Melatonin administration during the first half of pregnancy improves physiological response and reproductive performance of rabbits under heat stress conditions
Nesrein M. Hashem A B * , Elshymaa A. Abdelnaby C D , Mahmoud Madkour E and Hossam R. El-Sherbiny D
A B * , Elshymaa A. Abdelnaby C D , Mahmoud Madkour E and Hossam R. El-Sherbiny D
A
B
C
D
E
Handling Editor: Jose Alfonso Abecia
Abstract
Melatonin may have a heat-stress-alleviating role during pregnancy.
To investigate the effects of melatonin administration during the first half of pregnancy on heat-tolerance capacity and pregnancy outputs of naturally heat-stressed rabbits.
Forty female rabbits were stratified equally into two experimental groups and daily received 1 mg melatonin/kg body weight or not (control) for 15 consecutive days post-insemination. Heat tolerance indices, hormone profile, ovarian structures, and fetal loss were determined.
Treatment with melatonin significantly decreased respiration rate and rectal temperature, improved concentrations of nitric oxide, and tended to decrease malondialdehyde concentrations (P = 0.064) compared to control. Melatonin treatment significantly increased concentrations of high-density lipoprotein, oestradiol, and progesterone compared to control. No significant differences in the numbers of visible ovarian follicles, corpora lutea, and total implantation sites on day 18 of pregnancy were observed between experimental groups. However, melatonin treatment significantly reduced the number of absorbed implantation sites and significantly improved amniotic fluid volume and conception rate compared to control.
Melatonin administration during the first half of pregnancy can improve reproductive performance of heat-stressed female rabbits.
Melatonin can improve fetal survivability via improving heat-tolerance capacity of does and steroidogenesis.
Keywords: embryonic/fetal loss, melatonin, placenta, rabbit, reproduction, thermal stress, steroidogenesis.
Introduction
Rabbits are among the most sensitive farm animals to the negative impacts of heat stress. They have few sweat glands and depend on respiratory panting for heat loss. This main heat tolerance adaptive mechanism is not only energy consuming but also is associated with other physiological and hormonal imbalances (El-Desoky et al. 2022). Therefore, maternal exposure to heat stress has been found to decrease fertility and embryo development, and increase the stillborn rate in rabbits (Marco-Jiménez et al. 2017).
Melatonin (N-acetyl-5-methoxytryptamine) is an indolamine lipophilic hormone that is synthesised and secreted by the pineal gland. Studies have shown that the biological roles of melatonin are beyond synchronisation of circadian rhythms. It regulates several biological functions/pathways in mammals including oxidative and inflammatory reactions/pathways, cell differentiation/apoptotic pathways, and immune system functions. Moreover, at the level of reproductive processes, melatonin is distributed in various reproductive tissues and involved in folliculogenesis, oocyte maturation, luteal function, early embryonic development, and pregnancy maintenance in different mammalian species (Jiang et al. 2021). In pregnant animals, the release of melatonin in the ovary, placenta, uterus, embryo, and fetus is elevated, highlighting its pivotal role in pregnancy maintenance (Lanoix et al. 2008; Ejaz et al. 2021). Hashem et al. (2023) found that melatonin administration during the first half of pregnancy upregulated the expression of gonadotropin and steriod-related genes in the ovary and antioxidant-related genes in the ovary and placenta of day 18 pregnant rabbits, leading to better embryo survival and pregnancy outputs. Other studies on farm animals have highlighted the potential positive effects of melatonin on pregnant animals even under some environmental stresses such as heat stress (De Rensis et al. 2015). For example, heat-stressed ewes treated with melatonin expressed higher total antioxidant capacity and fertility rate compared to non-treated ewes (Bouroutzika et al. 2020). Similarly, heat-stressed dairy cows treated with melatonin implants before calving expressed reduced subsequent days open and fewer services per conception (artificial insemination semen doses; Garcia-Ispierto et al. 2013). Moreover, it has beneficial effects on blastocyst in vitro production from heat-stressed bovine oocytes (Cebrian-Serrano et al. 2013). As reported in these studies, the main mechanism by which melatonin can improve fertility in heat-stressed animals could be ascribed to its antioxidant effect. High ambient temperatures during summer increase oxidative stress in animals, and melatonin and its metabolites are considered indirect antioxidant agents and effective direct free radical scavengers (Bouroutzika et al. 2020). In this study, the effects of melatonin administration during the first half of pregnancy on the heat-tolerance capacity, hormone profile, ovarian structures, fetal development, and pregnancy outputs of heat-stressed rabbits were investigated.
Materials and methods
The present study was conducted in the Laboratory of Rabbit Physiology Research, Agricultural Experimental Station, Animal and Fish Production Department, Faculty of Agriculture, Alexandria University, Egypt (31°20′N, 30°E). The experimental protocols and procedures were checked and approved by the Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Cairo University (Ethical approval number: Vet CU 01122022584).
Animals, housing system, and experimental design
Forty sexually mature, nulliparous, V-line female rabbits (23 weeks old and 2.62 ± 0.13 kg of body weight, BW) were used. Each female rabbit was individually kept in a galvanised wire cage (60 cm × 55 cm × 40 cm, L × W × H) equipped with a feeder and automatic drinker. The female rabbits were kept under natural metrological conditions of the summer season, which is characterised by high ambient temperature and relative humidity. To monitor the meteorological data inside the rabbitry, an electronic digital thermo-hygrometer (Data logger-Log 100/110, Germany) was used to record ambient temperature (°C) and relative humidity (%) every hour during the entire experimental period. To calculate the temperature–humidity index (THI), temperature and relative humidity values were involved in the following equation: THI = db °C − [(0.31 − 0.31 × RH%) × (db °C − 14.4)], where db °C is dry bulb temperature in Celsius and RH% is the percentage of relative humidity. The THI values were categorised into four levels: <27.8 = absence of heat stress, 27.8–28.8 = moderate heat stress, 28.9–29.9 = severe heat stress, and >30.0 = very severe heat stress (Marai et al. 2002). The means of ambient temperature, relative humidity, and THI were 30.34 ± 1.29°C, 80.58 ± 3.75%, and 29.93 ± 1.33, respectively.
Females were fed a commercial pelleted diet that met their nutritional requirements (18.24% crude protein and 67.96% total digestible nutrients) and had free access to fresh clean water during the experimental period. To manage the females as one flock during the experimental period, each female was subjected to oestrous synchronisation by intramuscular (i.m.) injection of 25 IU equine chorionic gonadotropin (eCG, Gonaser®, Hipra, Girona, Spain) followed by an i.m. injection of 0.8 μg of gonadotropin releasing hormone (GnRH, Receptal, Boxmeer, Holland) 48 h later, and artificially inseminated with 20 × 106 fresh pooled sperm cells/female. Semen doses were collected with the aid of an artificial vagina from four proven-fertile rabbit males. Females were stratified equally into two experimental groups (n = 20/group) and daily received an oral dose of either 1 mL of corn oil (control, placebo) or 1 mL of corn oil containing 1 mg melatonin/kg BW (Puritan’s Pride Melatonin, Oakdale, New York, USA). The dose used in this study was based on the study of Mousa-Balabel and Mohamed (2011). The treatment started from the day of oestrous synchronisation to day 15 post-insemination. The schematic design of the study is depicted in Fig. 1.
Heat tolerance variables
For assessing the heat-tolerance capacity of the females, feed intake, respiration rate, and rectal temperature were recorded during the experimental period. The feed intake (g/day) was calculated weekly by subtracting the feed residues from the total amount offered feeds during this period. Respiration rate (breaths/min) and rectal temperature (°C) were recorded every other day in the morning. The number of flank movements was counted for 1 min and used for expressing respiration rate. A digital thermometer was used to measure rectal temperature of each female rabbit by introducing and attaching the thermometer to the rectal wall until a fixed reading was obtained (El-Desoky et al. 2022).
Determination of redox status biomarkers and hormones
Blood samples were collected in the morning before offering the diet, on the day of insemination, and 4, 7, and 18 days post-insemination from the ear vein of each female rabbit into blank tubes. Blood serum was obtained by centrifugation of blood samples at 700g for 20 min and stored at −80°C until subsequent analyses. The blood serum was colourimetrically analysed for redox status biomarkers (glutathione peroxidase, total antioxidant capacity, malondialdehyde, and nitric oxide). Concentrations of blood serum glutathione peroxidase, total antioxidant capacity, and malondialdehyde were determined using commercial kits at 340 nm, 505 nm, and 534 nm, respectively (Bio-diagnostic, Giza, Egypt). According to the procedures provided by the manufacturer, the linearity of the assay was up to 2 mM/L and up to 100 nmol/mL for total antioxidant capacity and malondialdehyde, respectively. Concentrations of blood serum nitric oxide were determined using a commercial kit (Cat# NB98, ClinicSciences, France) and the sensitivity of the method was 1 pmol/μL depending on the quantitation of nitrite using Griess reagent at 540 nm. Furthermore, the concentrations of blood serum high density lipoproteins (HDL) were determined at 500 nm (Biodiagnostic, Giza, Egypt).
Blood samples were also analysed for progesterone, oestradiol, prolactin, and melatonin using solid-phase enzyme immunoassay commercial kits. For progesterone determination, kits obtained from Monobind Inc., USA (Cat No: 8425-300A) were used. The lower limit of assay detection was 0.10 ng/mL and the intra- and inter-assay CVs were 10.3 and 11.6%, respectively. For oestradiol, antibodies-online GmbH kits (Cat No: ABIN365207, Aachen, Germany) were used with detection range: 62.5–1000 pg/mL. For prolactin, Antibodies.com kits (Cat No: A2703, Cambridge, UK) were used with detection range: 0.312–20 ng/mL and method sensitivity: 0.109 ng/mL. For melatonin, kits obtained from MyBioSource (Cat No. MBS2600264, CA, USA) with a detection range from 15.6 pg/mL to 1000 pg/mL were used.
Ovarian structures, feto-placental characteristics, and reproductive performance
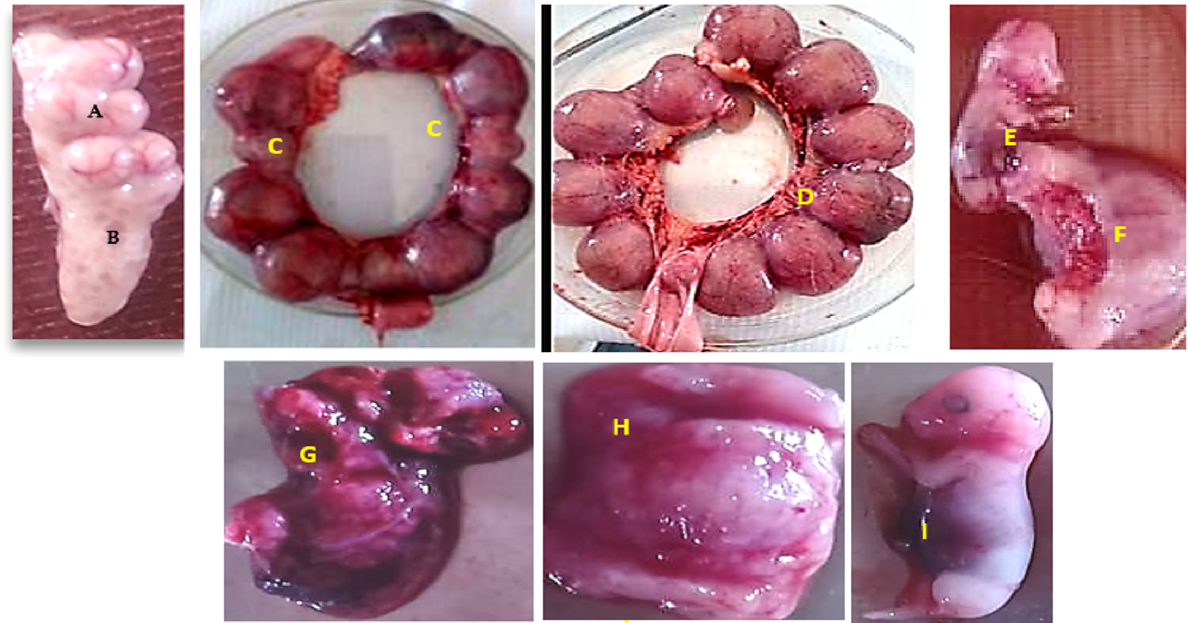
On day 18 of pregnancy, five pregnant female rabbits from the 10 pregnant female rabbits in the control group and 16 pregnant female rabbits in the melatonin group were randomly chosen and euthanised. The ovaries and reproductive tract of each female were dissected. The numbers of the visible ovarian follicles and corpora lutea were counted. Along each uterine horn, the numbers of implantation sites were counted and classified according to the presence or absence of fetus into developing implantation site (containing a differentiated/developed fetus) or absorbed implantation site/absorbed fetus (containing atrophic placenta without fetus). Each implantation site was separated and weighed (including weights of fetus, amniotic fluid, and placenta), and then the amniotic fluid was aspirated with a syringe and the remaining parts were dissected to separate fetus and placenta. For each implantation site, the weight of fetus and the maternal and fetal sides of the placenta were recorded. Placenta efficiency was determined by dividing the weight of fetus on the weight of correspondent placenta tissue (maternal and fetal sides) at each implantation site (Hashem and Aboul-Ezz 2018). Different ovarian structures and types of implantation site, developed or absorbed, are shown in Fig. 2.
Ovarian structures (A: corpus luteum; B: antral ovarian follicle) and fetal implantation sites (C: regressed/absorbed implantation sites; D: developed implantation site; E, F: fetus with placenta; G: fetal side of placenta; H: maternal side of placenta; I: fetus observed on day 18 of pregnancy.
Reproductive performance of the females was determined. Conception rate ([number of pregnant females on day 10 post-insemination/total number of inseminated females (20 females)] × 100) was determined on day 10 post-insemination by checking females for pregnancy using abdominal palpation procedure. Additionally, kindling rate ([number of females having birth/total number of females kept live to complete pregnancy term (15 females)] × 100) and litter size at birth ([number of bunnies at birth/number of females giving birth]) were estimated.
Statistical analysis
All statistical analyses were carried out by the aid of the Statistical Analysis Software Package (ver. 8, SAS Institute Inc., Cary, NC, USA). Data on reproductive tract characteristics and litter size and weight were analysed using one-way ANOVA using the General Linear Model Procedure. The model was as follows: Yij = μ + Ei + eij, where Yij is the observed value of the dependent variable, μ is the overall mean, Ei is the effect of ith experimental group (treatment, i: 1 and 2), and eij is the residual error. For data on redox status biomarkers and hormones, a mixed procedure, PROC MIXED, considering the time effect as repeated measurements was used. Treatment and time of blood collection were used as fixed factors, and each female rabbit was used as a random factor. The mixed model used was as follows: yijk = μ + Femi + Ei + Tj + (ET)ij + eijk, where yijk indicates the observed value of the dependent variable, μ is the overall mean, Femi is the effect of ith female ID, Ei is the fixed effect of the ith experimental group (treatment, i: 1 and 2), Tj is the fixed effect of the jth time of blood collection (j = 1:4), (E × T)ij is the interaction between experimental group and time of blood collection, and eijk is the residual error. Differences among the experimental means were tested using Duncan’s multiple range test. Conception and kindling rates (categorical data) were analysed using the chi-square test and are expressed as percentages. All results are expressed as means ± s.e.m. and the statistical significance was accepted at P < 0.05.
Results
Heat tolerance indicators
The effects of melatonin administration during the first half of pregnancy on the heat tolerance indicators of female rabbits compared to control are shown in Table 1. Compared to control, treatment with melatonin did not affect feed intake, but significantly decreased respiration rate and rectal temperature (P < 0.001).
| Variable | Experimental group, E | s.e.m. | P-value | ||||
|---|---|---|---|---|---|---|---|
| Control | Melatonin | E | Time (T) | E × T | |||
| Feed intake (g/day) | 150.03 | 141.66 | 5.44 | 0.315 | 0.514 | 0.027 | |
| Respiration rate (breaths/min) | 84.43a | 73.435b | 0.823 | <0.001 | <0.001 | 0.896 | |
| Rectal temperature (°C) | 39.65a | 39.21b | 0.078 | <0.001 | 0.591 | 0.166 | |
Means within a row without a common lowercase letter (a, b) differ at P < 0.05.
Blood serum redox status and hormone concentrations
The effects of melatonin administration during the first half of pregnancy on blood serum redox status and hormone concentrations of female rabbits compared to control are shown in Table 2. Melatonin treatment significantly improved concentrations of nitric oxide (P < 0.001) and tended to decrease malondialdehyde concentrations (P = 0.064) compared to control, whereas no significant differences in the other redox status indicators (total antioxidant capacity and glutathione peroxidase) were found between the two experimental groups. High-density lipoprotein concentrations were significantly increased due to melatonin treatment (P < 0.01). Melatonin treatment significantly increased concentrations of oestradiol, progesterone, and melatonin compared to control (P < 0.05) with no significant effect on prolactin concentration.
| Variable | Experimental group, E | s.e.m. | P-value | ||||
|---|---|---|---|---|---|---|---|
| Control | Melatonin | E | Time (T) | E × T | |||
| Redox status indicators | |||||||
| Total antioxidant capacity (μmol/L) | 417.67 | 418.23 | 0.882 | 0.669 | 0.265 | 0.457 | |
| Glutathione peroxidase (μmol/L) | 954.91 | 959.98 | 5.952 | 0.554 | 0.614 | 0.660 | |
| Malondialdehyde (μmol/L) | 11.02 | 10.30 | 0.286 | 0.064 | 0.155 | 0.715 | |
| Nitric oxide (μg/L) | 23.46b | 24.74a | 0.1995 | <0.001 | 0.521 | 0.112 | |
| High-density lipoprotein (mg/dL) | 37.03b | 41.46a | 0.901 | 0.002 | 0.396 | 0.219 | |
| Hormone concentrations | |||||||
| Oestradiol (pg/mL) | 544.44b | 560.57a | 4.3510 | 0.016 | 0.231 | 0.022 | |
| Progesterone (ng/mL) | 5.126b | 6.550a | 0.1260 | 0.046 | 0.007 | 0.093 | |
| Prolactin (ng/mL) | 3.174 | 3.358 | 0.1079 | 0.244 | 0.002 | 0.046 | |
| Melatonin (pg/mL) | 17.04b | 19.36a | 0.5440 | 0.007 | 0.424 | 0.726 | |
Means within a row without a common lowercase letter (a, b) differ at P < 0.05.
Ovarian structures, fetus/placenta characteristics, and reproductive performance
The effects of melatonin administration during the first half of pregnancy on ovarian structures, fetus/placenta characteristics, and reproductive performance of female rabbits compared to control are shown in Table 3. No significant differences were observed in the numbers of visible ovarian follicles, corpora lutea, and total implantation sites between both experimental groups. However, melatonin treatment significantly reduced number of absorbed implantation sites/fetuses compared to control (P < 0.01). Melatonin treatment significantly improved amniotic fluid weight (P < 0.05). Melatonin treatment improved both conception rate at day 10 of pregnancy (P < 0.05) and kindling rate (P = 0.071) compared to control.
| Variable A | Experimental group | s.e.m. | P-value | ||
|---|---|---|---|---|---|
| Control | Melatonin | ||||
| Ovarian structures (n = 5) | |||||
| No. of visible follicles | 24.80 | 24.40 | 1.594 | 0.874 | |
| No. of corpora lutea | 6.20 | 7.20 | 1.224 | 0.260 | |
| Fetus/placenta characteristics (n = 5) | |||||
| No. of total implantation sites | 6.20 | 7.20 | 0.200 | 0.260 | |
| No. of implantation sites with absorbed fetuses | 2.00a | 0.00b | 0.274 | 0.006 | |
| Implantation site weight (g) | 12.44 | 14.50 | 0.792 | 0.118 | |
| Fetus weight (g) | 5.03a | 4.43b | 0.139 | 0.025 | |
| Amniotic fluid volume (mL) | 4.64b | 6.93a | 0.597 | 0.042 | |
| Placenta weight (g) | 2.75 | 2.61 | 0.099 | 0.478 | |
| Fetus/placenta ratio | 1.83 | 1.70 | 0.057 | 0.175 | |
| Reproductive performance | |||||
| Conception rate (%) | 50.0b (10/20) | 80.0a (16/20) | – | 0.024 | |
| Kindling rate (%) | 26.67 (4/15) | 60.0 (9/15) | – | 0.071 | |
| Litter size at birth | 6.00 | 7.25 | 0.564 | 0.133 | |
Means within a row without a common lowercase letter (a, b) differ at P < 0.05.
Discussion
In rabbits, heat stress can negatively impact maintenance of pregnancy either at early or late pregnancy by its negative effects on embryo survival, implantation, maternal recognition of pregnancy, uterine microenvironment, and reproductive hormone balance (mainly sex steroids and gonadotropins). These effects may increase the incidence of early embryonic loss and/or abortion rate, interfering with animal welfare aspects and goals of the intensive production systems in rabbit farms (Mutwedu et al. 2021; Ebeid et al. 2023). The optimal ambient temperature range of rabbits is 15–25°C, and the optimal humidity is 55–65%. Heat stress occurs when the ambient temperature elevates above 30°C (Liang et al. 2022). Marco-Jiménez et al. (2017) reported that rabbits exposed to ambient temperatures between 25°C and 36°C compared to those kept between 14°C and 20°C during pregnancy and lactation had smaller litter sizes and kit weights at birth and had higher stillborn rates.
In this study, we aimed to explore the potential roles of melatonin administration during the first half of pregnancy in mitigating heat stress negative effects on pregnancy in rabbits. Melatonin is a potent antioxidant and an anti-stress agent, in addition to its roles in maintaining circadian rhythm of different physiological processes, particularly under stressful environmental conditions (Adah et al. 2020; Ayo and Ake 2022). Environmental factors such as ambient temperature, photoperiod, stress, and food availability are known to influence circadian rhythms to allow animals to adapt to environmental changes (Contreras-Correa et al. 2023). In a thermoneutral zone, the physiological rectal temperature of rabbit ranges from 38.5 to 39.5°C (Liang et al. 2022) and respiratory rate ranges from 32 to 60 breaths/min (Nowland et al. 2015). In contrast, under heat stress conditions (ambient temperature around 30.7°C), the average of rectal temperature ranges from 39.8 to 40.2°C (Marai et al. 2002). Consequently, rabbits have to increase respiratory panting by increasing respiration rates as a physiological thermoregulatory adaptive mechanism to lose excess body heat (El-Desoky et al. 2022; El-Raghi et al. 2023). In this study, melatonin treatment improved heat-tolerance capacity of pregnant females, as melatonin-treated females had respiration rates and rectal temperatures approaching normal physiological ranges compared to control females. These findings are in accordance with those obtained by Bouroutzika et al. (2020) who found that melatonin administration to heat-stressed pregnant ewes lowered their rectal temperatures and respiration rates compared to control ewes.
Melatonin is known as one of the potent antioxidant candidates that can efficiently maintain redox homeostasis by scavenging free radicals and preserving functional integrity of antioxidant enzymes (Contreras-Correa et al. 2023). Hashem et al. (2023) found that melatonin administration to rabbits during the first half of pregnancy upregulated the expression of antioxidant-related genes in the ovary and placenta. In this study, the antioxidant role of melatonin was not clearly evidenced as none of the antioxidant indicators, total antioxidant capacity or glutathion preroxidase enzyme, were affected by the treatment. The lack of the antioxidant effects of melatonin observed in this study may be related to the inadequacy of the melatonin dose. Higher doses of melatonin may be required to scavenge produced free radicals released due to heat stress and pregnancy, as both factors can increase the rate of free radical production. However, it is important to note that melatonin-treated rabbits tended to have lower concentrations of the free radical malondialdehyde, indicating reduced lipid peroxidation, which is associated with increased high density lipoprotein. Melatonin is a small lipophilic indoleamine; this chemical nature may enable melatonin to easily incorporate in lipid metabolism (Jin et al. 2017).
Melatonin treatment significantly increased concentrations of nitric oxide. Thakor et al. (2010) reported that, in sheep, treatment with melatonin during pregnancy increases umbilical blood flow via nitric oxide-dependent mechanisms, suggesting melatonin as a useful clinical tool for increasing or maintaining umbilical blood flow in complicated pregnancy. Although nitric oxide is classified as a free radical, the physiological level of nitric oxide plays a vital role in the regulation of blood vessel vasodilation and permeability, and has multiple regulatory functions within the reproductive system. It is involved in many cycle-dependent ovarian events (such as ovulation and luteal function modulation) and fetoplacental vascularisation (Abdelnaby and Abo El-Maaty 2021). During pregnancy, it acts locally on the vascular smooth muscle of the fetoplacental vessels and systemically by inhibiting the production of vasoconstriction in the maternal systemic circulation, resulting in increased uterine and fetoplacental blood flow (Tarrade et al. 2014).
In rabbits, heat stress results in many deleterious effects on reproductive organs and their functions. Exposure of female rabbits to heat stress weakens the responsiveness of ovarian granulosa cells to follicle-stimulating hormone, reduces ovarian weight, lowers plasma progesterone concentrations, and destroys the ovarian cell nucleoli (Mutwedu et al. 2021; Tang et al. 2022). As evidenced from our results, rabbits treated with melatonin had higher concentrations of blood serum oestradiol and progesterone. The elevation in steroid concentrations due to melatonin treatment may be related to the ability of melatonin to regulate the expression of several steroid-regulatory genes and related pathways. Melatonin has been found to upregulate the expression of the steroidogenic acute segulatory protein (STAR) gene in the ovaries of pregnant rabbits (Hashem et al. 2023). The protein encoded by the STAR gene plays a key role in the acute regulation of steroid hormone synthesis by permitting the cleavage of cholesterol into pregnenolone and by mediating the transport of cholesterol from the outer to the inner mitochondrial membranes. The improved concentrations of sex steroids observed in this study may be also due to the increased concentrations of high density lipoprotein, which is considered one of the sex steroid hormones precursors (Chang et al. 2017). Moreover, melatonin has been found to stimulate progesterone synthesis in bovine and ovine theca cells by activating the phosphatidylinositol 3-kinase and Akt (protein kinase B) pathway (Wang et al. 2019). It also stimulates the secretion of luteal and placental progesterone (Tamura et al. 2008), which reduces uterine contractility and prevents immunological rejection of the trophoblast.
In this study, the control rabbits had a significantly lower conception rate than melatonin-treated rabbits. Keeping in mind that conception rate was determined on day 10 of pregnancy, it can be said that heat stress can evoke early embryonic loss and hinder embryo implantation, leading to full lose of conceptus. Moreover, the results confirmed the continuous negative effects of heat stress on fetal growth and development as indicated by higher atrophic/absorbed implantation sites with dead/absorbed fetuses on day 18 of pregnancy in the control rabbits than those treated with melatonin. Thus, heat stress can lead to both complete and partial pregnancy losses in rabbits. The improved conception rate and number of developed/live fetuses due to melatonin treatment were associated with elevated plasma concentrations of oestradiol and progesterone. In rabbits, both oestradiol and progesterone play crucial roles in the maintenance of pregnancy and embryo/fetus survival and development. During the pre-implantation period, embryo survival is affected by the uterine micro-environment and the composition of its fluid. In this term, luteal progesterone is necessary to prepare the endometrium for embryo implantation, prevent uterine contractions, and stimulate the secretion of uterine proteins to maintain pregnancy. Interestingly, ovarian follicle-synthesised oestradiol plays a crucial role in the maintenance of luteal function and thus pregnancy via its luteotropic action (Hashem and Aboul-Ezz 2018).
The other reason for improved conception rate in melatonin-treated rabbits could be due to the ability of melatonin to improve placental functioning. Hashem et al. (2023) found that melatonin treatment during the first half of pregnancy upregulated the expression of several placental genes related to cell differentiation, functioning, and synthesis in rabbits. Melatonin can upregulate the expression of placental cyclin B1, cortexin 1, fibronectin 1, and Proliferation-Associated 2G4 genes which are involved in the cell invasion and cell adhesion process and provide structural support to tissues. It can also upregulate the placental expression of IMP metallopeptidase inhibitor 1, which plays a crucial role in the extracellular matrix remodelling process during implantation. Melatonin may also improve interactions between the conceptus and uterus by regulating sirtuin 1 during the early stages of pregnancy, which is important for decidualisation (Bae et al. 2020).
Improved placental functioning can be suggested in our study, as melatonin treatment significantly increased the weight of amniotic fluid. This effect may provide protection to the developing fetuses against the negative impacts of heat stress. It is now well established that the majority of in utero heat stress is transferred through the placenta via the fetal–placental circulation, and up to 20% of transferred heat can be dissipated via amniotic fluid (Kasiteropoulou et al. 2020). Amniotic fluid insulates the fetus and maintains a regular temperature. In addition, it contains antibodies against various infectious diseases, supports the development of fetal muscles and bones, and prevents the collapse of the umbilical cord, allowing efficient oxygen and nutrient transport to the fetuses (Khan et al. 2018). However, more studies are required to directly examine placental functioning (blood flow, nutrient or water transport, and vessel development) due to melatonin treatment under heat stress conditions for better elucidation of the mechanisms by which melatonin might affect placental functioning.
Conclusion
Heat stress has negative impacts on pregnancy outcomes in rabbits. High temperatures increased rectal temperatures of pregnant rabbits and reduced oestradiol and progesterone concentrations, which were associated with reduced numbers of live fetuses, and thus conception rates. Melatonin administration during the first half of pregnancy countered the negative effects of heat stress, leading to better fetal vitality and pregnancy outcomes. The positive effects of melatonin could be related to its thermoregulatory role and steroidogenesis-stimulating activity. Further studies are required to understand more clearly the mechanisms by which melatonin can act as a thermoprotective agent in rabbits during different stages of pregnancy.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Acknowledgements
N. M. Hashem is grateful for the Arab Fund Fellowship Program (Zamalat), Arab Fund for Economic and Social Development, Kuwait for offering the opportunity for executing a scientific mission at Cardenal Herrera University, Valencia, Spain.
References
Abdelnaby EA, Abo El-Maaty AM (2021) Melatonin and CIDR improved the follicular and luteal haemodynamics, uterine and ovarian arteries vascular perfusion, ovarian hormones and nitric oxide in cyclic cows. Reproduction in Domestic Animals 56(3), 498-510.
| Crossref | Google Scholar | PubMed |
Adah AS, Adah DA, Nwonuma CO, Aiyedun JO (2020) Modulatory effects of melatonin on erythrocyte indices in Arabian stallions following a 30-km sub-maximal exercise. Comparative Clinical Pathology 29, 921-926.
| Crossref | Google Scholar |
Ayo JO, Ake AS (2022) Modulatory roles of melatonin on respiratory and heart rates and their circadian rhythmicity in donkeys (Equus asinus) subjected to packing during the hot-dry season. Current Research in Physiology 5, 381-388.
| Crossref | Google Scholar | PubMed |
Bae H, Yang C, Lee J-Y, Park S, Bazer FW, Song G, Lim W (2020) Melatonin improves uterine-conceptus interaction via regulation of SIRT1 during early pregnancy. Journal of Pineal Research 69(2), e12670.
| Crossref | Google Scholar | PubMed |
Bouroutzika E, Kouretas D, Papadopoulos S, Veskoukis AS, Theodosiadou E, Makri S, Paliouras C, Michailidis M-L, Caroprese M, Valasi I (2020) Effects of melatonin administration to pregnant ewes under heat-stress conditions, in redox status and reproductive outcome. Antioxidants 9(3), 266.
| Crossref | Google Scholar | PubMed |
Cebrian-Serrano A, Salvador I, Raga E, Dinnyes A, Silvestre MA (2013) Beneficial effect of melatonin on blastocyst in vitro production from heat-stressed bovine oocytes. Reproduction in Domestic Animals 48(5), 738-746.
| Crossref | Google Scholar | PubMed |
Chang X-L, Liu L, Wang N, Chen Z-J, Zhang C (2017) The function of high-density lipoprotein and low-density lipoprotein in the maintenance of mouse ovarian steroid balance. Biology of Reproduction 97(6), 862-872.
| Crossref | Google Scholar | PubMed |
Contreras-Correa ZE, Messman RD, Swanson RM, Lemley CO (2023) Melatonin in health and disease: a perspective for livestock production. Biomolecules 13(3), 490.
| Crossref | Google Scholar | PubMed |
De Rensis F, Garcia-Ispierto I, López-Gatius F (2015) Seasonal heat stress: clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology 84(5), 659-666.
| Crossref | Google Scholar | PubMed |
Ebeid TA, Aljabeili HS, Al-Homidan IH, Volek Z, Barakat H (2023) Ramifications of heat stress on rabbit production and role of nutraceuticals in alleviating its negative impacts: an updated review. Antioxidants 12(7), 1407.
| Crossref | Google Scholar | PubMed |
Ejaz H, Figaro JK, Woolner AMF, Thottakam BMV, Galley HF (2021) Maternal serum melatonin increases during pregnancy and falls immediately after delivery implicating the placenta as a major source of melatonin. Frontiers in Endocrinology 11, 623038.
| Crossref | Google Scholar | PubMed |
El-Desoky NI, Hashem NM, Elkomy AG, Abo-Elezz ZR (2022) Improving rabbit doe metabolism and whole reproductive cycle outcomes via fatty acid-rich Moringa oleifera leaf extract supplementation in free and nano-encapsulated forms. Animals 12(6), 764.
| Crossref | Google Scholar | PubMed |
El-Raghi AA, Hassan MAE, Hashem NM, Abdelnour SA (2023) Struggling thermal stress impacts on growth performance and health status of newly weaned rabbits using nanoemulsion of Origanum majorana considering the economic efficiency of supplementation. Animals 13(11), 1772.
| Crossref | Google Scholar | PubMed |
Garcia-Ispierto I, Abdelfatah A, López-Gatius F (2013) Melatonin treatment at dry-off improves reproductive performance postpartum in high-producing dairy cows under heat stress conditions. Reproduction in Domestic Animals 48(4), 577-583.
| Crossref | Google Scholar | PubMed |
Hashem NM, Aboul-Ezz ZR (2018) Effects of a single administration of different gonadotropins on day 7 post-insemination on pregnancy outcomes of rabbit does. Theriogenology 105, 1-6.
| Crossref | Google Scholar | PubMed |
Hashem NM, El-Hawy AS, El-Bassiony MF, Saber A, Radwan MA, Ghanem N (2023) Melatonin administration during the first half of pregnancy improves the reproductive performance of rabbits: emphasis on ovarian and placental functions. Theriogenology 205, 40-49.
| Crossref | Google Scholar | PubMed |
Jiang Y, Shi H, Liu Y, Zhao S, Zhao H (2021) Applications of melatonin in female reproduction in the context of oxidative stress. Oxidative Medicine and Cellular Longevity 2021, 6668365.
| Crossref | Google Scholar |
Jin J-X, Lee S, Taweechaipaisankul A, Kim GA, Lee BC (2017) Melatonin regulates lipid metabolism in porcine oocytes. Journal of Pineal Research 62(2), e12388.
| Crossref | Google Scholar |
Kasiteropoulou D, Topalidou A, Downe S (2020) A computational fluid dynamics modelling of maternal-fetal heat exchange and blood flow in the umbilical cord. PLoS ONE 15, e0231997.
| Crossref | Google Scholar | PubMed |
Khan H, Wahab A, Kazi N, Muhammad Y, Kazi S, Kazi S, Khan A, Khan I, Ahmad S (2018) Biochemical attributes of amniotic fluid during fetal growth stage of successful pregnancy in rabbits. Pakistan Journal of Zoology 50(4), 1199-1600.
| Crossref | Google Scholar |
Lanoix D, Beghdadi H, Lafond J, Vaillancourt C (2008) Human placental trophoblasts synthesize melatonin and express its receptors. Journal of Pineal Research 45(1), 50-60.
| Crossref | Google Scholar | PubMed |
Liang Z-L, Chen F, Park S, Balasubramanian B, Liu W-C (2022) Impacts of heat stress on rabbit immune function, endocrine, blood biochemical changes, antioxidant capacity and production performance, and the potential mitigation strategies of nutritional intervention. Frontiers in Veterinary Science 9, 906084.
| Crossref | Google Scholar |
Marai IFM, Habeeb AAM, Gad AE (2002) Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: a review. Livestock Production Science 78(2), 71-90.
| Crossref | Google Scholar |
Marco-Jiménez F, García-Diego FJ, Vicente JS (2017) Effect of gestational and lactational exposure to heat stress on performance in rabbits. World Rabbit Science 25(1), 17-25.
| Crossref | Google Scholar |
Mousa-Balabel TM, Mohamed RA (2011) Effect of different photoperiods and melatonin treatment on rabbit reproductive performance. Veterinary Quarterly 31(4), 165-171.
| Crossref | Google Scholar | PubMed |
Mutwedu VB, Nyongesa AW, Oduma JA, Kitaa JM, Mbaria JM (2021) Thermal stress causes oxidative stress and physiological changes in female rabbits. Journal of Thermal Biology 95, 102780.
| Crossref | Google Scholar | PubMed |
Tamura H, Nakamura Y, Terron MP, Flores LJ, Manchester LC, Tan D-X, Sugino N, Reiter RJ (2008) Melatonin and pregnancy in the human. Reproductive Toxicology 25(3), 291-303.
| Crossref | Google Scholar | PubMed |
Tang L, Bai X, Xie X, Chen G, Jia X, Lei M, Li C, Lai S (2022) Negative effects of heat stress on ovarian tissue in female rabbit. Frontiers in Veterinary Science 9, 1009182.
| Crossref | Google Scholar | PubMed |
Tarrade A, Lecarpentier E, Gil S, Morel O, Zahr N, Dahirel M, Tsatsaris V, Chavatte-Palmer P (2014) Analysis of placental vascularization in a pharmacological rabbit model of IUGR induced by L-NAME, a nitric oxide synthase inhibitor. Placenta 35(4), 254-259.
| Crossref | Google Scholar | PubMed |
Thakor AS, Herrera EA, Serón-Ferré M, Giussani DA (2010) Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. Journal of Pineal Research 49(4), 399-406.
| Crossref | Google Scholar | PubMed |
Wang X, Meng K, He Y, Wang H, Zhang Y, Quan F (2019) Melatonin stimulates STAR expression and progesterone production via activation of the PI3K/AKT pathway in bovine theca cells. International Journal of Biological Sciences 15(2), 404-415.
| Crossref | Google Scholar | PubMed |



