2021), although the latter two genera are not monophyletic (Thompson and Fabillo 2021).
|
Thompson and Fabillo (2021) refined the distinction of taxa in the ‘the cleistogamy group’, comprising five putative genera and 10 species, by the inclusion of 37 characters that relate to the CL inflorescences and spikelets (Fig. 1, 2, Tables 1, 2). Prior to this, very few CL characters were used in keys and descriptions (Tothill and Hacker 1983; Clayton and Renvoize 1986; Webster 1987; Watson and Dallwitz 1992; Simon 2002; Simon and Alfonso, see http://ausgrass2.myspecies.info/, accessed 21 September 2020). For example, of the 157 inflorescence and spikelet characters used by Webster (1987), two relate to CL. Dimorphochloa rigida was subsumed in Cleistochloa s.s. because Dimorphochloa was considered to lack merit as a genus in the context of generic concepts used in Paniceae despite the five characters recognised by Blake (1941), Webster (1987), Clayton and Renvoize (1986) and Clayton (1987). Furthermore, only one of the characters used by Zuloaga et al. (2000) and Morrone et al. (2012) relates to CL.
The taxonomy of Ancistrachne maidenii has also vacillated. Vickery (1961a) considered the generic affinities of Eriochloa maidenii A.A.Ham. to be ambiguous but subsumed it in Ancistrachne on the basis of several characters (Table 1). Her opinion was that the generic circumscription of Ancistrachne should be broadened to encompass the specific variation found in A. maidenii. Vickery (1961a) considered that A. maidenii had some resemblance to Entolasia, Cleistochloa and Dimorphochloa whereas Simon and Alfonso (http://ausgrass2.myspecies.info/, accessed 21 September 2019) regarded A. maidenii to be of ‘questionable taxonomic status’ indicating that in Simon’s consideration of the morphology was inconsistent with the diagnosis of Ancistrachne by Blake (1941).
The study by Thompson and Fabillo (2021) provided a set of morphological characters that enabled recognition of putative taxa at several ranks and they examined the consistency of their results by analysing multiple datasets. Since their work, research on the morphology of ‘the cleistogamy group’ has been continued by the first author, resulting in expansion of the character set. In the present work, an updated dataset was statistically analysed with the aim of exploring the morphological similarities and dissimilarities of the taxa within ‘the cleistogamy group’ and among its allies (Ancistrachne s.s., Entolasia, Neurachne, Panicum and Thyridolepis). The consistency of results from analyses using different cluster algorithms and datasets, varying in composition of morphological characters and samples, were tested against a baseline. On the basis of the congruency of findings from this study and Thompson and Fabillo (2021), ‘the cleistogamy group’ and Ancistrachne maidenii were considered to have definitive taxonomic morphology and, as a consequence, a new subtribe and new genus respectively, are diagnosed herein.
Materials and methods
Taxon sampling
The baseline sample set was composed of 24 putative species modified from Thompson and Fabillo (2021) by (1) the addition of Dimorphochloa sp. (Mt Cooper R.J. Cumming 18623), and (2) the use of Panicum s.s. as the sole outgroup to which was added Panicum mitchellii Benth. and Panicum queenslandicum Domin (to provide morphological variation present in Australian species), and Walwhalleya subxerophylla (Domin) K.E.Wills & J.J.Bruhl. was removed (Appendix 1).
An image of the type specimen of Ancistrachne uncinulata was obtained from the Natural History Museum, London, and examined. For detailed examination of spikelet morphology, eight specimens from the Queensland Herbarium (BRI) were selected as topotypes for A. uncinulata (Appendix 2). Selection of topotypes was based on the location of the type collection of A. uncinulata from Keppel Bay, Queensland.
Ancistrachne ancylotricha was not sampled owing to restrictions placed on destructive sampling of the borrowed specimens. A. ancylotricha is known only from three collections and is presumed extinct.
Nomenclature and terminology
Botanical nomenclature follows Brown and Bostock (http://data.qld.gov.au/dataset/census-of-the-queensland-flora-2020/, accessed 15 May 2021).
General botanical terminology follows Harris and Harris (1994) and Beentje (2010). Terminology relating to inflorescences and spikelet morphology follows Tothill and Hacker (1983), Jacobs et al. (2008), Gibson (2009) and Thompson (2021). The spikelet is viewed here as a reduced inflorescence where florets are enveloped by bracts (Kellogg 2006; Endress 2010). Terminology relating to grass anatomy and micromorphology follows Ellis (1976, 1979) and Dengler et al. (1994).
Acquisition of data and classification of morphological characters
Characters scored for this study include macromorphological, anatomical and micromorphological characters of inflorescences, spikelets, leaves and culms (Table 3). Information was gathered from fresh material from cultivated and field plants, herbarium specimens and images. Character groups classified include the following:
inflorescence structure
spikelet bracts (glumes, lemmas, paleas), stigmas, anthers, lodicules and caryopses
leaf shape, transverse section, abaxial surface and ligules
culm transverse section and surface.

|
Plants were cultivated in pots under nursery conditions in semi-shade at a latitude of 27.5°S, in Brisbane, Australia, to provide fresh material of leaves, culms and spikelets. For A. maidenii, plants were propagated from portions of the root stock of ex situ plants, and for A. numaeensis, plants were established from caryopses obtained from New Caledonia. Plants of A. uncinulata were established from ex situ plants as well as propagated from caryopses taken from several herbarium specimens to examine potential variation over its broad latitudinal range (12.6–32.5°S).
Fresh material was used for free-hand transverse sections of leaves and inflorescence culms. Sections were prepared following the free-hand sectioning method used by Thompson (2017), modified from the method described by Frohlich (1984).
Leaf-surface micromorphology was examined from replicas of the abaxial surface of fresh leaves obtained using the ‘impression method’ described by Hilu and Randall (1984).
Leaf and culm anatomy and micromorphology including stomata, silica bodies and micro-hairs were classified and recorded following classifications used by other authors, including De Wet (1960), Metcalfe (1960), Twiss et al. (1969), Carolyn and Jacobs (1973), Ellis (1979), Renvoize (1987), Watson and Dallwitz (1992), Siqueiros-Delgado and Herrera-Arrieta (1996), Piperno and Pearsall (1998), Krishnan et al. (2000), Siqueiros-Delgado (2007), Lu et al. (2009) and Jattisha and Sabu (2015) (Table 3, 4, Appendix 1).
|
Silica bodies, stomata, epidermal long cell walls, microhairs and macrohairs on lower lemmas and upper lemmas and paleas were classified following other authors (Hsu 1965; Jirasek and Jozifova 1968; Ellis 1979; Valdes-Reyna and Hatch 1991; Snow 1996; Acedo and Llamas 2001; Liu et al. 2010; Mashau et al. 2015; Olonova et al. 2016; Neumann et al. 2017). Lodicules on fresh material were classified as plicate or non-plicate (Hsu 1965; Jirasek and Jozifova 1968; Guedes and Dupuy 1976).
Stigma characters included the position of emergence from the spikelet, overall shape and colour, and characteristics of the lobes such as shape of apex, relative length and tilt (Thiele et al. 1996; Table 3).
Caryopsis morphology was scored for characters relating to hilum, scutellum, spermaderm and stylopodium using characters and states by other authors (Kennedy 1899; Reeder 1957; Brown 1959, 1960; Watson and Dallwitz 1992; Klak 1994; Kosina 1995; Snow 1998; Liu et al. 2005, 2015; Table 3).
Classification of inflorescence types, ligules and leaf bases follows Jacobs et al. (2008), Tothill and Hacker (1983), and Webster (1987).
Imagery
Imagery was used as the major source of information, especially in the studies of anatomy and micromorphology and to illustrate differences across the taxa. Images were taken using light and scanning electron microscopy. Photographs for anatomy and micromorphology were taken using two light microscopes (Nikon SMZ25 binocular microscope with Nikon DS-Ri1 camera and images were viewed using imaging software NIS-Elements BR (ver. BR 5.11.000 64-bit, Laboratory Imaging, USA, see http://www.lim.cz, accessed 15 December 2019); Leica DMLB compound binocular microscope with an industrial digital camera and images were viewed using imaging software ToupView (ver. x64 4.7.14326.20190401, Touptek, China, see http://www.touptek.com, accessed 20 September 2019). Scanning electron micrographs (SEMs) were obtained using a Phenom G2 5kev SEM with backscatter detector, without sputter coating samples.
Data sets and phenetic analyses
Variations in inputs to analyses included algorithm (ordination, classification, association measure), sample composition in terms of number of samples and replications, and the format of characters following findings made by other authors (Clifford and Goodall 1967; ‘t Mannetje 1967; Clifford and Williams 1973; Austin and Belbin 1982; Hilu and Wright 1982; Johnson 1982; Stevens 1991; Thiele 1993; Wills et al. 2000; Scotland et al. 2003; Wortley et al. 2005; Pereira et al. 2007; Newmaster et al. 2008; Zuloaga et al. 2014; Peichoto et al. 2015; Aliscioni 2016; Table 5). Datasets were established from the baseline dataset composed of 24 samples and 161 morphological characters, modified from the data matrix and character list by Thompson and Fabillo (2021). The characters comprised 5 vegetative characters, 73 characters relating to CH spikelets and inflorescences, 38 characters relating to axillary CL spikelets, and 45 relating to leaf anatomy (Table 3, Appendix 1). The dataset consisted of 69 binary and 92 multistate characters. Characters and character states were evaluated in terms of homology, ambiguities, reliability, plasticity, practicality, repeatability and standardisation of assessment or measurement using considerations by other authors (Hillis 1987; Wagner 1989; Smith 1990; Lipscomb 1992; Hillis and Wiens 2000; Poe and Wiens 2000; Scotland et al. 2003; Wiens 2004; Smith and Turner 2005; Thompson and Fabillo 2021).
Phenetic analyses were conducted using PATN (ver. 4.00, Australia; Blatant Fabrications, see http://patn.org, accessed 20 February 2021). Cluster analyses used the unweighted pair method using arithmetic mean (UPGMA), and dendrograms were generated using agglomerative hierarchical fusion with unweighted pair group method with Beta value of −0.1. Classifications involved two association measures, Gower metric and Czekanowski (Somerfield 2008; Bray and Curtis), where the differences between states of polymorphic characters are considered equal and unequal respectively (L. Belbin, pers. comm.). Three-dimensional ordination plots were generated using semi-strong hybrid multidimensional scaling (SSH). Ordination stress value (OSV) was used as the measure of closeness of fit: stress values of <0.05 = excellent, <0.1 = good, <0.1–0.15 may be OK, <0.2 = not good (see https://patn.org/). Discriminating characters were generated for each analysis from Kruskal–Wallis (KW) values at two group levels, namely, 10-group level and three-group level respectively. The 10-group level corresponds to the 10 putative genera (Ancistrachne s.s., Ancistrachne maidenii, Calyptochloa, Cleistochloa s.s., Dimorphochloa rigida, Dimorphochloa spp., Entolasia, Neurachne, Panicum s.s., and Thyridolepis) and the three-group level was represented by the three main clusters identified by Thompson and Fabillo (2021), i.e. ‘the cleistogamy group’, Neurachninae s.s. and the ‘paniculate inflorescence group’.
Twenty-two variations in datasets for five types of tests were analysed to generate topologies for comparison with those from analysis of the baseline dataset (Table 5). The variations in datasets were as follows:
Test 1. Variation in sample size using 10 and 30 samples with the 161-character set. The sample of 10 was represented by a single species for each of the 10 putative genera and the 30 samples included replicates, so all genera have three samples.
Test 2. The 92 multistate characters expressed as binary, resulting in 365 characters
Test 3. Character set consisted of 102 characters and 21 samples from Thompson and Fabillo (2021).
Test 4. Fifteen datasets were established using a list of the most discriminating characters in a descending order, on the basis of KW values generated from analysis of the baseline dataset with Gower association measure and number of groups set at 10. Datasets were created by successively removing batches of 10 characters with the lowest KW values, starting from 150, followed by 140, 130, and so on (Table 6).
Test 5. An example of a priori character selection. Two tests were conducted, using the characters that overlap with Morrone et al. (2012). Of the 57 morphological characters used by Morrone et al. (2012), 15 apply to the seven genera that overlap with this study (Table 3). Two datasets were established using the 10 and 24 sample sets. The samples used in these analyses differ from Morrone et al. (2012) by the substitutions, Neurachne munroi for Neurachne alopecuroidea R.Br. and non-native species of Panicum s.s. with an Australian species. Analyses were also run using characters expressed as binary.
Results
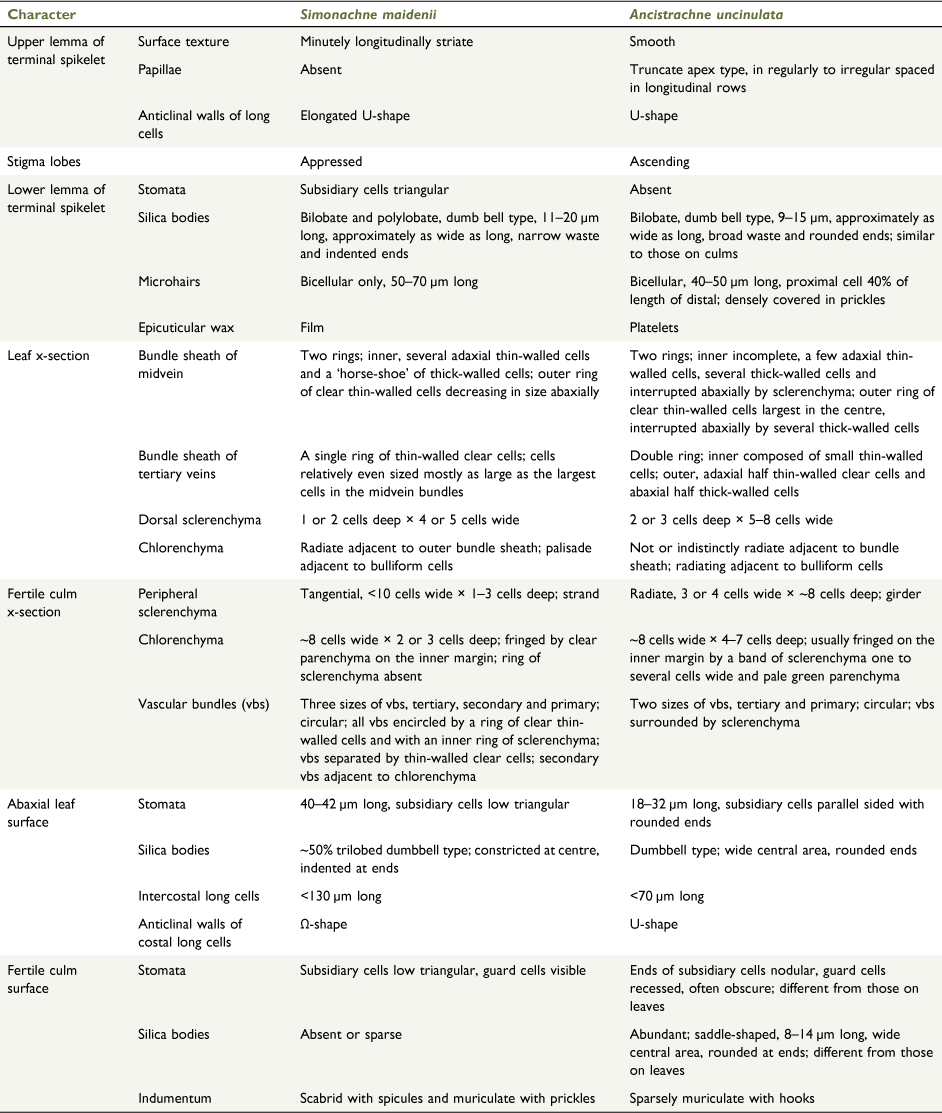
The three major clusters, viz. ‘the cleistogamy group’, the ‘paniculate inflorescence group’ and subtribe Neurachninae s.s. each with distinctive morphology, were consistently recovered from the cluster analyses. The topologies generated from the analyses showed that Ancistrachne s.l., Calyptochloa, Cleistochloa s.l. and Dimorphochloa s.l. are not monophyletic and Ancistrachne s.s. and A. maidenii are placed in separate clusters, viz. the ‘paniculate inflorescence group’ and ‘the cleistogamy group’ respectively (Table 5, Fig. 5, 6, Appendices 3–5). Ancistrachne maidenii and A. uncinulata differ in macro- and micromorphological and anatomical characters relating to upper and lower lemmas, surface of leaves and culms, stigmas, caryopses and leaf and culm cross-sections (Tables 1, 4, Fig. 4, Appendices 6–19). An unusual feature of A. uncinulata is the distinctly different stomata on the leaves and culm surfaces (Table 4, Appendices 14, 18).

|

|
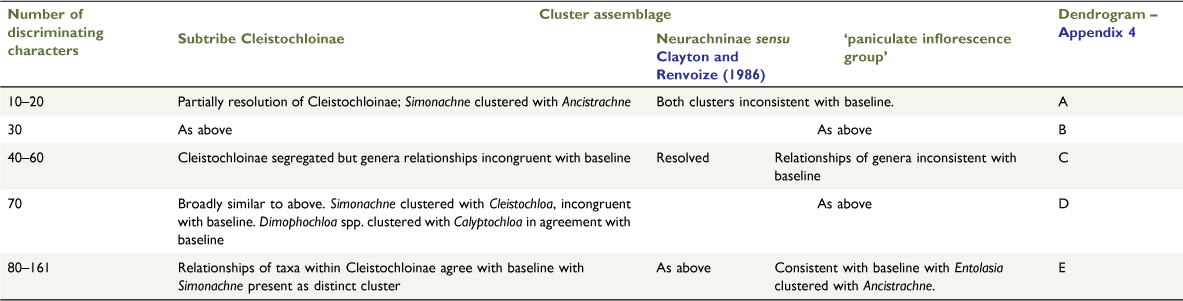
The topologies generated from cluster analyses in this study showed differences from that for the baseline, being affected by the algorithm (ordination, classification and association measure) used and the dataset with respect to variations in composition of samples and characters (Table 5). The dataset required the presence of only a single species for each of the 10 genera to achieve consistent topologies with the baseline (Fig. 5, Appendix 3). Consistency in resolution of subclusters within the ‘paniculate inflorescence group’ was achieved when the dataset included 130 or more most discriminating characters based on the highest KW values (Table 7, Appendix 4). The composition of the clusters generated from analysis at the three-group level was consistent with the baseline topology, except in the case of the small character sets used in Test 4 (Table 5).
Analysis of datasets for a priori characters and two sample sizes generated different topologies (Test 5; Table 7, Appendix 5). The topology for the eight-sample dataset was congruent with the baseline. However, in the topology for the 15-sample dataset, the position of some of the subclusters within the ‘paniculate inflorescence group’ was affected (Appendix 5).
The most discriminating characters generated from the analyses were dependent of the composition of the sample and the group level. From analysis of the baseline dataset, the following were recorded for the top 40 most discriminating characters (Table 8):
The list comprised six categories of characters, viz. vegetative, terminal inflorescences, macromorphology of spikelets in the terminal inflorescences, micromorphology of obligately cleistogamous spikelets in axillary racemes, culm anatomy and surface micromorphology, and spikelet micromorphology.
Of the 10 most discriminating characters generated at the 3- and 10-group levels (corresponding to the three major clusters and the 10 putative genera respectively), there were three characters common to both, viz. terminal inflorescence type, characters of the upper glume and the germination lid on the upper lemma.
Nine of the characters were additions to the 104-character set from Thompson and Fabillo (2021).
Three of the fifteen characters from Morrone et al. (2012) were represented.

|
Discussion
The combination of processes in this study with Thompson and Fabillo (2021) provided a balance of information for alpha, beta and gamma taxonomy (Guerra-Garcia et al. 2008; Wood 2010). The comprehensive dataset of characters and samples permitted detailed description of the taxa at various ranks, and the erection of classifications that acknowledge natural groups (Clayton and Renvoize 1986; Wiens 2004; Kellogg 2006; (Guerra-Garcia et al. 2008; Wood 2010). The processes also provided information for accountable and transparent taxonomic decision making that included conducting impact assessments to determine the consistency of results from analyses of multiple datasets using different algorithms. Furthermore, the process provided information about the content of datasets required to achieve repeatable results. The phenetic analyses generated topologies that show the morphological similarities among taxa of various ranks and provided lists of the most discriminating characters to elucidate differences in the taxa.
The results from this study and Thompson and Fabillo (2021) support the intuitive evaluation of the taxonomic status of A. maidenii by Simon and Alfonso (http://ausgrass2.myspecies.info/, accessed 21 September 2019) by revealing that Ancistrachne s.l. is not monophyletic. A. maidenii and Ancistrachne s.s. each bear combinations of characters that are distinctive in Poaceae and that distinguish them from each other. The distinguishing characters possessed by A. maidenii include a dimorphic reproductive system with similar CH and CL spikelet morphology, woolly indumentum on the upper glume and lower lemma, and presence of a contraligule (Campbell et al. 1983; Webster 1987; Watson and Dallwitz 1992; Thompson and Fabillo 2021; Appendix 7). Distinctive characters for Ancistrachne s.s. that are not shared by A. maidenii but are found in other taxa include the following:
Uncinate tuberculate-based hairs on the spikelets are rare in Panicoideae but are very similar to those found in the Australian genera Leptaspis R.Br. and Scrotochloa Judz. (subfamily Pharoideae). However, the African genus Pseudechinolaena Stapf (subtribe Boivinellinae) has hooked hairs (Clayton 1974; Clayton and Renvoize 1982), also referred to as hooked spines (Watson and Dallwitz 1992), on the upper glumes of mature spikelets. These trichomes differ from those of Ancistrachne s.s. by their development (immature vs mature), shape (flattened when dry) and size (longer and thicker). Observations of specimens for this study showed that hairs on immature spikelets of Pseudechinolaena have an appearance different from mature the ones with the former appressed and without the hook.
Spikelets tilted on the pedicel also occurs in Lasiacis A.Hitchc. (Boivinellinae), Panicum hirtum Lam. (subtribe Panicinae), and Tatianyx Zuloaga & Soderstr. (tribe Paspaleae);
The rachillar stipe below the upper floret (Appendix 8), referred to as a proximal beak on the upper lemma by Thompson and Fabillo (2021), is very similar to that found in Arundinella Raddi (tribe Arundinelleae) and similar to Entolasia (Boivinellinae) but different from that found in Yakirra Lazarides & R.Webster and some species of Panicum (Lazarides and Webster 1984; Zuloaga 1986; Zuloaga et al. 2018).
The broad range of morphological characters used in this study showed similarities and differences in the taxa that support the definition of groups at several taxonomic ranks recognised by Thompson and Fabillo (2021). Nevertheless, although the results from the phenetic analyses indicate that small character sets such as the a priori set of 15 characters can be sufficient for segregation of clusters in topologies, they were inadequate for satisfying identity of taxa at all ranks.
‘The cleistogamy group’ is diagnosed here as the new subtribe, Cleistochloinae E.J.Thomps., and Ancistrachne maidenii is transferred to a new genus, Simonachne E.J.Thomps. Furthermore, Cleistochloa sp. (Duaringa K. B. Addison 42) is recognised as a new species of Calyptochloa and the three species of Dimorphochloa with phrase names are considered new species of a new genus that will be circumscribed elsewhere. It is suggested from interpretation of the results that Ancistrachne s.s. should be placed in Boivinellinae with Entolasia.
Taxonomic treatment
Cleistochloinae E.J.Thomps. subtrib. nov.
‘The cleistogamy group’ of Thompson and Fabillo (2021).
Plants stoloniferous, rhizomatous or decumbent. Collar of leaves with or without a contraligule. Inflorescences of two types; Type 1 terminal, a reduced spike-like panicle or raceme, and Type 2, reduced racemes, clandestine axillary, or exposed apical on short sub-branches. Spikelets of corresponding inflorescences relatively similar or markedly dissimilar; terminal spikelets chasmogamous or uncommonly mixed with cleistogamous spikelets; Type 2 spikelets cleistogamous. Spikelets slightly dorsi-ventrally compressed; lower glume absent or vestigial, upper glume and lower lemma, chartaceous, usually as long as the spikelet; lower floret barren without a palea; upper lemma chartaceous to cartilaginous, slightly hardened, papery texture, loosely overlapping the caryopsis. Anatomical type: C3.
Key to the genera of CleistochloinaeThree species with phrase names: D. sp. (Charters Towers E. J. Thompson + CHA554), D. (Miles E. J. Thompson EJT906), D. (Mt Cooper R. J. Cumming 18623).
|
1. Spikelets from the two types of inflorescences strongly dimorphic |
|
2. Plants rhizomatous with wiry erect tufted culms; CL spikelets usually solitary, apical on leafy subordinate branches |
|
3. Upper lemmas mucronate; CL spikelets abaxial |
|
4. Plants not stoloniferous, bushy with some decumbent branching; upper lemma subequal to lower lemma. |
Simonachne E.J.Thomps., gen. nov.
Stoloniferous perennials with ascending fertile culms. Culm with pith. Leaf sheath with one margin pilose. Ligule and contraligule a fringe of hairs. Leaf blades lanceolate, base truncate, pseudopetiolate, proximal margins white, ciliate and one margin undulate. Inflorescences of two types, the terminal ones spike-like panicles with short proximal branches or raceme, and axillary racemes partly concealed within leaf sheath. Axes of inflorescence branches lacking pulvinii. Pedicel apices a shallow cup with thin walls. Spikelets of two similar types, falling entire except for proximal ones of axillary racemes trapped in leaf sheath terminal spikelets chasmogamous and axillary spikelets cleistogamous; adaxial, slightly dorsally compressed, elliptical in outline. Lower glume much reduced; upper glume 7-veined, woolly with mostly appressed tubercular-based hairs. Lower lemma 5-veined, similar in shape, size and indumentum to upper glume. Lower palea absent. Upper lemma subequal to lower lemma; 5-veined, chartaceous, body glabrous; margins hyaline, apex with flattened cilia; minutely longitudinally ridged, without papillae; apex mucronate; germination lid a crescent-shaped depression. Upper palea 2-veined. Anthers 3. Caryopsis dorsi-ventrally compressed; hilum punctiform.
Etymology
The genus is named in memory and honour of Bryan Kenneth Simon (1943–2015), curator of Poaceae at BRI for nearly 40 years and author of numerous publications on grasses, and achne from the Greek for scale in reference to the spikelets.
Simonachne maidenii (A.A.Ham.) E.J.Thomps., comb. nov.
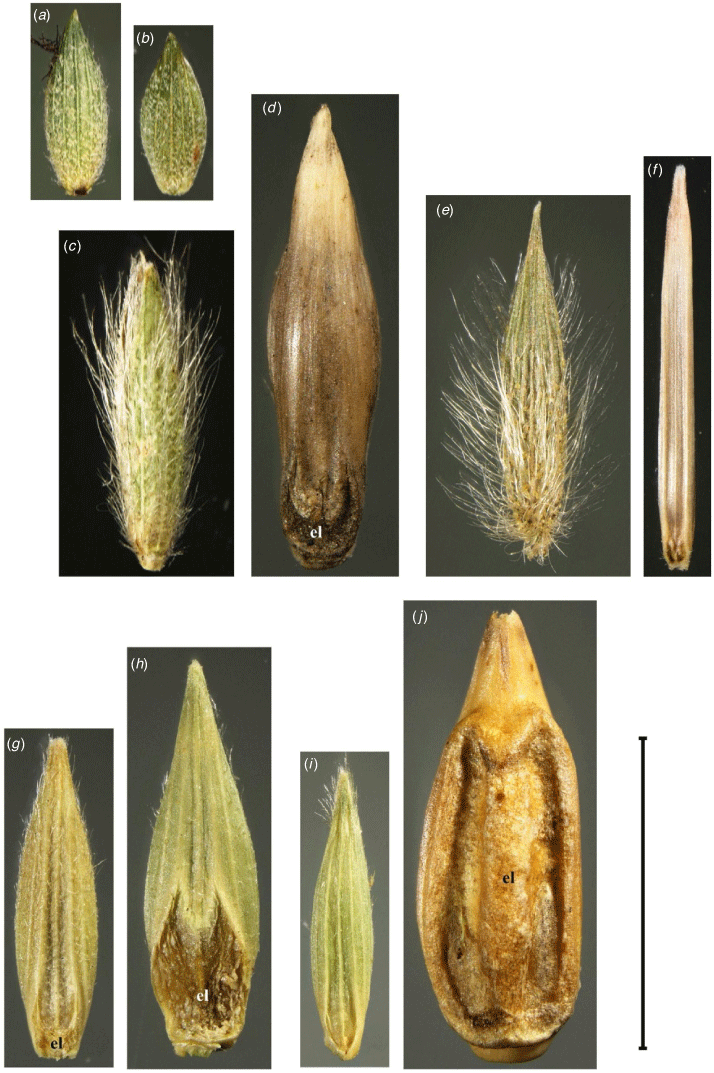
Fertile branches ascending to 40 cm high, copiously branched with up to ~12 nodes. Fertile culm internodes up to 4.5 cm long. Mature fertile leaf sheaths retained, convolute, pilose; outer margin ciliate with ascending tuberculate-based simple trichomes upto 0.5 mm long. Leaf blades upto 4.5 cm long and 7 mm wide; lower margin with tuberculate-based simple trichomes upto 3 mm long. Ligule 0.3 mm long. Contraligule 0.1 mm long. Mid-culm leaf blades 2.5–4.5 cm long, 2.5–4.0 mm wide, apex tapering, base truncate, margins white; both surfaces sparsely pubescent with simple trichomes upto 0.8 mm long. Terminal inflorescences on axes 1.5–5.5 cm long, 8–32-flowered; 0–several branches, appressed, rachis upto 1 cm long, 1–6-flowered. Spikelets 2.6–2.9 mm long, 1.0–1.1 mm wide; lateral pedicels 0.4–1.0 mm long, ultimate pedicel 0.5–2.0 mm long. Lower glume lunar, ~0.2 mm long, apex obtuse. Upper glume ovate, 2.6–2.9 mm long, apex acute, margins inrolled. Lower lemma ovate, 2.6–3.0 mm long; apex acute, margins inrolled. Upper lemma 2.4–2.6 mm long; apical cilia 60–90 µm long, mucronate to shortly awned. Lodicules ∼0.2 mm long. Upper palea 2.4–2.6 mm long; apex acute. Anthers 1.5 mm long. Caryopsis ~1.7 mm long, ~0.8 mm wide, rarely present. Axillary inflorescences usually present below apical 2 or 3 internodes; 3- or 4-flowered, lowest 1 or 2 enclosed in leaf sheath. Spikelets 2.8–3.2 mm long, 1.2–1.3 mm wide. Lower glume vestigial upto 0.1 mm long. Upper glume ovate, 2.8–3.2 mm long, chartaceous, villous with woolly hairs upto 0.2 mm long; apex obtuse. Lower lemma ovate, 2.7–3.2 mm long; apex revolute. Upper lemma 2.2–2.4 mm long; apex revolute, cilia 60–90 µm long, mucronate to shortly awned. Upper palea 2.2–2.4 mm long; apex revolute. Anthers 0.2 mm long. Caryopsis 1.4–1.9 mm long, 0.7–0.9 mm wide; surface minutely longitudinally striate; light brown. Fig. 1, 2, Appendix 19.
Illustrations
D. J. B. Wheeler, S. W. L. Jacobs and B. E. Norton, Grasses of New South Wales 93 (1982); S. W. L. Jacobs and C. A. Wall in G. J. Harden (ed.), Flora of New South Wales 4: 452-3 (1993).
Additional specimens examined
Distribution
Occurs in two disjunct populations on the New South Wales coastline (Fig. 3).
Habitat and ecology
Under a canopy of trees or shrubs on sandy soils derived from sandstone.
Phenology
Dates of herbarium collections indicate that S. maidenii flowers throughout the year. Cultivated plants flower mostly in summer.
Conservation status
Listed as Vulnerable under the Biodiversity Conservation Act 2016, New South Wales.
Notes
Simonachne maidenii shares morphological characters with all of the other taxa in subtribe Cleistochloinae (Table 1). Notable similarities include S. maidenii and Calyptochloa spp. having a contraligule and the stoloniferous growth habit, giving plants a very similar appearance in the field.
Breeding system
The type of CL manifested by Simonachne maidenii fits the category of dimorphic anthers with amphigamy in the classification of CL by Thompson (2017). Axillary CL in S. maidenii is obligate, whereas in Ancistrachne, CL is facultative with the CL and CH anthers, the same size fitting the type ‘monomorphic CH and CL anthers’ on the same plant as defined by Thompson (2017).
Micromorphology and macromorphology of the lemmas and palea
Simonachne maidenii and Ancistrachne s.s. differ in the lower lemmas by S. maidenii having absence of prickles and film of epicuticular wax. Upper lemmas of S. maidenii have minute longitudinal ridges and lack papillae. Upper lemmas of the terminal spikelets of S. maidenii are chartaceous to slightly hardened and loosely clasping the caryopsis, whereas for Ancistrachne s.s. they are hardened, glossy and tightly clasping the caryopsis. Simonachne maidenii, as for other species in Cleistochloinae, lacks a lower palea, whereas species of Ancistrachne, members of Neurachninae s.s. and the species of Panicum have a lower palea. Simonachne maidenii differs from Ancistrachne by the indumentum on the upper glume and lower lemma and differs from other members of Cleistochloinae by having an indistinct germination flap on the upper lemma (Tables 1, 3, Fig. 4; Appendices 8, 10–13).
Abaxial leaf-blade epidermis
Costal−intercostal zonation conspicuous. Papillae absent. Costal long cells rectangular, much narrower than intercostal; anticlinal walls of intercostal long cells Ω-shaped. Anticlinal walls of intercostal long cells moderately undulating, often irregular with short-wave length. Stomata 38–43 µm long with low triangular subsidiaries, in 2 rows separated by 5–6 files of long cells. Bicellular microhairs 52–56 µm long, proximal cell longer than distal, occasional. Silica bodies in single rows, bilobate and polylobate, 16–25 µm long, common. Hooks present (Appendices 14, 15).
The shape of the anticlinal walls of long cells in the upper lemmas and the abaxial leaf surface in A. maidenii differ from those in A. uncinulata, and, in A. maidenii, the shapes of these walls differ from each other (Table 3). Such differences in the shape of anticlinal walls of long cells have been reported for some other panicoid grasses (Lu et al. 2009; Harun et al. 2020).
Transverse section of leaf blade
C3; XyMS+. Mesophyll with radiate chlorenchyma; adaxial pallisade chlorenchyma present. Midrib not prominent; with a double bundle sheath; outer complete ring of parenchyma cells and partial inner ring of thick-walled cells with adaxial arc of clear parenchyma cells. Bulliform cells in discrete regular groups; in simple fans. Sclerenchyma accompanying all vascular bundles as adaxial strands and abaxial girders (Appendix 16).
Transverse section of culm
Culm examined 0.6 mm in diameter. Outer smallest vascular bundles adjacent to tangential girder sclerenchyma and imbedded in large-celled sclerenchyma. Vascular bundles with a ring of clear parenchyma; three sizes in separate circles, smallest to the periphery. Chlorenchyma in rectangular blocks, 2 or 3 cells deep by up to 10 cells wide; cells with regular size and shape, more or less circular. Inner ground tissue consisting of large thin-walled cells. (Appendix 17).
The distribution of the bundle sheath parenchyma of S. maidenii has similarities to the fresh culm sections of Entolasia spp. prepared for this study.
Surface of inflorescence culm
Pilose with tuberculate-based macrohairs up to 2 mm long, muriculate with hooks and scabridulous with prickles. Stomata frequent, similar to those on the abaxial leaf surface. Bicellar microhairs, ~44 μm long, occasional. Silica bodies absent. (Appendix 18).
Data availability
The data that support this study are available in Appendix 1.
Conflicts of interest
The author declares that he has no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
I am very grateful to Jacek Wager from the Natural History Museum, London, who supplied images of the type specimen of Panicum uncinatum R.Br. I am most thankful to Guillame Lannuzel and Bruno Fogliani, Axe II ‘Diversités biologique et fonctionnelle des écosystèmes terrestres’, Paita, New Caledonia, for providing leaf material and caryopses of Ancistrachne numaeensis. Many thanks go to Dr Gordon Guymer for his continuous support at BRI.
References
Acedo C, Llamas F (2001) Variation of micromorphology characters of lemma and palea in the genus Bromus (Poaceae). Annales Botanici Fennici 38, 1–14.Aliscioni S, Ospina JC, Gomiz NE (2016) Morphology and leaf anatomy of Setaria s.l. (Poaceae: Panicoideae) and its taxonomic significance. Plant Systematics and Evolution 302, 173–185.
| Morphology and leaf anatomy of Setaria s.l. (Poaceae: Panicoideae) and its taxonomic significance.Crossref | GoogleScholarGoogle Scholar |
Austin MP, Belbin L (1982) A new approach to the species classification problem in floristic analysis. Australian Journal of Ecology 7, 75–89.
| A new approach to the species classification problem in floristic analysis.Crossref | GoogleScholarGoogle Scholar |
Beentje H (2010) ‘The Kew Plant Glossary an illustrated dictionary of plant terms.’ (Kew Publishing, Kew: London, UK)
Blake ST (1941) New genera of Australian grasses. University of Queensland Papers, Department of Biology 1, 1–12.
Brown WV (1959) The epiblast and coleoptile in the grass embryo. Bulletin of the Torrey Botanical Club 86, 13–16.
| The epiblast and coleoptile in the grass embryo.Crossref | GoogleScholarGoogle Scholar |
Brown WV (1960) The morphology of the grass embryo. Phytomorphology 10, 215–223.
Campbell CS, Quinn JA, Cheplick GP, Bell TJ (1983) Cleistogamy in grasses. Annual Review of Ecology and Systematics 14, 411–441.
| Cleistogamy in grasses.Crossref | GoogleScholarGoogle Scholar |
Carolyn RC, Jacobs SLW (1973) The structure of the cells of the mesophyll and parenchymous bundle sheath of the Gramineae. Botanical Journal of Linnean Society 66, 295–275.
Chase A (1918) Axillary cleistogenes in some American grasses. American Journal of Botany 5, 254–258.
| Axillary cleistogenes in some American grasses.Crossref | GoogleScholarGoogle Scholar |
Christin PA, Wallace MJ, Clayton H, Furbank RT, Hattersley PW, Sage RF, Macfarlane TD, Ludwig M (2012) Multiple photosynthetic transitions, polypoidy, and lateral gene transfer in the grass subtribe Neurachninae. Journal of Experimental Botany 63, 6297–6308.
| Multiple photosynthetic transitions, polypoidy, and lateral gene transfer in the grass subtribe Neurachninae.Crossref | GoogleScholarGoogle Scholar | 23077201PubMed |
Clayton WD (1974) Gramineae. In ‘Flora of West Tropical Africa: Vol. 3, Part 2 Juncaceae-Gramineae’. (Eds J Hutchison, JM Dalziel) (Royal Botanic Gardens, Kew: London, UK)
Clayton WD (1987) Miscellaneous notes on panicoid grasses. Kew Bulletin 42, 401–403.
| Miscellaneous notes on panicoid grasses.Crossref | GoogleScholarGoogle Scholar |
Clayton WD, Renvoize SA (1982). Gramineae (Part 3). XXIX Paniceae. In ‘Flora of Tropical East Africa’. (Ed. RM Polhill) (Royal Botanic Gardens, Kew: London, UK)
Clayton WD, Renvoize DW (1986) ‘Genera Graminum Grasses of the World.’ (Her Majesty’s Stationery Office: London, UK)
Clifford HT, Goodall DW (1967) A numerical contribution to the classification of the Poaceae. Australian Journal of Botany 15, 499–519.
Clifford HT, Williams WT (1973) Classificatory dendrograms and their interpretation. Australian Journal of Botany 21, 151–162.
| Classificatory dendrograms and their interpretation.Crossref | GoogleScholarGoogle Scholar |
De Wet JM (1960) Culm anatomy in relation to taxonomy. Bothalia 7, 311–316.
| Culm anatomy in relation to taxonomy.Crossref | GoogleScholarGoogle Scholar |
Dengler NG, Dengler RE, Donnelly PM, Hattersley PW (1994) Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): bundle sheath and mesophyll surface area relationships. Annals of Botany 73, 241–255.
| Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): bundle sheath and mesophyll surface area relationships.Crossref | GoogleScholarGoogle Scholar |
Ellis RP (1976) A procedure for standardizing comparative leaf anatomy in the Poaceae: 1. The leaf-blade as viewed in transverse section. Bothalia 12, 65–109.
Ellis RP (1979) A procedure for standardizing comparative leaf anatomy in the Poaceae: 2. The epidermis as seen in surface view. Bothalia 12, 641–671.
Endress PK (2010) Disentangling confusions in inflorescence morphology. Journal of Systematics and Evolution 48, 225–239.
| Disentangling confusions in inflorescence morphology.Crossref | GoogleScholarGoogle Scholar |
Frohlich MW (1984) Freehand sectioning with parafilm. Stain Technology 59, 61–62.
| Freehand sectioning with parafilm.Crossref | GoogleScholarGoogle Scholar | 6548052PubMed |
Gibson DJ (2009) ‘Grasses & Grassland Ecology.’ (Oxford University Press: Oxford, UK)
(2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytologist 193, 304–312.
| New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins.Crossref | GoogleScholarGoogle Scholar |
Guedes M, Dupuy P (1976) Comparative morphology of lodicules in grasses. Botanical Journal of the Linnean Society 73, 317–331.
| Comparative morphology of lodicules in grasses.Crossref | GoogleScholarGoogle Scholar |
Guerra-Garcia JM, Espinosa F, Garcia-Gȯmez JC (2008) Trends in taxonomy today: an overview about the main topics in taxonomy. Zoologica Baetica 19, 15–49.
Hamilton AA (1913) A new species of Eriochloa from the Hawksbury River. Proceedings of the Linnean Society of New South Wales 37, 709–711.
| A new species of Eriochloa from the Hawksbury River.Crossref | GoogleScholarGoogle Scholar |
Harris JG, Harris MW (1994) ‘Plant identification terminology: an illustrated glossary.’ (Spring Lake Publishing: Spring Lake, UT, USA)
Harun N, Shaheen S, Ahmad M, Shahid MNS (2020) Light and scanning microscopy-based foliar micro morphological tools for the identification of fodder grass taxa. Microscopy Research and Technique 83, 953–978.
| Light and scanning microscopy-based foliar micro morphological tools for the identification of fodder grass taxa.Crossref | GoogleScholarGoogle Scholar | 32378268PubMed |
Hattersley PW, Watson L, Johnson CR (1982) Remarkable leaf anatomical variations in Neurachne and its allies (Poaceae) in relation to C3 and C4 photosynthesis. Botanical Journal of the Linnean Society 84, 265–272.
| Remarkable leaf anatomical variations in Neurachne and its allies (Poaceae) in relation to C3 and C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Hillis DM (1987) Molecular versus morphological approaches to systematics. Annual Review of Ecology and Systematics 18, 23–42.
| Molecular versus morphological approaches to systematics.Crossref | GoogleScholarGoogle Scholar |
Hillis DM, Wiens, JJ (2000) Molecules versus morphology in systematics: conflicts, artifacts, and misconceptions. In ‘Phylogenetic Analysis of Morphological Data’. (Ed. JJ Wiens) pp. 1–19. (Smithsonian Institution Press: Washington, DC, USA)
Hilu KW, Randall JL (1984) Convenient method for studying grass leaf epidermis. Taxon 33, 413–415.
| Convenient method for studying grass leaf epidermis.Crossref | GoogleScholarGoogle Scholar |
Hilu KW, Wright K (1982) Systematics of Gramineae, a cluster analysis study. Taxon 31, 9–36.
| Systematics of Gramineae, a cluster analysis study.Crossref | GoogleScholarGoogle Scholar |
Hsu CC (1965) The classification of Panicum (Gramineae) and its allies with special reference to the characters of lodicule, style-base and lemma. Journal of the Faculty of Science University of Tokyo, Section III, Botany 9, 43–150.
Jacobs SWL, Whalley RDB, Wheeler DJB (2008) ‘Grasses of New South Wales.’ (University of New England: Armidale, NSW, Australia)
Jattisha PI, Sabu M (2015) Foliar phytoliths as an aid to the identification of Paniceae (Panicoideae: Poaceae) grasses in South India. Webbia 70, 115–131.
| Foliar phytoliths as an aid to the identification of Paniceae (Panicoideae: Poaceae) grasses in South India.Crossref | GoogleScholarGoogle Scholar |
Jirasek V, Jozifova M (1968) Morphology of lodicules, their variability and importance in the taxonomy of the Poaceae family. Boletin de la Siciedad Argentina de Botanica 12, 324–349.
Johnson RJ (1982) Effect of weighting and the size of the attribute set in numerical analysis. Australian Journal of Botany 30, 161–174.
| Effect of weighting and the size of the attribute set in numerical analysis.Crossref | GoogleScholarGoogle Scholar |
Kellogg EA (2006) Beyond taxonomy: prospects for understanding morphological diversity in the grasses (Poaceae). Darwiniana 44, 7–17.
Kellogg EA (2015) ‘The Families and Genera of Vascular Plants. Flowering Plants: Monocots: Poaceae.’ (Springer: Cham, Switzerland)
Kennedy PB (1899) The structure of the caryopsis of grasses with reference to their morphology and classification. U.S. Department of Agriculture Division of Agrostology Bulletin 19, 1–14.
Klak C (1994) Embryo and caryopsis morphology of danthonioid grasses (Arundinoideae: Poaceae): important characters for their systematics? Honours thesis, University of Cape Town, South Africa. Available at http://hdl.handle.net/11427/25967
Kosina R (1995) Remarks on taxonomy of some species of Elytrigia s.l. (Tritceae) in the light of embryo morphology. Acta Societatis Botanicorum Poloniae 64, 295–302.
| Remarks on taxonomy of some species of Elytrigia s.l. (Tritceae) in the light of embryo morphology.Crossref | GoogleScholarGoogle Scholar |
Krishnan S, Samson NP, Ravichandran P, Narasimhan D, Dayasnandan P (2000) Phytoliths of Indian grasses and their potential use in identification. Botanical Journal of the Linnean Society 132, 241–252.
| Phytoliths of Indian grasses and their potential use in identification.Crossref | GoogleScholarGoogle Scholar |
Lazarides M, Webster RD (1984) Yakirra (Paniceae, Poaceae), a new genus for Australia. Brunonia 7, 289–296.
| Yakirra (Paniceae, Poaceae), a new genus for Australia.Crossref | GoogleScholarGoogle Scholar |
Lipscomb DL (1992) Parsimony, homology and the analysis of multistate characters. Cladistics 8, 45–65.
| Parsimony, homology and the analysis of multistate characters.Crossref | GoogleScholarGoogle Scholar | 34929948PubMed |
Liu Q, Zhao N-X, Hao G, Hu X-Y, Liu Y-X (2005) Caryopsis morphology of the Chloridoideae (Gramineae) and its systematic implications. Botanical Journal of the Linnean Society 148, 57–72.
| Caryopsis morphology of the Chloridoideae (Gramineae) and its systematic implications.Crossref | GoogleScholarGoogle Scholar |
Liu Q, Zhang DX, Peterson PM (2010) Lemma micromorphological characters in the Chloridoideae (Poaceae) optimized on a molecular phylogeny. South African Journal of Botany 76, 196–209.
| Lemma micromorphological characters in the Chloridoideae (Poaceae) optimized on a molecular phylogeny.Crossref | GoogleScholarGoogle Scholar |
Liu H, Hu XY, Liu YX, Liu Q (2015) Caryopsis micromorphology survey of Sorghum (Poaceae): taxonomic implications. South African Journal of Botany 99, 1–11.
| Caryopsis micromorphology survey of Sorghum (Poaceae): taxonomic implications.Crossref | GoogleScholarGoogle Scholar |
Lu H, Zhang J, Wu N, Liu K-B, Xu D, Li Q (2009) Phytoliths analysis for the discrimination of foxtails millet (Setaria italica) and common millet (Panicum miliaceum). Plos One 4, 1–15.
| Phytoliths analysis for the discrimination of foxtails millet (Setaria italica) and common millet (Panicum miliaceum).Crossref | GoogleScholarGoogle Scholar |
Mashau AC, Fish L, van Wyk AE (2015) Taxonomic significance of the abaxial lemma surface in southern African members of Helictotrichon (Poaceae). Bothalia 45, 1–8.
| Taxonomic significance of the abaxial lemma surface in southern African members of Helictotrichon (Poaceae).Crossref | GoogleScholarGoogle Scholar |
Metcalfe CR (1960) ‘Anatomy of the Monocotyledons 1. Gramineae.’ (Oxford University Press: London, UK)
Morrone O, Aagesen L, Scataglini MA, Salariato DL, Denham SS, Chemisquy MA, Sede SM, Giussani LM, Kellogg EA, Zuloaga FO (2012) Phylogeny of the Paniceae (Poaceae: Panicoideae): integrating plastid DNA sequences and morphology into a new classification. Cladistics 28, 333–356.
| Phylogeny of the Paniceae (Poaceae: Panicoideae): integrating plastid DNA sequences and morphology into a new classification.Crossref | GoogleScholarGoogle Scholar | 34836451PubMed |
Neumann K, Fahmy AG, Muller-Scheeβel N, Schmidt M (2017) Taxonomic, ecological and paleoecological significance of leaf phytoliths in West African grasses. Quaternary International 434B, 15–32.
Newmaster SG, Balasubramaniam V, Murugesan M, Ragupathy S (2008) Tripogon cope (Poaceae: Chloridoideae), a new species supported by morphometric analysis and a synopsis of Tripogon in India. Systematic Botany 33, 695–701.
| Tripogon cope (Poaceae: Chloridoideae), a new species supported by morphometric analysis and a synopsis of Tripogon in India.Crossref | GoogleScholarGoogle Scholar |
Olonova MV, Barkworth ME, Gudkova PD (2016) Lemma micromorphology and the systematics of Siberian species of Stipa (Poaceae). Nordic Journal of Botany 34, 322–334.
| Lemma micromorphology and the systematics of Siberian species of Stipa (Poaceae).Crossref | GoogleScholarGoogle Scholar |
Peichoto MC, Welker CAD, Neffa VGS (2015) Morphometric analysis of Schizachyrium (Poaceae – Andropogoneae) reveals two new species from South America. Systematic Botany 40, 461–473.
| Morphometric analysis of Schizachyrium (Poaceae – Andropogoneae) reveals two new species from South America.Crossref | GoogleScholarGoogle Scholar |
Pereira MP, Perez GE, Balbuena ES (2007) European sweet vernal grasses (Anthoxanthum: Pooideae: Aveneae): a morphometric taxonomic approach. Systematic Biology 32, 43–59.
Piperno DR, Pearsall DM (1998) The silica bodies of the tropical American grasses: morphology, taxonomy, and implications for grass systematics and fossil phytolith identification. Smithsonian Contributions to Botany 85, 1–40.
| The silica bodies of the tropical American grasses: morphology, taxonomy, and implications for grass systematics and fossil phytolith identification.Crossref | GoogleScholarGoogle Scholar |
Poe S, Wiens JJ (2000) Character selection and the methodology of morphological phylogenetics. In ‘Phylogenetic Analysis of Morphological Data’. (Ed. JJ Wiens) pp. 20–36. (Smithsonian Institution Press: Washington, DC, USA)
Reeder JR (1957) The embryo in grass systematics. American Journal of Botany 44, 756–768.
| The embryo in grass systematics.Crossref | GoogleScholarGoogle Scholar |
Renvoize SA (1987) A survey of leaf-blade anatomy in grasses XI Paniceae. Kew Bulletin 42, 739–768.
| A survey of leaf-blade anatomy in grasses XI Paniceae.Crossref | GoogleScholarGoogle Scholar |
Scotland RW, Olmstead RG, Bennett JR (2003) Phylogenetic reconstruction: the role of morphology. Systematic Biology 52, 539–548.
| Phylogenetic reconstruction: the role of morphology.Crossref | GoogleScholarGoogle Scholar | 12857644PubMed |
Simon BK (2002) Key to genera of Australian grasses. In ‘Flora of Australia. Poaceae 1: Introduction and Atlas’, Vol. 43. (Eds K Mallett, AE Orchard) pp. 263–277. (ABRS: Canberra, ACT, Australia; and CSIRO: Melbourne, Vic., Australia)
Siqueiros-Delgado ME (2007) Culm anatomy of Bouteloua and relatives (Gramineae: Chloridoideae: Boutelouinae). Acta Botanica Mexicana 78, 39–59.
| Culm anatomy of Bouteloua and relatives (Gramineae: Chloridoideae: Boutelouinae).Crossref | GoogleScholarGoogle Scholar |
Siqueiros-Delgado ME, Herrera-Arrieta Y (1996) Taxonomic value of culm anatomical characters in the species of Bouteoua Lagasca (Poaceae: Eragrostoideae). Phytologia 81, 124–141.
Smith, GR (1990) Homology in morphometrics and phylogenetics. In ‘Proceedings of the Michigan Morphometrics Workshop’. (Eds FJ Rohif, FL Bookstein) pp. 325–338. (The University of Michigan Museum of Zoology: Ann Arbor, MI, USA)
Smith ND, Turner AH (2005) Morphology’s role in phylogenetic reconstruction: perspective from paleontology. Systematic Biology 54, 166–173.
| Morphology’s role in phylogenetic reconstruction: perspective from paleontology.Crossref | GoogleScholarGoogle Scholar |
Snow N (1996) The phylogenetic utility of lemmatal micromorphology in Leptochloa s.l. and related genera in subtribe Eleusininae (Poaceae, Chloridoideae, Eragrostideae). Annals of the Missouri Botanical Garden 83, 504–529.
| The phylogenetic utility of lemmatal micromorphology in Leptochloa s.l. and related genera in subtribe Eleusininae (Poaceae, Chloridoideae, Eragrostideae).Crossref | GoogleScholarGoogle Scholar |
Snow N (1998) Caryopsis morphology of Leptochloa sensu lato (Poaceae, Chloridoideae). Sida 18, 271–282.
Somerfield PJ (2008) Identification of the Bray-Curtis similarity index: comment on Yoshioka (2008). Marine Ecology Progress Series 372, 303–306.
| Identification of the Bray-Curtis similarity index: comment on Yoshioka (2008).Crossref | GoogleScholarGoogle Scholar |
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Davis JI, Morrone O (2015) A worldwide phylogenetic classification of the Poaceae (Gramineae). Journal of Systematics and Evolution 53, 117–137.
| A worldwide phylogenetic classification of the Poaceae (Gramineae).Crossref | GoogleScholarGoogle Scholar |
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Teisher JK, Clark LG, Barbera P, Gillespie LJ, Zuloaga FO (2017) A worldwide phylogenetic classification of the Poaceae (Gramineae) II: and update and a comparison of the two 2015 classifications. Journal of Systematics and Evolution 55, 259–290.
| A worldwide phylogenetic classification of the Poaceae (Gramineae) II: and update and a comparison of the two 2015 classifications.Crossref | GoogleScholarGoogle Scholar |
Stevens PF (1991) Character states, morphological variation, and phylogenetic analysis: a review. Systematic Botany 16, 553–583.
| Character states, morphological variation, and phylogenetic analysis: a review.Crossref | GoogleScholarGoogle Scholar |
‘t Mannetje L (1967) A comparison of eight numerical procedures applied to the classification of some African Trifolium taxa based on Rhizobium affinities. Australian Journal of Botany 15, 521–528.
| A comparison of eight numerical procedures applied to the classification of some African Trifolium taxa based on Rhizobium affinities.Crossref | GoogleScholarGoogle Scholar |
Thiele K (1993) The holy grail of the perfect character: the cladistic treatment of morphometric data. Cladistics 9, 275–304.
| The holy grail of the perfect character: the cladistic treatment of morphometric data.Crossref | GoogleScholarGoogle Scholar | 34929957PubMed |
Thiele HL, Clifford HT, Rogers RW (1996) Diversity in the grass pistil and its taxonomic significance. Australian Systematic Botany 9, 903–912.
| Diversity in the grass pistil and its taxonomic significance.Crossref | GoogleScholarGoogle Scholar |
Thompson EJ (2017) Elionurus purpureus (Panicoideae: Andropogoneae: Rottboelliinae), a new species for Queensland: circumscription and breeding system. Austrobaileya 10, 139–162.
Thompson EJ (2021) A review of the classification and taxonomic and geographic distribution of cleistogamy in Australian grasses. Australian Journal of Botany
| A review of the classification and taxonomic and geographic distribution of cleistogamy in Australian grasses.Crossref | GoogleScholarGoogle Scholar | In press
Thompson EJ, Fabillo M (2021) The impact of multiple molecular and morphological data sets on the phylogenetic reconstruction of subtribe Neurachninae (Poaceae: Panicoideae: Paniceae). Australian Systematic Botany 34, 227–251.
| The impact of multiple molecular and morphological data sets on the phylogenetic reconstruction of subtribe Neurachninae (Poaceae: Panicoideae: Paniceae).Crossref | GoogleScholarGoogle Scholar |
Tothill JC, Hacker JB (1983) ‘The Grasses of Southern Queensland.’ (University of Queensland Press: Brisbane, Qld, Australia)
Twiss PC, Suess E, Smith RM (1969) Morphological classification of grass phytoliths. Soil Science Society of America Journal 31, 109–115.
| Morphological classification of grass phytoliths.Crossref | GoogleScholarGoogle Scholar |
Valdes-Reyna J, Hatch SL (1991) Lemma micromorphology in the Eragostoideae (Poaceae). Sida 14, 531–549.
Vickery JW (1961a) Contributions to the taxonomy of Australian grasses. Contributions from the New South Wales National Herbarium 3, 83–84.
Vickery JW (1961b) 19. Gramineae. Contributions from the New South Wales National Herbarium, Flora Series 19, 1–124.
Wagner GP (1989) The origin of morphological characters and the biological basis of homology. Evolution 43, 1157–1171.
| The origin of morphological characters and the biological basis of homology.Crossref | GoogleScholarGoogle Scholar | 28564509PubMed |
Watson L, Dallwitz MJ (1992) ‘The Grass Genera of the World.’ (University Press: Cambridge, UK)
Webster RD (1987) ‘The Australian Paniceae (Poaceae).’ (J Cramer: Berlin, Germany)
Wiens JJ (2004) The role of morphological data in phylogenetic reconstruction. Systematic Biology 53, 653–661.
| The role of morphological data in phylogenetic reconstruction.Crossref | GoogleScholarGoogle Scholar |
Wills KE, Whalley RDB, Bruhl JJ (2000) Systematic studies in Paniceae (Poaceae): Homopholis and Whalleya gen. et sp. nov. Australian Systematic Botany 13, 437–468.
| Systematic studies in Paniceae (Poaceae): Homopholis and Whalleya gen. et sp. nov.Crossref | GoogleScholarGoogle Scholar |
Wood BA (2010) Systematics, taxonomy, and phylogenetics: ordering life, past and present. Chapter 3. In ‘A Companion to Biological Anthropology’. (Ed. CS Larsen) pp. 56–73. (Wiley-Blackwell: Chichester, UK)
Wortley AH, Rudall PJ, Harris DJ, Scotland RW (2005) How much data are needed to resolve a difficult phylogeny? Case study in Lamiales. Systematic Biology 54, 697–709.
| How much data are needed to resolve a difficult phylogeny? Case study in Lamiales.Crossref | GoogleScholarGoogle Scholar | 16195214PubMed |
Zuloaga FO (1986) Systematics of the New World species of Panicum (Poacaeae: Psaniceae). In ‘Grass Systematics and Evolution’. (Eds TR Soderstom, KW Hilu, CS Campbell, ME Barkworth) pp. 277–286. (Smithsonian Institute Press: Washington, DC, USA)
Zuloaga FO, Morrone O, Giussani LM (2000) A cladistic analysis of the Paniceae: a preliminary approach. In ‘Grasses: Systematics and Evolution’. (Eds SWL Jacobs, JE Everett) pp. 123–135. (CSIRO Publishing: Melbourne, Vic., Australia)
Zuloaga FO, Salomon L, Scataglini MA (2014) Phylogeny of sections Clavelligerae and Pectinatae of Panicum (Poaceae, Panicoideae, Paniceae): Establishment of the new subtribe Dichantheliinae and the genus Adenochloa. Plant Systematics and Evolution 301, 1693–1711.
| Phylogeny of sections Clavelligerae and Pectinatae of Panicum (Poaceae, Panicoideae, Paniceae): Establishment of the new subtribe Dichantheliinae and the genus Adenochloa.Crossref | GoogleScholarGoogle Scholar |
Zuloaga FO, Salariato DL, Scataglini MA (2018) Molecular phylogeny of Panicum s. str. (Poaceae, Panicoideae, Paniceae) and insights into its biogeography and evolution. Plos One 13, 1–39.
| Molecular phylogeny of Panicum s. str. (Poaceae, Panicoideae, Paniceae) and insights into its biogeography and evolution.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. Taxon and character-state data matrix for the 161 morphological characters for 24 samples
A number sign (#) indicates samples not used by Thompson and Fabillo (2021).


Appendix 2. Topotypes for Ancistrachne uncinulata
PORT CURTIS DISTRICT: Fingerfield Rd Rules Beach – Private Holding, April 2003, Sankowsky and Sankowsky 1966; Yeppoon, ~2 km N of Township on road to Bayfield, June 1978, Sharpe 2366; between Lotus and Marlborough, June 1955, Beauglehole 3578; Pine Inlet, Percy Islands, 87 miles (~140 km) SE of Mackay, May 1956, Lazarides 5677A; Deep Water National Park, 40 km E of Miriam Vale, March 1990, Gibson TOI919.
SOUTH KENNEDY DISTRICT: Cape Hillsborough National Park, near Hidden Valley, July 1994, Bvatianoff & Dillewaard 9407140; Shoalwater Bay Training Area, Pine Mountain sector, western slope of Pine Mountain, April 2011, Halford & Bean QM329; Calder Island, 60 km NE of Mackay, May 1992, Halford & Crombie Q1346.
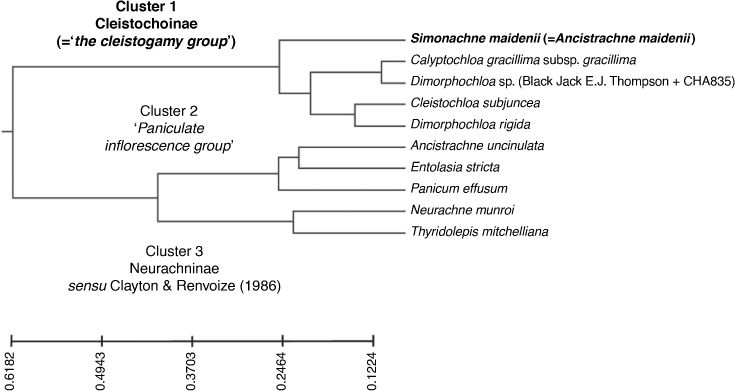
Appendix 3. Dendrogram generated from analysis of dataset composed of 10 samples corresponding to the 10 putative genera
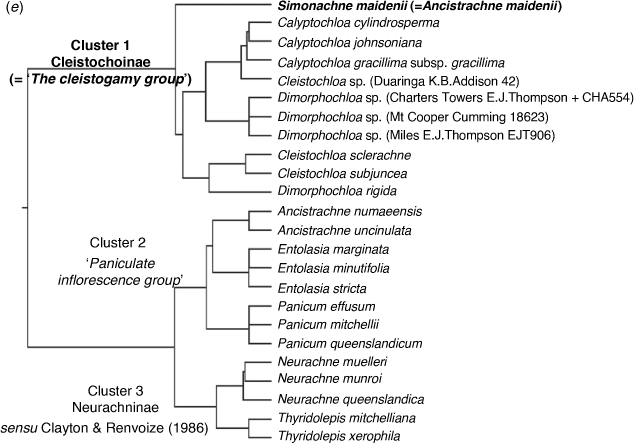
Dendrogram generated from PATN analysis using GOWER association measure and a subset of 10 samples and 161 morphological characters. Three main clusters, subtribes Cleistochloinae and Neurachninae sensu Clayton & Renvoize (1986) and the ‘paniculate inflorescence group’, congruent with the result using the baseline dataset comprising 24 samples and 161 characters. Classification strategy set at flexible UPGMA agglomerative hierarchical fusion technique with Beta = −0.10.
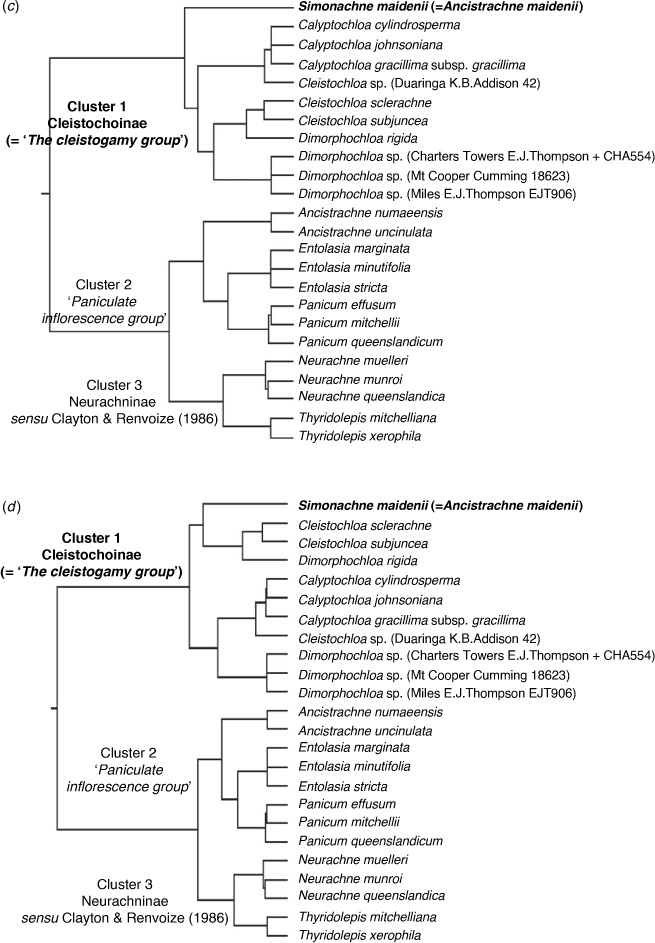
Appendix 4. Dendrograms generated from PATN analyses using various datasets and analyses run using GOWER association measure
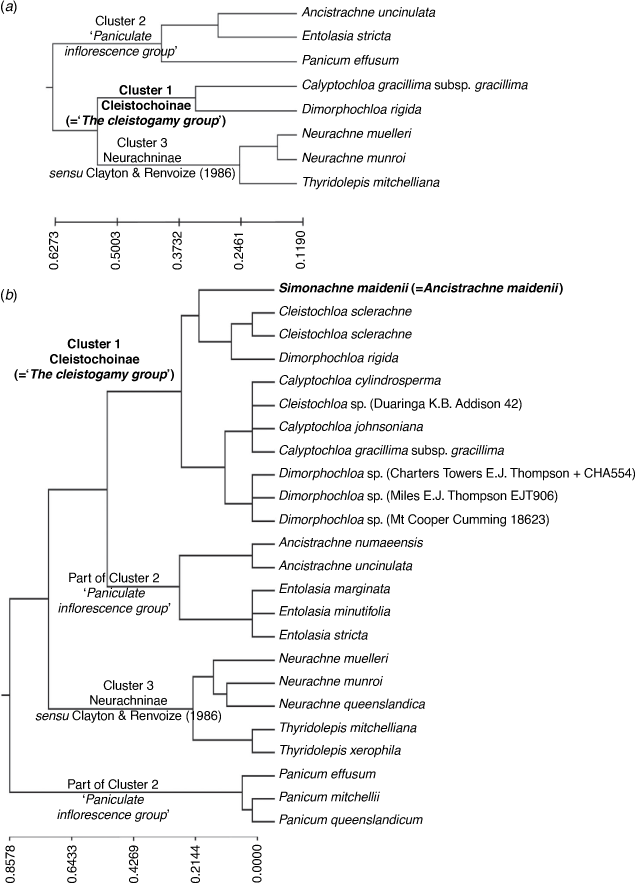
Datasets comprise 24 samples and sets of most discriminating characters based on Kruskal–Wallis values obtained from the analysis of the baseline dataset of 24 samples and 161 morphological characters. (a) Top 10–20 characters. (b) Top 30 characters. (c) Top 40–60 characters. (d) Top 70 characters. (e) Top 80–161 characters. Topologies consistent with the baseline for datasets with 80 or more characters. Classification strategy set at flexible UPGMA agglomerative hierarchical fusion technique with Beta = −0.10.

Appendix 5. Dendrograms generated from analysis of datasets comprising predetermined sample composition and character set as used by Morrone et al. (2012)
Dendrograms generated from PATN analyses using GOWER association measure and two subsets of data based on 15 characters used Morrone et al. (2012). (a) Overlapping samples from Neurachninae sensu Morrone et al. (2012) using Australian species of Panicum s.s. and Neurachne munroi substituted for Neurachne alopecuroidea. Three main clusters congruent with result from baseline dataset but similarity relationships different. (b) All 24 samples. The topology is not congruent with the result from the baseline dataset. Classification strategy set at flexible UPGMA agglomerative hierarchical fusion technique with Beta = −0.10.
Appendix 6. Stigmas of some of the species from rehydrated and fresh samples
(a, f) Simonachne maidenii (rehydrated) from Thompson EJT936 (BRI). (b, g) Ancistrachne uncinulata from Thompson EJT1018 (BRI). (c, h) Panicum effusum from Thompson EJT916 (BRI). (d, i) Neurachne muelleri from Thompson GAL354 (BRI). (e, j) Dimorphochloa sp. (Miles E.J.Thompson EJT906) from Thompson + EJT906 (BRI). Images: E. J. Thompson. Scale bar: 2 mm for a–e and 50 µm for f–j.

Appendix 7. Contraligule of Simonachne maidenii
From Thompson EJT936 (BRI). Photo: E. J. Thompson. Scale bar: 1 mm.

Appendix 8. Lemmas and paleas of upper florets
(a, b) Cleistogamous spikelet from axillary inflorescence of Simonachne maidenii. (a) Upper lemma. (b) Upper palea. (c, d) Ancistrachne uncinulata. (c) Upper lemma. (d) Upper palea. (e) Cleistogamous spikelet from axillary inflorescence of Cleistochloa subjuncea, upper lemma. (f) Upper palea. (a, b) From Thompson EJT936 (BRI), (c, d) from Thompson EJT1018 (BRI) and (e, f) from Thompson HUG815 & Simon (BRI). Photos: E. J. Thompson. c1, transverse callus; ca, creased apex; c2, oblique callus; gl1, germination lid with inconspicuous crescent-shaped depression between raised veins; gl2, lid inconspicuous; gl3, lid with crescent-shaped depression between raised veins; m1, mucronate to shortly awned; m2, mucronate; pb, proximal beak. Scale bar: 2 mm.

Appendix 9. Caryopses of Simonachne maidenii and Ancistrachne uncinulata
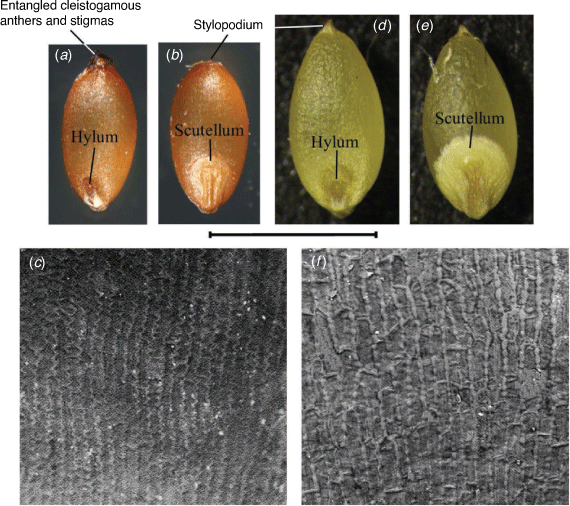
(a–c) Simonachne maidenii. (a) View of hylum. (b) View of scutellum. (c) Scanning electron micrograph of endosperm. (a–f) Ancistrachne uncinulata. (d) View of hylum. (e) View of scutellum. (f) Scanning electron micrograph of endosperm. (a–c) From Thompson EJT936 (BRI) and (d–f) from Thompson EJT1018 (BRI). Photos and micrographs (captured at 500×): E. J. Thompson. Scale bar: 2 mm for a, b, d, e and 200 µm for c, f.
Appendix 10. Scanning electron micrographs of surface of upper lemmas
(a) Simonachne maidenii, from terminal spikelet. (b) S. maidenii from axillary spikelet. (c) Ancistrachne uncinulata. (d) Ancistrachne numaeensis. (a, b) From Thompson EJT936 (BRI), (c) from Thompson EJT1018 (BRI) and (d) from van Drot 567 (NOU). Micrographs (captured at 500×): E. J. Thompson. Scale bar: 200 µm.
Appendix 11. Scanning electron micrographs of surface of upper lemmas
(a) Simonachne maidenii, longitudinal ridges, papillae absent, elongated U-shaped anticlinal walls of long cells. (b) Ancistrachne uncinulata, lacking ridges, papillae with beak-like apex to nipple-shaped, irregularly spaced in longitudinal rows, U-shaped anticlinal walls of long cells. (a) From Thompson EJT936 (BRI) and (b) from Thompson EJT1018 (BRI). Micrographs (captured at 4000×): E. J. Thompson. p, papillae, r, ridge. Scale bar: 30 µm.

Appendix 12. Scanning electron micrographs of surface of upper paleas
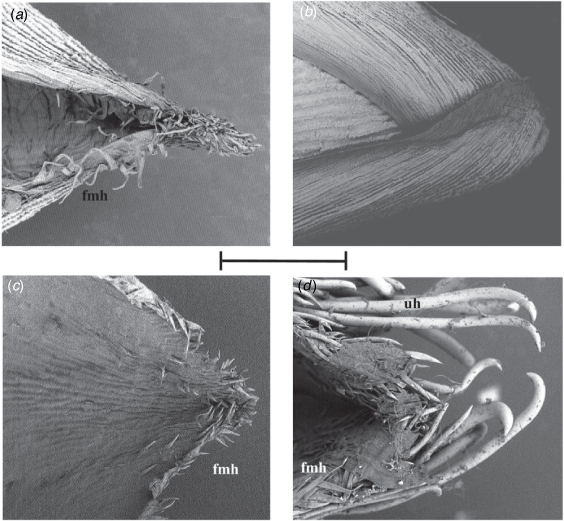
(a) From chasmogamous spikelet of Simonachne maidenii. (b) From spikelet of Ancistrachne uncinulata. (a) From Thompson EJT936 (BRI), (b) from Thompson EJT1018 (BRI). Micrographs (captured at 1000×): E. J. Thompson. fmh flattened microhairs on margin. Scale bar: 50 µm.
Appendix 13. Scanning electron micrographs of apex of lower lemmas
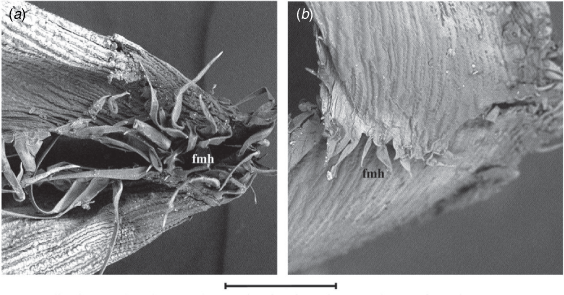
(a, c) Simonachne maidenii, silica bodies with narrow waist and indented ends. (b, d) Ancistrachne uncinulata, silica bodies with broad waist and rounded ends. (a, c) From Thompson EJT936 (BRI) and (b, d) from Thompson EJT1018 (BRI) (BRI). Micrographs (captured at 1000× & 5000×): E. J. Thompson. bmh, bicellular microhair; ewf, film of epicuticular wax; ewp, platelets of epicuticular wax; h, hook; p, prickle; sb, silica body; tmh, tuberculate-based macrohair. Scale bars: 200 µm for a, b and 20 µm for c, d.

Appendix 14. Scanning electron micrographs of abaxial surface of leaf blade
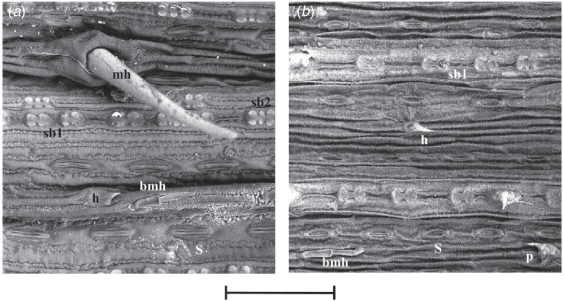
(a) Simonachne maidenii. (b) Ancistrachne uncinulata. (a) From Thompson EJT936 (BRI); (b) from Thompson EJT1018 (BRI). Micrographs: E. J. Thompson, captured at 1000×. bmh, bicellular microhair; h, hook; mh, macrohair; sb1, bilobate silica body; sb2, polylobate silica body; S, stoma. Scale bar: 100 µm.
Appendix 15. Replicas of abaxial surface of fresh leaf blade
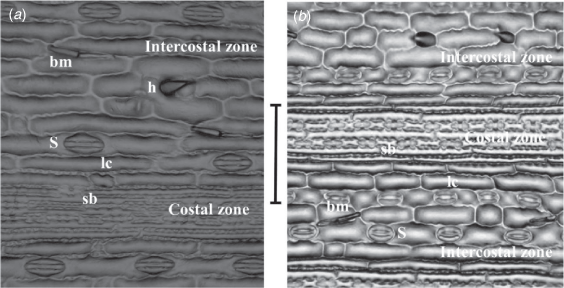
(a) Simonachne maidenii. (b) Ancistrachne uncinulata. (a) From Thompson EJT936 (BRI); (b) from Thompson EJT1018 (BRI). Photos: E. J. Thompson. bm, bicellular microhair; h, hook; lc, long cell; sb, silica body; S, stoma. Scale bar: 100 µm.
Appendix 16. Transverse section of fresh leaf at mid-vein
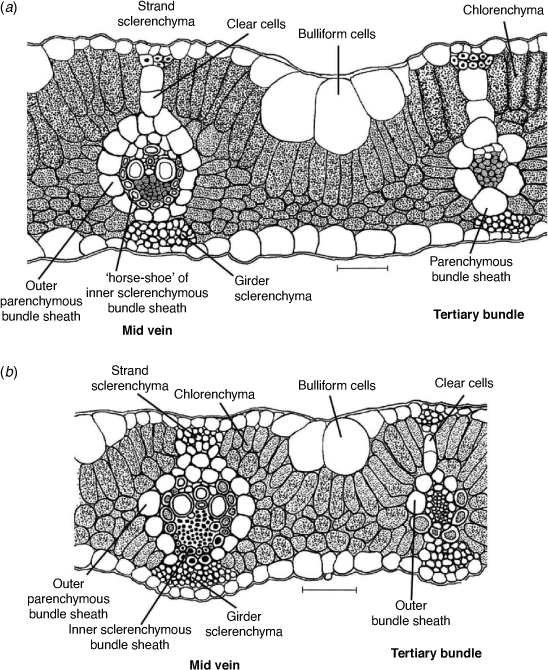
(a) Simonachne maidenii. (b) Ancistrachne uncinulata. (a) From Thompson EJT936 (BRI); (b) from Thompson EJT1018 (BRI). Del. E. J. Thompson. Scale bar: 50 µm.
Appendix 17. Transverse section of fresh terminal inflorescence culm
(a) Simonachne maidenii, vascular bundles with parenchymous sheath. (b) Ancistrachne uncinulata, example with vascular bundles surrounded by sclerenchyma. (a) From Thompson EJT936 (BRI); (b) from Thompson EJT1018 (BRI). Photos: E. J. Thompson. 1, primary vascular bundle (vb); 2, secondary vb; 3, tertiary vb. Scale bar: 50 µm.

Appendix 18. Scanning electron micrograph of surface of terminal inflorescence culm
(a) Simonachne maidenii. (b) Ancistrachne uncinulata. (a) From Thompson EJT936 (BRI); (b) from Thompson EJT1018 (BRI). Micrographs: E. J. Thompson captured at 1000×. bmh, bicellular microhair; h, hook; mh, macrohair; p, prickle; sb3, saddle-shaped silica body; sp, spicule; S, stoma. Scale bar: 100 µm.

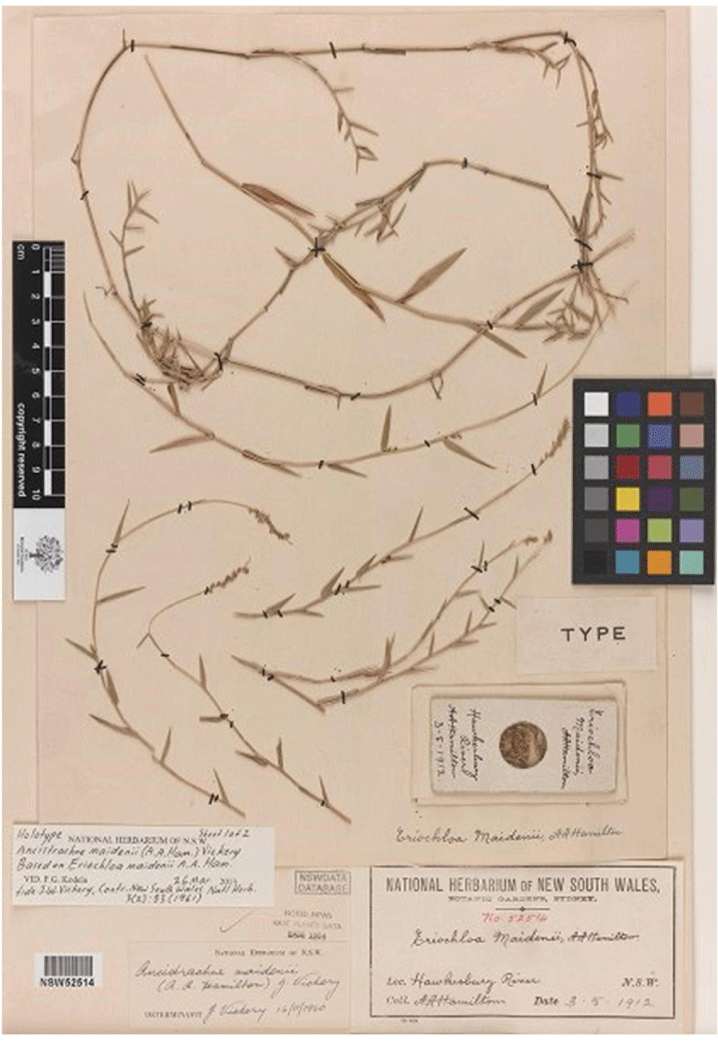
Appendix 19. Holotype of Eriochloa maidenii (=Simonachne maidenii) (A.A. Hamilton s.n. (NSW 52514))