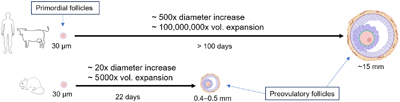
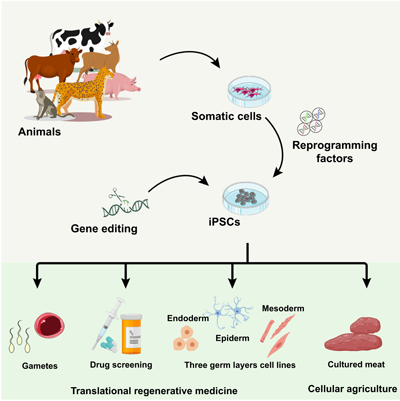
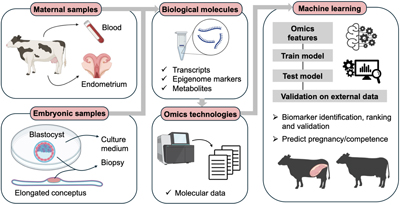
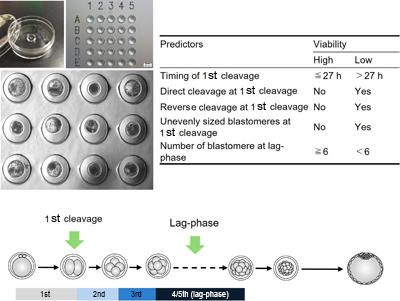
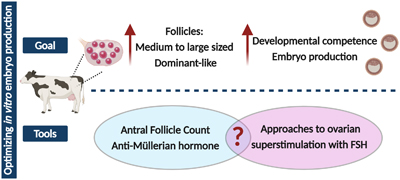
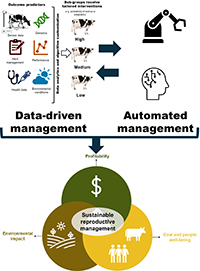
This collection of Reproduction, Fertility and Development contains full papers and abstracts from the 2025 IETS annual conference. The content focuses on this year’s conference theme of 'Emerging technologies for healthy reproduction and sustainability'.
This collection was published for the International Embryo Technology Society by CSIRO Publishing.
Edited on behalf of the Society by Program Co-Chairs Paula Rodriguez-Villamil (Genus plc, DeForest, WI, USA) Kiho Lee (University of Missouri, Columbia, MO, USA)
Last Updated: 03 Mar 2025