Maternal high-fat diet during pregnancy and lactation affects factors that regulate cell proliferation and apoptosis in the testis of adult progeny
Helen Viotti A , Daniel Cavestany
A , Daniel Cavestany  A , Graeme B. Martin
A , Graeme B. Martin  B * , Mark H. Vickers
B * , Mark H. Vickers  C , Deborah M. Sloboda
C , Deborah M. Sloboda  D E F G and Graciela Pedrana
D E F G and Graciela Pedrana  A
A
A
B
C
D
E
F
G
Abstract
A maternal high-fat diet is thought to pose a risk to spermatogenesis in the progeny.
We tested whether a maternal high-fat diet would affect Sertoli cell expression of transcription factors (insulin-like growth factor I (IGF-I); glial-cell line-derived neurotrophic factor (GDNF); Ets variant 5 (ETV5)) and cell proliferation and apoptotic proteins, in the testis of adult offspring.
Pregnant rats were fed ad libitum with a standard diet (Control) or a high-fat diet (HFat) throughout pregnancy and lactation. After weaning, male pups were fed the standard diet until postnatal day 160. Males were monitored daily from postnatal day 34 to determine onset of puberty. On postnatal day 160, their testes were processed for morphometry and immunohistochemistry.
The HFat diet increased seminiferous-tubule diameter (P < 0.03), the numbers of Sertoli cells (P < 0.0001) and Ki-67-positive spermatogonia (P < 0.0006), and the areas immunostained for ETV5 (P < 0.0001), caspase-3 (P < 0.001) and Bcl-2 (P < 0.0001). By contrast, the HFat diet reduced the areas immunostained for IGF-I (P < 0.01) and GDNF (P < 0.0001).
A maternal high-fat diet alters the balance between spermatogonia proliferation and spermatid apoptosis.
A maternal high-fat diet seems to ‘program’ adult male fertility.
Keywords: apoptosis, cell proliferation, developmental programming, high-fat diet, rat, reproduction, Sertoli cell, spermatogenesis, testis.
Introduction
The ‘developmental programming’ hypothesis suggests that the maternal environment during prenatal or early postnatal life influences the subsequent health and performance of the progeny, including susceptibility to the development of obesity and related metabolic disorders. An adverse maternal environment during fetal life would also be expected to alter germ cell production and thus compromise the fertility of the next generation (Vautier and Cadaret 2022). Indeed, recent studies in the rat by our group (Pedrana et al. 2020, 2021) have shown that maternal undernutrition significantly impacts on several molecular factors that control testis function in developing male offspring.
Among those factors, the Sertoli cells produce insulin-like growth factor I (IGF-I) (Escott et al. 2014) and two important transcription factors, transcription variant 5 (ETV5) and glial-cell line-derived neurotrophic factor (GDNF) that are essential to achieve a testicular microenvironment and the development of normal spermatogenesis (Yang and Han 2010; Alves et al. 2016). These proteins that act upon receptors on spermatogonial stem cells (SSCs), in turn induce critical genes for SSC self-renewal. Particularly, ETV5 acts also in an autocrine manner to contribute to the production of Sertoli chemoattraction factors for germ cells (Eo et al. 2012) and induce spermatogonial cell proliferation of ETV5 (Schlesser et al. 2008).
Furthermore, apoptotic proteins, cysteinyl-aspartate protease 3 (caspase-3), Bcl-2 associated X protein (Bax) and B-cell lymphoma-2 (Bcl-2) determine the balance between apoptosis and proliferation, which determines sperm output. The apoptosis process is a strategy to limit the number of germ cells that a Sertoli cell must support, and Sertoli cells themselves control germ cell apoptosis through paracrine signalling that balances pro-apoptotic factors (caspase-3, Bax) and anti-apoptotic factors such as Bcl-2 (Murphy and Richburg 2014).
The effects of maternal diets on molecular and cellular programming in the testes of offspring are probably not confined to maternal undernutrition. For example, we have shown previously that a maternal obesogenic diet increases the risk of obesity and a variety of cardiometabolic disorders, including postnatal hyperglycemia, hyperinsulinemia and dyslipidemia, in male offspring (Sloboda et al. 2009; Howie et al. 2013; Langley-Evans 2015). Moreover, a maternal high-fat diet can impair the testicular antioxidant defence system, thus increasing oxidative stress (Bautista et al. 2017; Billah et al. 2022), and suppress apoptosis, reduce sperm count and impair sperm function (Ruiz Valderrama et al. 2016; Mao et al. 2018). Many of these factors are associated with premature aging of the reproductive system and poor fertility (Williams et al. 2014; Bautista et al. 2017). In female offspring, there are comparable consequences for ovarian function (Tsoulis et al. 2016).
Despite the fact that we have made significant advances in our understanding of testicular development after prenatal dietary manipulation, we still do not have a complete understanding of the mechanisms that underlie the effects of a maternal high-fat diet on testis development in the progeny. One of the key points to consider is the balance between cell proliferation and apoptosis in the developing testis because it is a critical aspect of the regulation of spermatogenesis and determination of sperm output, and thus probably ‘programs’ future fertility. Therefore, in the present study, we focused on how a maternal obesogenic diet affects: (1) the pro- and anti-apoptotic proteins in germ cells (Shaha et al. 2010; Rodrigo et al. 2022); (2) the Sertoli cell-produced transcription factors that regulate self-renewal of spermatogonial stem cells (Parekh et al. 2019; Zhang et al. 2021); and (3) IGF-I, also produced by Sertoli cells as an autocrine regulator of spermatogonial renewal, cell growth, proliferation and differentiation (Griffeth et al. 2014). In our rat model, we used immunoexpression to test whether a maternal high-fat diet during pregnancy and lactation would increase the Sertoli cell GDNF and ETV5, decrease active caspase-3 and Bax, increase Bcl-2, and decrease IGF-I in the testis of post-pubertal progeny. We also measured plasma concentrations of androgens because maternal obesity during pregnancy and lactation leads to Leydig cell atrophy and impaired steroidogenesis in pups when they reach adulthood (Pinto-Fochi et al. 2016).
Materials and methods
Animal model
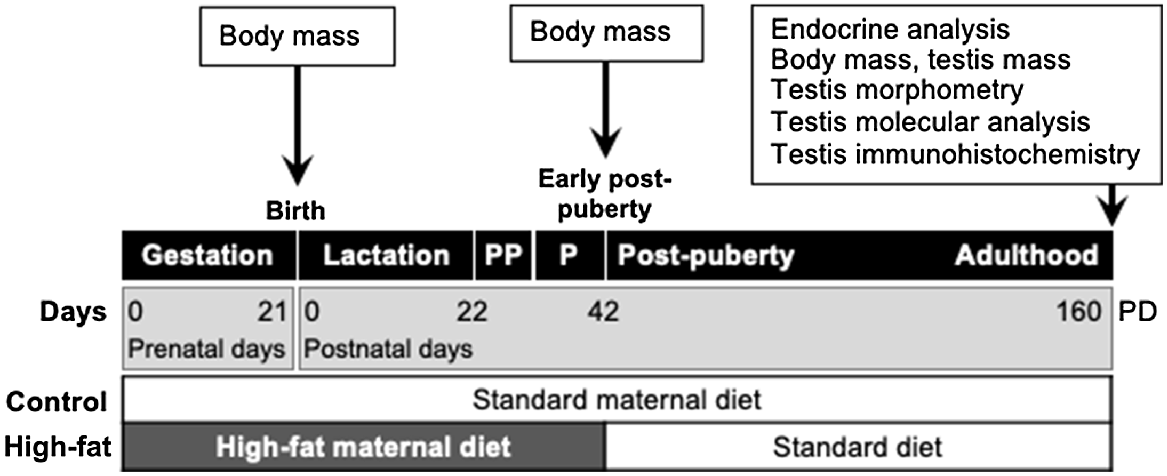
All animal procedures were performed under the guidelines of the Animal Ethics Committee of the University of Auckland (R402). An established model of developmental programming based on manipulation of maternal nutrition was used (Howie et al. 2012). Female Wistar rats (120 days old; n = 10) from the Vernon Jansen Unit at the University of Auckland were time-mated using a rat oestrous cycle monitor (Fine Science Tools, USA). Thereafter, they were housed individually in standard rat cages in the same room, with free access to water, a constant temperature of 25°C and a 12:12 light:dark cycle. Pregnant dams were randomly allocated to two dietary treatments (five per group): (1) Control – dams fed ad libitum throughout pregnancy and lactation with a standard diet (fat 6.2%, protein 18.6%; carbohydrate 44.2%; Teklad Global 18% Protein Diet, Diet 2018, calories (kcals/g) from fat 18%, from protein 24%, calories from carbohydrate 58%, https://insights.envigo.com/hubfs/resources/data-sheets/2018-datasheet-0915.pdf); and (2) HFat – dams were fed a high-fat diet throughout pregnancy and lactation (fat 24%, protein 24%, carbohydrate 41%; calories (kcals/g) from fat 45%, calories from protein 20%, calories from carbohydrate 35%, High fat D12451, Research Diets, Inc New Brunswick, NJ, USA, http://67.20.83.195/china/pdf/Data%20Sheets/D12451.pdf). After birth, pups were weighed, and litters were adjusted to comprise four males plus four females to ensure standardised nutrition until weaning (day 22). After weaning, male pups were housed in pairs and fed the standard chow diet ad libitum until the end of the study (day 160). Offspring body weight were recorded at birth, early post-puberty (day 42) and at the end of the trial on postnatal day 160.
Testis sampling and histological processing
On postnatal day 160, male pups (n = 20; 10 per dietary group) were fasted overnight, weighed, anaesthetised with pentobarbital (60 mg kg−1 i.p.) and then killed by decapitation. Blood was collected into heparinised tubes, and plasma was separated by centrifugation (2400g, 20 min) and stored at −20°C until later analysis. Testes were weighed and the data were used to calculate relative testis mass (g testis mass per g body mass) and thus the gonadosomatic index (relative testis mass × 100). Left testes were frozen in liquid nitrogen and stored at −80°C for molecular analyses. Right testes were immersed in fixative (4% formaldehyde phosphate buffer, pH 7.4) for 24 h for histology and immunohistochemistry (Fig. 1).
Schematic representation of the experimental design. There were two treatments for maternal nutrition (Control and High-fat) for gestation days (GD) 0–21, and for lactation, from birth (prenatal day 21) to weaning on postnatal day (PD) 22. After weaning, male pups were fed ad libitum with a control diet until the end of the study (PD 160) when blood and testes were sampled. Puberty (P) was observed over the period 35-37 PD. PP, pre-puberty.
Fixed testes were progressively dehydrated by immersion in increasing concentrations of ethanol (70%, 95%, 100%), embedded in paraffin resin (Historesin; Leica, Germany) and sectioned at 5 μm by microtome (Leica 149 Reichert Jung Biocut 2030, Wetzlar, Germany) in preparation for hematoxylin–eosin staining and immunohistochemistry.
Endocrine assays
Commercially available assays from Diagnostic Systems Laboratories Inc. (Webster, TX, USA) were used to measure the plasma concentrations of testosterone (DSL-4100) and androstenedione (DSL-4200). We followed the manufacturer’s instructions and included all samples within a single assay. For testosterone, the limit of detection was 0.10 ng/mL and the within-assay coefficient of variation (WACV) was 2.8%. For androstenedione, the limit of detection was 0.10 ng/mL and the WACV was 5.7%.
Immunohistochemistry
Slides were processed for the detection by immunoexpression of ETV5, GDNF, IGF-I, caspase-3 Bax, Bcl-2 and Ki-67 using the streptavidin–biotin–peroxidase protocol, as described previously (Pedrana et al. 2020). In brief: the tissue sections were immersed in 250 mL of 0.01 M citrate buffer solution (pH 6.0) with Tween-20 (5 mL) at a concentration of 2% for dewaxing, hydration and epitope retrieval by heating for 5 min in microwave oven (100% power); slides were then washed with distilled water and phosphate-buffered saline (PBS, pH 7.4); endogenous peroxidases were inactivated by treatment with 3% hydrogen peroxide 200 (20 min), followed by washing in PBS; non-specific binding proteins were blocked by incubation with normal rat serum; each slide was then incubated for 30 min with a different primary antibody (Table 1), for 30 min with biotinylated secondary antibody (rabbit-specific HRP/DAB; ab 64261; Abcam, USA), and for 30 min with streptavidin–peroxidase enzyme complex; to visualize the antigen, slides were incubated for 5 min with diaminobenzidine (DAB) chromogen solution (1 mL hydrogen peroxide + 30 μL DAB); sections were counterstained with Mayer’s hematoxylin, dehydrated, and mounted with Entellan® (Merck) on glass coverslips. All assays contained a negative control for the immunohistochemistry using different testicular sections incubated with rat serum diluted 1:100 in PBS.
| Target protein | Antibody | Clonality | Catalogue number, supplier | Concentration | |
|---|---|---|---|---|---|
| ETV5 | Anti-ETV5 | Polyclonal IgG | Ab 102010, Abcam, USA | 1:100 | |
| GDNF | Anti-glial cell line-derived neurotrophic factor | Polyclonal IgG | ab18956, Abcam, USA | 1:200 | |
| IGF-I | Anti-IGF1 | Polyclonal IgG anti-IGF-I | ab9572, Abcam, USA | 4 μg/mL | |
| Caspase-3 | Anti-active caspase-3 | Polyclonal IgG | AF835 R&D Systems | 5 μg/mL | |
| Bax | Anti-Bax | Monoclonal IgG Clone E63 | ab32503, Abcam, USA | 1:200 | |
| Bcl-2 | Anti-Bcl-2 | Polyclonal IgG | ab7973, Abcam, USA | 1:200 | |
| Ki-67 | Anti-Ki-67 antibody | Monoclonal SP6 IgG | ab16667, Abcam, USA | 1:200 |
All antibodies were raised in the rabbit.
Morphometry, immunohistochemistry and image analysis
Following hematoxylin-eosin staining, seminiferous tubule diameters were measured in 50 transverse cross-sections of seminiferous tubules at a final magnification of 100×, and Sertoli cells were counted in each of 50 transverse cross-sections of seminiferous tubules at a final magnification of 400×. Digital images were retrieved using a light microscope (Olympus CX23, Olympus Scientific Solutions Americas Corp., MA, USA) connected to a digital camera (Dino-Eyepiece Edge, AM-7025X) and then captured with DinoCapture 2.0 software (https://www.dinolite.us/es/features/dinocapture/).
Immunohistochemistry images showing the immunostained area (IA) for ETV5, GDNF, IGF-I, caspase-3, Bax, Bcl-2 and the number of Ki-67-positive spermatogonial cells per transverse section of seminiferous tubules were analysed using ImageJ software (Ver. 1.52b 6 May 2018; Wayne Rasband, National Institutes of Health, USA; https://imagej.net/ij/index.html). Immunostained areas were measured using a locally written macro that included colour threshold values from processing RGB images and conversion to binary images. The threshold values were verified and normalised with controls carried across several runs for calibration. This process provided quantitative values for percentage (%) IA in 1000 digital images per testis, per animal and per immunohistochemically stained factor.
Gene expression analysis by qPCR
Total RNA was extracted using commercially available kits (AllPrep DNA/RNA mini kit; cat 80204; Qiagen, Germantown, MD, USA). Genomic DNA was removed from each sample by treatment with RNase-free DNase (Invitrogen Life Technologies, New Zealand) according to the manufacturer’s instructions. RNA quantity and purity were analysed using a NanoDrop spectrophotometer (ND-1000; BioLab Ltd) and NanoDrop software (ver. 3.1.2). All RNA samples were stored at −80°C until required. For first-strand cDNA synthesis, we used 5 mg total RNA, Moloney Murine Leukaemia Virus Reverse Transcriptase (MMLV- RT; Promega Corp., WI, USA) and a standard thermocycler (GeneAmp PCR System 9700; Applied Biosystems). A master mix was prepared containing 5 mL M-MLV 5x (cat M531A; In Vitro Technologies, Madison, WI, USA), 0.5 mL M-MLV-RT (cat. M170B; In Vitro Technologies) and 1.25 mL 10 mM deoxynucleoside-triphosphates (cat. R0181, Thermo Fisher Scientific, USA). The cycling conditions were: initial denaturation for 5 min at 96°C followed by 30 cycles (30 s each) at 96°C (denaturation), 60°C (annealing) and 72°C (extension). The cDNAwas stored at −20°C until assay by quantitative polymerase chain reaction (qPCR). Testicular gene expression for caspase-3, Bax and Bcl-2, as well as the endogenous reference (b-actin), was measured by qPCR using the ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems, New Zealand). All primers were designed using Primer 3 software (Primer3, 0.4.0, Whitehead Institute for Biomedical Research, https://bioinfo.ut.ee/primer3-0.4.0/) and manufactured by Invitrogen Life Technologies. Primer set controls were b-actin (Table 2). Optimal primer conditions were adjusted to the cycling conditions: length, 20 bp (range 17–23 bp); temperature, 63°C (range 60–65°C); and amplicon length, 100–300 bp. Dissociation analyses were used to ensure specificity and only samples producing a single peak in the dissociation curves were retained. Amplified products were visualised on an agarose gel using the E-Gel CloneWell 0.8% SYBR Safe gel (cat. G6618-08; Invitrogen, Burlington, ON, Canada), run on the E-Gel iBaseTM Power System (cat. G6400; Invitrogen, CA, USA) and sequenced by spectrophotometry (Allan Wilson Centre, Massey University, New Zealand). The resulting sequences were evaluated using NCBI BLAST to ensure specificity. Transcript levels were quantified by qPCR under the following conditions: an initial 2-min hold period at 50°C for normalisation (Stage 1), followed by enzyme activation at 95°C for 2 min (Stage 2); amplification of the gene product through 40 successive cycles of 95°C for 15 s and then 60°C for 1 min (Stage 3); a dissociation stage of 15 s at 95°C, 15 s at 60°C, and 2 min at 99°C (Stage 4). A standard curve was generated from the mean cycle threshold (Ct) of eight standards (1:5 serial dilution) of a known concentration in triplicate and amplification and dissociation curves were generated for all standards and samples (Applied Biosystems, CA, USA). Each sample was run in triplicate.
| Rat mRNA | Forward primer | Reverse primer | Amplicon length (bp) | NCBI reference sequence | |
|---|---|---|---|---|---|
| Caspase-3 | GAGCTTGGAACGCGAAGAAA | TCCACGGAGGTTTCGTTGT | 59 | NM_012922.2 | |
| Bax | TGACGGCAACTTCAACTGGG | GCAGCCGATCTCGAAGGAA | 143 | NM_017059.2 | |
| Bcl-2 | GGATCCAGGATAACGGAGGC | ATGCACCCAGAGTGATGCAG | 141 | NM_016993.1 | |
| Beta-actin | CACCAACTGGGACGATATGGA | CAGCCTGGATGGCTACGTACAT | 188 | NM_031144 |
Statistical analysis
The mother was used as the biological replicate. The pups were derived from the various litters, and they were compared statistically, not the mothers. With 10 litters and 2 males per litter, we obtained 10 males per dietary group (two males from each of five litters). Although the numbers of Sertoli cells and germ cells are ordinal and discrete, we treated them as continuous variables. The data were checked for normal distribution and met the criteria for parametric testing. For each dependent variable, differences between Control and HFat were compared using two-sample Student’s t-tests, aided by PROC TTEST and SAS statistical analysis (SAS, v. 9.1, SAS Institute Inc., Cary, NC, USA). The level of significance was P < 0.05. All data are expressed as mean ± standard error of mean (s.e.m.).
Results
Treatment had no significant effect on litter size (13.9 pups per litter in the Control vs 14.0 pups per litter in the HFat group) or on the male:female ratio (1:1.013 in the Control vs 1:1.050 in the HFat group).
Physiological parameters
The data are summarised in Table 3. There were no differences between treatments in plasma concentrations of androstenedione and testosterone. Compared to the Control group, the HFat group showed a decrease in birth weight, an earlier onset of puberty and a decrease in body weight at puberty. However, as adults at age 160 days, HFat animals were heavier than Control animals. At 160 days, testis weight did not differ between groups but, due to the differences in body mass, relative testis weight, and thus gonadosomatic index, were smaller in the HFat group than in the Control group (Table 3).
| Control | HFat | P-value | ||||
|---|---|---|---|---|---|---|
| Body mass at birth (g) | 6.3 ± 0.7 | 5.5 ± 0.4 | 0.01 | * | ||
| Body mass at puberty (g) | 166.0 ± 4.3 | 144.0 ± 4.0 | 0.0078 | ** | ||
| Age at puberty (days) | 37.0 ± 0.2 | 35.0 ± 0.2 | 0.008 | ** | ||
| Adult body mass at 160 days (g) | 575.0 ± 10.0 | 771.0 ± 35.0 | 0.0001 | *** | ||
| Testis mass (g) | 4.3 ± 0.2 | 4.5 ± 0.1 | 0.4 | |||
| Relative testis mass (g/g) | 0.0075 ± 0.0003 | 0.0060 ± 0.0003 | 0.008 | ** | ||
| Gonadosomatic index (%) | 0.75 ± 0.03 | 0.60 ± 0.03 | 0.008 | ** | ||
| Plasma androstenedione (ng/mL) | 0.18 ± 0.02 | 0.20 ± 0.01 | 0.4 | |||
| Plasma testosterone (ng/mL) | 1.47 ± 0.22 | 1.71 ± 0.38 | 0.5 | |||
| Testis morphometry | ||||||
| Seminiferous tubule diameter (μm) | 198.0 ± 6.3 | 211.0 ± 4.0 | 0.03 | * | ||
| Sertoli cells (number per transverse section of seminiferous tubule) | 9.5 ± 0.2 | 16.0 ± 0.3 | 0.001 | *** | ||
| Spermatogonia (Ki-67-positive cells per transverse section of seminiferous tubule) | 12.8 ± 0.9 | 18.9 ± 1.1 | 0.0006 | *** | ||
| Testis protein immunoexpression (% area immunostained) | ||||||
| Transcription factors | ETV5 (%) | 3.5 ± 0.2 | 6.1 ± 0.4 | 0.0001 | *** | |
| GDNF (%) | 14.3 ± 0.6 | 7.6 ± 0.6 | 0.0001 | *** | ||
| IGF-1 (%) | 4.9 ± 0.3 | 3.3 ± 0.3 | 0.01 | * | ||
| Apoptosis proteins | Caspase-3 (%) | 13.0 ± 0.5 | 15.6 ± 0.6 | 0.001 | ** | |
| Bax (%) | 14.0 ± 0.6 | 14.2 ± 0.6 | 0.9 | |||
| Bcl-2 (%) | 21.8 ± 0.7 | 29.8 ± 0.8 | 0.0001 | *** | ||
| Testis gene expression (mRNA; ng/μL) | ||||||
| Apoptosis factors | Caspase-3 | 0.50 ± 0.04 | 0.40 ± 0.09 | 0.4 | ||
| Bax | 0.38 ± 0.02 | 0.35 ± 0.01 | 0.26 | |||
| Bcl-2 | 0.33 ± 0.03 | 0.19 ± 0.03 | 0.01 | * | ||
Data are expressed as mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001.
Testis morphometry
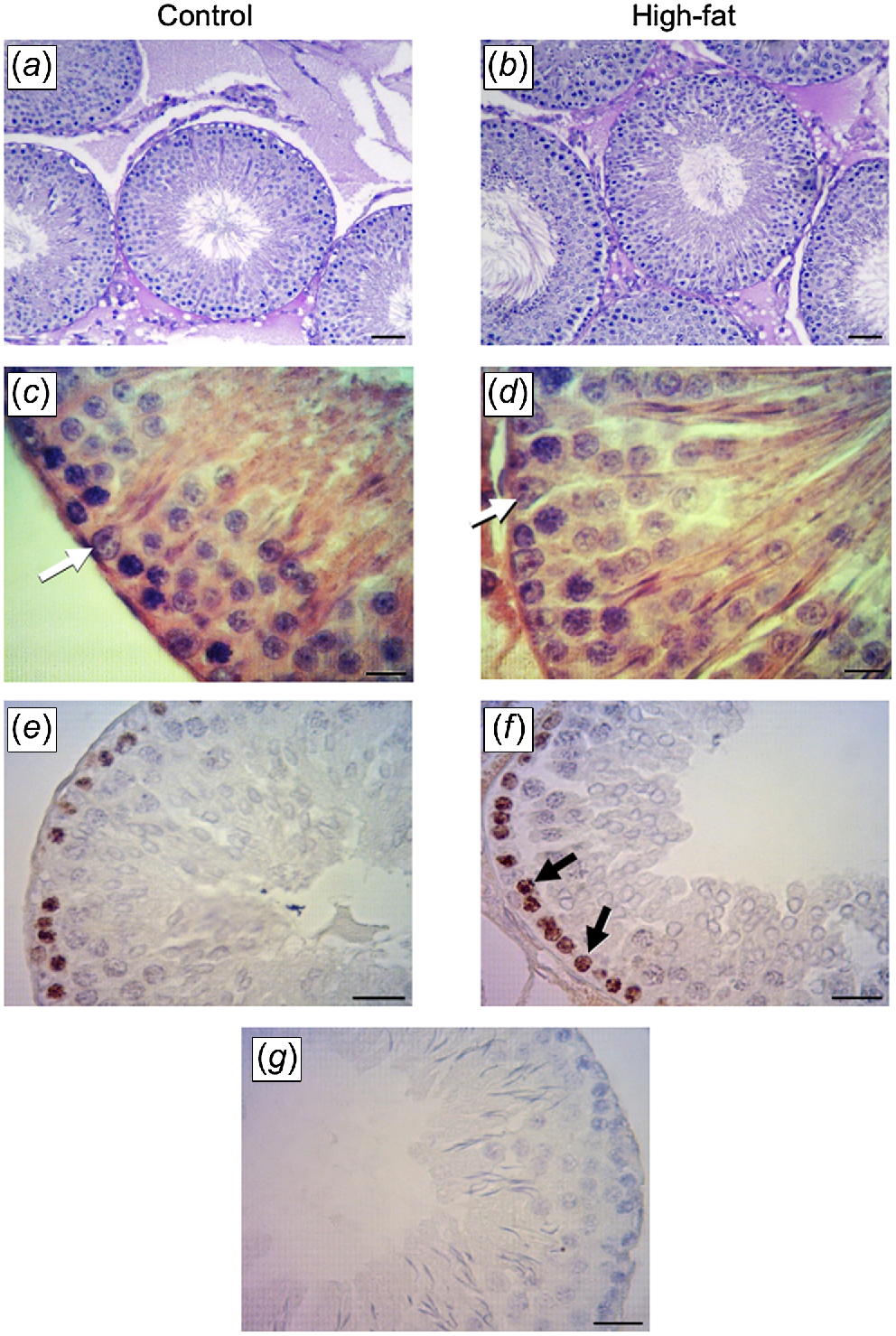
In both groups, the testicular parenchyma showed a normal structure with seminiferous tubules and interstitial tissue, although some images show alterations in seminiferous tubules in the HFat group. Seminiferous tubule diameter was greater in the HFat group than in the Control group (Table 3, Fig. 2a, b). Compared to the Control group, the HFat group had greater numbers of Sertoli cells (Table 3, Fig. 2c, d) and Ki-67-positive spermatogonial cells (Table 3, Fig. 2e, f).
Testis histology in male rats born to mothers fed either the Control or the High-fat diet during pregnancy and lactation. (a, b) Hematoxylin–eosin-stained seminiferous tubules (magnification, 100×; scale bar, 50 μm). (c, d) Sertoli cells (white arrows) in hematoxylin–eosin-stained seminiferous tubule cross-sections (magnification, 600×; scale bar, 20 μm). (e, f) Ki-67-positive spermatogonia (black arrows; magnification 400×; scale bar, 20 μm. (g) Negative control for immunohistochemistry.
Testis protein immunoexpression
Immunoexpression of the transcription factors, ETV5 and GDNF, was clearly evident in both groups. The ETV5 was localised in the spermatogonia, spermatocytes and the acrosome region of round spermatids, elongated spermatids and Sertoli cells (Fig. 3a–d). The IA for ETV5 was greater in the HFat group than in the Control group. For GDNF, immunoexpression was localised in cytoplasm of Sertoli cells, spermatogonia and Leydig cells (Fig. 3e–h). The IA for GDNF was lower in the HFat group than in the Control group.
Immunohistochemical localisation transcription factors in the testis in rats born to mothers fed either the Control or the High-fat diet during pregnancy and lactation. (a–d) Brown immunostaining for transcription variant 5 (ETV5) evident in the acrosome region in round spermatids (black arrow), spermatocytes (black arrowhead) and Sertoli cells (white arrows). (e–h) Brown immunostaining for glial-cell line-derived neurotrophic factor (GDNF) evident in elongated spermatids (black triangle), spermatocytes (black arrowhead) and Leydig cells (asterisk). (i–l) Brown immunostaining for insulin-like growth factor I (IGF-1) in Leydig cells (asterisk) and in Sertoli cells (intense immunostaining; white arrow) and spermatids (segmented arrow). Negative controls for immunohistochemistry are presented in the three panes on the right (magnification, 400×; scale bar, 20 μm).
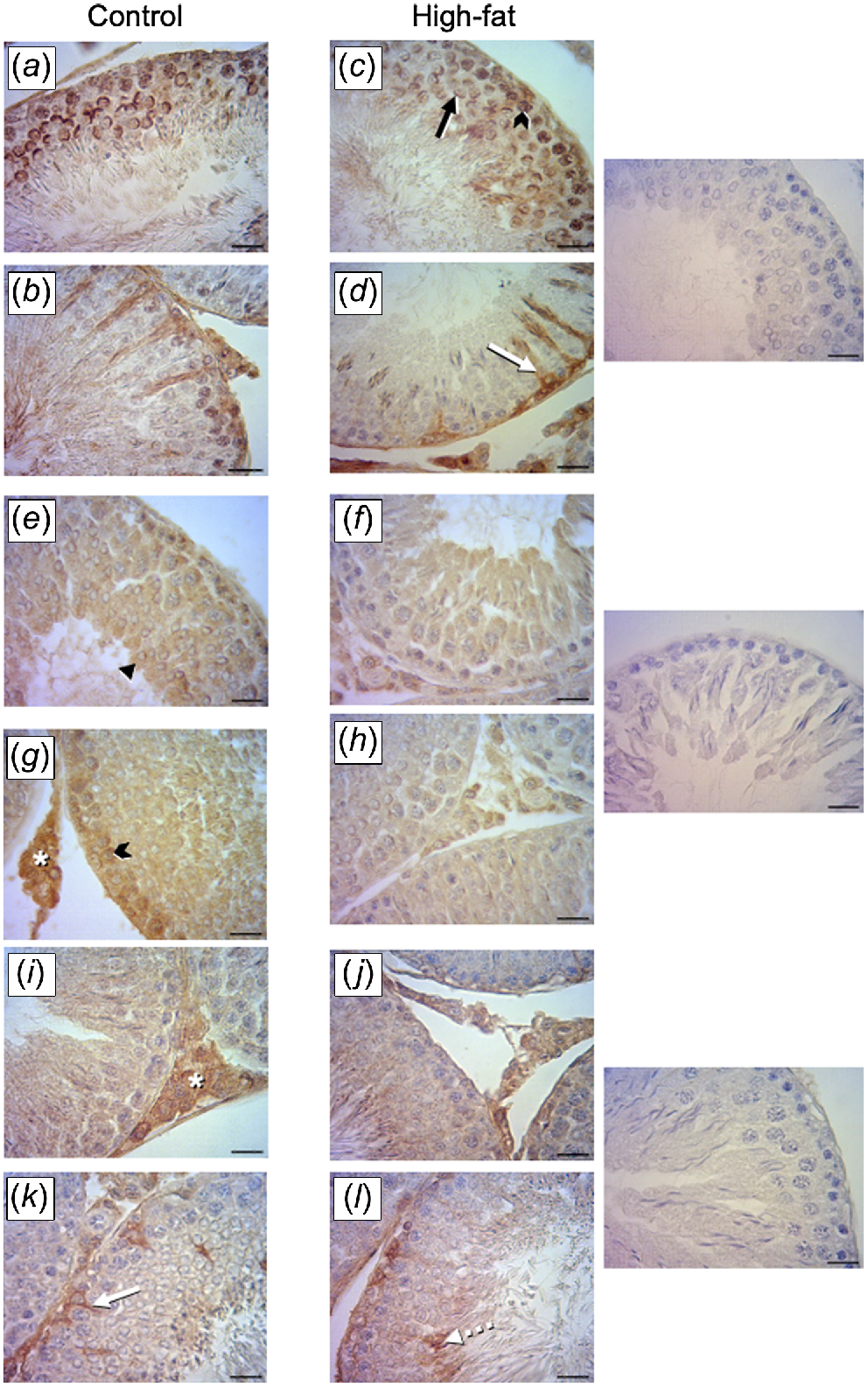
The IGF-I immunoexpression was localised in the Leydig cells, the acrosome region of sperm and in Sertoli cells (Fig. 3i–l). The IA for IGF-I was lower in the HFat group than in the Control group.
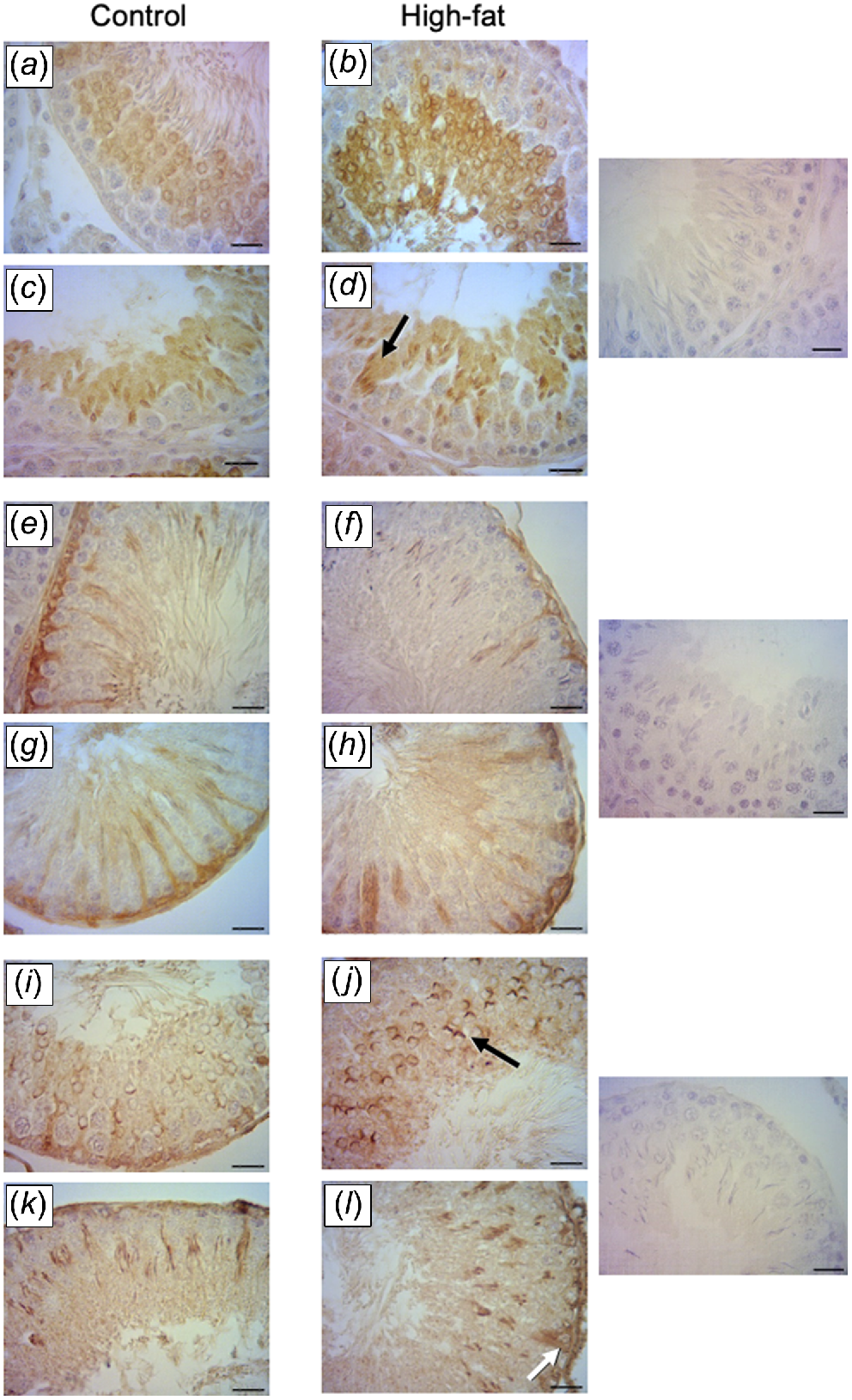
Active caspase-3 immunoexpression was evident in round and elongated spermatids (Fig. 4a–d). The IA for caspase-3 was greater in the HFat group than in the Control group. For Bax, immunoexpression was localised in the cytoplasm of spermatogonia, elongated spermatids and Sertoli cells (Fig. 4e–h). The IA for Bax did not differ between dietary treatments. For Bcl-2, immunoexpression was detected in round spermatids, elongated spermatids and Sertoli cells (Fig. 4i–l). The IA for Bcl-2 was greater in the HFat group than in the Control group (Table 3).
Effects of maternal high-fat diet during pregnancy and lactation in apoptosis proteins in the testis. (a–d) Brown immunostaining for caspase-3 in spermatids (black arrows) and spermatozoa in the lumen. (e–h) Brown immunostaining for Bax evident in Sertoli cells and spermatogonia. (i–l) Brown immunostaining for Bcl-2 evident in acrosome region of round spermatids (black arrow) and in Sertoli cells (white arrow). Negative controls for immunohistochemistry are presented in the three panes on the right (magnification, 400×; scale bar, 20 μm).
Testis gene expression
The testis levels of caspase-3 mRNA and Bax mRNA did not differ between dietary treatments. In contrast to Bcl-2 protein, the concentration of Bcl-2 mRNA was lower in the HFat group than in the Control group (Table 3).
Discussion
The maternal high-fat diet had significant consequences for the adult’s male offspring including an earlier onset of puberty at a lighter body mass, followed by rebound growth leading to a heavier adult body mass. These outcomes were accompanied by major impacts on testis function, with significant alterations in testicular morphometry, including an increase in the diameter of seminiferous tubules that was caused, at least in part, by increases in the numbers of Sertoli cells and spermatogonial germ cells. In addition, apoptosis was increased in spermatocytes and spermatids. Thus, the maternal high-fat diet disrupted the balance of apoptosis and proliferation in the germ cells, challenging the ability of Sertoli cells to support germ cell development.
The body mass of the offspring was affected, with the HFat group being lighter at birth and at puberty, although the difference reversed as adulthood approached. These outcomes probably reflect general metabolic effects of the high-fat maternal diet on the offspring as seen in recent studies (Rodríguez-González et al. 2023). A generalised effect on metabolism could thus have contributed to effects in the developing testis.
Testis mass at adulthood (160 days) did not differ between groups but body mass was approximately 35% greater in the HFat group than in the Control group. Therefore, relative testis weight, and thus gonadosomatic index, was smaller in the HFat group than in the Control group. This outcome was accompanied by changes in morphometry, including an increase in the diameter of the seminiferous tubules, as reported for a previous study of rats fed high-fat diets during pregnancy (Campos-Silva et al. 2018). The increase in tubule diameter can be explained by increases in the number of Ki-67-positive spermatogonia (evidence of proliferation) and increases in the anti-apoptotic Bcl-2 protein. Germ cell proliferation would also be promoted in the high-fat group by an increase in the production by Sertoli cells of ETV5 that is essential for spermatogonial stem cell self-renewal (Morrow et al. 2007; Tyagi et al. 2009).
The production of IGF-I, a key growth factor involved in the regulation of spermatogenesis, is reduced by a maternal high-fat diet, leading to negative consequences for spermatocytes, early spermatids and spermatozoa, where IGF-I receptors have been previously reported for a variety of species (Vannelli et al. 1988; Griffeth et al. 2014). It is of interest to compare the outcomes of the present study with those from our recent work on the effects of maternal undernutrition during pregnancy or lactation. Notably, undernutrition evoked an opposite effect by increasing IGF-I production, whereas both dietary treatments evoked a decrease in GDNF (Pedrana et al. 2020, 2021).
With respect to apoptosis, Bcl-2 is known to play an anti-apoptotic role in the regulation of the balance between proliferation and programmed cell death. The maternal high-fat diet increased the amounts of Bcl-2 protein in Sertoli cells, spermatocytes and spermatids, but decreased the amount of Bcl-2 mRNA in testis of the adult offspring. This apparent contradiction can be explained by the well-established post-transcriptional modification of Bcl-2 protein (Greenbaum et al. 2003; Ramazi and Zahiri 2021). Indeed, we had previously shown that immunoexpression of Bcl-2 protein caspase-3 increase in parallel (Pedrana et al. 2021), consistent with Bcl-2 preventing apoptosis by blocking the release of cytochrome C from the mitochondria. In turn, pores induced by Bax permit the release of mitochondrial cytochrome C into the cytosol, subsequently activating the caspase cascade that leads to apoptosis (Singh et al. 2019).
Therefore, the increase in active caspase-3 protein in the spermatocytes and spermatids in the adult offspring in the high-fat diet treatment would be expected to reduce sperm output. Indeed, a similar dietary treatment was shown to reduce spermatid number and daily sperm production in offspring in early adulthood (Sertorio et al. 2022) and, recently, TUNEL was used to demonstrate an increased testicular apoptosis in 60 day old offspring of mothers fed a maternal ‘cafeteria’ diet (Meneghini et al. 2022).
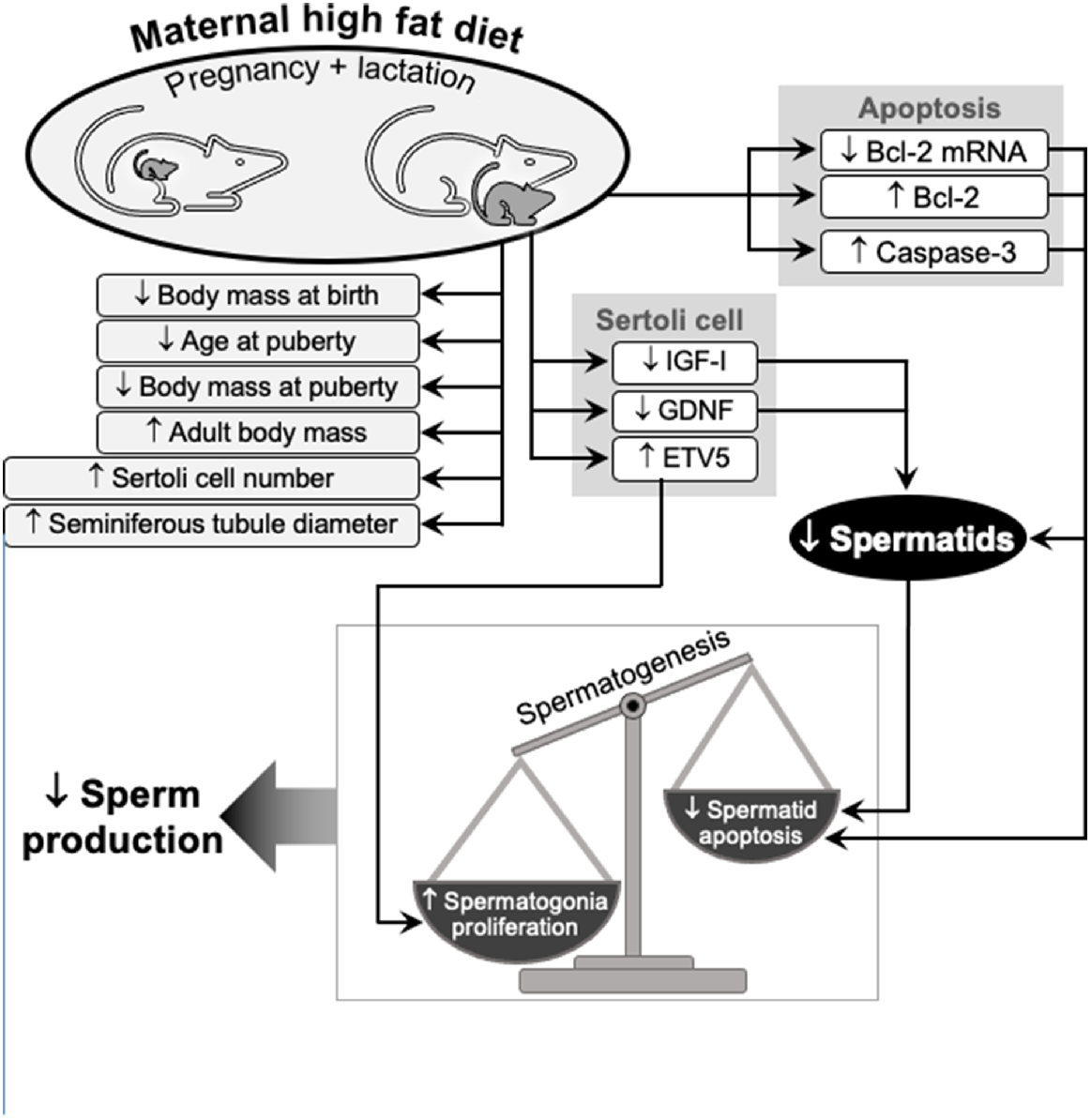
We conclude that a maternal high-fat diet, fed during pregnancy and lactation, has major and persistent effects on a variety of factors that regulate spermatogenesis, and therefore the production of spermatozoa, in the adult offspring (Fig. 5). Changes in the production by Sertoli cells of growth factors and transcription factors lead to an acceleration of stem cell renewal and therefore excessive proliferation of germ cells. At the same time, changes in apoptotic proteins in spermatocytes and spermatids lead to a decrease in the rate of apoptosis. This disruption in the balance of proliferation and apoptosis increases the germ cell population and this challenging the capacity of Sertoli cells to provide support for germ cell development. Ultimately, there is a reduction in the production of spermatozoa in the adult pups. The ’fetal programming’ hypothesis, previously applied to the effects of maternal undernutrition during prenatal and postnatal life, with consequences for the reproductive health of the next generation, is also clearly relevant to other types of maternal malnutrition.
Schematic summary of the effects of a maternal high-fat diet on the testis in adult offspring: morphometry and growth; factors that affect germ cell proliferation and apoptosis; and the consequences for Sertoli cell regulation of spermatogenesis. Essentially, a maternal high-fat during pregnancy and lactation leads to an imbalance between proliferation and apoptosis in germ cells, affecting the number of spermatogonia produced, with flow-on effects to spermiogenesis and fertility.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Graeme Martin is the Editor-in-Chief of Reproduction, Fertility and Development, but was blinded from the peer-review process for this paper. There are no other conflicts of interest.
Declaration of funding
This research was supported by Health Research Council of New Zealand, Gravida: National Centre for Growth and Development. DMS holds a Canada Research Chair in Perinatal Programming. GP is supported by a Dedicación Total from The University of the Republic, Uruguay.
Acknowledgements
We thank Silvana Soria, the technician in the Histology Laboratory, for her help with microtome sectioning of rat testis.
References
Alves MG, Jesus TT, Sousa M, Goldberg E, Silva BM, Oliveira PF (2016) Male fertility and obesity: are ghrelin, leptin and glucagon-like peptide-1 pharmacologically relevant? Current Pharmaceutical Design 22, 783-791.
| Crossref | Google Scholar | PubMed |
Bautista CJ, Rodríguez-González GL, Morales A, Lomas-Soria C, Cruz-Pérez F, Reyes-Castro LA, Zambrano E (2017) Maternal obesity in the rat impairs male offspring aging of the testicular antioxidant defence system. Reproduction, Fertility and Development 29, 1950-1957.
| Crossref | Google Scholar | PubMed |
Billah MM, Khatiwada S, Lecomte V, Morris MJ, Maloney CA (2022) Ameliorating high-fat diet-induced sperm and testicular oxidative damage by micronutrient-based antioxidant intervention in rats. European Journal of Nutrition 61, 3741-3753.
| Crossref | Google Scholar | PubMed |
Campos-Silva P, Costa WS, Sampaio FJB, Gregorio BM (2018) Prenatal and/or postnatal high-fat diet alters testicular parameters in adult Wistar albino rats. Histology and Histopathology 33, 407-416.
| Crossref | Google Scholar | PubMed |
Eo J, Song H, Lim HJ (2012) Etv5, a transcription factor with versatile functions in male reproduction. Clinical and Experimental Reproductive Medicine 39, 41-45.
| Crossref | Google Scholar | PubMed |
Escott GM, de Castro AL, Jacobus AP, Loss ES (2014) Insulin and IGF-I actions on IGF-I receptor in seminiferous tubules from immature rats. Biochimica et Biophysica Acta (BBA) – Biomembranes 1838, 1332-1337.
| Crossref | Google Scholar | PubMed |
Greenbaum D, Colangelo C, Williams K, Gerstein M (2003) Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biology 4, 117.
| Crossref | Google Scholar | PubMed |
Griffeth RJ, Bianda V, Nef S (2014) The emerging role of insulin-like growth factors in testis development and function. Basic and Clinical Andrology 24, 12.
| Crossref | Google Scholar |
Howie GJ, Sloboda DM, Vickers MH (2012) Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. British Journal of Nutrition 108, 298-307.
| Crossref | Google Scholar |
Howie GJ, Sloboda DM, Reynolds CM, Vickers MH (2013) Timing of maternal exposure to a high fat diet and development of obesity and hyperinsulinemia in male rat offspring: same metabolic phenotype, different developmental pathways? Journal of Nutrition and Metabolism 2013, 517384.
| Crossref | Google Scholar |
Langley-Evans SC (2015) Nutrition in early life and the programming of adult disease: a review. Journal of Human Nutrition and Dietetics 28, 1-14.
| Crossref | Google Scholar |
Mao J, Pennington KA, Talton OO, Schulz LC, Sutovsky M, Lin Y, Sutovsky P (2018) In utero and postnatal exposure to high fat, high sucrose diet suppressed testis apoptosis and reduced sperm count. Scientific Reports 8, 7622.
| Crossref | Google Scholar | PubMed |
Meneghini MA, Galarza RA, Flores QJP, Faletti AG (2022) Diet-induced maternal obesity and overnutrition cause a decrease in the sperm quality of the offspring. The Journal of Nutritional Biochemistry 103, 108966.
| Crossref | Google Scholar | PubMed |
Morrow CMK, Hostetler CE, Griswold MD, Hofmann M-C, Murphy KM, Cooke PS, Hess RA (2007) ETV5 is required for continuous spermatogenesis in adult mice and may mediate blood–testes barrier function and testicular immune privilege. Annals of the New York Academy of Sciences 1120, 144-151.
| Crossref | Google Scholar | PubMed |
Murphy CJ, Richburg JH (2014) Implications of Sertoli cell induced germ cell apoptosis to testicular pathology. Spermatogenesis 4, e979110.
| Crossref | Google Scholar | PubMed |
Parekh PA, Garcia TX, Hofmann M-C (2019) Regulation of GDNF expression in Sertoli cells. Reproduction 157, R95-R107.
| Crossref | Google Scholar |
Pedrana G, Viotti H, Lombide P, Cavestany D, Martin GB, Vickers MH, Sloboda DM (2020) Maternal undernutrition during pregnancy and lactation affects testicular morphology, the stages of spermatogenic cycle, and the testicular IGF-I system in adult offspring. Journal of Developmental Origins of Health and Disease 11, 473-483.
| Crossref | Google Scholar |
Pedrana G, Larrañaga C, Diaz A, Viotti H, Lombide P, Cavestany D, Vickers MH, Martin GB, Sloboda DM (2021) Maternal undernutrition during pregnancy and lactation increases transcription factors, ETV5 and GDNF, and alters regulation of apoptosis and heat shock proteins in the testis of adult offspring in the rat. Reproduction, Fertility and Development 33, 484-496.
| Crossref | Google Scholar | PubMed |
Pinto-Fochi ME, Pytlowanciv EZ, Reame V, Rafacho A, Ribeiro DL, Taboga SR, Góes RM (2016) A high-fat diet fed during different periods of life impairs steroidogenesis of rat Leydig cells. Reproduction 152, 795-808.
| Crossref | Google Scholar |
Ramazi S, Zahiri J (2021) Post-translational modifications in proteins: resources, tools and prediction methods. Database 2021, baab012.
| Crossref | Google Scholar |
Rodrigo N, Saad S, Pollock C, Glastras SJ (2022) Diet modification before or during pregnancy on maternal and foetal outcomes in rodent models of maternal obesity. Nutrients 14, 2154.
| Crossref | Google Scholar | PubMed |
Rodríguez-González GL, Vega CC, Boeck L, Vázquez M, Bautista CJ, Reyes-Castro LA, Saldaña O, Lovera D, Nathanielsz PW, Zambrano E (2015) Maternal obesity and overnutrition increase oxidative stress in male rat offspring reproductive system and decrease fertility. International Journal of Obesity 39, 549-556.
| Crossref | Google Scholar |
Rodríguez-González GL, de Los Santos S, Méndez-Sánchez D, Reyes-Castro LA, Ibáñez CA, Canto P, Zambrano E (2023) High-fat diet consumption by male rat offspring of obese mothers exacerbates adipose tissue hypertrophy and metabolic alterations in adult life. British Journal of Nutrition 130, 783-792.
| Crossref | Google Scholar | PubMed |
Ruiz Valderrama L, Cervantes RE, Fragoso I, Rodríguez Tobón A, González Márquez H, Arrieta I, Tarragó MR, Arteaga M, Arenas E (2016) Efecto de la obesidad en la fertilidad masculina. Estudios en modelos animales. Revista Iberoamericana de Ciencias. 1a Jornadas de Reproducción UAM-Iztapalapa 3, 44-51.
| Google Scholar |
Schlesser HN, Simon L, Hofmann M-C, Murphy KM, Murphy T, Hess RA, Cooke PS (2008) Effects of ETV5 (ets variant gene 5) on testis and body growth, time course of spermatogonial stem cell loss, and fertility in mice. Biology of Reproduction 78, 483-489.
| Crossref | Google Scholar | PubMed |
Sertorio MN, César H, de Souza EA, Mennitti LV, Santamarina AB, De Souza Mesquita LM, Jucá A, Casagrande BP, Estadella D, Aguiar O, Jr, Pisani LP (2022) Parental high-fat high-sugar diet intake programming inflammatory and oxidative parameters of reproductive health in male offspring. Frontiers in Cell and Developmental Biology 10, 867127.
| Crossref | Google Scholar |
Shaha C, Tripathi R, Mishra DP (2010) Male germ cell apoptosis: regulation and biology. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 1501.
| Crossref | Google Scholar |
Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nature Reviews Molecular Cell Biology 20, 175-193.
| Crossref | Google Scholar | PubMed |
Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH (2009) Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS ONE 4, e6744.
| Crossref | Google Scholar | PubMed |
Tsoulis MW, Chang PE, Moore CJ, Chan KA, Gohir W, Petrik JJ, Vickers MH, Connor KL, Sloboda DM (2016) Maternal high-fat diet-induced loss of fetal oocytes is associated with compromised follicle growth in adult rat offspring. Biology of Reproduction 94, 1-11.
| Crossref | Google Scholar |
Tyagi G, Carnes K, Morrow C, Kostereva NV, Ekman GC, Meling DD, Hostetler C, Griswold M, Murphy KM, Hess RA, Hofmann M-C, Cooke PS (2009) Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biology of Reproduction 81, 258-266.
| Crossref | Google Scholar | PubMed |
Vannelli BG, Barni T, Orlando C, Natali A, Serio M, Balboni GC (1988) Insulin-like growth factor-1 (IGF-I) and IGF-I receptor in human testis: an immunohistochemical study. Fertility and Sterility 49, 666-669.
| Crossref | Google Scholar |
Vautier AN, Cadaret CN (2022) Long-term consequences of adaptive fetal programming in ruminant livestock. Frontiers in Animal Science 3, 778440.
| Crossref | Google Scholar |
Williams L, Seki Y, Vuguin PM, Charron MJ (2014) Animal models of in utero exposure to a high fat diet: a review. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1842, 507-519.
| Crossref | Google Scholar | PubMed |
Yang Y, Han C (2010) GDNF stimulates the proliferation of cultured mouse immature Sertoli cells via its receptor subunit NCAM and ERK1/2 signaling pathway. BMC Cell Biology 11, 78.
| Crossref | Google Scholar |
Zhang X, Zhao X, Li G, Zhang M, Xing P, Li Z, Chen B, Yang H, Wu Z (2021) Establishment of Etv5 gene knockout mice as a recipient model for spermatogonial stem cell transplantation. Biology Open 10, bio056804.
| Crossref | Google Scholar |


