Taxonomic revision of the southern hemisphere pygmy forget-me-not group (Myosotis; Boraginaceae) based on morphological, population genetic and climate-edaphic niche modelling data
Jessica M. Prebble A B C * , V. Vaughan Symonds
A B C * , V. Vaughan Symonds  A , Jennifer A. Tate
A , Jennifer A. Tate  A and Heidi M. Meudt
A and Heidi M. Meudt  B
B
A School of Natural Sciences, Massey University, Private Bag 11222, Palmerston North 4442, New Zealand.
B Museum of New Zealand Te Papa Tongarewa, PO Box 467, Cable Street, Wellington 6140, New Zealand.
C Present address: Manaaki Whenua–Landcare Research, PO Box 69040, Lincoln 7640, New Zealand.
Australian Systematic Botany 35(1) 63-94 https://doi.org/10.1071/SB21031
Submitted: 24 August 2021 Accepted: 17 February 2022 Published: 5 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
A taxonomic revision of the southern hemisphere pygmy forget-me-not group (Myosotis L.; Boraginaceae) is presented here. Climate-edaphic niches are modelled and compared for five species in the pygmy group, namely, M. antarctica Hook.f., M. brevis de Lange & Barkla, M. drucei (L.B.Moore) de Lange & Barkla, M. pygmaea Colenso and M. glauca (G.Simpson & J.S.Thomson) de Lange & Barkla, and one unnamed putative taxon, M. “Volcanic Plateau”. In this case, niche-modelling data mostly do not aid species delimitation, but morphological and genetic data provide evidence for recognising the following three species within the group: M. brevis and M. glauca (both endemic to New Zealand), and an enlarged M. antarctica (native to New Zealand, Campbell Island and Chile). Myosotis antarctica is here circumscribed to include M. antarctica sens. strict., M. drucei and M. pygmaea. The following two allopatric subspecies of M. antarctica are recognised on the basis of minor morphological differences: subsp. antarctica (formerly M. antarctica from Campbell Island and Chile, M. drucei and M. “Volcanic Plateau”) and subsp. traillii Kirk (formerly known by New Zealand botanists as M. pygmaea Colenso, an illegitimate name). For all three species, which are considered Threatened or At Risk, most of their genetic variation is partitioned between rather than within populations, meaning that conserving as many populations as possible should be the priority to minimise risk of extinction.
Keywords: Boraginaceae, microsatellite markers, morphometrics, Myosotis, population genetics, species radiation, taxonomic revision, New Zealand.
Introduction
The southern hemisphere species of Myosotis L. (Boraginaceae) are an example of a recent and rapid species radiation in need of taxonomic revision (Winkworth et al. 2002; Meudt et al. 2015), and work is ongoing to that end (Lehnebach 2012a, 2012b; Meudt et al. 2013, 2020; Prebble et al. 2015, 2018, 2019; Meudt 2016, 2021; Meudt and Prebble 2018). It can be challenging to determine species boundaries in such recent and rapid radiations, but delimiting species after analysing multiple sources of data, as per the general lineage concept (de Queiroz 2007), has recently been undertaken with success for several groups of southern hemisphere Myosotis (e.g. the M. petiolata Hook.f. complex by Meudt et al. 2013; bracteate-prostrate species excluding the pygmy species group by Meudt and Prebble 2018; and the M. australis R.Br. species complex by Meudt et al. 2020).
The main aim of this paper is to undertake a taxonomic revision of the pygmy species group (often called the Myosotis pygmaea Colenso species group in previous studies; Prebble et al. 2015, 2018, 2019), by synthesising evidence from morphological data (Prebble et al. 2018), molecular data (Prebble et al. 2019), and climate-edaphic niche modelling data (this paper). The pygmy species group includes small, self-pollinating, herbaceous plants with petiolate obovate rosette leaves, and decumbent inflorescences with many flowers, each being associated with a cauline leaf. The white, cream or blue corollas are up to 4 mm in diameter, with cylindric corolla tubes and included stamens (Prebble et al. 2018). Currently, five published species make up this group, namely, M. antarctica Hook.f. (native to the New Zealand subantarctic Campbell Island and Chile), M. brevis de Lange & Barkla, M. drucei (L.B.Moore) de Lange & Barkla, M. glauca (G.Simpson & J.S.Thomson) de Lange & Barkla, and M. pygmaea Colenso (the latter four are endemic to New Zealand), plus multiple unnamed putative taxa (known as tag-named taxa in New Zealand, or phrase-named taxa in Australia; listed in detail in table 1 of Prebble et al. 2018).
With the goal of delimiting species within the pygmy forget-me-not group, morphological data (Prebble et al. 2018) and population genetic data from microsatellite markers (Prebble et al. 2015, 2019) have both been analysed. The morphological data recovered four morph-groups that corresponded to Myosotis brevis, M. pygmaea, M. glauca and M. antarctica + M. drucei. None of the unnamed putative taxa could be distinguished morphologically (Prebble et al. 2018). Of the four morph-groups, only M. brevis was strongly supported by the population genetic data. Although some genetic evidence was found to distinguish the M. glauca morph-group, the M. pygmaea morph-group was not separated genetically from the M. antarctica + M. drucei morph-group (Prebble et al. 2019). None of the unnamed putative taxa could be distinguished genetically. It was hypothesised that incorporating ecological data might be useful to aid in species delimitation (Prebble et al. 2019) and, so, we here model the climate-edaphic niches of the pygmy forget-me-nots.
Climate-edaphic niche models estimate a species’ niche across a geographical area by relating presence records of the species to environmental variables to generate predictions. These models estimate the probability that species occur in areas where they have not been observed, given the environmental variables (Elith et al. 2006). The field of niche modelling has grown rapidly in recent years and the methods have been used in a variety of applications, such as, for example, to test for ecological speciation (Joly et al. 2014), to compare lineage diversification with niche divergence (Wooten and Gibbs 2012), to assess evidence for glacial refugia (Buckley et al. 2010), to find previously unrecorded populations of threatened species (Bourg et al. 2005), and, most relevant here, to aid in species delimitation (e.g. Raxworthy et al. 2007; Rissler and Apodaca 2007; Reeves and Richards 2011; Ahmadzadeh et al. 2013; Prata et al. 2018). Although we do not think that the climate-edaphic niche is a property of the species, as it might be considered if following the ecological species concept originally propounded by Van Valen (1976), niche-modelling data can still be useful for species delimitation. To use climate-edaphic niche modelling data in species delimitation, niches are modelled for each hypothesised species, and then measures of niche overlap are calculated (e.g. Warren et al. 2008). Niche modelling provides useful data for species delimitation in cases where the distributions of the two taxa are overlapping, and their niches are shown to differ (Godsoe 2010).
Species delimitation requires both a species concept and evidence to assess species boundaries. For our species concept, we use the general lineage concept whereby species can be thought of as separately evolving metapopulation lineages (de Queiroz 2007). To assess whether hypothesised metapopulation lineages are separately evolving, and thus test their species limits, multiple lines of evidence are useful. Our taxonomic decisions are based on synthesising all available information, including recently generated morphological, genetic, and climate-edaphic niche modelling data, but also considering other information where available, including previously published taxonomic treatments, pollen morphology, chromosome counts, life-history information and other field observations. The general lineage concept provides a flexible and easy-to-apply framework for understanding, recognising and interpreting species (including those that are recently evolved) in a way that is compatible with a unifying theoretical context, as well as with our broader understanding of evolution. In the simplest case, we would expect species to be represented by morphologically and genetically distinct clusters, and if their ranges are overlapping and niches differ, this would provide additional evidence that speciation has occurred. Decisions about recognising species or subspecies follow advice in Stuessy (2009), whereby species rank is recommended when multiple data sources agree, especially in cases of sympatry, and subspecies rank is recommended for allopatric taxa with minor morphological differences, as has been used in other New Zealand studies, such as, for example, Edgar (1986), de Lange et al. (1999), Bayly et al. (2003) and Meudt (2006). These criteria are congruent with the common practise identified by Hamilton and Reichard (1992), whereby the rank of subspecies is most often used for lineages united by morphological and either evolutionary or ecogeographic data.
In addition to climate-edaphic niche modelling and a taxonomic revision, this paper assesses the genetic diversity and conservation status of each of the pygmy forget-me-not species as circumscribed here. Understanding patterns of genetic diversity in rare and threatened species, as evidenced by structure and variation within and among populations, is of fundamental importance to their conservation (Ellstrand and Elam 1993). Although none of the species recognised here is newly described, the new circumscription of the species present in the pygmy group outlined here requires a re-assessment of the threat classification of each species.
Therefore, the aims of the present study are to
Model the climate-edaphic niches of the pygmy species group, and assess the utility of this data type to aid species delimitation for this group;
Undertake a taxonomic revision of the pygmy species group;
Compare the population genetic variation within and between each species and subspecies as circumscribed here and consider the implications for conservation; and
Assess the threat status of the newly circumscribed species.
For ease of understanding, existing published names and unnamed putative taxon names as currently applied (hereafter called ‘a priori’ names) are used throughout most of this paper until the taxonomic treatment presented at the end of this paper, where the justifications for the name changes are provided. In the taxonomic revision, three entities within the pygmy species group are recognised at the rank of species, one with two allopatric subspecies.
Materials and methods
Climate-edaphic niche modelling
Latitude and longitude points for niche modelling were obtained from 290 herbarium specimens (Fig. 1, Supplementary Table S1) from AK, CHR, K, OTA, UPS, and WELT (herbarium acronyms follow Index Herbariorum, see http://sweetgum.nybg.org/ih/, accessed 11 June 2021). There are over 700 specimens of the pygmy species group housed across these herbaria; however, to include a specimen in the niche modelling, the identification was assessed by J. M. Prebble, and all latitude and longitude points were individually checked. Collections that could not be plotted precisely (i.e. to within 100 m) were not georeferenced, and only one specimen from each collection location for each taxon (e.g. ‘Lake Lyndon’) was included. The known geographic range of each species was well represented, including specimens from the only two known localities of M. antarctica from Chile (Punta Arenas and Puerto Altamirano; J. M. Prebble, pers. obs., based on study of specimens from AK, BM, CHR, CONC, K, OTA, S, UPS, and WELT).
We modelled the climate-edaphic niches and investigated the similarity and differences between these niches for the five a priori species (Myosotis antarctica, M. brevis, M. drucei, M. pygmaea and M. glauca), four morph-groups identified in Prebble et al. (2018) (M. antarctica + M. drucei, M. brevis, M. pygmaea and M. glauca), and three genetically supported morph-groups identified in Prebble et al. (2019) (M. antarctica + M. drucei + M. pygmaea, M. brevis and M. glauca) that make up the pygmy forget-me-not group. The niche of one putative un-named taxon, M. “Volcanic Plateau” (see table 1 in Prebble et al. 2019 for voucher information for informally named taxa) was also modelled. This meant that the individuals identified as M. “Volcanic Plateau” were excluded from the niche modelling of M. drucei (the species), M. antarctica + M. drucei (the morph-group) and M. antarctica + M. drucei + M. pygmaea (the genetically supported morph-group) to which they would otherwise have contributed. This name for a putative taxon was used by Robertson (1989) as well as by A. P. Druce on some herbarium specimen annotations and unpublished plant lists. It was ecologically distinguished because it is found in a unique habitat of ‘periodically scoured, shallowly incised flood channels or runnels within red tussock covered valley floors’ (G. Rogers, pers. comm., August 2012). As M. “Volcanic Plateau” is putatively a distinct taxon based on its climate-edaphic niche, a niche modelling approach is therefore considered an appropriate method to test its distinctiveness, even though this taxon was not recovered as a separate group in either morphological data (Prebble et al. 2018) or genetic data (Prebble et al. 2019) analyses.
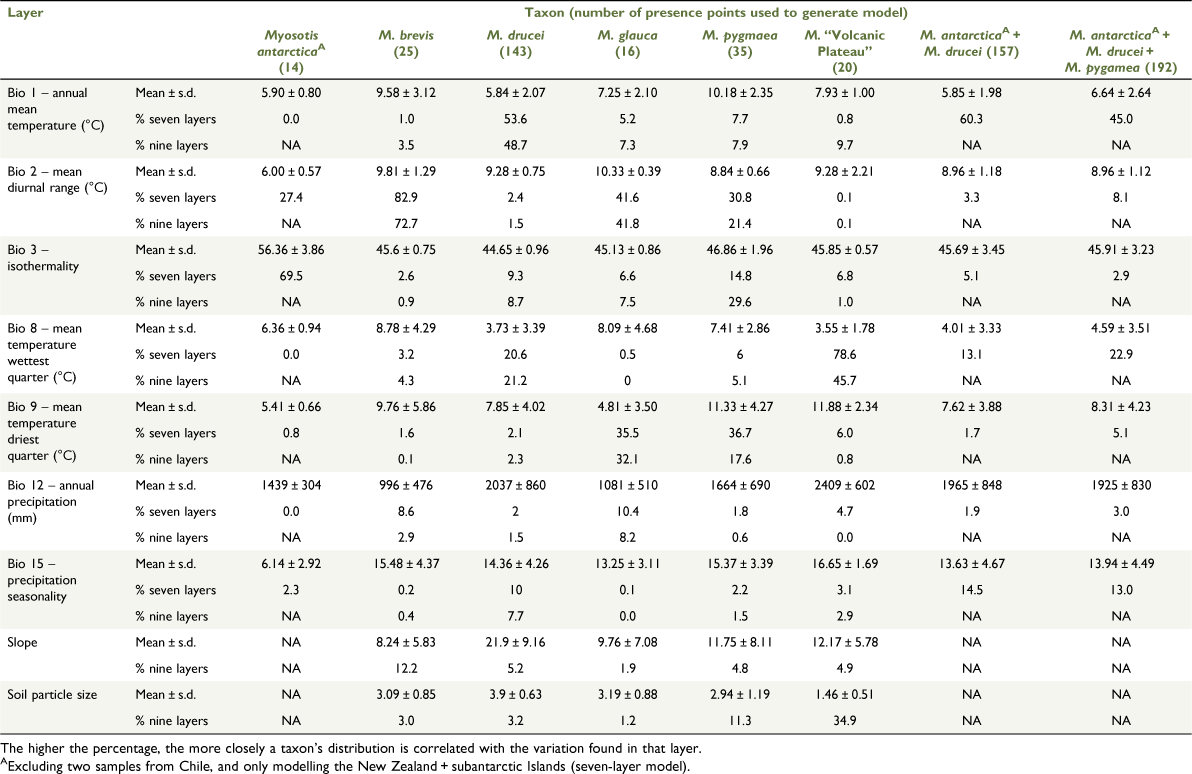
For the niche modelling, 33 environmental layers were considered (Table 1), including elevation, 19 WorldClim bioclimatic variables (see https://worldclim.org/data/worldclim21.html, accessed 19 August 2021; Hijmans et al. 2005), and 13 layers developed for Land Environments New Zealand (LENZ; https://lris.scinfo.org.nz/, accessed 19 August 2021; Leathwick et al. 2002). Raster layers from WorldClim are available in a maximum of a 30-arc-second quadrat resolution (~1-km grid squares at the equator). The LENZ layers were generated at a higher resolution (100-m2 grid and are available at an even higher resampled resolution of 25 m2); however, the data in these layers have not been modelled for the New Zealand subantarctic islands, nor for Chile, and therefore do not encompass the entire geographic range of M. antarctica. To co-analyse the LENZ and WorldClim datasets, the resolution and projection of the LENZ layers were transformed to match those of the WorldClim data. This was undertaken in R (ver. 3.1.3, R Foundation for Statistical Computing, Vienna, Austria) and RStudio (ver. 0.97, RStudio, Inc., Boston, MA, USA, see http://www.rstudio.com, accessed 15 April 2021), using the function spatial_sync_raster from the package spatial.tools (ver. 1.4.8, J. A. Greenberg, see http://CRAN.R-project.org/package=spatial.tools). We selected nine uncorrelated (Pearson’s correlation < 0.8) variables on the basis of our knowledge of the species’ ecology (LENZ layers were slope and soil; WorldClim layers were annual mean temperature (Bio 1), mean diurnal range (Bio 2), isothermality (Bio 3), mean temperature of the wettest quarter (Bio 8), mean temperature of the driest quarter (Bio 9), annual precipitation (Bio 12), and precipitation seasonality (Bio 15)). Because of the incorporation of the two LENZ layers, the New Zealand subantarctic islands and southern Chile were excluded from this dataset, called the ‘nine-layer model’. To assess New Zealand, the subantarctic islands and the region of southern Chile that comprises the range of M. antarctica, the seven WorldClim bioclimatic data layers were also downloaded for southern South America, and a second dataset, called the ‘seven-layer model’ with this expanded geographic range that excluded the two LENZ layers was created.

|
Climate-edaphic niche modelling was undertaken using presence-only data and the maximum entropy model as implemented by the program MaxEnt (ver. 3.3.3, see https://biodiversityinformatics.amnh.org/open_source/maxent/, accessed 14 April 2022; Phillips et al. 2004, 2006). The ‘maximum iterations’ threshold was set to 5000 and the ‘convergence threshold’ was left at the default (0.00001); these two parameters determine the stopping point for the maximisation algorithm. The ‘regularisation multiplier’, which controls the degree of over- or under-fitting of the model, was set to the default of 1. Five independent runs of each niche model were combined to get the average. Model performance was evaluated by cross-validation using the area under the receiving operating characteristic curve (AUC). The AUC varies from 0.5 for a model that performs no better than random, to 1.0 for a model that always predicts presence v. absence. There are known issues with using the AUC for testing climate-edaphic niche models obtained from presence-only data, not least because AUC values are affected in a range of ways by the extent of the background from which pseudo-absences are drawn (VanDerWal et al. 2009). For example, AUC values are usually higher for species with narrow ranges in comparison to the study area (Phillips 2010). This problem can be avoided by reducing the background points to a fixed area surrounding occurrence points (VanDerWal et al. 2009). The background region was delimited in three different ways, namely, using (1) the MaxEnt default of the whole region of interest, (2) the union area of circles of radius of 80 km around each occurrence (following Joly et al. 2014), and (3) the union area of circles of radius of 200 km (following VanDerWal et al. 2009). One thousand pseudo-absences were sampled at random within each background region to train the model.
Niche overlap for pairwise comparisons between each species generated under the same conditions was calculated in ENMTools (Warren et al. 2010), by using the ‘D’ similarity statistic, developed by Schoener (1968), and applied to environmental niche models by Warren et al. (2008). The D statistic describes the difference between two niche models in the predicted probability of presence across a study area, scaled from 0 (no overlap) to 1 (identical models). Two measures were calculated to test whether different species’ niches were statistically differentiated by using ENMTools (Warren et al. 2010). Both tests were run using 100 pseudo-replicates. First, the niche identity test assesses whether the environmental niches of two species are indistinguishable by comparing the observed overlap. Niche identity is a stringent test for niche similarity and usually can find two niches to be identical only if the ranges of the two species being compared are also fully overlapping. The background similarity test attempts to overcome this limitation and assesses whether the observed niche overlap can be attributed to the general environmental conditions that are available within the accessible area of one species. It is a two-tailed test, which is therefore calculated for each species pair separately. However, owing to computer-memory constraints, the background test was run only to compare between the niches of the M. antarctica + M. drucei morph-group and of M. “Volcanic Plateau” for the LENZ + Worldclim niche estimates. Background points used in this analysis were a random subsample of the background points from the union area of circles of a radius of 80 km around each occurrence.
Taxonomic treatment
The taxonomic treatment was assembled in R by using the function tableToDescription from the package ‘MonographaR’, which facilitates writing parallel descriptions (see https://CRAN.R-project.org/package=monographaR; Reginato 2016). Descriptions are based on morphological data measured or observed as detailed in Prebble et al. 2018 on herbarium specimens from WELT (55), CHR (33), OTA (4), AK (5), K (3), and UPS (2; see appendix 1 in Prebble et al. 2018). The description of M. antarctica is based on a total of 73 specimens, M. brevis on a total of 13 specimens and M. glauca on a total of 16 specimens. The description of M. antarctica is based on more specimens originally identified as M. drucei (19), M. pygmaea (18), M. antarctica sens. strict. (i.e. 15 from Campbell Island and 4 from Chile), M. “Volcanic Plateau” (9) and M. “intermedia” (8; see table 1 in Prebble et al. 2019 for more information about unnamed taxa). Morphological terminology follows the latest world Boraginaceae treatment (Weigend et al. 2016). Specifically, the appendages found between the corolla lobes are called ‘faucal scales’ here (v. ‘corolla scales’ in Moore 1961); ‘distal cauline leaves’ is here used to refer to what Moore (1961) called ‘bracts’; ‘trichomes’ is used here instead of ‘hairs’; and the word ‘ribbed’ is used to describe the nutlet margins, rather than ‘winged’ (Webb and Simpson 2001). Phenology and habitat information were taken from all databased pygmy forget-me-not herbarium specimens housed at AK, CHR, CONC, K, OTA, S, UPS, and WELT. Pollen morphology information (Meudt 2016) and chromosome counts were taken from published papers (Beuzenberg and Hair 1983; Murray and de Lange 2013).
Assessing genetic structure and variation
Previously, microsatellite markers were developed for the pygmy forget-me-not group (Prebble et al. 2015) and 497 individuals were genotyped at 12 loci (Prebble et al. 2019). Population genetic metrics were calculated for individual populations in that paper. To assess genetic variation for each species and subspecies newly circumscribed in this paper, the following were calculated in GenAlEx 6 (ver. 6.5, see https://biology-assets.anu.edu.au/GenAlEx/Download.html, accessed 14 April 2022; Peakall and Smouse 2006, 2012): average observed number of alleles (NA), the effective number of alleles (NE), expected heterozygosity (HE), observed heterozygosity (HO), expected heterozygosity of individuals within a subpopulation relative to the total expected heterozygosity of individuals across all populations (FST), and the percentage of polymorphic loci (% P). Pairwise FST using the method of Weir and Cockerham (1984) was also calculated among the species (and subspecies), and among populations of each species (and subspecies). This was calculated using R (ver. 4.0.3, R Foundation for Statistical Computing, Vienna, Austria) and RStudio (ver. 1.3.1093, RStudio, Inc., Boston, MA, USA, see http://www.rstudio.com, accessed 15 April 2021) using the function genet.dist from the package hierfstat (ver. 0.5.7, see https://cran.r-project.org/package=hierfstat; Goudet 2005). These data were used here to assess differences in population genetic variation among species with different threat levels.
Revising conservation status
The conservation status of each newly circumscribed species and subspecies was assessed following the guidelines of the New Zealand Threat Classification System (NZTCS; Townsend et al. 2008). Data required to determine the threat status, i.e. population size and area of occupancy of each population, were recorded in the field when on collecting trips between 2011 and 2015. Each species’ extent of occurrence (EOO) and overall area of occupancy (AOO) were measured using the GeoCAT online tool developed by Kew’s Spatial Analysis team (http://geocat.kew.org/editor, accessed 15 April 2021). The niche-modelling occurrence points were uploaded to the website in .CSV file format, and the ‘grid size’, which defines how large an area around each occurrence point the species inhabits, was estimated to be the smallest unit allowed by the online tool (100 m2) on the basis of the average area of occupancy of populations in the field. The number of additional populations not visited was estimated on the basis of herbarium records from AK, CHR, OTA, and WELT. Levels of predicted ongoing decline were estimated on the basis of the number of historical locations visited at which target plants could no longer be located. On the basis of these analyses, a recommendation to maintain the most recently published conservation status in de Lange et al. (2018, which was based on recommendations submitted to the assessment panel by two of us, H. M. Meudt and J. M. Prebble), or to revise it, is outlined and justified for each recircumscribed taxon in the taxonomic treatment.
As another measure of potential risk of extinction, the percentage of populations for each species that are protected by growing on land managed by the New Zealand Department of Conservation (DOC) was calculated. The GIS layer ‘Protected Areas’ is available from https://data.linz.govt.nz/layer/53564-protected-areas/ (accessed 18 November 2020).
Results
Climate-edaphic niche modelling
The projected maps of modelled niches for each taxon, built using default background sampling points and the nine-layer model being the LENZ + WorldClim layers (in most cases), can be seen in Fig. 2. For most taxa, the modelled niche is similar to the known distribution, which is reflected by good AUC values, as described below. Confidence in the AUC values is gained by all three background extents trialled showing similar AUC values for most taxa (Table 2), and so we discuss only the default background sampling further. As a rough guide, AUC scores from 0.7 to 0.8 are often considered ‘fair’, 0.8–0.9 ‘good’ and 0.9–1 ‘excellent’ and, for these models, all AUC values ranged from 0.75 to 0.98 (Table 2). There were two models where the niches did not match the known distribution well, namely, Myosotis antarctica from Chile and M. pygmaea (Fig. 2). For M. pygmaea, this is likely to be due to a small number of inland populations (most populations are coastal), meaning that the modelled niche of M. pygmaea is broad. For M. antarctica from Chile, this is likely to be due to the very small number of localities (two) that contribute to the model. As a result of the poor fit of the model in Chile, we instead report model statistics for M. antarctica, including the New Zealand subantarctic islands but excluding Chile (e.g. Tables 2–4). As predicted, taxa with narrower ranges tended to have higher AUC values. For example, the average AUC for M. “Volcanic Plateau” was 0.98 v. 0.76 for the more widely distributed M. pygmaea (Table 2). However, in some cases the taxa with the highest AUC values, for example, M. “Volcanic Plateau”, actually showed a poor fit to the model as assessed by commission and omission rates (Fig. 3), probably owing to the small sample size. The nine-layer models usually had a higher AUC score than the values for the same taxa modelled just with the WorldClim layers (seven-layer model), although the differences were small. However, the niches modelled sometimes differed markedly, for example, for M. “Volcanic Plateau” (see maps in Fig. 2). This is likely to be due to the importance of one of the LENZ layers (soil particle size) to building the model when that environmental layer was included (Table 3). The differences in AUC values between niches modelled using the different background sampling methods were small, with AUC values either increasing or decreasing as the area the background points were sampled from was reduced (Table 2).

|

|

|
Different environmental variables contributed differently to each taxon’s modelled niche (Table 3). Overall, the niche of Myosotis antarctica reflects the subantarctic climate of Campbell Island, being cold and wet throughout the year. The temperature and rainfall are consistent both over a day and over the year, which is illustrated by the higher isothermality score, which is the layer that contributed most to building the niche model for M. antarctica on Campbell Island (Bio 3 contributed 69.5%, Table 3). The niche of M. brevis is drier, with the lowest mean rainfall relative to the niches of the other pygmy group taxa (Bio 12, Table 3), and warmer both when considered annually and considering the wettest and driest quarters (Bios 1, 8, and 9; Table 3). However, mean diurnal range contributed most to building the niche model of M. brevis (Bio 2 contributed 82.9% to the seven-layer model and 72.7% to the nine-layer model; Table 3). The niche of M. drucei is characterised by being cold (Bios 1 and 8), wet (Bio 12), and steep with coarse soils (Slope and Soil particle size; Table 3). Mean annual temperature (Bio 1) contributed most to both the seven-layer model (53.6%) and the nine-layer model (48.7%) for M. drucei. Myosotis glauca plants grow in locations that are colder when it is wet (Bio 8) and warmer when it is dry (Bio 9), and the niche of M. glauca has the highest mean diurnal range (Bio 2) of the niches of the remaining pygmy taxa. Bio 2 is also the layer that contributes most to building its niche model (contributing 41.6 and 41.8% to the seven- and nine-layer models respectively; Table 3). The niche of M. pygmaea is the warmest of the pygmy group (Bio 1) and is particularly warm during the driest quarter (Bio 9). Three layers are equally important to building this niche model, namely Bio 2, Bio 3, and Bio 9 (Table 3). The unnamed putative taxon M. “Volcanic Plateau” has a niche characterised by low temperatures during the wettest quarter (Bio 8), high temperatures during the driest quarter (Bio 9) and small soil particle size (Table 3). This model is one that changes the most in terms of the percentage contribution of layers between the seven- and nine-layer models. When soil particle size is included in the nine-layer model, it contributes 34.9% and Bio 8 contributes 45.7%, but when soil particle size is excluded in the seven-layer model, Bio 8 contributes 78.6%. When data points from M. antarctica and M. drucei are combined (both with and without M. pygmaea), their combined niche becomes an average of the individual niches, and the mean annual temperature (Bio 1) is the most important character in terms of the layer that contributes most to building the model.
Overlap between the niches modelled using the nine-layer model ranged from 0.11 to 0.62 (Table 4). Niche overlap between Myosotis “Volcanic Plateau” and M. drucei increased from 0.17 to 0.56 when analysing the seven-layer model only, as compared to the nine-layer model (Table 4). The niche identity test (based on the nine-layer model data) found that the niches of M. brevis and M. glauca were identical, but no other tested pairs were (Table 4). Even though the niches of M. brevis and M. glauca were found to be identical, their projected distributions were different, particularly around the coastal areas of Southern North Island (Fig. 2). The niche background similarity test (which could be performed only on one species pair because of computer processing constraints) found that the M. “Volcanic Plateau” niche was not significantly different from that of M. drucei + M. antarctica. However, this is a pairwise test, and the opposite test found the niche of M. drucei + M. antarctica to be significantly different from that of M. “Volcanic Plateau”. This means that the niche of M. “Volcanic Plateau” is a subset of that of M. drucei + M. antarctica.
Assessing genetic structure and variation
The genetic variation contained in each newly circumscribed species is detailed in Table 5. All species or subspecies had lower observed heterozygosity than expected, and, overall, Myosotis brevis had the lowest observed heterozygosity, and highest F-statistics (Table 5). Myosotis glauca had the lowest percentage of polymorphic loci, but also the smallest number of sampled populations (Table 6). Pairwise FST values among the a priori taxa were low (0.12–0.38), with the lowest value being between M. drucei + M. antarctica and M. pygmaea. In comparison, the pairwise FST values among the populations of each taxon were high (M. drucei + M. antarctica 0.23–0.98; M. pygmaea −0.1–1; M. brevis 0.42–0.99; M. glauca 0.88–0.93; see Supplementary Tables S2–S7).

|
Discussion
Assessing the utility of climate-edaphic niche modelling of pygmy forget-me-nots for species delimitation
The modelled niches of the pygmy forget-me-not group were found to be very similar (but not identical), as evidenced by high niche overlap scores and niche background similarity tests (Table 3). For niche modelling to contribute useful data for species delimitation, geographic distributions must be contiguous and niches different, so that interruption of gene flow and species-level differentiation can be inferred (Tocchio et al. 2015). When geographic distributions are contiguous, but niches are similar, conclusions are limited; either no ecological differentiation has taken place, or ecological differentiation exists that has not been expressed in the niche model or captured by the variables fed into the model.
The geographic ranges of the pygmy forget-me-nots overlap in most cases (Fig. 1), meaning that it is theoretically possible for niche modelling to contribute useful data for species delimitation if their niches were found to differ. Apart from Myosotis antarctica (from Campbell Island and Chile), the geographic ranges of the remaining pygmy group species (all from mainland New Zealand) overlap. As expected, the niche of M. antarctica was found to be different from the rest of the pygmy group (niche overlap scores of zero or near zero; see Table 4), reflecting the difference between the climate of Campbell Island and that of mainland New Zealand. Although this demonstrates the potential for climate-mediated divergent selection on the Campbell Island population, given the lack of range overlap in this case, it does not provide evidence either way when considering whether M. antarctica should be recognised at species rank. The geographic ranges of the morph-groups M. antarctica + M. drucei, M. pygmaea, M. glauca, and M. brevis overlap, and their niches also overlap (niche overlap scores range from 0.30 to 0.61; Table 4). Climate-edaphic niche differences therefore have not manifested or were not captured by the environmental layers assessed here, and climate-edaphic niche modelling does not contribute any data to the question of species delimitation for these morph-groups. The ranges of M. “Volcanic Plateau” and M. antarctica + M. drucei do not overlap, so in this case niche differences (if found) may represent vicariant speciation in allopatry, or they may simply indicate that the species inhabits a broad niche (Godsoe 2010). However, we found no evidence for niche differences between the two; rather, the background test for niche similarity showed that the niche of M. “Volcanic Plateau” is a subset of that of M. drucei. This could point to no ecological differentiation of the putative taxon, or it could indicate that the environmental layers assessed here have not captured real ecological differentiation.
The success and accuracy of niche modelling depends heavily on the underlying data (Warren 2012). Both the occurrence points and the environmental layers used bring their own sources of error. Deciding which environmental layers to use, and whether these are a good estimation of the niche of the species of interest, is difficult to assess. Furthermore, each environmental layer has itself been modelled, which brings additional sources of error. The size of the grid used can also have important implications. For organisms that are large and mobile (such as a large mammal), ~1-km2 grids could well be a good estimation, but for organisms that are small and less mobile (such as pygmy forget-me-nots), such a scale is most likely concealing some of the important micro-habitat variation. In cases where the niche has been modelled well, the projected inhabited area can still be very different from a species’ geographic distribution. This difference can be due to dispersal barriers, or the difference between the fundamental (or ideal) and realised niche (Warren 2012). Nevertheless, the climate-edaphic niches modelled here do appear to have some biological meaning in that they mostly match the current known geographic distributions (as confirmed by the generally high AUC scores (Table 2) and the maps in Fig. 2). However, the grids that the models are based on are likely to be too coarse. The problem of scale has been noted by others attempting to model the niches of New Zealand herbaceous plants, even when a 25-m2 scale is being used (Lehnebach 2008; Pufal 2010), although others have had success using the coarser scale (e.g. Pachycladon Hook.f.; Joly et al. 2014)
Given that niche modelling has been shown to provide little useful data for species delimitation in the pygmy forget-me-not group, future niche-modelling research could focus on gathering more fine-scale ecological data. For example, data-recording boxes could be installed at relevant sites to help assess whether the WorldClim and LENZ layers used in this study are adequately describing the niches of the pygmy forget-me-not group. Additionally, it could be that climate-edaphic niche modelling is more relevant to population differentiation rather than species delimitation in this group, and with more fine-scale ecological data this would be an interesting question to explore further.
Taxonomic conclusions
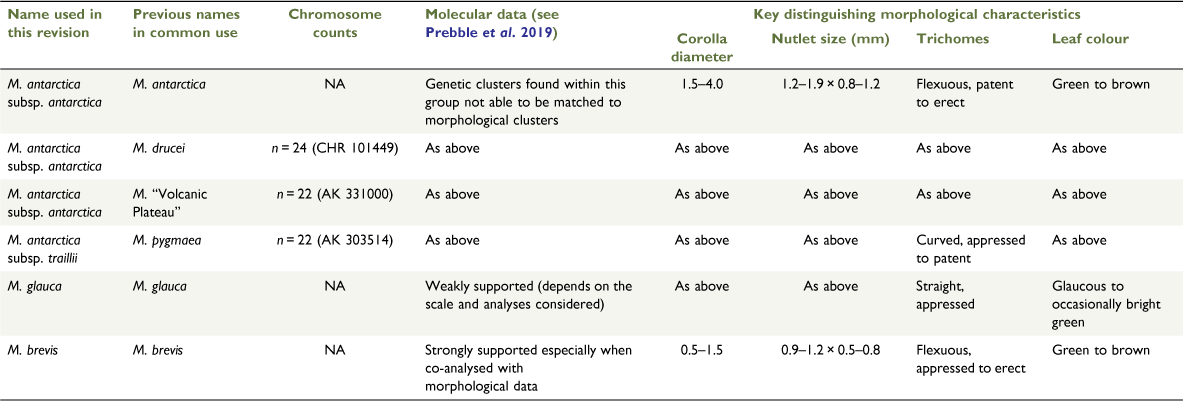
Of the five named a priori species that make up the pygmy forget-me-not group, four are distinguished using morphological data (i.e. the four morph-groups recovered in Prebble et al. 2018 correspond to M. antarctica + M. drucei, M. brevis, M. glauca and M. pygmaea). As previously discussed, there is neither morphological (Prebble et al. 2018) nor molecular (Prebble et al. 2019) evidence to support recognition of any of the unnamed putative taxa, and the climate-edaphic niche modelling we have undertaken does not support the recognition of one putative taxon primarily considered different because of its habitat (M. “Volcanic Plateau”).
There is evidence that Myosotis brevis can be distinguished from the rest of the pygmy species group by morphological data (Prebble et al. 2018), molecular data (Prebble et al. 2019), and through the co-analysis of these two data types. Taken in conjunction with differing life histories (M. brevis is usually a spring annual v. the remainder of the pygmy species group, which are usually perennial), different times of peak flowering (see Taxonomic notes sections below), and pollen morphology differences (Meudt 2016), we take this as strong evidence to support recognising M. brevis at the rank of species.
The remaining species of the pygmy species group are less easily distinguished. Prebble et al. (2019) discussed the morphological and molecular data and concluded that additional data in the form of niche modelling may be useful, but this has not proven to be the case. Given that the integrated analyses of morphological and molecular data showed only two groups (M. brevis v. the remainder of the pygmy forget-me-nots; Prebble et al. 2019), recognising only two species within the pygmy species group is an option that we considered. Nevertheless, there are three morphological entities within this group (M. antarctica + M. drucei, M. glauca, and M. pygmaea) that can be distinguished using a few minor characters (rosette-leaf colour and trichome type). Trichome characters are important in Myosotis and are commonly used in keys and species descriptions (e.g. trichome density, distribution, orientation, type: Moore 1961; Meudt and Prebble 2018; Meudt et al. 2020; H. M. Meudt, unpubl. data). Because only minor genetic changes are required for changes in trichome features (e.g. density of trichomes in Arabidopsis is altered by any of several quantitative trait loci; Bloomer et al. 2014), these characters are best considered as part of a suite of evidence when considering species delimitation. To be explicit, if there were major morphological differences and the taxa were sympatric or allopatric, we would likely recognise these at species rank; if it were only these minor morphological differences and the taxa were sympatric, then we would be unlikely to recognise the taxa as distinct; whereas if it were only these morphological differences and the taxa were allopatric, then we would likely consider it appropriate to recognise these taxa at subspecies rank.
However, as touched on in Prebble et al. (2019), there are some correlations between the morphological and molecular data at fine scales, that in conjunction with range overlaps, suggest that speciation has occurred or is occurring. Specifically, populations identified as Myosotis glauca are genetically similar across a large distance, and genetically distinguished from populations of other pygmy species group taxa that are present at nearby localities (Prebble et al. 2019). In an analysis of microsatellite data, all populations of M. glauca formed a cluster in a Structure analysis above K = 10 (fig. 3a in Prebble et al. 2019), and these populations also group together in a NeighbourNet network (fig. 5A in Prebble et al. 2019). Because the ranges of M. glauca and other pygmy group species overlap, it would not be appropriate to recognise this morphologically and genetically distinct taxon at subspecies rank (Stuessy 2009), and so we continue to recognise this taxon at species rank.
By contrast, the two remaining morph-groups (Myosotis antarctica + M. drucei and M. pygmaea) are distinguished only by morphological differences; they were not genetically differentiated on the basis of microsatellite data, and their ranges are mostly allopatric (Chile, Campbell Island and usually inland North and South Islands v. usually coastal North and South Islands), meaning that subspecies rank is more appropriate (Stuessy 2009). As M. antarctica is the earliest published name of the three, it has priority at species rank (Art. 11.2 of the International Code of Nomenclature for Algae, Fungi, and Plants, ‘ICN’; Turland et al. 2018). Regarding the subspecies epithets, ‘antarctica’ and ‘traillii’ should be used instead of ‘pygmaea’ and ‘drucei’. In the non-metric multidimensional scaling (nMDS) analysis of the morphological data, the type specimen of M. pygmaea groups with the M. antarctica + M. drucei morph-group instead of the morph-group called M. pygmaea (see fig. 6 in Prebble et al. 2018). By contrast, the type specimen for the previously published M. antarctica subsp. traillii Kirk clusters with the M. pygmaea morph-group. Furthermore, Myosotis pygmaea Colenso, published in 1884 for a New Zealand taxon, is an illegitimate name. The name has been occupied since at least 1840 (see Alessandrini 1840, p. 439; Bertoloni 1842, p. 13; Edmondson 2017). Myosotis pygmaea Bertol is based on the type specimen BM 000900913 and is currently considered to be a synonym of M. ramosissma (http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:119209-1, accessed 24 August 2021). That specimen was collected ‘near the mouth of the Sedjour’ (the Sejour River enters the Euphrates River in what is currently Syria) on the Euphrates expedition led by F. R. Chesney in 1836. John Lindley processed the specimens on that trip and made them available to Antonio Bertoloni of Bologna, who described several new species from the material (Edmondson 2017). The name Myosotis pygmaea Colenso has been commonly used in New Zealand since the late 19th century, and thus we have used it here when discussing the a priori species in the Introduction, Materials and methods, Results, and Discussion. However, M. pygmaea can no longer be applied for this native New Zealand taxon, and the name M. antarctica subsp. traillii Kirk, which is available and appropriate on the basis of the results from the nMDS analysis mentioned above, is used instead in the Taxonomic treatment below.
Integrating data from multiple sources is once again shown to be a useful method for delimiting species, even in recently radiating species groups. Multiple lines of evidence have been analysed to study the New Zealand native pygmy forget-me-not group, and, as a result, a taxonomic revision in which three species are recognised (one comprising two subspecies) has been produced (Table 7). This recognises fewer taxa than in the taxonomy currently in use, which comprises five species (e.g. de Lange et al. 2010) and several unnamed putative taxa (see table 1 in Prebble et al. 2019), and is a change to the taxonomy in the latest treatment in the Flora of New Zealand (Moore 1961), which included five taxa (two species and three varieties).
|
Genetic variation present in the pygmy forget-me-nots: implications for conservation
The three species constituting the pygmy forget-me-not group are all self-fertilising, which contributes to the patterns seen in their morphological (Prebble et al. 2018) and genetic data (Prebble et al. 2019). For all three species, the same pattern is evident, whereby most of the genetic variation is partitioned among, rather than within, subpopulations (i.e. high FST values, Table 5 and low among species v. high among populations pairwise FST values, Supplementary Tables S2–S7), which is characteristic of selfing species (Frankham 1995; Frankham et al. 2010). Note that although the FST values are low among all species, they are lowest between the two newly circumscribed subspecies, which supports the new taxonomy. The levels of genetic variation partitioned among populations seen here are high, even compared with other self-fertilising plants (Nybom 2004), which suggests low levels of dispersal. The seed dispersal mechanisms of the pygmy forget-me-nots are most likely water splash, wind, or possibly ‘foliage as fruit’ (Thorsen et al. 2009), which refers to seeds being eaten and dispersed incidentally by herbivores. Evidence for this scenario has been found in Myosotis seeds found in coprolites from moa (Dinornithidae, Megalapterygidae; Wood et al. 2012). Because moa are extinct, it is possible that seed dispersal in New Zealand Myosotis has declined as a result.
The observed heterozygosity of an individual relative to the expected heterozygosity of individuals in the population (FIS) was calculated and published for each population previously (see table 2 in Prebble et al. 2019). Considering those data with regard to the new species circumscriptions shows considerable variation among populations within each species and subspecies. The species with the least variation is Myosotis brevis, for which the majority of the populations included in that study show zero observed heterozygosity, leading to FIS values of 1.00 for 7 of the 10 sampled populations. The populations of the other three taxa all have a mix in FIS values ranging from −1.00 (indicating fixed heterozygosity at certain markers) to 1.00. The most common FIS result for all populations is a high positive value, indicating most populations have lower than expected heterozygosity. However, there is a quite different pattern when considering allelic diversity and unique alleles, and M. brevis is the species whose populations show the highest number of both.
The population genetic metrics are similar between the two subspecies of Myosotis antarctica, despite the differences in their overall census sizes (Table 5). A reduction in the percentage of polymorphic loci is evident in M. glauca as compared to M. antarctica, although this could be due to the small number of M. glauca populations sampled. Myosotis brevis has a very low observed heterozygosity compared with the other species and subspecies. This could indicate an even higher rate of selfing than in the other pygmy forget-me-not species; M. brevis has even smaller corollas than the already miniscule corollas of the other species (0.5–1.5 v. 1.5–4.0 mm in diameter). Alternatively, or additionally, the patterns of genetic variation in M. brevis could be influenced by their often annual life cycle, which in conjunction with their fluctuating population sizes (Rogers et al. 2002; hence the use of the qualifier Extreme Fluctuations for the conservation status of this species in de Lange et al. 2010), could lead to high levels of genetic drift and corresponding fixation of alleles (Ellstrand and Elam 1993). Fixation of alleles leading to a low level of genetic variation is concerning, as in conjunction with small population size this has been linked with lower fitness and, hence, an increased risk of extinction (Leimu et al. 2006).
Selfing species require a greater emphasis on conservation of multiple populations than do outcrossers, owing to most of their genetic variation being partitioned among populations. Additionally, mutational accumulation owing to genetic drift in smaller populations is a greater threat (Paland and Lynch 2006), meaning that larger population sizes should be conserved when possible (Frankham et al. 2010). This could be challenging for the threatened pygmy forget-me-nots, given their usually small population sizes (Table 6). Another potential challenge to managing the conservation of pygmy forget-me-nots is that not all populations are found on land managed by DOC (Table 6). The percentage of populations growing on DOC-managed land ranged from 24 to 72% (Table 6). The taxon that is least threatened, Myosotis antarctica subsp. antarctica, has the highest proportion of populations growing on land managed by DOC (75%; Table 6). Myosotis antarctica is also the species with the highest elevational range (from sea level to 2300 m). This is not an unexpected pattern; it has often been recognised that lowland plants are most at risk in New Zealand, owing to greater levels of habitat modification (Rogers and Walker 2002). Most of the protected land in New Zealand is at higher elevation and is less attractive for development. The pygmy forget-me-not that has the highest rate of decline, M. antarctica subsp. traillii (Table 6), inhabits the lowland (sea level to 250 m) for most of its range, and has a low percentage (~25%) of populations growing on DOC-managed land. Populations that are at particular risk, or may be particularly important to conserve because of being genetically or morphologically unusual, are indicated in the Notes section of the Taxonomic treatment below.
Taxonomic treatment
The pygmy forget-me-not subgroup is one part of the bracteate-prostrate group (Robertson 1989; Meudt et al. 2015), the limits of which have been addressed recently elsewhere (Meudt and Prebble 2018) as part of an ongoing project to revise the taxonomy of all native New Zealand Myosotis. The key presented here is for plants in the pygmy forget-me-not subgroup only, is based on herbarium specimens, and requires flowering or fruiting material.
All pygmy forget-me-not plants have the following characteristics: decumbent annual, biennial or perennial rosette herbs, with multiple prostrate flowering and fruiting inflorescences (sometimes branching) on which each flower is associated with a cauline leaf (i.e. ‘bracteate-prostrate’). Rosette leaves are obovate, with lamina (excluding the petiole) ranging from 1.0 to 26.0 mm long, 0.9–11.0 mm wide. Flowers, calyces and nutlets are small: corolla diameter 0.5–4.0 mm, corolla-lobe length <1.5 mm, corolla tube length <3.0 mm; anther placement usually wholly (but at least partly) below the faucal scales, anthers <1.0 mm long, anthers (sub)sessile (i.e. filament length of 0–0.3 mm); style length < calyx length, calyx lobed approximately half way to the base, calyx length at flowering <4.0 mm; pedicel length at fruiting <2.0 mm. Nutlets 4, 0.9–1.9 mm long 0.5–1.2 mm wide, margins scarcely forming ribs, sometimes only at apex, glossy brown to black when mature. Trichomes are densely distributed and antrorse on leaves, stems and calyces; they can be straight, flexuous or curved, and can vary from appressed to erect. Retrorse trichomes are never present on the leaves but are occasionally a feature of the calyces. Hooked trichomes are not present anywhere on pygmy forget-me-nots. Number of inflorescences and flowers per inflorescence are not distinguishing characteristics, and leaf size, inflorescence length and internode length are also highly plastic. The plasticity of these characters was revealed when growing plants in a common garden experiment (Prebble et al. 2018), whereby plants in the common garden grew much larger than did their parent plants in the field. Additional observations on live plants in the field and growth room (e.g. WELT SP111285; photos available to view online at https://collections.tepapa.govt.nz/object/1952936, accessed 8 June 2021) indicate that petals sometimes have pink or blue lines down the middle of them; however, this character was not observed on dried specimens.
Key to pygmy forget-me-not group|
1 Corollas 1.5–4.0 mm in diameter; calyx at flowering 1.7–3.5 mm long, at fruiting 3.0–7.8 mm long; nutlets 1.2–1.9 × 0.8–1.2 mm |
|
2. Trichomes on leaves, calyces and stems flexuous or curved, patent to erect, densely distributed and often overlapping; leaves green to brown; throughout New Zealand (North, South, Stewart, and Campbell Islands), also southern Chile |
Myosotis brevis de Lange & Barkla in P. J. de Lange et al. Threat. Pl. New Zealand 437 (2010) [nom. nov., non M. minutiflora Boiss. & Reut., Pugill. Pl. Afr. Bor. Hispan. 80 (1852)]
Description
Rosette plants with multiple prostrate branches up to 5 cm long. Rosette leaves 1–9; petioles (0–)0.7–7.0 mm long; lamina usually flat, oblanceolate to broadly obovate, 1–9 mm long, 0.9–4 mm wide (length:width ratio 1.2–2.5:1), green to brown; apex obtuse (or occasionally acute), with hydathode on abaxial side; trichomes densely distributed and often overlapping, flexuous, antrorse, appressed to erect, spreading or sometimes appressed on leaf margins, distributed evenly (on leaf adaxial surface), but sparsely distributed, or on leaf midribs only, or absent (on leaf abaxial surface), (0.2–)0.4–0.9(–1.6) mm long, deciduous with age. Basal cauline leaves not subtending flowers, 1–5 per branch, lamina similar in size and shape to the rosette leaves, with petioles up to 2.7 mm; distal cauline leaves subtending flowers up to 17 per branch, lamina 1.0–6.5 mm long, 0.5–2.3 mm wide, usually sessile. Pedicels up to 0.7 mm long (flowering) or 0.8 mm long (fruiting). Calyx 0.7–1.7 mm long (flowering), increasing to 1.7–3.7 mm long (fruiting), 0.9–3.2 mm wide at the top at fruiting, lobed to 1/3–2/3 the length of the calyx; with trichomes usually of uniform length, denser along calyx ribs, occasionally of two different lengths, longer and antrorse on ribs v. shorter and retrorse between ribs and near the base. Corolla 0.5–1.5(–2.0) mm in diameter, white or cream, occasionally pale blue or cream striped with blue; faucal scales yellow; corolla lobes 0.2–0.5(–0.7) mm long, 0.2–0.4(–0.7) mm wide; corolla tube 0.3–0.5(–0.9) mm wide at faucal scales, 0.8–1.6 mm long from base to faucal scales, narrow cylindric. Stamens 5, included; filaments attached below faucal scales, filaments 0–0.1 mm long; anthers 0.2–0.3(–0.5) mm long, subsessile. Style 0.5–1.2 mm long (flowering) to 0.5–1.6(–2) mm long (fruiting). Nutlets 4, 0.9–1.2(–1.4) mm long, 0.5–0.8 mm wide.
Illustration citations
Fig. 4; Webb and Simpson (2001, p. 142), as M. pygmaea var. minutiflora; de Lange et al. (2010, pp. 300–301).
Distribution
NEW ZEALAND: North Island: Taranaki, Southern North Island; South Island: Canterbury and Otago (Fig. 4).

|
Habitats
North Island, Southern North Island: shore platforms, cliff-top herb fields and turfs, or beach gravels. South Island, Canterbury: in shingle or mud at seasonally inundated lake or tarn edges; Otago: dry, exposed, sunny, sometimes seasonally moist, alpine fell field, cushion field, eroded pasture, or turf. Elevation sea level to 1900 m.
Phenology
Flowering September–April. Fruiting October–April. Peak flowering and fruiting October–December.
Notes
Identification. Myosotis brevis is the smallest New Zealand forget-me-not. Plants of this species can be distinguished from all other Myosotis, including other pygmy forget-me-nots, on the basis of their smaller corolla diameter of 0.5–1.5(–2.0) mm, smaller calyx length at flowering of 0.7–1.7 mm and smaller nutlet size of 0.9–1.2(–1.4) mm long, 0.5–0.8 mm wide. When not in flower or fruit, plants of M. brevis can be difficult to distinguish from small plants of M. antarctica subsp. antarctica, because plants of both species have flexuous trichomes. However, plants of M. brevis are usually spring annuals (Rogers et al. 2002, as M. pygmaea var. minutiflora; de Lange et al. 2010), and it is rare to find plants that are not in either flower or fruit.
Taxonomic history. Myosotis brevis was first described as a variety of M. pygmaea (as var. minutiflora; Simpson and Thomson 1943). It was then elevated to species rank on the basis of its morphological distinctiveness (de Lange et al. 2010) and because the name M. minutiflora was already in use for a species with a European type, the replacement name of M. brevis was chosen. Species rank is considered to be appropriate for this taxon, given the multiple discrete morphological characters and molecular evidence that unites it (see below).
Patterns in the data. Myosotis brevis specimens can be distinguished both morphologically (Prebble et al. 2018) and genetically (Prebble et al. 2019). In an nMDS analyses of morphological characters measured on herbarium specimens, all samples of M. brevis group together (fig. 6A in Prebble et al. 2018). Multiple morphological characters were found to significantly distinguish M. brevis from other pygmy forget-me-not species in both the herbarium and growth-room datasets, for example, length of calyx at fruiting, floral lobe length and nutlet length. When molecular data from microsatellites was integrated with the morphological data from herbarium specimens, all populations identified as M. brevis formed a significantly differentiated cluster (fig. 6 in Prebble et al. 2019). When analysing just the molecular data, all M. brevis populations fall into a single cluster in the Structure analyses of K = 3 (fig. 3 in Prebble et al. 2019), and most populations of M. brevis form a group in the NeighbourNet network (fig. 5 in Prebble et al. 2019).
However, plants from three locations not identified as Myosotis brevis also grouped genetically with M. brevis in the Structure analyses of K = 3, including three populations of M. antarctica subsp. traillii from one area (North Island, Southern North Island: WELT SP090629, WELT SP090631 and WELT SP090634) and three populations of M. antarctica subsp. antarctica from two locations (South Island, Canterbury, Lake Tennyson: WELT SP100425; Campbell Island, Mt Honey: WELT SP102779 and WELT SP102780). Five of the six populations (the three populations of M. antarctica subsp. traillii and two populations of M. antarctica subsp. antarctica from Campbell Island) are likely to group with M. brevis because of an artefact of the analyses. Specifically, these five populations, together with populations of M. brevis, all have high numbers of unique alleles and allelic diversity (see scored microsatellite data in Prebble et al. 2020). In the Structure analysis of this dataset at K = 3, in which three groups must be recovered, these five populations and the populations of M. brevis cluster together on the basis of this, thus forming the group at K = 3 with the highest number of unique alleles and highest allelic diversity. Put another way, these five populations cluster together and with M. brevis because they are the most different from one another and from all other sampled populations, rather than because they are most similar to each other or to M. brevis. For the remaining population (from Lake Tennyson, Canterbury WELT SP100425) there may be a different explanation, because this specimen was identified by J. M. Prebble as M. antarctica subsp. antarctica (not M. brevis) only because of nutlet size (1.4 × 1.0 mm v. the range for M. brevis nutlets of 0.9–1.2 × 0.5–0.8 mm; flowers absent). However, given that it shares a habitat and morphology otherwise similar to M. brevis, and plants at this location have been identified as M. brevis previously (Rogers et al. 2002), this is a difficult population to classify. Nevertheless, when microsatellite and morphological data were co-analysed, this population did not group with M. brevis (fig. 6 in Prebble et al. 2019) and, on that basis, we identify this population as M. antarctica subsp. antarctica here.
Pollen morphology. Pollen of Myosotis brevis has the M. discolor morphology type, and although it fell into Cluster 1 in an nMDS analyses with other pollen identified as the M. australis type (see fig. 2 in Meudt 2016), its pollen is nevertheless the most distinctive of all bracteate-prostate species (Meudt 2016). This pollen morphology type was previously not known from New Zealand Myosotis and was hypothesised to represent parallel evolution owing to a shared annual habit between M. brevis and other Myosotis species with the M. discolor pollen type. Some individuals of M. australis and M. saxatilis have also been found to have this pollen type (Meudt et al. 2020).
Chromosome number. Unknown.
Recommended conservation status
Previously, Myosotis brevis was assessed as Threatened – Nationally Vulnerable C(3) with the qualifiers Extreme Fluctuations and Sparse (de Lange et al. 2018). Of all the pygmy forget-me-nots, Myosotis brevis was found to have the largest estimated census size (17 600), even though it had the smallest number of populations (35) (Table 6). Myosotis brevis has a large enough census size that it could be considered At Risk – Naturally Uncommon; however, its small area of occupancy (Table 6) and fluctuating population size (Rogers et al. 2002) mean that it is better placed in the Threatened, Naturally Vulnerable category (see Table 6 for details). Therefore, we recommend leaving the current conservation status and qualifiers unchanged from those of de Lange et al. (2018).
Threats. The main threats to Myosotis brevis are habitat loss, and invasive weeds leading to overshading (de Lange et al. 2010). North Island populations of M. brevis are more at risk than the South Island populations. The North Island populations are smaller on average (190 v. 1000 plants per population) and cover a smaller area on average (34 × 34 m v. 83 × 83 m). The M. brevis bare pavement habitat in Otago may be increasing (Rogers et al. 2002), whereas the coastal habitat in the North Island is at risk; for example, populations around Taranaki (WELT SP090361), Wairarapa (WELT SP090545) and Wellington (WELT SP090550) grow in habitat types that are themselves considered acutely threatened (Department of Conservation 2014). Furthermore, none of the North Island populations inhabits DOC-managed land (Supplementary Table S1). The populations around the North Island Taranaki coast appear particularly precarious; for example, the population visited at Puketapu Road (WELT SP090361) is less than 2 m from the edge of an eroding cliff, but the population cannot migrate inland because of farmland (J. M. Prebble, pers. obs., 2011). At least one population that has recently (c. 2005) gone extinct in the East Cape was most likely to be M. brevis; its habitat is thought to have been destroyed by wild goats (G. Atkins, pers. comm. 2012). The two most genetically distinct M. brevis populations are one from the North Island (Taranaki, St Road; WELT SP090543) and one from the South Island (Otago, Bendigo; WELT SP102760); these could be prioritised when it comes to potential conservation effort.
Representative specimens (58 specimens examined)
NEW ZEALAND. North Island: Taranaki: Puketapu Rd end, Nov. 1971, A. P. Druce s.n. (CHR 245911); Stent Rd, 5 Oct. 2011, H. M. Meudt HMM311, J. M. Prebble, C. Ogle, E. King, K. Eaton, G. La Cock, B. Clarkson, M. Parsons & B. Hartley (WELT SP090543). Southern North Island: Kawakawa Rocks, near Ngawi, 1 Nov. 2011, H. M. Meudt HMM313, J. M. Prebble, B. Sneddon & T. Silbery (WELT SP090545/A); Te Ikaamaru Bay, 7 Nov. 2011, H. M. Meudt HMM317, J. M. Prebble, P. Garnock-Jones & E. Robertson (WELT SP090549); Te Ohau Bay, 7 Nov. 2011, H. M. Meudt HMM318, J. M. Prebble, P. Garnock-Jones & E. Robertson (WELT SP090550/A). South Island: Canterbury, Lake Lyndon; 21 Feb. 2012, J. M. Prebble JMP12009 & M. Thorsen (WELT SP093294); Lake Lyndon, Nov. 1971, A. P. Druce s.n. (CHR 208536). Otago: Bannockburn, 8 Oct. 2013, J. M. Prebble JMP13045 (WELT SP102761); Bendigo, 8 Oct. 2013, J. M. Prebble JMP13044 (WELT SP102760); Chapman Rd Reserve, 9 Oct. 2013, J. M. Prebble JMP13046 (WELT SP102762); Hawkdun Range, 8 Dec. 2011, J. Barkla s.n. (WELT SP093498); Nevis Valley, Feb. 1992, A. P. Druce s.n. (CHR 476031); Springvale Reserve, 9 Oct. 2013, J. M. Prebble JMP13047 (WELT SP102763).
Myosotis glauca (G.Simpson & J.S.Thomson) de Lange & Barkla in P. J. de Lange et al. Threat. Pl. New Zealand 438 (2010)
Typification notes
The type citation mentions ‘specimens’ plural, but there is only one specimen at CHR (formerly BD) that matches the type citation, CHR 75722. However, AK 210591 has identical collection information. The AK specimen is considered to be a duplicate of the CHR specimen (E. Cameron, Auckland Museum, pers. comm.), so we can assume that it was once held at CHR and subsequently sent to AK, making this an isolectotype.
Description
Rosette plants with multiple prostrate branches up to 12 cm long. Rosette leaves 4–15; petioles 1.5–9.0 mm long; lamina usually flat, narrowly oblanceolate to broadly obovate, 3.7–17.0 mm long 1.5–7.0 mm wide (length:width ratio 1.3–3.5:1), dull greyish-green (glaucous) or occasionally bright green; apex obtuse, with hydathode on abaxial side; trichomes sparsely distributed, straight, antrorse, appressed to patent, appressed on margins, distributed evenly (on adaxial surface), and usually absent or occasionally sparsely distributed and on midrib (of abaxial surface), (0.2–)0.4–0.8(–1.2) mm long, deciduous with age. Basal cauline leaves not subtending flowers, 1–5 per branch, lamina similar in size and shape to the rosette leaves, with petioles up to 7.5 mm; distal cauline leaves subtending flowers up to 19 per branch, lamina 2.0–11.5 mm long, 1.0–5.0 mm wide, usually sessile. Pedicels up to 1.0 mm long (flowering) or 1.8 mm long (fruiting). Calyx 1.6–3.3 mm long (flowering) increasing to 2.5–7.8 mm long (fruiting), 1.3–4.3 mm wide at the top at fruiting, lobed to 1/4–1/2 the length of the calyx, with trichomes usually only along ribs both inside and outside the calyx, but occasionally present in between ribs. Corolla (1.0–)1.4–4.0 mm in diameter, white; faucal scales yellow; corolla lobes 0.3–1.3 mm long 0.2–1.0 mm wide; corolla tube 0.4–1.1 mm wide at faucal scales, 1.2–2.5(–3.2) mm long from base to faucal scales, narrow cylindric. Stamens 5, included; filaments attached below faucal scales, 0.0–0.1 mm long; anthers 0.4–0.9 mm long, subsessile; style 0.8–2.3 mm long (flowering) to 0.9–2.8 mm long (fruiting). Nutlets 4, (1.0–)1.2–1.5 mm long, (0.7–)0.8–1.2 mm wide.
Illustration citations
Fig. 5; Moore (1961, p. 808), as M. pygmaea var. glauca; Webb and Simpson (2001, p. 142), as M. pygmaea var. glauca; de Lange et al. (2010, pp. 404–405); Mark (2012, p. 257).
Distribution
NEW ZEALAND: South Island: Canterbury and Otago (Fig. 5).

|
Habitats
Fine semi-consolidated gravels on lake, tarn or stream edges, erosion fans, the base of tors, or old mine tailings. Depleted tussock-grassland, low grass turf. Elevation 180–1500 m.
Phenology
Flowering September–March. Fruiting October–April. Peak flowering and fruiting December–January.
Notes
Identification. Myosotis glauca plants can be distinguished from other pygmy forget-me-nots by their straight, appressed, non-overlapping trichomes and (usually) glaucous grey leaves. M. glauca as here circumscribed is known only from Central Otago and southern Canterbury. Specimens identified as M. glauca collected from the North Island Central Plateau previously identified as M. glauca (e.g. CHR 252337) do not solely have the straight, appressed leaf trichomes that characterise all other plants that fall under this species. Instead, a small number of straight, appressed trichomes are mixed with flexuous, patent trichomes, and therefore these specimens are better included in M. antarctica subsp. antarctica. Although most plants of M. glauca have glaucous green to grey leaves, some plants with brighter green leaves from the Pisa Range (previously identified as M. aff. glauca, e.g. WELT SP089898) cannot be distinguished from the remainder of M. glauca. Leaf colour variation is known from other pygmy forget-me-nots, notably M. brevis (Fig. 4), and thus these M. aff. glauca specimens are considered here to be M. glauca sens. str., which is variable in leaf colour. Recent collections of M. aff. glauca (a) “Mata-Au” (WELT SP104520) from the Clutha outwash are difficult to place owing to their unusual combination of glaucous leaf colour with flexuous trichomes, and require further study (more details below).
Taxonomic history. Myosotis glauca was first described as a variety of M. pygmaea (as var. glauca; Simpson and Thomson 1942). It was then elevated to species rank owing to its morphological distinctiveness (de Lange et al. 2010). Species rank is considered appropriate for this taxon, given the morphological and molecular evidence that defines it and distinguishes it from other species (see below). The morphological description given here differs subtly from that given by de Lange et al. (2010, p. 405). Specifically, two characters they identified as distinguishing M. glauca were not found here to be diagnostic, i.e. ‘…inner calyx surface midline of M. glauca is furnished with 4–5 shortly erect, stiff hairs’, and ‘broadly ovate rather than narrowly ovate nutlets (seeds)’. The surface of the inner calyx of M. glauca specimens was found to be sometimes glabrous, sometimes covered in short stiff hairs, and sometimes as described above by de Lange et al. (2010) (data not shown). The length to width ratio of M. glauca nutlets was not found to differ from that of M. antarctica, although nutlets of M. brevis did have a slightly higher length: width ratio on average (visible in Fig. 4 v. 5).
Patterns in the data. Specimens of Myosotis glauca are united by morphological (Prebble et al. 2018) and genetic (Prebble et al. 2019) data. In the nMDS analyses of morphological characters measured on herbarium specimens, all samples of M. glauca group together (fig. 6 in Prebble et al. 2018). Qualitative morphological characters distinguish M. glauca from all other pygmy forget-me-nots, i.e. leaf colour (usually glaucous-green to grey), and trichomes that are straight and appressed on the leaf blade and leaf margins (Fig. 5). In the analyses of microsatellite data, all populations of M. glauca form a cluster in the Structure analyses above K = 10 (fig. 3 in Prebble et al. 2019), and these populations also group together in the NeighbourNet network (fig. 5 in Prebble et al. 2019).
Five specimens identified as Myosotis aff. glauca (see appendix 1 in Prebble et al. 2018) cluster with those identified as M. glauca sens. str. on the basis of morphological data (fig. 6 in Prebble et al. 2018), and appear to differ only by having brighter green leaves than is usual for M. glauca. Only one individual identified as M. aff. glauca was included in the genetic dataset (WELT SP093282); so, little is known regarding genetic relationships, except that this one individual does not cluster with other M. glauca populations. Specimens identified as M. aff. glauca are therefore here considered part of M. glauca on the basis of morphological similarity. Recent collections identified as M. aff. glauca (a) “Mata-Au” (WELT SP104520), WELT SP108906 and WELT SP104520) are from a single locality from the terraces of glacial outwash gravels of the Clutha River/Mata-Au below Lake Wanaka, Otago, and appear to possess a unique suite of character traits compared with other pygmy Myosotis, i.e. a more erect or decumbent habit, purple stems, two leaf colour morphs (glaucous grey-green or brown) and flexuous, patent to erect trichomes. They have been observed to have a spring annual life cycle like many M. brevis (Geoff Rogers, formerly of DOC, pers. comm., July 2016). Apart from the more erect habit, they are somewhat morphologically intermediate between M. glauca and M. antarctica subsp. antarctica or M. brevis. Measurements of a single plant (that at the time was the only material available for study) was included in the nMDS analyses of morphological characters measured on herbarium specimens, which placed this sample within the cluster containing M. antarctica subsp. antarctica + M. brevis and not M. glauca, although with high uncertainty (fig. 6 in Prebble et al. 2018). Four individuals were included in the microsatellite dataset, and genetically M. aff. glauca (a) “Mata-Au” (WELT SP104520) does not appear to be affiliated with M. glauca on the basis of the Structure, nMDS and NeighbourNet network of microsatellite data (figs 3–5 in Prebble et al. 2019), but may be similar to the single included specimen of M. aff. glauca (WELT SP093282; on the basis of the Structure and NeighbourNet but not nMDS analyses). Although we do not consider M. aff. glauca (a) “Mata-Au” (WELT SP104520) to be included within M. glauca, with only a few collections from a single location, we are not yet confident in describing this putative taxon as a species. Further research into the pollen morphology and genetic affinities would be beneficial.
Pollen morphology. Pollen of Myosotis glauca has the M. australis morphology type, the most common pollen type for bracteate-prostrate Myosotis species (Meudt 2016) and the ebracteate-erect species sampled so far (Meudt et al. 2020). Representative specimens were recovered in both Clusters 1 and 2 in an nMDS analysis (see fig. 2. in Meudt 2016), along with all other pollen of the M. australis morphology type. The separation between Clusters 1 and 2 was not high, with several samples, including four of the five M. glauca specimens, having high uncertainty in their placement intermediate between the two clusters. The main morphological difference between the two clusters is that those specimens in Cluster 1 usually had eight pollen apertures, and those in Cluster 2 usually had 10 apertures, but this is not always a simple character to assign because some individuals are polymorphic for aperture number.
Chromosome number. Unknown.
Recommended conservation status
De Lange et al. (2018) listed Myosotis glauca as Threatened – Nationally Vulnerable C(3) with the qualifiers Data Poor and Sparse. It is clear from the data that Myosotis glauca indeed fits the criteria for Threatened – Nationally Vulnerable on the basis of both census size and small areas of occupancy and should maintain that conservation status (Table 6). The data qualifier Sparse should also be maintained, but the qualifier Data Poor is no longer applicable and should be replaced with Range Restricted (see Table 6 for more details).
Threats. The main threat to Myosotis glauca is considered to be weed invasion (de Lange et al. 2010). Myosotis glauca is the least common pygmy forget-me-not on the basis of estimated census size; it is found only in Central Otago and southern Canterbury, and only five of its populations (31%) grow on DOC-managed land (Table 6). At one of those sites (Lake Ohau, AK 280800), plants of M. glauca could not be found in 2013, and further searches are recommended. Populations from two locations included in the microsatellite analysis of Prebble et al. (2019) (Nevis valley: WELT SP093284 & WELT SP093285, and Macraes: WELT SP100497) are from areas both managed by DOC. With the decision to reject a proposal to dam the Nevis Valley (Environment Court decision, 2013, available at http://www.nzlii.org/cgi-bin/sinodisp/nz/cases/NZEnvC/2013/131.html?query=nevis, accessed 11 November 2020), the future of populations there has become more secure.
Representative specimens (39 specimens examined)
NEW ZEALAND. South Island: Canterbury: Lake Ohau, 27 Oct. 2002, A. E. Wright 12963 (AK 280800). Otago: base of Mt Ida, s. dat., G. Simpson & J. S. Thomson s.n. (AK 210591); Dunstan, 17 Jan. 2006, M. Thorsen s.n. (WELT SP089837); Kyeburn Diggings, 9 Dec. 2006, M. Thorsen s.n. (WELT SP089838); Macraes flats, 1 Mar. 2013, J. M. Prebble JMP13039 & K. Pilkington (WELT SP100497); mountains of Vincent County, s. dat., D. Petrie s.n. (WELT SP081871); Nevis Valley, 25 Apr. 2004, M. Thorsen s.n. (WELT SP089836); School House Flat, Nevis Valley, 15 Feb. 2012, J. M. Prebble JMP12003 & JMP12004 (WELT SP093284 & WELT SP093285); Tourist Spur on Mt Ida, 26 Apr. 1969, L. B. Moore s.n. (CHR 191750); Pisa Range, around snowfarm, 23 Jan. 2006, M. Thorsen s.n. (WELT SP089898); Pisa Range, Roaring Meg, 14 Feb. 2012, J. M. Prebble JMP12002 (WELT SP093282); western Pisa Range, 26 Jan. 2006, M. Thorsen s.n. (CHR 586018); western slopes of The Remarkables, 25 Jan. 1972, C. Meurk s.n. (OTA 34535).
Myosotis antarctica Hook.f., Fl. Antarct. 1(4): 57, t. 38 (1844)
Typification notes
Moore (1961) cited the type of the name Myosotis antarctica as being held at ‘K’ but did not cite a specimen. This is here treated as effective (first-step) lectotypification by Moore in accordance with ICN Art. 7.11 (Turland et al. 2018). Because there are two Hooker specimens at K collected from Campbell Island, second-step lectotypification is here effected. K 000787899 has a ‘Herbariorum hookerianum 1867’ stamp, and a note that reads ‘1609 Myosotis antarctica Hook.f. On rocky debris near the sea and at considerable elevation (1000 ft) Campbells Island December 1840’. There is also a pencil illustration pinned to the sheet with the number ‘1609’ in the corner. The drawing consists of recognisable drafts of the colour plate published in the protologue (reproduced here as Fig. 7). ‘TYPE specimen!’ is written on the sheet in a different pen. There are five plants making up the specimen, which is clearly distinguishable from the other specimen on the sheet (K 000787898; collected by T. Kirk in 1884 from ‘Dog Island’). The second specimen, K 000787901, which is on a separate sheet, has a ‘Herbariorum benthamianum’ stamp on it, and a note that reads ‘Myosotis antarctica Hook.f. Fl. Ant. p. 57 & 305 Campbell Island Hooker 1845’. Because it is less clear whether this second specimen has exactly the same collection information as the first, it is better excluded from the lectotype.
Description
Rosette plants with multiple prostrate branches up to 15(–31) cm long. Rosette leaves 4–22; petioles 1.0–20.0 mm long; lamina margins and apex sometimes curling under, narrowly oblanceolate to very broadly obovate, 3.0–26.0 mm long, 1.5–11.0 mm wide (length:width ratio 1.0–4.0(–6.0):(1), bright to dull green to reddish-brown, often with red–brown petioles and mid-veins; apex obtuse, with hydathode on abaxial side; trichomes densely distributed and often overlapping, curved or flexuous, antrorse, patent to erect, appressed or spreading on margins, distributed evenly (on leaf adaxial surface), and sparsely distributed, or on midrib only, or absent (on abaxial surface), (0.2–)0.5–1.1(–2.0) mm long, deciduous with age. Basal cauline leaves not subtending flowers, 1–5 per branch, lamina similar in size and shape to the rosette leaves, with petioles up to 8.8 mm; distal cauline leaves subtending flowers up to 46 per branch, lamina 1.4–16.0(–25) mm long, 0.8–7.0 mm wide, usually sessile. Pedicels up to 1.2 mm long (flowering) or 1.9 mm long (fruiting). Calyx 1.0–3.5 mm long (flowering) increasing to (2.0–)3.0–6.5 mm long (fruiting), 1.5–6.0 mm wide at the top at fruiting, lobed to 1/3–3/4 the length of the calyx; with trichomes sometimes of two lengths, longer and antrorse on ribs v. shorter and retrorse in between ribs and near the base (in other instances the two length classes are not so obvious, and retrorse trichomes are not always present). Corolla (1.0–)1.5–4.0 mm in diameter, white, cream, blue, faucal scales yellow; corolla lobes 0.5–1.5 mm long (0.2–)0.4–1.1(–1.3) mm wide; corolla tube 0.5–1.2(–1.5) mm wide at faucal scales, 1.2–2.8(–3.3) mm long from base to faucal scales, narrow cylindric. Stamens 5, included; filaments attached below faucal scales, 0–0.3 mm long; anthers 0.3–0.9 mm long, subsessile; style (0.7–)1.1–2.3 mm long (flowering) to (0.8–)1.1–2.8(–4.8) mm long (fruiting). Nutlets 4, (1.1–)1.2–1.9 mm long, (0.7–)0.8–1.2 mm wide.
Two subspecies of M. antarctica are recognised|
Trichomes on rosette leaves curved, and appressed to patent on lamina surfaces and margins; coastal localities of North, South and Stewart Islands (rarely inland) |
Myosotis antarctica Hook.f. subsp. antarctica
Typification notes
Moore (1961) cited the type of the name Myosotis pygmaea as ‘W, 4743, W. Colenso’, which is treated here as effective lectotypification. (Note that W was the old herbarium code for what is now WELT.) One other Colenso specimen of M. pygmaea also matches the type citation (WELT SP004744!) and may be original material; however, because it has a different collection date, it is excluded from the lectotype. Material at K mentioned by Moore (1961) was collected earlier and posted to W. J. Hooker in 1848 and 1850, so is not part of the type material (Colenso 1733, K 000787896!; Colenso 2499, K 000787897!: St George 2009, pp. 235, 251).
Description
Rosette plants with multiple prostrate branches up to 15(–31) cm long. Rosette leaves 4–22; petioles 1.0–20.0 mm long; lamina margins and apex sometimes curling under, narrowly oblanceolate to very broadly obovate, 3.0–26.0 mm long, 1.5–11.0 mm wide (length:width ratio 1.0–4.0(–6.0):1), bright to dull green to reddish-brown, often with red–brown petioles and mid-veins; apex obtuse, with hydathode on abaxial side; trichomes densely distributed and often overlapping, flexuous, antrorse, patent to erect, spreading at the margins, distributed evenly (on leaf adaxial surface), and sparsely distributed, or on midrib only, or absent (on abaxial surface), (0.2–)0.5–1.1(–2.0) mm long, deciduous with age. Basal cauline leaves not subtending flowers, 1–5 per branch, lamina similar in size and shape to the rosette leaves, with petioles up to 8.8 mm; distal cauline leaves subtending flowers up to 46 per branch, lamina 1.4–16.0(–25) mm long, 0.8–7.0 mm wide, usually sessile. Pedicels up to 1.2 mm long (flowering) or 1.9 mm long (fruiting). Calyx 1.0–3.5 mm long (flowering) increasing to (2.0–)3.0–6.5 mm long (fruiting), 1.5–6.0 mm wide at the top at fruiting, lobed to 1/3–3/4 the length of the calyx; with trichomes sometimes of two lengths, longer and antrorse on ribs v. shorter and retrorse in between ribs and near the base (in other instances, the two length classes are not so obvious, and retrorse trichomes are not always present). Corolla (1.0–)1.5–4.0 mm in diameter, white, cream, blue, faucal scales yellow; corolla lobes 0.5–1.5 mm long (0.2–)0.4–1.1(–1.3) mm wide; corolla tube 0.5–1.2(–1.5) mm wide at faucal scales, 1.2–2.8(–3.3) mm long from base to faucal scales, narrow cylindric. Stamens 5, included; filaments attached below faucal scales, 0–0.3 mm long; anthers 0.3–0.9 mm long, subsessile; style (0.8–)1.1–2.3 mm long (flowering) to (0.8–)1.1–2.8(–4.8) mm long (fruiting). Nutlets 4, (1.1–)1.2–1.9 mm long, (0.7–)0.8–1.2 mm wide.
Illustration citations
Fig. 6, 7; Hooker (1844, pl. 38), reproduced here as Fig. 7; Dusén (1900, p. 134), as M. albiflora; Moore (1961, p. 808), as M. pygmaea var. drucei; Mark and Adams (1973, p. 87), as M. pygmaea s.l.; Wilson (1994, p. 245), as M. pygmaea var. drucei; Wilson (1996, p. 221), as M. pygmaea var. drucei; Webb and Simpson (2001, p. 142), as M. pygmaea var. drucei; Mark (2012, p. 245), as Myosotis drucei.

|
Distribution
NEW ZEALAND: North Island: Gisborne, “Volcanic Plateau”, Southern North Island; South Island: Western Nelson, Sounds-Nelson, Marlborough, Westland, Canterbury, Otago, Southland, Fiordland; Stewart Island; Campbell Island; CHILE: Magallanes; Fig. 6.
Habitats
From coastal turf to subalpine damp semi-stable scree, cliff faces, incised runnels and fell-fields. Elevation from sea level to 2200 m.
Phenology
Flowering August–April. Fruiting September–April. Peak flowering and fruiting December–January.
Notes
Identification. Myosotis antarctica subsp. antarctica can be distinguished from M. glauca and M. antarctica subsp. traillii on the basis of its flexuous, patent to erect trichomes. It can be separated from M. brevis because of its generally larger size, for example, corolla diameter of (1.0–)1.5–4.0 mm, calyx length at flowering of (1.2–)2.0–3.0(–3.5) mm long and nutlets of (1.0–)1.2–1.9 mm long (0.7–)0.8–1.2 mm wide.
Taxonomic history. The name Myosotis antarctica was first published by Hooker (1844), for plants he collected on Campbell Island. Soon after, the name was also applied to specimens collected from elsewhere in New Zealand, for example, T. Kirk, 1877, Otago, Dart Valley (WELT SP043359) and J. Hector, s. dat. (but likely 1860s), Mount Aspiring Range (UPS V-702353). The name was first applied to Chilean specimens by Skottsberg (1915). The name M. pygmaea was published by Colenso (1884), on the basis of specimens he collected in the North Island. Although a few specimens collected from mainland New Zealand after this time were still identified as M. antarctica (e.g. Western Nelson, Mount Arthur, F. Gibbs, 1894, WELT SP002665), it became common usage to reserve the name M. antarctica for plants from Campbell Island and use M. pygmaea for all plants from the North, South and Stewart Islands, a convention which was formalised in the treatment by Moore (1961). Several varietal names within M. pygmaea were also published in the 1940s and 1960s and elevated to species rank by de Lange and Barkla in de Lange et al. (2010), i.e. M. pygmaea var. glauca was elevated to M. glauca, M. pygmaea var. minutiflora to M. brevis and M. pygmaea var. drucei to M. drucei. Two of those taxa are accepted here at species rank (see M. glauca and M. brevis above), but M. pygmaea, M. drucei and M. antarctica are considered to comprise a single species, for which M. antarctica is the earliest validly published name. The specimens that were previously identified as M. antarctica and M. drucei are considered best recognised as a single taxon at the rank of subspecies, as M. antarctica subsp. antarctica, given that they are united by morphological (Prebble et al. 2018) but not genetic data (Prebble et al. 2019), and they are mostly allopatric with respect to M. antarctica subsp. traillii (which encompasses specimens that were previously identified as M. pygmaea, except for the type; see below).
Not only does this circumscription of Myosotis antarctica subsp. antarctica include several previously described species, but it also subsumes two unnamed putative taxa. Those examined that are here considered to be part of this enlarged concept of M. antarctica subsp. antarctica are M. “Volcanic Plateau” (e.g. Volcanic Plateau; Kaimanawa Mountains: CHR 244442) and M. “intermedia” (e.g. Otago; Rock and Pillar Range, WELT SP089911; see table 1 in Prebble et al. 2019 for more details on these putative taxa). An additional species name, which has not been applied since its publication, M. ramificata (Simpson 1952), could not be distinguished morphologically from M. antarctica subsp. antarctica (fig. 6 in Prebble et al. 2018).
Patterns in the data. Specimens of Myosotis antarctica subsp. antarctica are united morphologically (Prebble et al. 2018) but not genetically (Prebble et al. 2019). In the nMDS analyses of morphological characters measured on herbarium specimens, all samples of M. antarctica subsp. antarctica group together (identified as M. antarctica, M. drucei, M. “intermedia” and M. “Volcanic Plateau”; fig. 6 in Prebble et al. 2018). Qualitative morphological characters found in both the herbarium and growth-room datasets distinguish M. antarctica subsp. antarctica from M. glauca and M. antarctica subsp. traillii, i.e. trichomes that are flexuous and patent to erect on the leaf blade and leaf margins. Despite the similarity in trichome types, multiple quantitative morphological characters distinguish this taxon from M. brevis (see Notes under that species).
Trichome density was the sole morphological character found to distinguish specimens identified as Myosotis drucei from an earlier narrow circumscription of M. antarctica that referred to plants from Campbell Island and Chile only. However, the ranges in trichome density overlap considerably, and so this character is not considered useful in this context (Prebble et al. 2018). Furthermore, no morphological characters were found to distinguish specimens identified as M. “Volcanic Plateau” or M. “intermedia” from M. antarctica subsp. antarctica.
Regarding the microsatellite data, some (but not all) populations of Myosotis antarctica subsp. antarctica group together in the Structure (fig. 3 in Prebble et al. 2019) and NeighbourNet network (fig. 5 in Prebble et al. 2019) analyses. There is geographic structure in the genetic data, whereby populations growing closer together are often more closely related, but this pattern is not universal. For example, some of the populations from Central Otago group together in the Structure and NeighbourNet network (e.g. WELT SP091599, WELT SP093286 and WELT SP093291), but the populations collected from Campbell Island (WELT SP102775, WELT SP102777, WELT SP102779, and WELT SP102780) are not more closely related to each other than they are to other populations on the North and South Islands. Some of the populations identified as M. “Volcanic Plateau” do cluster together genetically (WELT SP089738, WELT SP089909; see fig. 3 in Prebble et al. 2019, K = 24), but given the lack of morphological differentiation, and the presence of geographic clustering in the genetic data discussed already, this is not considered sufficient evidence to recognise this taxon. The two populations identified as M. “intermedia” (WELT SP093292 and WELT SP100498) included in the microsatellite dataset do not cluster together genetically (fig. 3 in Prebble et al. 2019).
Pollen morphology. Pollen of Myosotis antarctica subsp. antarctica has the M. australis morphology type, the most common pollen type for bracteate-prostrate Myosotis species (Meudt 2016) and for the ebracteate-erect species sampled so far (Meudt et al. 2020). Representative specimens were recovered in both Clusters 1 and 2 in an nMDS analyses (see fig. 2 in Meudt 2016) along with all other specimens with pollen of the M. australis morphology type. The majority of M. antarctica subsp. antarctica pollen samples were in Cluster 1 (8 of 10), with only two (as M. sp. “intermedia”) falling into Cluster 2, one with high uncertainty. The main morphological difference between the two clusters is that those specimens in Cluster 1 usually had eight pollen apertures, and those in Cluster 2 usually had 10 apertures; however, this is not always a simple character to assign because some individuals are polymorphic for aperture number.
Chromosome number. n = 24 (CHR 101449, as Myosotis pygmaea s.l.; Beuzenberg and Hair 1983); n = 22 (AK 331000, as M. aff. drucei–M. “Volcanic Plateau”, Murray and de Lange 2013). Although this could potentially be a character used to distinguish M. “Volcanic Plateau”, we consider that we do not know enough about chromosome number variation in Myosotis to know whether this difference is meaningful, particularly given M. antarctica subsp. traillii also has a count of n = 22 (see below) and disploidy appears to be a common feature of New Zealand Myosotis on the basis of the counts undertaken so far (e.g. 2n = 36, 40, 44, 46 and 48; Murray and de Lange 2013).
Recommended conservation status
Myosotis antarctica subsp. antarctica as circumscribed here was previously listed as two different species in de Lange et al. (2018). In that publication, it was assessed as Not Threatened (as M. drucei) and as At Risk – Naturally Uncommon with qualifiers Data Poor, Sparse and Threatened Overseas (as M. antarctica). Of all the pygmy forget-me-nots, M. antarctica subsp. antarctica has the highest number of estimated number of populations (299), but the smallest estimated average population size (40) (Table 6). Taking into account evidence of census size and area of occupation, it is recommended that Myosotis antarctica subsp. antarctica maintain the same conservation status as M. antarctica in de Lange et al. (2018), i.e. At Risk – Naturally Uncommon, Threatened Overseas (see Table 6 for more details).
When considering the Chilean populations and using the IUCN criteria (IUCN Species Survival Commission 2001), Data Deficient would be the most appropriate IUCN category for Myosotis antarctica subsp. antarctica in Chile. In total, nine herbarium specimens are known from southern Chile (Magallanes Region), and these have been collected only from two locations. Six of these specimens represent two collection events of M. antarctica from Punta Arenas (Lechler s.n. 1852, S15-37467, S15-37492 and K000573650; Dusén s.n. 1895, UPS V-702363, UPS V-702365 and UPS V-702371). Therefore, the most recent collection of M. antarctica from Punta Arenas was by Per Dusén in 1895. Punta Arenas is now a city with over 100 000 inhabitants and this population and any suitable habitat may no longer exist. There is a later collection possibly from the same area but with the less precise locality information of ‘Magellans Land’ collected by Andersson in 1905 (S15-37494). By contrast, when Carl Skottsberg visited the second known location, Puerto Altamirano, and collected two specimens in 1908 (UPS V-702372 and S15-37481), he encountered ‘…a resident (at that time the only one) in Puerto Altamirano…’ (Skottsberg 1941, p. 20), and the area is still sparsely populated today. The modelled niche for this species, projected into southern Chile, does not fit the known distribution in that region well at all, but, nevertheless, does suggest there may be additional suitable habitat for this species (Fig. 2). Because the most recent herbarium specimen was collected over 100 years ago in Magallanes, M. antarctica is potentially at a severe risk of extinction there. Botanists, landowners and conservation staff working in the area are encouraged to look for populations of M. antarctica at Puerto Altamirano and other locations nearby. Further survey work is essential to determine whether the species is still extant in Chile; its population sizes, extent and quality of habitat, and population-size trends are all unknown.
Threats. Over 70% of the populations of Myosotis antarctica subsp. antarctica on the North, South and Campbell Islands are growing on DOC-managed land, and can therefore be considered protected to some degree. Additionally, some of the other populations are in reserves managed by other organisations (e.g. local councils) and therefore can also be considered protected. However, the diminutive nature of these plants, like the other pygmy forget-me-nots, means that they are at risk of weed invasion. Little is known regarding threats to the Chilean populations.
Representative specimens (415 specimens examined)
NEW ZEALAND. North Island: Gisborne: Mt Hikurangi, Jan. 1954, A. P. Druce s.n. (CHR 86262). “Volcanic Plateau”: Waipakihi, 15 Feb. 2011, N. Singers s.n. (WELT SP089738); Moawhango, Kaimanawa Ranges, Jan. 1974, A. P. Druce s.n. (CHR 252337); Mt Tihia, South West of Lake Taupo, Apr. 1974, A. P. Druce s.n. (CHR 273143). Southern North Island: Ruahine Range, Te Hekenga, Feb. 1968, A. P. Druce s.n. (CHR 190683); Ruahine Range, Makirikiri tarns, Jan. 1977, A. P. Druce s.n. (CHR 310160). South Island: Marlborough: Tapuae-o-Uenuku, Feb. 1981, B. Molloy s.n. (CHR 386879); Mt Altimarlock, 8 Feb. 2013, J. M. Prebble JMP13026 & C. Jones (WELT SP100428). Western Nelson, Cobb Valley, near Lake Sylvester, 13 Jan. 1962, R. Melville 5961 & H. Telbot (CHR 142854). Canterbury: Banks Peninsula, Port Hills, Trig O, 19 Feb. 2012, J. M. Prebble JMP12007 (WELT SP093292). Otago, Macraes flat, 1 Mar. 2013, J. M. Prebble JMP13040 & K. Pilkington (WELT SP100498); Taieri River, Beaumont Station, 15 Dec. 2009, M. Thorsen 129/09 (WELT SP089916); Rock and Pillar Range, 2 Feb. 2014, J. M. Prebble JMP14002 & E. Connor (WELT SP102783). Fiordland: Beansburn, s. dat., B. D. Rance s.n. (WELT SP104524). Stewart Island / Rakiura: Mt Anglem, 6 Jan. 2000, P. J. de Lange 4109, (AK 251910). Campbell Island: Mt Azimuth; 27 Dec. 2013, J. M. Prebble JMP13065 & A. J. Fergus (WELT SP102777); Mt Honey, 28 Dec. 2013, J. M. Prebble JMP13067 & A. J. Fergus (WELT SP102779) and JMP13068 & A. J. Fergus (WELT SP102780). CHILE: Magallanes: Punta Arenas, 12 Dec. 1895, P. K. H. Dusén 173 (UPS V-702363); Skyring Sound, Puerto Altamirano, 22 Apr. 1908, C. Skottsberg s.n. (UPS V-702372).
Myosotis antarctica subsp. traillii Kirk, Trans. & Proc. New Zealand Inst. 16: 373 (1884)
Description
Rosette plants with multiple prostrate branches up to 20 cm long. Rosette leaves 4–22; petioles 1.0–20.0 mm long; lamina margins and apex sometimes curling under, oblanceolate to obovate, 6.5–22.0 mm long, 3.0–15.0 mm wide (length:width ratio 1.0–2.5:1), bright to dull green to reddish-brown; apex obtuse, with hydathode on abaxial side; trichomes densely distributed, curved, antrorse, appressed to patent, appressed at the margins, distributed evenly (on leaf adaxial surface), and sparsely distributed, or on midrib only, or absent (on abaxial surface), (0.2–)0.4–0.7(–1.2) mm long, deciduous with age. Basal cauline leaves not subtending flowers, 1–5 per branch, lamina similar in size and shape to the rosette leaves, with petioles up to 5.4 mm; distal cauline leaves subtending flowers up to 33 per branch, lamina 2.0–9.0 mm long, 1.0–5.5 mm wide, usually sessile. Pedicels up to 0.9 mm long (flowering) or 1.6 mm long (fruiting). Calyx 1.0–3.0 mm long (flowering) increasing to (2.0–)3.0–5.5 mm long (fruiting), 1.5–5.5 mm wide at the top at fruiting, lobed to 1/3–2/3 the length of the calyx; with trichomes usually of uniform length but denser along ribs, sometimes of two lengths, longer and antrorse on ribs v. shorter and retrorse in between ribs and near the base (in other instances, the two length classes are not so obvious, and retrorse trichomes are not always present). Corolla (1.0–)1.5–3.5 mm in diameter, white, cream, faucal scales yellow; corolla lobes 0.5–1.3 mm long (0.2–)0.4–1.0 mm wide; corolla tube 0.5–1.0 mm wide at faucal scales, 1.2–2.4 mm long from base to faucal scales, narrow cylindric. Stamens 5, included; filaments attached below faucal scales, 0–0.3 mm long; anthers 0.4–0.8 mm long, subsessile; style (0.7–)1.1–2.1 mm long (flowering) to 1.4–2.7 mm long (fruiting). Nutlets 4, 1.2–1.8 mm long, 0.8–1.2 mm wide.
Illustration citations
Fig. 8; Moore (1961, p. 808), as M. pygmaea var. pygmaea; Wilson (1994, p. 245), as M. pygmaea var. pygmaea [with note ‘=M. antarctica var. traillii’]; Webb and Simpson (2001, p. 142), as M. pygmaea var. pygmaea; Mark (2012, p. 256), as Myosotis pygmaea.
Distribution
NEW ZEALAND: North Island: Auckland, Taranaki, Southern North Island; South Island: Western Nelson, Canterbury, Otago, Southland; Stewart Island: Rakiura; mostly coastal (Fig. 8).

|
Habitats
Coastal turfs, sand dunes, fell fields, river terraces, and rock tors. Elevation from sea level to 250(–1500) m.
Phenology
Flowering August–April. Fruiting September–April. Peak flowering and fruiting December–January.
Notes
Identification. Plants of Myosotis antarctica subsp. traillii can be distinguished from M. glauca and M. antarctica subsp. antarctica on the basis of their curved, appressed to patent trichomes. Like M. antarctica subsp. antarctica, this subspecies can be separated from M. brevis because of its generally larger size, for example, corolla diameter of (1.0–)1.5–4.0 mm, calyx length at flowering of (1.2–)2.0–3.0(–3.5) mm long and nutlets of (1.0–)1.2–1.9 mm long and (0.7–)0.8–1.2 mm wide. Plants of M. antarctica subsp. traillii usually grow coastally (Fig. 8), but 18 specimens collected from inland populations with curved, appressed to patent trichomes have been identified; these are the populations that reach the higher elevations indicated above.
Taxonomic history. The name Myosotis antarctica subsp. traillii was first published by Kirk (1884). The name was not often applied to herbarium specimens (although see CHR 357370 collected by L. Cranwell in 1940), and in the Flora of New Zealand treatment, Moore (1961) considered it to be a synonym of M. pygmaea. However, most of specimens identified as M. pygmaea do not match the type of the species (WELT SP004743!), which has flexuous trichomes (fig. 6 in Prebble et al. 2018). The names M. pygmaea Colenso and M. antarctica subsp. traillii were published in two different papers in the same volume but different issues of the same journal, and Moore (1961, p. 815) noted the apparent similarities in the descriptions of these two taxa but did not discuss the differences in the trichomes between the two type specimens themselves. Thus, the epithet ‘pygmaea’ is unable to be used for this subspecies, because the type specimen for the name falls within the circumscription of the other subspecies, M. antarctica subsp. antarctica. In any case, M. pygmaea Colenso is an illegitimate name (see Discussion). The original description of M. antarctica subsp. traillii mentions the trichomes are ‘appressed’, which matches those plants generally identified as M. pygmaea in recent years. Furthermore, in nMDS analyses of morphological characters, the type of M. antarctica subsp. traillii clusters with all other specimens identified as ‘M. pygmaea’ apart from the type specimen of M. pygmaea (fig. 6 in Prebble et al. 2018).
Additional characters identified by Moore (1961) as characteristic of Myosotis pygmaea var. pygmaea v. M. pygmaea var. drucei (e.g. protruding nutlets) were found to not vary significantly between these taxa (here called M. antarctica subsp. traillii and M. antarctica subsp. antarctica respectively). Given that there is only a single morphological character that distinguishes these two subspecies, the possibilities of not recognising them as distinct or recognising them at the rank of variety were considered. However, given that the morphological differentiation seen here is correlated with allopatry (i.e. inland v. coastal on the North and South Islands), it was decided that it was appropriate to recognise these taxa at subspecies rank (e.g. Hamilton and Reichard 1992; Meudt 2006; Stuessy 2009).
Patterns in the data. All specimens of Myosotis antarctica subsp. traillii are united morphologically (Prebble et al. 2018) but not genetically (Prebble et al. 2019). In the nMDS analyses of morphological characters measured on herbarium specimens, all samples of M. antarctica subsp. traillii group together (fig. 6 in Prebble et al. 2018, identified as M. pygmaea, excluding the M. pygmaea type specimen). Qualitative morphological characters found in both the herbarium and growth-room datasets distinguish M. antarctica subsp. antarctica from M. glauca and M. antarctica subsp. traillii, i.e. trichomes that are curved and appressed to patent on the leaf blade and leaf margins.
In the Structure analyses of microsatellite data, not all populations of Myosotis antarctica subsp. traillii form a cluster (fig. 3 in Prebble et al. 2019, as M. pygmaea), and neither do these populations group together in the NeighbourNet network (fig. 5 in Prebble et al. 2019, as M. pygmaea). There is geographic structuring present in the genetic data, whereby populations that grow closer together are often more closely related, although this pattern is not universal. Five populations from Western Nelson in the South Island and coastal Taranaki in the North Island are united genetically (WELT SP100460, WELT SP100462, WELT SP090542, WELT SP090544 and WELT SP090540); these land areas would have been connected during the last glacial maxima (Lewis et al. 1994); so, this can be interpreted as a geographic pattern. No morphological characters were found to unite these five populations.
Pollen morphology. Pollen of Myosotis antarctica subsp. traillii has the M. australis morphology type, the most common pollen type for bracteate-prostrate species of Myosotis (Meudt 2016) and the ebracteate-erect species sampled so far (Meudt et al. 2020). Representative specimens were recovered in Cluster 1 in an nMDS analyses (see fig. 2 in Meudt 2016), along with other specimens with pollen of the M. australis morphology type.
Chromosome number. A count from one individual (identified as Myosotis pygmaea) has been undertaken, i.e. n = 22, AK 303514 (Murray and de Lange 2013).
Recommended conservation status
Myosotis antarctica subsp. traillii is listed as At Risk – Declining B(1) with the qualifier Sparse in de Lange et al. (2018, as M. pygmaea). Taking into account evidence of census size and small area of occupation, we recommend the conservation status of M. antarctica subsp. traillii be amended to Threatened – Nationally Vulnerable with the qualifier Sparse (see Table 6 for more details).
Threats. It has been recognised that Myosotis antarctica subsp. traillii is declining (de Lange et al. 2018, as M. pygmaea). As is the case with M. brevis (see above), the North Island populations are most at risk, as none of them inhabits DOC-managed land, and the same pressures of cliff-edge erosion and farmland proximity were seen at coastal Taranaki populations (i.e. WELT SP090540, WELT SP090542, and WELT SP090544). Two populations previously collected from the Wairarapa and Taranaki coasts (e.g. CHR 245912 and WELT SP095607) were not relocated when searching for them in 2011. The most genetically distinct M. antarctica subsp. traillii populations that could be considered a priority for conservation are from the North Island Hawke’s Bay region (WELT SP090629, WELT SP090631 and WELT SP090634), where they grow on rock outcrops on privately owned farmland.
Representative specimens (94 specimens examined)
NEW ZEALAND. North Island: Taranaki: Arawhata Rd end, 5 Oct. 2011, H. M. Meudt HMM310, J. M. Prebble, C. Ogle, E. King, K. Eaton, G. La Cock, B. Clarkson, M. Parsons & B. Hartley (WELT SP090542); Manihi Rd end, 6 Oct. 2011, H. M. Meudt HMM312, J. M. Prebble, E. King, K. Eaton, B. Clarkson, & B. Hartley (WELT SP090544); Opunake water treatment ponds, 5 Oct. 2011, H. M. Meudt HMM309, J. M. Prebble, C. Ogle, E. King, K. Eaton, G. La Cock, B. Clarkson, M. Parsons & B. Hartley (WELT SP090540); Puketapu Rd end, Nov. 1971, A. P. Druce s.n. (CHR 245912). Southern North Island: Te Waka Range, Jan. 1972, A. P. Druce s.n. (CHR 246383); Waipuna Station, 13 Dec. 2011, H. M. Meudt HMM333, J. M. Prebble, M. Thorsen and P. Carswell (WELT SP090631). South Island: Western Nelson, Cape Farewell, Whararkiki Beach, Nov. 1971, A. P. Druce s.n. (CHR 245193); Gordon’s Knob, 5 Feb. 1910, D. Petrie s.n. (WELT SP002650A); Hoary Head, 21 Jan. 2013, J. M. Prebble JMP13007 (WELT SP100472); north of Heaphy River, Nov. 1977, A. P. Druce s.n. (CHR 313155); near Sandhill Ck river mouth, 26 Jan. 2013, J. M. Prebble JMP13022 (WELT SP100460); ridge track to Mt Arthur, 22 Jan. 2013, J. M. Prebble JMP13009 (WELT SP100477); south of Paturau River mouth, 26 Jan. 2013, J. M. Prebble JMP13020 (WELT SP100462). Otago: Chrystall’s Beach, 27 Dec. 2004, M. Thorsen s.n. (WELT SP089920); Eyre Creek, headwaters of Little Jungle Creek, 6 Jan. 1987, A. F. Mark s.n. (OTA 044898). Southland, Oraka Point, 17 Jan. 2000, B. D. Rance s.n. (CHR 541256); Omaui, Three Sisters Dune, 8 Jan. 1995, P. J. de Lange s.n. (AK 231694); Tiwai Point, 25 Feb. 2013, J. M. Prebble JMP13031 & K. Pilkington (WELT SP100487). Stewart Island: Rakiura: Mason Bay, 13 Jan. 1882, T. Kirk s.n. (AK 7443).
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
Jennifer Tate is an editor for Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This research was supported by core funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment’s Science and Innovation Group, and by funding from Massey University, the Royal Society of New Zealand, Project Tongariro, The Eastbourne Bays Community Trust, the Enderby Trust and the Museum of New Zealand Te Papa Tongarewa.
Supplementary material
Supplementary material is available online.
Acknowledgements
This paper forms part of the Massey University PhD thesis of the first author, Jessica M. Prebble (2016). For collecting samples or help in the field, thanks go to Graeme Atkins, John Barkla, Jesse Bythell, Jan Clayton-Green, Shannel Courtney, Peter de Lange, Alex Fergus, Rowan Hindmarsh-Walls, Cathy Jones, Carlos Lehnebach, Graeme Low, Colin Ogle, Kay Pilkington, Brian Rance, Geoff Rogers, Neill and Barbara Simpson, Tony Silbury, Nick Singers, Mike Thorsen and Hugh Wilson. For helping with permits, location information, or assisting with access, thanks go to Castle Hill Station, Joy Comrie, Jacob Dexter, Paul Jansen, Graeme La Cock, Darren Peters, Viv McGlynn, Kiersten McKinley, New Zealand’s Aluminium Smelter, Rodney Russ, Te iwi o Ngātiwai, Bev and Allan Potts, Ryonier Forests, Catherine Warren, Tama Wipaki and the Aorangi Awarua Trust, and many additional New Zealand Department of Conservation staff. All field collections on New Zealand Department of Conservation land were made under Global Concession CA-5169-OTH. For funding thanks go to Massey University for a doctoral scholarship, the Royal Society of New Zealand for a grant from the Hutton Fund, the Australasian Systematic Botany Society for a Hansjörg Eichler Research Grant, Project Tongariro for a Memorial Award, the Eastbourne Bays Community trust for the Eastbourne Freemason’s Scholarship, and the Enderby Trust for a scholarship to join the Forgotten Islands of the South Pacific cruise with Heritage Expeditions. For comments on an earlier version thanks go to Mike Bayly, Peter de Lange, Gary Houliston, Anna Munro, Matt Renner, Rob Smissen and Richard Winkworth. For facilitating the transfer of many herbarium specimens thanks go to the curators and staff of AK, CHR, K, OTA, U, WELT and MPN. Thanks go to the Te Papa Botany team for hosting, mentoring and logistical support of J. M. Prebble.
References
Ahmadzadeh F, Flecks M, Carretero MA, Mozaffari O, Bohme W, Harris DJ, Freitas S, Rodder D (2013) Cryptic speciation patterns in Iranian rock lizards uncovered by integrative taxonomy. PLoS One 8, e80563| Cryptic speciation patterns in Iranian rock lizards uncovered by integrative taxonomy.Crossref | GoogleScholarGoogle Scholar | 24324611PubMed |
Alessandrini A (1840) Rendiconto delle sessione dell’accademia dell scienze dell’istituto di Bologna, 14a Sessione, 13 Febbraio 1840. Nuovi Annali delle Scienze Naturali 4, 435–443.
Bayly MJ, Kellow AV, de Lange PJ, Mitchell KA, Markham KR, Garnock-Jones PJ, Brownsey PJ (2003) Geographic variation in morphology and flavonoid chemistry in Hebe pubescens and Hebe bollonsii (Scrophulariaceae), including a new infraspecific classification for H. pubescens. New Zealand Journal of Botany 41, 23–53.
| Geographic variation in morphology and flavonoid chemistry in Hebe pubescens and Hebe bollonsii (Scrophulariaceae), including a new infraspecific classification for H. pubescens.Crossref | GoogleScholarGoogle Scholar |
Bertoloni A (1842) ‘Miscellanea Botanica 1.’ (ex typographaeo emygdii ab Ulmo: Bononiae)
Beuzenberg EJ, Hair JB (1983) Contributions to a chromosome atlas of the New Zealand flora—25. Miscellaneous species. New Zealand Journal of Botany 21, 13–20.
Bloomer RH, Lloyd AM, Symonds VV (2014) The genetic architecture of constitutive and induced trichome density in two new recombinant inbred line populations of Arabidopsis thaliana: phenotypic plasticity, epistasis, and bidirectional leaf damage response. BMC Plant Biology 14, 119
| The genetic architecture of constitutive and induced trichome density in two new recombinant inbred line populations of Arabidopsis thaliana: phenotypic plasticity, epistasis, and bidirectional leaf damage response.Crossref | GoogleScholarGoogle Scholar | 24885520PubMed |
Bourg NA, McShea WJ, Gill DE (2005) Putting a cart before the search: Successful habitat prediction for a rare forest herb. Ecology 86, 2793–2804.
| Putting a cart before the search: Successful habitat prediction for a rare forest herb.Crossref | GoogleScholarGoogle Scholar |
Buckley TR, Marske K, Attanayake D (2010) Phylogeography and ecological niche modelling of the New Zealand stick insect Clitarchus hookeri (White) support survival in multiple coastal refugia. Journal of Biogeography 37, 682–695.
| Phylogeography and ecological niche modelling of the New Zealand stick insect Clitarchus hookeri (White) support survival in multiple coastal refugia.Crossref | GoogleScholarGoogle Scholar |
Colenso W (1884) A further contribution towards making known the botany of New Zealand. Transactions and Proceedings of the New Zealand Institute 16, 325–344.
de Lange PJ, Cameron EK, Murray BG (1999) Alectryon excelsus subsp. grandis (Sapindaceae): a new combination for an uncommon small tree endemic to the Three Kings Islands, New Zealand. New Zealand Journal of Botany 37, 7–16.
| Alectryon excelsus subsp. grandis (Sapindaceae): a new combination for an uncommon small tree endemic to the Three Kings Islands, New Zealand.Crossref | GoogleScholarGoogle Scholar |
de Lange PJ, Heenan P, Norton D, Rolfe J, Sawyer J (2010) ‘Threatened Plants of New Zealand.’ (Canterbury University Press: Christchurch, New Zealand)
de Lange PJ, Rolfe JR, Barkla JW, Courtney SP, Champion PD, Perrie LR, Beadel SM, Ford KA, Breitwieser I, Schönberger I, Hindmarsh-Walls R, Heenan PB, Ladley K (2018) ‘Conservation status of New Zealand indigenous vascular plants, 2017.’ (Department of Conservation: Wellington, New Zealand)
de Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879–886.
| Species concepts and species delimitation.Crossref | GoogleScholarGoogle Scholar | 18027281PubMed |
Department of Conservation (2014) ‘Department of Conservation biodiversity indicators: 2014 assessment: supplementary material.’ (Department of Conservation: Wellington, New Zealand)
Dusén P (1900) ‘Die Gefässpflanzen der Magellansländer; nebst einem Beitrage zur Flora der Ostküste von Patagonien.’ (Norstedt: Stockholm, Sweden)
Edgar E (1986) Poa L. in New Zealand. New Zealand Journal of Botany 24, 425–503.
| Poa L. in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Edmondson JR (2017) The flora and fauna of the Euphrates Expedition of 1836. Israel Journal of Plant Sciences 64, 224–238.
Elith J, Graham C, Anderson R, Dudík M, Ferrier S, Guisan A, Hijmans RJ, Huettmann F, Leathwick JR, Lehmann A, Li J, Lohmann LG, Loiselle BA, Manion G, Moritz C, Nakamura M, Nakazawa Y, Peterson AT, Phillips SJ, Richardson K, Scachetti‐Pereira R, Schapire RE, Soberón J, Williams S, Wisz MS, Zimmermann NE (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29, 129–151.
| Novel methods improve prediction of species’ distributions from occurrence data.Crossref | GoogleScholarGoogle Scholar |
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population-size: implications for plant conservation. Annual Review of Ecology and Systematics 24, 217–242.
| Population genetic consequences of small population-size: implications for plant conservation.Crossref | GoogleScholarGoogle Scholar |
Frankham R (1995) Conservation Genetics. Annual Review of Genetics 29, 305–327.
| Conservation Genetics.Crossref | GoogleScholarGoogle Scholar | 8825477PubMed |
Frankham R, Ballou JD, Briscoe DA (2010) ‘Introduction to Conservation Genetics.’ (Cambridge University Press: Cambridge, UK)
Godsoe W (2010) Regional variation exaggerates ecological divergence in niche models. Systematic Biology 59, 298–306.
| Regional variation exaggerates ecological divergence in niche models.Crossref | GoogleScholarGoogle Scholar | 20525637PubMed |
Goudet J (2005) Hierfstat, a package for R to compute and test hierachical F-statistics. Molecular Ecology Notes 5, 184–186.
| Hierfstat, a package for R to compute and test hierachical F-statistics.Crossref | GoogleScholarGoogle Scholar |
Hamilton W, Reichard SH (1992) Current practice in the use of subspecies, variety, and forma in the classification of wild plants. Taxon 41, 485–498.
| Current practice in the use of subspecies, variety, and forma in the classification of wild plants.Crossref | GoogleScholarGoogle Scholar |
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25, 1965–1978.
| Very high resolution interpolated climate surfaces for global land areas.Crossref | GoogleScholarGoogle Scholar |
Hooker JD (1844) ‘The Botany of the Antarctic Voyage of H.M. discovery ships Erebus and Terror in the Years 1839–1843: under the command of Captain Sir James Clark Ross.’ (Reeves Brothers: London, UK)
IUCN Species Survival Commission (2001) ‘IUCN Red List categories and criteria.’ (International Union for Conservation of Nature: Gland, Switzerland)
Joly S, Heenan PB, Lockhart P (2014) Species radiation by niche shifts in New Zealand’s rockcresses (Pachycladon, Brassicaceae). Systematic Biology 63, 192–202.
| Species radiation by niche shifts in New Zealand’s rockcresses (Pachycladon, Brassicaceae).Crossref | GoogleScholarGoogle Scholar | 24335427PubMed |
Kirk T (1884) Description of new plants collected on Stewart Island. Transactions and Proceedings of the New Zealand Institute 16, 371–374.
Leathwick J, Morgan F, Wilson G, Rutledge D, McLeod M, Johnston K (2002) ‘Land environments of New Zealand: a technical guide.’ (Ministry for the Environment: Wellington, New Zealand)
Lehnebach CA (2008) Phylogenetic affinities, species delimitation and adaptive radiation of New Zealand Ranunculus. PhD thesis, Massey University, Manawatū, Palmerston North, New Zealand.
Lehnebach CA (2012a) Lectotypification of three species of forget-me nots (Myosotis: Boraginaceae) from Australasia. Tuhinga 23, 17–28.
Lehnebach CA (2012b) Two new species of forget-me-nots (Myosotis, Boraginaceae) from New Zealand. PhytoKeys 16, 53–64.
| Two new species of forget-me-nots (Myosotis, Boraginaceae) from New Zealand.Crossref | GoogleScholarGoogle Scholar |
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? Journal of Ecology 94, 942–952.
| How general are positive relationships between plant population size, fitness and genetic variation?Crossref | GoogleScholarGoogle Scholar |
Lewis KB, Carter L, Davey FJ (1994) The opening of Cook Strait: interglacial tidal scour and aligning basins at a subduction to transform plate edge. Marine Geology 116, 293–312.
| The opening of Cook Strait: interglacial tidal scour and aligning basins at a subduction to transform plate edge.Crossref | GoogleScholarGoogle Scholar |
Mark A (2012) ‘Above the Treeline: a Nature Guide to Alpine New Zealand.’ (Craig Potton Publishing: Nelson, New Zealand)
Mark AF, Adams NM (1973) ‘New Zealand Alpine Plants.’ (Reed Methuen Publishers: Auckland, New Zealand)
Meudt HM (2006) Monograph of Ourisia (Plantaginaceae). Systematic Botany Monographs 77, 1–188.
Meudt HM (2016) Pollen morphology and its taxonomic utility in the southern hemisphere bracteate-prostrate forget-me-nots (Myosotis, Boraginaceae). New Zealand Journal of Botany 54, 475–497.
| Pollen morphology and its taxonomic utility in the southern hemisphere bracteate-prostrate forget-me-nots (Myosotis, Boraginaceae).Crossref | GoogleScholarGoogle Scholar |
Meudt HM (2021) Taxonomic revision of five species groups of ebracteate-erect Myosotis (Boraginaceae) endemic to New Zealand based on morphology, and description of new subspecies. Australian Systematic Botany 34, 252–304.
| Taxonomic revision of five species groups of ebracteate-erect Myosotis (Boraginaceae) endemic to New Zealand based on morphology, and description of new subspecies.Crossref | GoogleScholarGoogle Scholar |
Meudt HM, Prebble JM (2018) Species limits and taxonomic revision of the bracteate-prostrate group of southern hemisphere forget-me-nots (Myosotis, Boraginaceae), including description of three new species endemic to New Zealand. Australian Systematic Botany 31, 48–105.
| Species limits and taxonomic revision of the bracteate-prostrate group of southern hemisphere forget-me-nots (Myosotis, Boraginaceae), including description of three new species endemic to New Zealand.Crossref | GoogleScholarGoogle Scholar |
Meudt HM, Prebble JM, Stanley RJ, Thorsen MJ (2013) Morphological and amplified fragment length polymorphism (AFLP) data show that New Zealand endemic Myosotis petiolata (Boraginaceae) comprises three rare and threatened species. Australian Systematic Botany 26, 210–232.
| Morphological and amplified fragment length polymorphism (AFLP) data show that New Zealand endemic Myosotis petiolata (Boraginaceae) comprises three rare and threatened species.Crossref | GoogleScholarGoogle Scholar |
Meudt HM, Prebble JM, Lehnebach CA (2015) Native New Zealand forget-me-nots (Myosotis, Boraginaceae) comprise a Pleistocene species radiation with very low genetic divergence. Plant Systematics and Evolution 301, 1455–1471.
| Native New Zealand forget-me-nots (Myosotis, Boraginaceae) comprise a Pleistocene species radiation with very low genetic divergence.Crossref | GoogleScholarGoogle Scholar |
Meudt HM, Thorsen MJ, Prebble JM (2020) Taxonomic revision of the Myosotis australis group (Boraginaceae) native to Australia, New Zealand and New Guinea. Australian Systematic Botany 33, 477–524.
| Taxonomic revision of the Myosotis australis group (Boraginaceae) native to Australia, New Zealand and New Guinea.Crossref | GoogleScholarGoogle Scholar |
Moore LB (1961) Boraginaceae. In ‘Flora of New Zealand. Vol. 1’. (Ed. HH Allan) pp. 806–833. (PD Hasselberg, Government Printer: Wellington, New Zealand)
Murray BG, de Lange PJ (2013) Contributions to a chromosome atlas of the New Zealand flora: 40. Miscellaneous counts for 36 families. New Zealand Journal of Botany 51, 31–60.
| Contributions to a chromosome atlas of the New Zealand flora: 40. Miscellaneous counts for 36 families.Crossref | GoogleScholarGoogle Scholar |
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Molecular Ecology 13, 1143–1155.
| Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants.Crossref | GoogleScholarGoogle Scholar | 15078452PubMed |
Paland S, Lynch M (2006) Transitions to asexuality result in excess amino acid substitutions. Science 311, 990–992.
| Transitions to asexuality result in excess amino acid substitutions.Crossref | GoogleScholarGoogle Scholar | 16484491PubMed |
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6, 288–295.
| GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research.Crossref | GoogleScholarGoogle Scholar |
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research: an update. Bioinformatics 28, 2537–2539.
| GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research: an update.Crossref | GoogleScholarGoogle Scholar | 22820204PubMed |
Phillips S (2010) A brief tutorial on MaxEnt. Lessons in Conservation 3, 107–135.
Phillips SJ, Dudik M, Schapire RE (2004) A maximum entropy approach to species distribution modeling. In ‘Proceedings of the 21st International Conference on Machine Learning’, 4–8 July 2004, Bamff, AB, Canada. (Ed. C Brodley) pp. 655–662. (ACM Press: New York, NY, USA)
| Crossref |
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecological Modelling 190, 231–259.
| Maximum entropy modeling of species geographic distributions.Crossref | GoogleScholarGoogle Scholar |
Prata EMB, Sass C, Rodrigues DP, Domingos FMCB, Specht CD, Damasco G, Ribas CC, Fine PVA, Vicentini A (2018) Towards integrative taxonomy in Neotropical botany: disentangling the Pagamea guianensis species complex (Rubiaceae). Botanical Journal of the Linnean Society 188, 213–231.
| Towards integrative taxonomy in Neotropical botany: disentangling the Pagamea guianensis species complex (Rubiaceae).Crossref | GoogleScholarGoogle Scholar |
Prebble JM, Tate JA, Meudt HM, Symonds VV (2015) Microsatellite markers for the New Zealand endemic Myosotis pygmaea species group (Boraginaceae) amplify across species. Applications in Plant Sciences 3, 1500027
| Microsatellite markers for the New Zealand endemic Myosotis pygmaea species group (Boraginaceae) amplify across species.Crossref | GoogleScholarGoogle Scholar |
Prebble JM, Meudt HM, Tate JA, Symonds VV (2018) Bolstering species delimitation in difficult species complexes by analysing herbarium and common garden morphological data: a case study using the New Zealand native Myosotis pygmaea species group (Boraginaceae). Systematic Botany 43, 266–289.
| Bolstering species delimitation in difficult species complexes by analysing herbarium and common garden morphological data: a case study using the New Zealand native Myosotis pygmaea species group (Boraginaceae).Crossref | GoogleScholarGoogle Scholar |
Prebble JM, Symonds VV, Meudt HM, Tate JA (2019) Comparing and co-analysing microsatellite and morphological data for species delimitation in the New Zealand native Myosotis pygmaea species group (Boraginaceae). Taxon 68, 731–750.
| Comparing and co-analysing microsatellite and morphological data for species delimitation in the New Zealand native Myosotis pygmaea species group (Boraginaceae).Crossref | GoogleScholarGoogle Scholar |
Prebble JM, Meudt HM, Tate JA, Symonds VV (2020) Data from: Comparing and co-analysing microsatellite and morphological data for species delimitation in the New Zealand native Myosotis pygmaea species group (Boraginaceae), Dryad, [Dataset].
| Data from: Comparing and co-analysing microsatellite and morphological data for species delimitation in the New Zealand native Myosotis pygmaea species group (Boraginaceae), Dryad, [Dataset].Crossref | GoogleScholarGoogle Scholar |
Pufal G (2010) The evolution and ecology of hygrochastic capsule dehiscence. PhD thesis, Victoria University of Wellington, Wellington, New Zealand.
Raxworthy C, Ingram C, Rabibisoa N, Pearson R (2007) Applications of ecological niche modeling for species delimitation: a review and empirical evaluation using day geckos (Phelsuma) from Madagascar. Systematic Biology 56, 907–923.
| Applications of ecological niche modeling for species delimitation: a review and empirical evaluation using day geckos (Phelsuma) from Madagascar.Crossref | GoogleScholarGoogle Scholar | 18066927PubMed |
Reeves PA, Richards CM (2011) Species delimitation under the general lineage concept: an empirical example using wild North American hops (Cannabaceae: Humulus lupulus). Systematic Biology 60, 45–59.
| Species delimitation under the general lineage concept: an empirical example using wild North American hops (Cannabaceae: Humulus lupulus).Crossref | GoogleScholarGoogle Scholar | 21088008PubMed |
Reginato M (2016) MonographaR: an R package to facilitate the production of plant taxonomic monographs. Brittonia 68, 212–216.
| MonographaR: an R package to facilitate the production of plant taxonomic monographs.Crossref | GoogleScholarGoogle Scholar |
Rissler LJ, Apodaca JJ (2007) Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus). Systematic Biology 56, 924–942.
| Adding more ecology into species delimitation: ecological niche models and phylogeography help define cryptic species in the black salamander (Aneides flavipunctatus).Crossref | GoogleScholarGoogle Scholar | 18066928PubMed |
Robertson A (1989) Evolution and pollination of New Zealand Myosotis (Boraginaceae). PhD thesis, University of Canterbury, Christchurch, New Zealand.
Rogers G, Walker S (2002) Taxonomic and ecological profiles of rarity in the New Zealand vascular flora. New Zealand Journal of Botany 40, 73–93.
| Taxonomic and ecological profiles of rarity in the New Zealand vascular flora.Crossref | GoogleScholarGoogle Scholar |
Rogers G, Walker S, Tubbs M, Henderson J (2002) Ecology and conservation status of three ‘spring annual’ herbs in dryland ecosystems of New Zealand. New Zealand Journal of Botany 40, 649–669.
| Ecology and conservation status of three ‘spring annual’ herbs in dryland ecosystems of New Zealand.Crossref | GoogleScholarGoogle Scholar |
Rolfe J, Makan T, Tait A (2021) Supplement to the New Zealand threat classification manual 2008: new qualifiers and amendments to qualifier definitions, 2021. (Department of Conservation: Wellington, New Zealand) Available at https://www.doc.govt.nz/globalassets/documents/science-and-technical/nztcs-supplement-2021.pdf
Schoener TW (1968) The Anolis Lizards of Bimini: resource partitioning in a complex fauna. Ecology 49, 704–726.
| The Anolis Lizards of Bimini: resource partitioning in a complex fauna.Crossref | GoogleScholarGoogle Scholar |
Simpson G (1952) Notes on some New Zealand plants and descriptions of new species (number 5). Transactions and Proceedings of the Royal Society of New Zealand 79, 426
Simpson G, Thomson JS (1942) Notes on some New Zealand plants and descriptions of new species (number 2). Transactions and Proceedings of the Royal Society of New Zealand 72, 21–38.
Simpson G, Thomson JS (1943) Notes on some New Zealand plants and descriptions of new species. Transactions and Proceedings of the Royal Society of New Zealand 73, 155–171.
Skottsberg C (1915) Notes on the relations between the floras of Subantarctic America and New Zealand. Plant World 18, 129–142.
Skottsberg C (1941) Communities of marine algae in Subantarctic and Antarctic waters. Kungliga Svenska Vetenskapsakademiens Handlingar Ser. 3 19, 1–92.
St George I (2009) ‘Colenso’s collections.’ (New Zealand Native Orchid Group: Wellington, New Zealand)
Stuessy TF (2009) ‘Plant Taxonomy: the Systematic Evaluation of Comparative Data.’ (Columba University Press: New York, NY, USA)
Thorsen MJ, Dickinson KJM, Seddon PJ (2009) Seed dispersal systems in the New Zealand flora. Perspectives in Plant Ecology, Evolution and Systematics 11, 285–309.
| Seed dispersal systems in the New Zealand flora.Crossref | GoogleScholarGoogle Scholar |
Tocchio LJ, Gurgel-Goncalves R, Escobar LE, Peterson AT (2015) Niche similarities among white-eared opossums (Mammalia, Didelphidae): is ecological niche modelling relevant to setting species limits? Zoologica Scripta 44, 1–10.
| Niche similarities among white-eared opossums (Mammalia, Didelphidae): is ecological niche modelling relevant to setting species limits?Crossref | GoogleScholarGoogle Scholar |
Townsend AJ, de Lange PJ, Duffy CAJ, Miskelly CM, Molloy J, Norton DA (2008) ‘New Zealand Threat Classification System Manual.’ (Science and Technical Publishing, Department of Conservation: Wellington, New Zealand)
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (Eds) (2018) ‘International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code)’, adopted by the Nineteenth International Botanical Congress, July 2017, Shenzhen, PR China. Regnum Vegetabile, vol. 159. (Koeltz Botanical Books: Glashütten, Germany)
| Crossref |
Van Valen L (1976) Species, multispecies, and oaks. Taxon 25, 233–239.
| Species, multispecies, and oaks.Crossref | GoogleScholarGoogle Scholar |
VanDerWal J, Shoo LP, Graham C, Williams SE (2009) Selecting pseudo-absence data for presence-only distribution modeling: How far should you stray from what you know? Ecological Modelling 220, 589–594.
| Selecting pseudo-absence data for presence-only distribution modeling: How far should you stray from what you know?Crossref | GoogleScholarGoogle Scholar |
Warren DL (2012) In defense of ‘niche modeling’. Trends in Ecology & Evolution 27, 497–500.
| In defense of ‘niche modeling’.Crossref | GoogleScholarGoogle Scholar |
Warren D, Glor R, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883.
| Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution.Crossref | GoogleScholarGoogle Scholar | 18752605PubMed |
Warren DL, Glor RE, Turelli M (2010) ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 33, 607–611.
| ENMTools: A toolbox for comparative studies of environmental niche models.Crossref | GoogleScholarGoogle Scholar |
Webb CJ, Simpson MJA (2001) ‘Seeds of New Zealand Gymnosperms & Dicotyledons.’ (Manuka Press: Christchurch, New Zealand)
Weigend M, Selvi F, Thomas DC, Hilger HH (2016) Boraginaceae. In ‘Flowering Plants. Eudicots, the Families and Genera of Vascular Plants’, Vol 14. (Eds JW Kadereit, V Bittrich)pp. 41–102. (Springer International Publishing: Cham, Switzerland)
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370.
Wilson H (1994) ‘Field Guide: Stewart Island Plants.’ (Manuka Press: Christchurch, New Zealand)
Wilson H (1996) ‘Wild Plants of Mt Cook National Park.’ (Manuka Press: Christchurch, New Zealand)
Winkworth R, Grau J, Robertson A, Lockhart P (2002) The origins and evolution of the genus Myosotis L. (Boraginaceae). Molecular Phylogenetics and Evolution 24, 180–193.
| The origins and evolution of the genus Myosotis L. (Boraginaceae).Crossref | GoogleScholarGoogle Scholar | 12144755PubMed |
Wood JR, Wilmshurst JM, Worthy TH, Cooper A (2012) First coprolite evidence for the diet of Anomalopteryx didiformis, an extinct forest ratite from New Zealand. New Zealand Journal of Ecology 36, 164–170.
Wooten JA, Gibbs HL (2012) Niche divergence and lineage diversification among closely related Sistrurus rattlesnakes. Journal of Evolutionary Biology 25, 317–328.
| Niche divergence and lineage diversification among closely related Sistrurus rattlesnakes.Crossref | GoogleScholarGoogle Scholar | 22111895PubMed |