Floods, fires and phytoplankton: some perspectives on water quality in the Gippsland Lakes
Perran Cook A * and Jonathan Smith B
A * and Jonathan Smith B
A
B
Abstract
The Gippsland Lakes form Australia’s largest estuary and suffer periodic blooms of toxic cyanobacteria that close the waterway for recreation and fishing. Here we discuss the underlying causes and sequence of events that lead to bloom formation, and the consequences as well as the history of these blooms prior to European colonisation. Efforts to prevent blooms have focused on catchment nutrient reduction efforts and we believe this is the most sensible approach, albeit one that is costly and long term.
Keywords: algal bloom, cyanobacteria, diatom, dinoflagellate, Gippsland Lakes, nitrogen, Nodularia, phosphorus.
Bloom succession
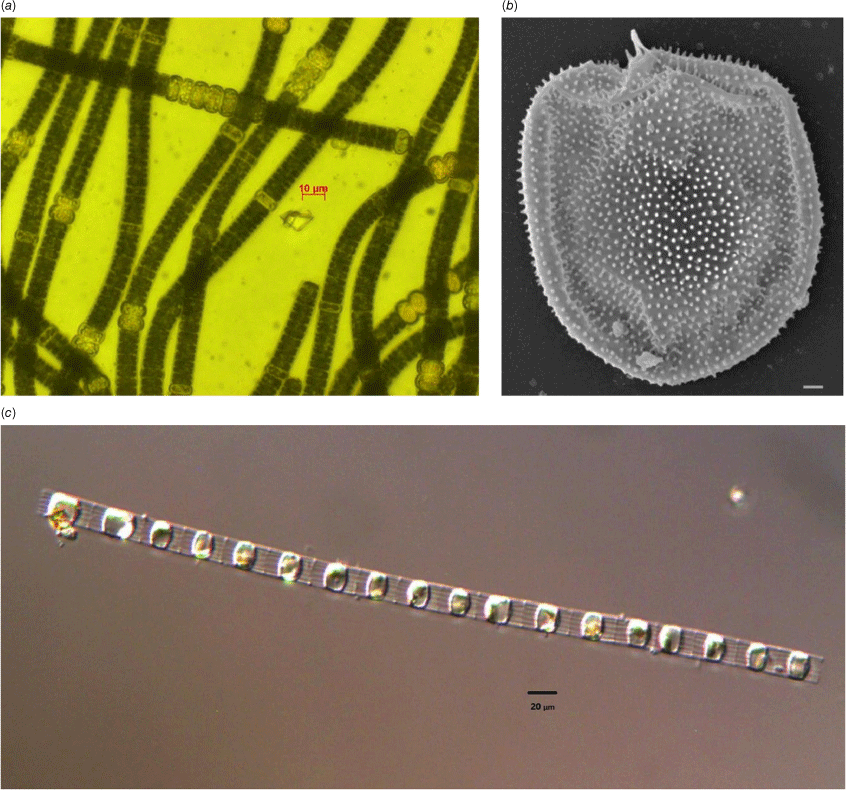
The Gippsland Lakes are well known for their recurring blooms of cyanobacteria, which mostly comprise the species Nodularia spumigena (Fig. 1a). Underlying these blooms, however, is a complex interaction between river flows, nutrient biogeochemistry and phytoplankton succession that supports the productivity of the Gippsland Lakes. Nodularia blooms typically take place in years of above average river flow, which generally occur over the winter months and into late spring (Cook and Holland 2012). These flows bring nitrogen and phosphorus into the lakes, as well as lowering the salinity of the lakes’ surface layer which leads to stratification. Over the winter months, this stimulates blooms of dinoflagellates such as Prorocentrum cordatum (Fig. 1b) and Gymnodinium sp., as well as ciliates such as Favella spp. and diatoms such as Skeletonema sp. (Fig. 1c) The size of these blooms is closely linked to the amount of nutrient inputs from the rivers entering the Gippsland Lakes, highlighting the close link between the catchments and system productivity (Cook and Holland 2012). These phytoplankton are rapidly grazed by zooplankton and have turnover times of ~0.5–2 days−1 (Holland et al. 2012). Additionally, recent work has highlighted the importance of flagellates in juvenile fish nutrition (McNaughton et al. 2022). These winter and spring blooms of diatoms, ciliates and flagellates play a critical role in supporting the food webs of the Gippsland Lakes.
Phytoplankton commonly observed in the Gippsland Lakes including (a) Nodularia spumigena, (b) Prorocentrum cordatum (scale bar 1 μm), (c) Skeletonema costatum. Images by J. Smith.
As the season progresses from spring into summer, river flows typically decline, which means that nutrient recycling is the dominant source of nitrogen and phosphorus for phytoplankton. Stratified conditions lead to low oxygen levels in the bottom waters of the Gippsland Lakes, stimulating phosphorus release into the water from the sediment (Cook et al. 2010). This is a critical process in the Gippsland Lakes, and our own research, as well as that of CSIRO, suggests that phosphorus releases can, at times, be more rapid than might be expected from diffusion alone (Webster et al. 2001; Zhu et al. 2016). We believe that this process can be enhanced by the presence of benthic fauna such as capitellid worms. The burrowing action of these organisms greatly enhances oxygenation of the sediment leading to the accumulation of iron oxide and phosphorus during oxic periods in the water column. Upon the onset of low oxygen or anoxic conditions, these large pools of phosphorus are released into the water column (Scicluna et al. 2015; Zhu et al. 2016). Nitrogen recycling processes, in contrast, lead to a loss of nitrogen through the process of denitrification. This in turn leads to the depletion of nitrogen relative to phosphorus to well below the critical ‘Redfield Ratio’ of 16N:1P required for phytoplankton growth (Cook et al. 2010). Stratified and nitrogen-limited conditions are highly conducive to Nodularia blooms because these organisms are capable of fixing nitrogen from the atmosphere, thereby alleviating nitrogen limitation. In addition, these organisms can control their buoyancy, accumulating at the surface to meet their light requirements and sinking to the bottom to meet their phosphorus requirements. The exact trigger for the bloom initiation (and akinete spore germination) is unclear; however, blooms seem to occur during periods of prolonged warm and calm weather.
Once initiated, the size and duration of Nodularia blooms are most likely controlled by the period of favourable weather conditions. The size of the bloom appears unrelated to catchment nutrient inputs, reflecting the importance of internal recycling (Cook and Holland 2012). Nodularia produces the hepatotoxin nodularin which results in the Gippsland Lakes being closed for recreational purposes during blooms. In addition, studies have shown nodularin makes its way into fish and crustaceans both within the Gippsland Lakes as well as in Bass Strait (Victorian Health Department, pers. comm.). The exact mechanism of this transfer remains unclear; however, both passive uptake from the water column and via the food web are possible. Although cyanobacteria are generally unpalatable to grazing organisms, we have found that nitrogen from Nodularia can make its way into the food web. Nitrogen that has been newly ‘fixed’ by Nodularia has a unique isotope signature that allows us to trace the progression of this nitrogen into other phytoplankton and fish such as bream. While making these isotope measurements, we simultaneously conducted grazing experiments that showed Nodularia were not directly grazed by zooplankton. Instead, it seems likely that zooplankton such as Oncaea sp. were scraping epibiont bacteria off the surface of Nodularia (Woodland et al. 2013). It is unclear if this mechanism could be a vector for nodularin transfer. Further work is required to understand the movement of nodularin through the food web of the Gippsland Lakes.
The impact of fire
Not all high-flow years follow the above progression, with some wet years having no blooms of Nodularia. Of particular note were the years 2007‒2008, which experienced extensive fires in the catchment during the summer of 2007. In June, a flood led to the highest loads ever recorded of nitrogen and phosphorus into the lakes. Nitrate loads were particularly high and high concentrations of the nitrogen and chlorophyll persisted in the water column over the summer and into the winter of 2008. These high concentrations were associated with a bloom of the pico-cyanobacteria Synechococcus sp. (Cook and Holland 2012). The small size of this cyanobacterium means that it does not settle to the sediment and it also appears to be a poor food source for grazers, resulting in its long persistence. The ‘Black Summer’ fires of 2019–2020 also affected the Gippsland Lakes catchments, in particular for the Mitchell, Tambo and Nicholson rivers. In this instance, a key impact was extremely large loads of suspended sediments (Kirono et al. 2022), which led to localised fish kills within these river estuaries. No notably large algal blooms were recorded after this event.
The history of blooms
While reports of Nodularia blooms in the Gippsland Lakes have existed since the 1960s, it is less clear whether these are a modern phenomenon or have a longer history. Anecdotes from the 1880s of fishermen being poisoned by toxic water exist in fisherman Jock Carstairs’ diary (Synan 1989). To explore this, we used paleo-proxies in cores retrieved from Lake King. This work suggested that prior to the opening of the entrance in 1880, the lakes were fresher, as expected, but that they were also in a more eutrophic state as indicated by higher sediment organic carbon content, and the presence of cyanobacteria pigments. Nitrogen isotopes also suggest more nitrogen fixation by cyanobacteria. The fresher state of the Gippsland Lakes is likely a key factor that promoted the apparently higher abundance of cyanobacteria during this period. In addition, we observed much higher levels of charcoal in the sediment prior to the 1880s. As noted previously, bushfires can lead to increased nutrient loads to the lakes and it is possible that the lakes were maintained in a eutrophic state by cultural burning practices (or possibly burning as a retaliation response to colonisation). After the opening of the entrance, there was a period of relative oligotrophy with lower sediment organic carbon contents and cyanobacteria pigments. From the 1950s onwards, proxies of eutrophication began to increase, coincident with a rapid increase in urbanisation and agricultural intensity in the catchment (Cook et al. 2016).
Prevention of blooms
Given the severe socio-economic impacts of Nodularia blooms, there has been much interest their prevention. In the Peel–Harvey Estuary, Western Australia, the magnitude of the bloom size has been greatly reduced by the construction of a second artificial entrance. This raised salinities above the threshold suitable for Nodularia (Huang et al. 2020) which is typically in the range of 9–20 PSU (Cook and Holland 2012). This approach has also been explored using models, but these studies have concluded that a second entrance into the Gippsland Lakes is unlikely to have a significant impact on bloom size (Webster et al. 2001). Other engineering approaches such as destratification, which may reduce sediment phosphorus release, have been discussed, but the size of the Gippsland Lakes means the costs are most likely prohibitive. At present, the major focus is on reducing loads of nitrogen and phosphorus to the lakes through catchment revegetation and farm nutrient management strategies. Although this is an expensive and long-term program, the numerous other water quality and ecological co-benefits mean this is probably the most prudent approach.
Declaration of funding
Current funding for research for P. Cook comes from the West Gippsland CMA, the Australian Research Council, DEECA, Gippsland Water, EPA and Southern Rural Water as part of a Linkage Grant LP190101250.
References
Cook PLM, Holland DP (2012) Long term nutrient loads and chlorophyll dynamics in a large temperate Australian lagoon system affected by recurring blooms of cyanobacteria. Biogeochemistry 107, 261-274.
| Crossref | Google Scholar |
Cook PLM, Holland DP, Longmore AR (2010) Effect of a flood event on the dynamics of phytoplankton and biogeochemistry in a large temperate Australian lagoon. Limnology & Oceanography 55, 1123-1133.
| Crossref | Google Scholar |
Cook PLM, Jennings M, Holland DP, et al. (2016) Blooms of cyanobacteria in a temperate Australian lagoon system post and prior to European settlement. Biogeosciences 13, 3677-3686.
| Crossref | Google Scholar |
Holland D, Van Erp I, Beardall J, Cook P (2012) Environmental controls on the nitrogen-fixing cyanobacterium Nodularia spumigena in a temperate lagoon system in SE Australia. Marine Ecology Progress Series 461, 47-57.
| Crossref | Google Scholar |
Huang P, Hennig K, Kala J, Andrys J, Hipsey MR (2020) Climate change overtakes coastal engineering as the dominant driver of hydrological change in a large shallow lagoon. Hydrology and Earth System Sciences 24, 5673-5697.
| Crossref | Google Scholar |
McNaughton C, Cook PLM, Wong WW, et al. (2022) Environmental flows stimulate estuarine plankton communities by altered salinity structure and enhanced nutrient recycling. Estuarine, Coastal and Shelf Science 279, 108157.
| Crossref | Google Scholar |
Scicluna TR, Woodland RJ, Zhu Y, Grace MR, Cook PLM (2015) Deep dynamic pools of phosphorus in the sediment of a temperate lagoon with recurring blooms of diazotrophic cyanobacteria. Limnology & Oceanography 60, 2185-2196.
| Crossref | Google Scholar |
Woodland RJ, Holland DP, Beardall J, Smith J, Scicluna T, Cook PL (2013) Assimilation of diazotrophic nitrogen into pelagic food webs. PLoS One 8, e67588.
| Crossref | Google Scholar | PubMed |
Zhu Y, Hipsey MR, McCowan A, Beardall J, Cook PLM (2016) The role of bioirrigation in sediment phosphorus dynamics and blooms of toxic cyanobacteria in a temperate lagoon. Environmental Modelling & Software 86, 277-304.
| Crossref | Google Scholar |

