Reproduction, Fertility and Development
Volume 37 Number 1 2025
Proceedings of the Annual Conference of the International Embryo Technology Society, 18–22 January 2025
Fort Worth, Texas, USA
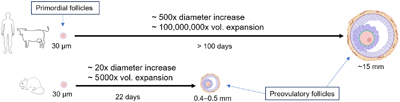
Producing eggs and sperm in the laboratory is a novel technology that has the potential to reduce the impact of animal agriculture on our planet and accelerate the genetic improvement of cattle. However, there are many pieces of information that we still need to learn about how cows reproduce before we can take advantage of this technology. Recent scientific advances are helping fill these gaps and should lead us toward more efficient and sustainable agriculture. Image by Anna C. Denicol.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
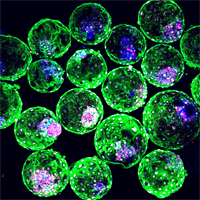
Advancements in stem cell and developmental biology have propelled the generation of stem-cell-derived tools that mirror early mammalian development. These fascinating structures, exemplified by the human blastoids shown here that mimic blastocyst formation, have offered unparalleled opportunities to unveil the mysteries of life’s genesis and explore limitless potential applications beyond our imaginations. Image sourced from Yu et al. (2023) with permission (https://doi.org/10.1016/j.stem.2023.08.002).
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
RD24138 Abstract | RD24138 Full Text | RD24138PDF (4.1 MB) Open Access Article
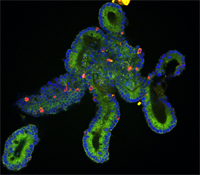
Identifying methods to improve animal health, production, and resilience is critical to create a sustainable food supply to meet global nutritional demand. Recent advancements in gene editing tools, cell culture methods, and in vitro phenotyping can accelerate the identification of variants or novel alleles that are transformative for livestock health and resilience. The use of organoids and gene editing can provide a model to link the genome to the phenome and decrease the number of animals needed for research. Image by Eun Su Jeon and the University of Missouri Molecular Cytology Core.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
RD24135 Abstract | RD24135 Full Text | RD24135PDF (1.7 MB) Open Access Article
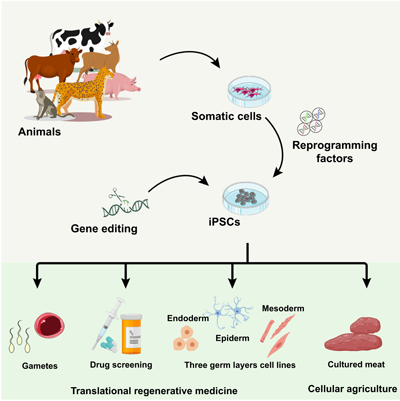
RD24136Use of induced pluripotent stem cells for regenerative medicine and understanding of cell biology
Induced pluripotent stem cells (iPSCs) are a valuable tool not only for in vitro disease modeling and in vivo therapies but also emerging as a new alternative for improving animal production and breeding. Recent potential uses of stem cells, focusing on generating and using iPSCs from diverse species, are presented and discussed. Genetic conservation or dissemination using in vitro gametogenesis, cellular therapies, and cellular agriculture are examples. Image created with BioRender.com.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
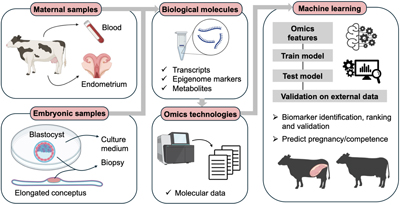
RD24141The role of machine learning in decoding the molecular complexity of bovine pregnancy: a review
High embryo losses in cattle negatively impact the livestock industry. Modern techniques can measure the biological molecules involved in embryo–maternal communication at a large scale. These datasets are usually analysed through traditional pipelines. However, machine learning (ML) tools can learn from data to make predictions, helping decipher the complexity of biological data. Here, we review the contribution of ML in understanding pregnancy establishment in cattle, as well as the current challenges and future potential of ML in this field. Diagram created with BioRender.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
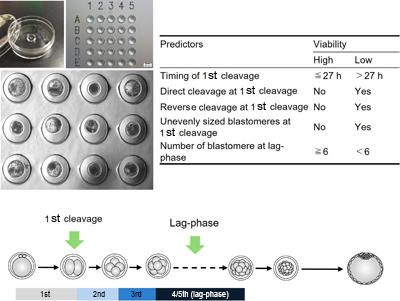
RD24139Morphokinetic prediction of embryo viability in cattle
Conventionally, the quality of bovine IVP embryos is evaluated around day 7–8, and the process is subjective and operator-dependent. A new approach using time-lapse monitoring in specialized culture dishes improves accuracy by continuously tracking individual embryo development. When predicting embryo viability, it is crucial to emphasize the importance of early cleavage stage morphokinetics rather than relying on morphological evaluation based on blastocyst snapshots at the time of transfer. Image by Satoshi Sugimura.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
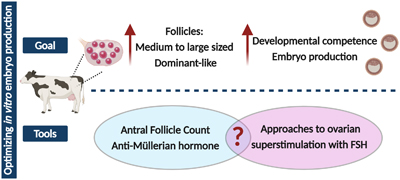
RD24143Advances in synchronization and superstimulation for OPU/IVEP: optimizing oocyte quantity and quality
In vitro embryo production plays a critical role in the genetic improvement of cattle. Efficient in vitro embryo production requires the collection of multiple oocytes of optimal quality. This review describes the contribution of ovarian physiology to oocyte quantity and quality as well as the current status of ovarian superstimulation treatments directed at improving the efficacy of in vitro embryo production. In addition, the possibility of developing targeted ovarian superstimulation treatments based on distinct ovarian physiological characteristics is explored. Image by J. C. L. Motta and A. García-Guerra using Biorender.com.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
RD24143 Abstract | RD24143 Full Text | RD24143PDF (2.2 MB) Open Access Article
Nonsurgical embryo transfer in pigs is now a reality. This procedure was previously deemed impossible because of the complex anatomy of the porcine female reproductive tract. However, advancements have overcome these anatomical challenges and allowed embryos to be nonsurgically deposited deep into the uterine horn. The technique is simple and safe, and the promising results with stored and vitrified–warmed embryos mark a significant advance. This technology facilitates genetic exchange with minimal disease transmission, which benefits the pig industry. Photograph by E. A. Martinez.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
RD24137 Abstract | RD24137 Full Text | RD24137PDF (686 KB) Open Access Article
Current dairy breeding systems in tropical zones rely on crossbreeding between native, adapted breeds, and foreign high-performance genetics to increase milk yield. When the proportion of foreign-derived genetics becomes too high in subsequent generations of breeding, the animals typically fail to fit in the production environment due to unmanageable heat stress and susceptibility to endemic diseases. To overcome these types of genetic by environmental challenges, we bred animals with traits delivered through a multiplex gene editing platform that will contribute to significant and sustainable production gains for tropical dairy production systems. Photographs by Haley Jo.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
RD24145 Abstract | RD24145 Full Text | RD24145PDF (325 KB) | RD24145Supplementary Material (502 KB) Open Access Article
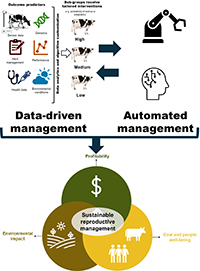
RD24148Data-driven technologies and management practices for improving the sustainability of reproductive management
Dairy farms must constantly evolve to achieve sustainability goals, including profitability, minimal environmental impacts, and improve well-being of cows and people. Data-driven management practices and automated technologies are a growing opportunity for improving the sustainability of reproductive management. Data-driven targeted reproductive interventions can shorten interbreeding intervals, increase fertility, and reduce interventions on cows. Likewise, advances in technology enable automation of management tasks including detection of estrus, ovulation synchronization, and pregnancy testing. These innovations are reshaping reproductive management of dairy cattle. Image created by Julio Giordano.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.
RD24148 Abstract | RD24148 Full Text | RD24148PDF (1.8 MB) Open Access Article
RD24144Organs-on-a-chip to understand reproduction
Organs-on-a-chip technology is transforming reproductive health research by offering unprecedented insights into complex reproductive physiology. These micro-engineered systems aim to recreate the conditions of the body, providing scientists new knowledge which can be used to address reproductive issues. This technology could significantly enhance fertility treatments, drug testing, and our understanding of human and animal reproduction. Image created with BioRender.com.
This article belongs to the Collection: Proceedings of the Annual Conference of the International Embryo Technology Society, Fort Worth, TX, USA, 18–22 January 2025.