Inland aquatic systems and their fauna at Two Peoples Bay Nature Reserve, Western Australia, with emphasis on microinvertebrates
Stuart A. Halse A and Andrew W. Storey B *
B *
A
B
Abstract
Decisions about fauna conservation priorities in a region, and management actions required to facilitate conservation objectives, require as much information as possible about the ecology of fauna species present.
This paper summarises information on the inland aquatic fauna of Two Peoples Bay Nature Reserve as part of the process of documenting the Reserve’s conservation values.
Surveys in the early 1990s, and subsequently, show that rivers, creeks, and lakes of the Reserve support a rich aquatic invertebrate fauna. The microinvertebrate community is unusually rich in rotifers and cladocerans. Probably nine Indigenous fish species occur in the Reserve, including the vulnerable Nannatherina balstoni, restricted Galaxias maculatus and critically endangered Galaxias truttaceus. The installation of a vertical slot fish ladder on the Goodga River weir has likely helped these species by expanding available habitat. Although the low nutrient status and relatively deep water of the lakes limit their waterbird value, considered together the Reserve’s lakes fall just outside the top 10% of nature reserves in Western Australian for number of waterbird species supported.
The richness of aquatic invertebrate species in the Reserve’s lakes contrasted with previous results from rivers in south-western Australia and has led to a changed understanding of both the diversity and conservation significance of the inland aquatic invertebrate fauna in Western Australian lakes.
Invertebrate community composition and conservation values are not recognised unless surveyed by people with appropriate taxonomic expertise. Without such surveys the focus of conservation effort and management may be, at least partially, misdirected.
Keywords: Cladocera, conservation value, fish, macroinvertebrates, microinvertebrates, Rotifera, waterbirds, wetlands.
Introduction
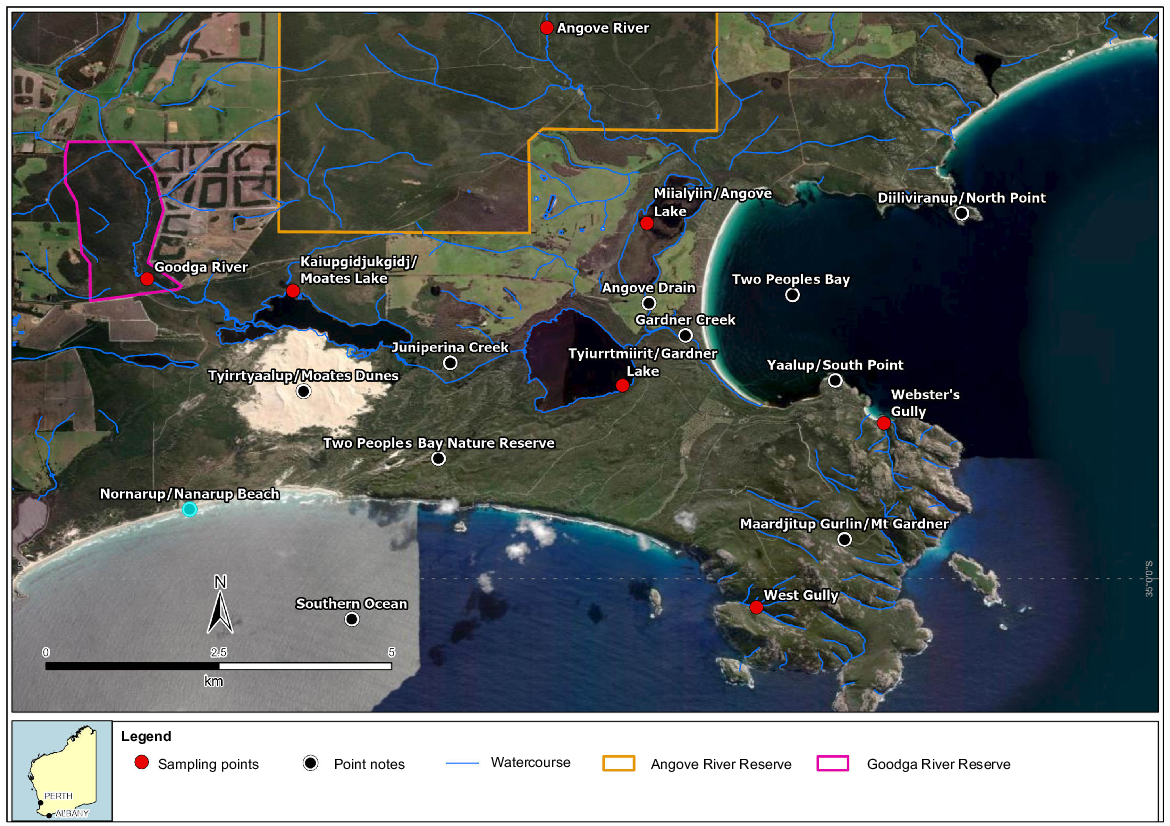
This paper is part of a collection of Pacific Conservation Biology documenting the natural history of the Two Peoples Bay Nature Reserve in Western Australia (Hopkins et al. 2024) and provides information about the drainage systems and their non-marine aquatic fauna. Wetlands, including creeks, are prominent features of the Reserve. Three distinct drainage systems occur, associated with the Goodga River, Angove River, and the upland streams of the Maardjitup Gurlin/Mt Gardner headland (Fig. 1) (Noongar names are from Knapp et al. 2024). Two of the systems (Angove and Goodga) arise outside the Reserve and are influenced by external land practices.
Lakes, rivers, and streams of Two Peoples Bay Nature Reserve and sampling points in the water bodies.
Data on water depth, salinity, and discharge of the Goodga and Angove systems have been recorded for a number of years (Lane et al. 2015) and the invertebrate fauna has been surveyed relatively intensively (Storey et al. 1993), with a range of authors sampling fish (current authors in 1990; Morgan et al. 1998, 2006; Morgan and Beatty 2006; N. Coy, unpubl. data). Our paper describes the physico-chemical characteristics and vegetation (emergent and riparian) of the aquatic ecosystems of the Reserve area, provides the results of some surveys of fish and waterbirds, and discusses the significance of the aquatic invertebrate fauna.
The data reported in this paper are mostly several decades old and, with the exception of physico-chemical data for the lakes, were collected to provide an inventory of the biological values of the Reserve, which was gazetted in 1966 and 1967 for the purpose of flora and fauna conservation (Chatfield and Saunders 2024). The protection thus provided to the aquatic systems of the Reserve and their fauna represents early recognition of the value of formal protection processes by government as an effective conservation tool, which is overlooked in much conservation policy-making (Tickner et al. 2020). Detailed inventory of aquatic invertebrates in south-western Australia was in its early stages in 1990 and 1991, when the invertebrate work reported here was undertaken, and the results from the Reserve have been important for highlighting the value of sampling all aquatic invertebrate groups and also in providing taxonomic focus for much of the more recent aquatic invertebrate work in Western Australia.
Background to aquatic fauna surveys in south-western Australia
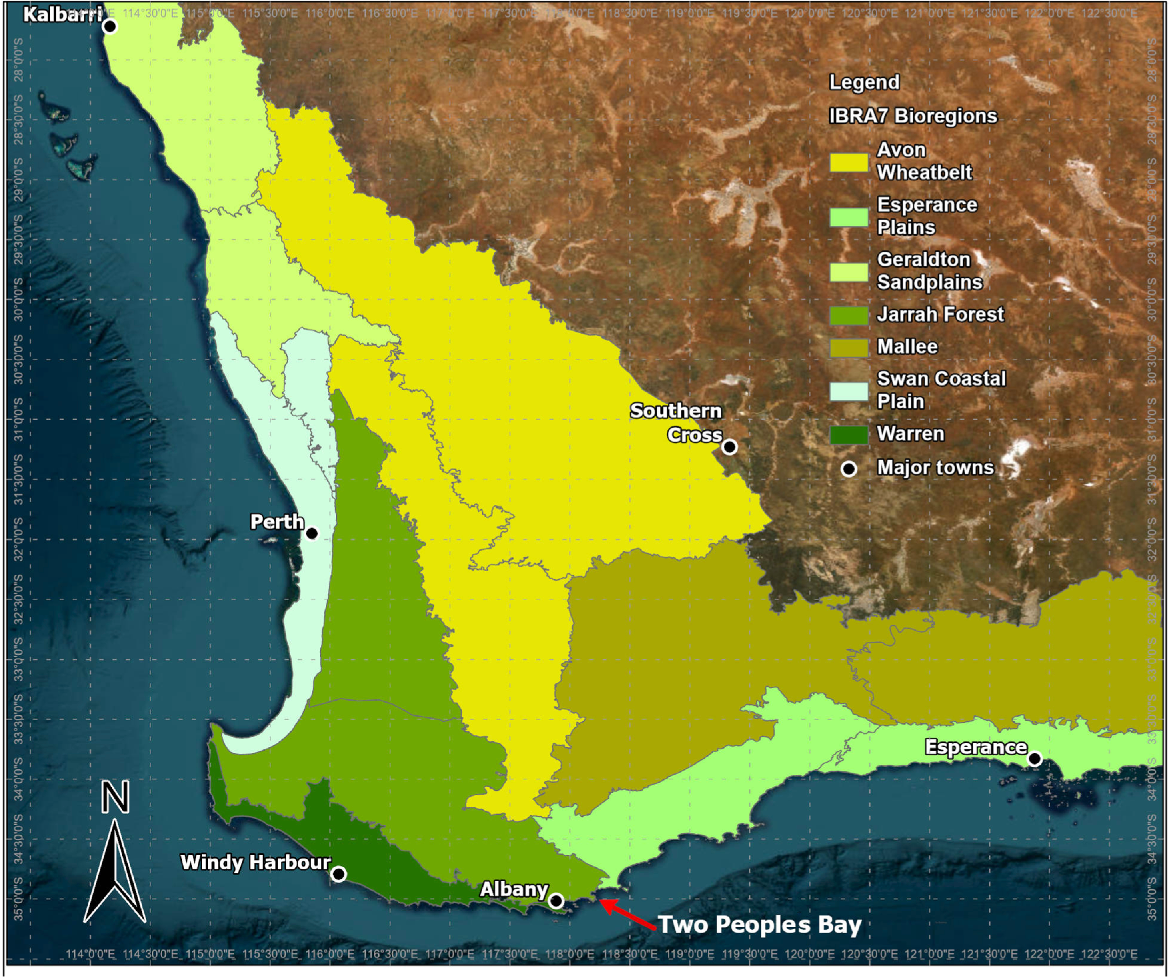
This review of inland aquatic fauna surveys focuses on south-western Australia, comprising a zone extending approximately from Kalbarri to Esperance and comprising seven bioregions (Fig. 2). Two Peoples Bay Nature Reserve lies in the southern part of the Jarrah Forest bioregion. Permanent or near permanent lakes are mostly restricted to the Swan Coastal Plain, Warren, southern Jarrah Forest, and Esperance bioregions, and are uncommon even in these regions. South-western Australia has a Mediterranean climate, albeit with less pronounced seasonality at Two Peoples Bay on the south coast. Albany, just west of Two Peoples Bay, has a mean monthly maximum temperature of 16°C in the coldest winter month and 23°C in the warmest summer month, and an annual rainfall of 921 mm (Table 1). Across most of the south-west, rivers flow for limited periods in winter and spring but near the coast may contain permanent water, especially in the wetter Warren region.
Interim biogeographical regions of south-western Australia and towns/sites used in rainfall statistics.
| July (°C) | February (°C) | Annual rainfall (mm) | ||
|---|---|---|---|---|
| Albany | 15.9 | 22.9 | 920.9 | |
| Esperance | 17.3 | 26.2 | 617.4 | |
| Kalbarri | 21.9 | 34.1 | 341.8 | |
| Perth | 18.5 | 31.7 | 727.3 | |
| Southern Cross | 16.3 | 34.5A | 295.4 | |
| Windy Harbour | 16.8 | 23.7 | 1061.1 |
There is a long history of waterbird surveys at wetlands in Western Australia, often by amateur ornithologists. Large-scale, coordinated surveys with multiple surveys of sites and a clear conservation purpose did not begin until the 1980s. The main surveys have included inventory of waterbirds in nearly all nature reserves containing wetlands in the 1980s (Jaensch et al. 1988), censuses of ducks, coots, and swans across the south-west from 1986 to 1992 (Halse et al. 1995), and surveys of 251 wetlands on the coastal plain around Perth in 1990 and 1991 to assist water allocation planning (Storey et al. 1993). Waterbirds were surveyed across all of Western Australia in 2008 as part of a national census (Kingsford et al. 2012) and regular monitoring of the effects of salinisation at 25 wetlands began in the mid-1990s (Pinder et al. 2023). These broadscale surveys provide a context for the many surveys that have been conducted at individual wetlands or groups of wetlands, including the surveys at Two Peoples Bay Nature Reserve.
Important contributions to knowledge of inland fish in south-western Australia have been made by Gerry Allen (Allen et al. 2002) and Ian Potter, David Morgan, Luke Pen, and Howard Gill (Pen and Potter 1991; Morgan et al. 1998). Among the many benefits, surveys of fish in the past 30 years have led to new species being discovered (Morgan et al. 2013), the habitats and distributions of south-western Australian inland aquatic fish being known in detail (Morgan and Gill 2000) and fitted into a state-wide picture (Morgan et al. 2014), and managers being able to predict the likely impacts of increasing salinity on different fish species (Beatty et al. 2011). Dryland salinity is extensive in south-western Australia and has affected all aquatic life (Halse et al. 2003). The impact of invasive fish species has also been studied (Lymbery et al. 2010).
Several people have made outstanding taxonomic contributions that have supported inventory of aquatic invertebrates across Western Australia. They include Tony Watson for dragonflies (Watson et al. 1991), Chris Watts for beetles (Watts 1978), Peter Cranston and Don Edward for dipterans (Leung et al. 2013; Cranston 2019), Ian Bayly for calanoid copepods (Bayly 1992), Patrick De Deckker for ostracods (De Deckker 1983), Michael Geddes and Brian Timms for anostracans (Geddes 1981; Timms 2012), Adrian Pinder for oligochaetes (Pinder and Brinkhurst 1994), and Russell Shiel for rotifers and cladocerans (Shiel 1995).
Large-scale surveys of aquatic invertebrates have been undertaken on the coastal plain around Perth for water allocation planning (Davis et al. 1993), wetlands across the wheatbelt region of south-western Australia for conservation planning (Pinder et al. 2004), in rivers across Western Australia to provide the basis for using invertebrates as indicators of the ecological health of wetlands (Smith et al. 1999), and in rivers of the forested areas for conservation planning and monitoring of ecological condition (Pennifold et al. 2017). Many smaller scale surveys, including those along the south coast by Edward et al. (1994), Stewart (2009, 2011) and Timms (2008), have provided inventories and examined the effect of water chemistry on the invertebrate species present. Ecological processes in small streams were studied during the 1980s and 1990s in jarrah forest (Bunn 1988; Storey et al. 1990) and karri forest (Trayler and Davis 1998). The invertebrates of granite rock pools have also been a focus of attention (Pinder et al. 2000; Bayly et al. 2011).
Several reviews or analyses of the aquatic fauna of the south-west have been undertaken, including a comprehensive review of all groups of aquatic fauna and flora using rivers by Davies and Stewart (2013), who considered 43% of species in rivers to be endemic to south-western Australia. Reviewing stream studies in south-western Australia in the 1980s, Bunn and Davies (1990) highlighted the depauperate nature of the recorded fish and macroinvertebrate faunas and suggested this is associated with the isolation of south-western Australian streams from other stream systems (the south-west is surrounded by arid regions), the occurrence of much greater aridity in the south-west in the past than occurs now (hence the fauna reflects a more arid situation), and a very low level of primary productivity. In an analysis of available information on aquatic invertebrates, fish and frogs in the Warren bioregion (Fig. 2), Trayler et al. (1996) found records of 156 described invertebrate species, 22 frogs, and 14 native fish in lotic and lentic systems and concluded 86% of the species were protected by their occurrence in various forms of nature reserves, although external impacts (including salinisation) may reduce the protection provided by some reserves. Analysing results of sampling for aquatic invertebrates in peatlands and shrublands of southern parts of the Warren and Jarrah Forest bioregions, Horwitz (1997) found endemism to be higher in the coastal plain of the Warren region than elsewhere, perhaps because this area was less impacted by salinity, both currently and historically, than further north. A similar analysis by Horwitz et al. (2009) for aquatic invertebrates in lentic waterbodies around Perth in the Swan Coastal Plain bioregion found 520 species had been recorded, with the coastal wetlands having higher average richness than wetlands further inland in more arid areas. However, only 16% of species recorded by Horwitz et al. (2009) are endemic to south-western Australia.
The aquatic systems at Two Peoples Bay Nature Reserve
The wetland area in the Two Peoples Bay Nature Reserve fluctuates seasonally between ~400 ha (8.5% of the 4745 ha reserve) in normal summer conditions and ~750 ha (15.8%) during average winter/spring flooding in August/September. In very wet winters an estimated 830 ha (17.5%) is inundated, with an additional 170 ha (3.5%) of waterlogged land. The widespread occurrence of peaty sands in the area suggests it was much wetter historically than now. Extraction of water from the Angove River for a potable water supply for Albany (Chatfield and Saunders 2024) and construction of drains on the coastal plain are probably partly responsible for this drying. Components of the wetland systems are described below.
The Goodga drainage system covers 21 km and runs from the headwaters of the Goodga River through Kaiupgidjukgidj/Moates Lake, Juniperina Creek, Tyiurrtmiirity/Gardner Lake and Gardner Creek into the ocean in Two Peoples Bay (Fig. 1). Physico-chemical and depth data for the Goodga system are summarised in Table 2 and Fig. 3; much of this information comes from Lane et al. (2015).
| Physico-chemical parameter | Angove River | Goodga River | West Gully | Webster’s Gully | Angove Lake | Gardner Lake | Moates Lake | |
|---|---|---|---|---|---|---|---|---|
| Depth (m) | 0.5 | 0.4 | 0.4 | 0.3 | 1.8 | 2.6 | 4.4 | |
| Salinity (mg/L TDS A) | 240 | 560 | 440 | 180 | 580 | 880 | 470 | |
| pH | 5.9 | 6.3 | 6.5 | 6.8 | 6.8 | 7.6 | 6.7 | |
| Temperature (°C) | 13.1 | 11.4 | 10.8 | 11.4 | 13.3 | 10.7 | 11.5 | |
| Dissolved oxygen (% sat.) | 72 | 96 | 93 | 93 | 96 | 100 B | 96 | |
| Total phosphorus (mg/L) | – | – | – | – | 0.03 | 0.02 | 0.03 |
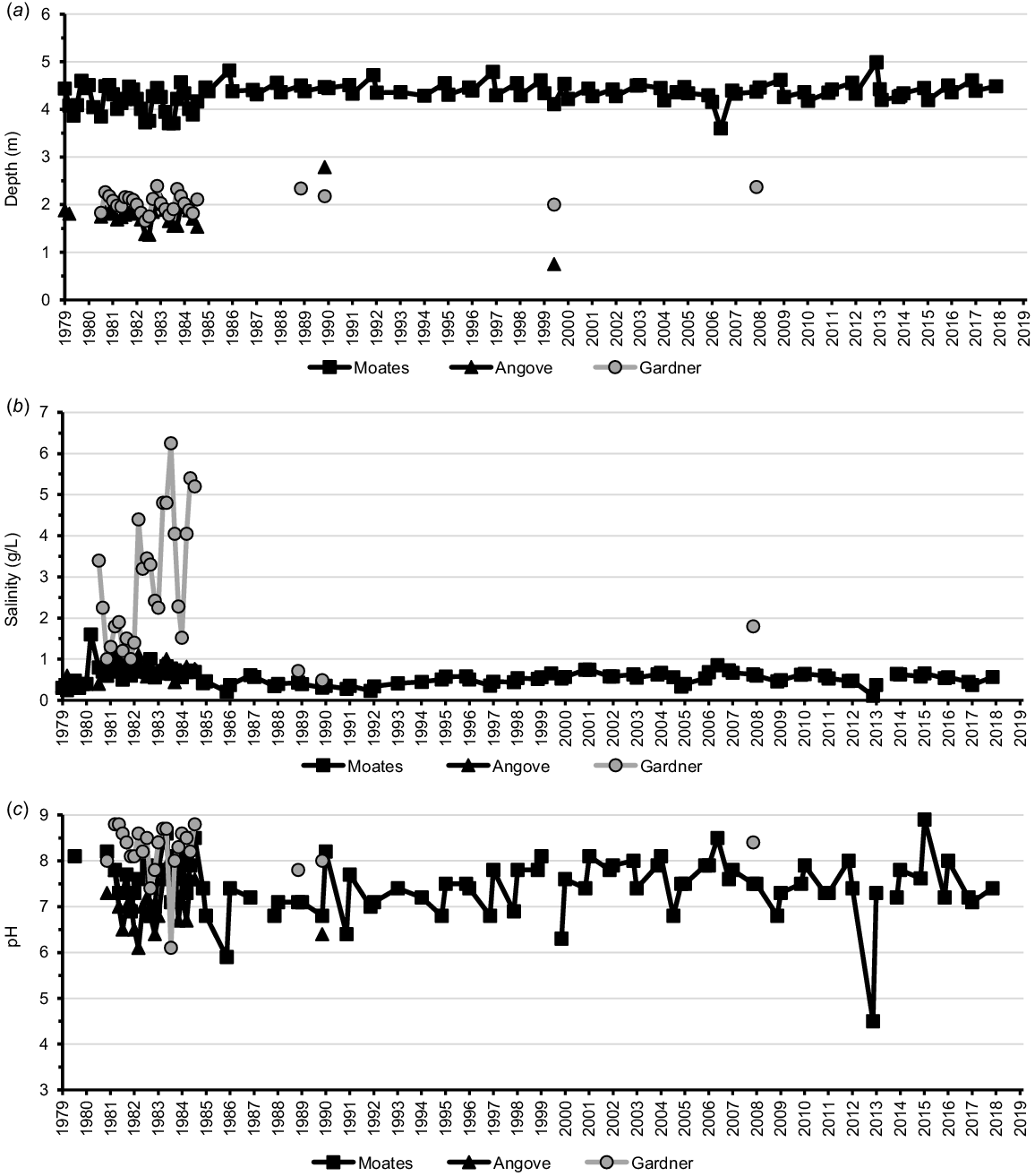
Long-term data on physico-chemistry of the lakes of Two Peoples Bay Nature Reserve, (a) depth, (b) salinity and (c) pH.
The Goodga River arises ~80 m above sea level (a.s.l.) in Water Catchment Reserve No. 13802, which currently has an area of 4006 ha. The Catchment Reserve was first gazetted on 27 October 1911 with an area of 20,000 ha, then gazetted to its present size in 1953 and vested in the Department of Planning, Lands and Heritage.
Water from the headwater swamps in the Catchment Reserve descends 30 m in the first 2 km as it passes between low granite hills before flowing for 6 km in a broad, swampy valley through cleared farmland (Churchward et al. 1988) and being joined by a smaller tributary from the west. One kilometre downstream from this confluence the river enters National Park/Water Reserve No. 24991, which was gazetted on 16 May 1958.
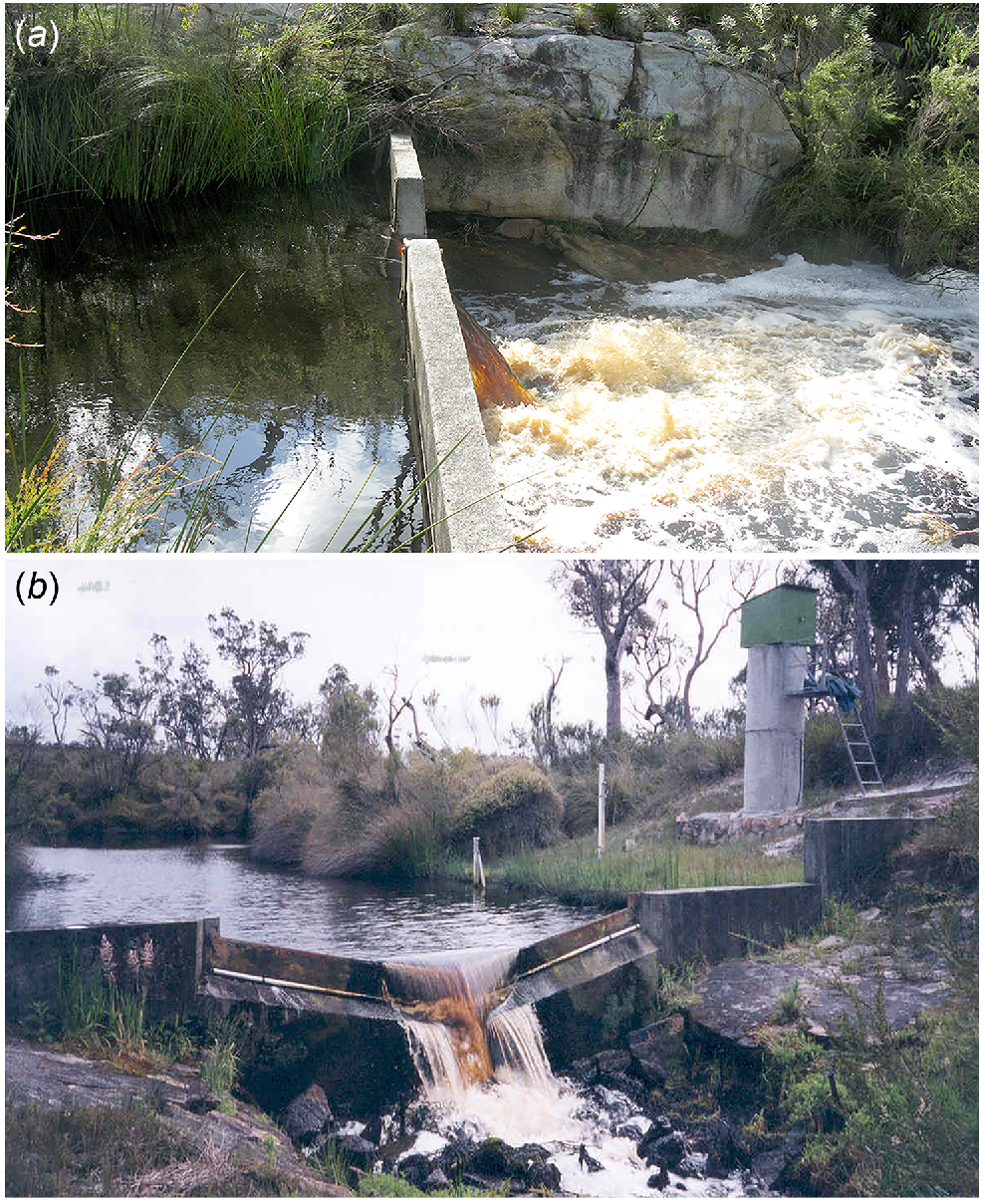
Throughout Reserve No. 24991, what is now the Goodga River is slightly acidic (Table 2) and characterised by a series of long, highly coloured pools with silty substrates that are connected by riffles flowing over laterised rock. Wetland plants along the watercourse include Triglochin procera, Machaerina juncea, Leptocarpus scariosus, Juncus holoschoenus, and Lepyrodia monoica. The Department of Water and Environmental Regulation maintains a V-notch gauging weir 2.5 km upstream from the Two Peoples Bay Road (Fig. 4).
V-notch weirs on (a) Angove Creek (Credit: WA Department of Water and Environmental Regulation) and (b) Goodga Creek (Credit: J. Oates).
As the river gets closer to Kaiupgidjukgidj/Moates Lake, there are a series of sedge swamps along the water course dominated by Evandra aristata, Leptocarpus scariosus, Hypolaena exsulca, and Xyris lantana. The tannin-stained Kaiupgidjukgidj/Moates Lake is one of the deepest natural lakes in south-western Australia, with depth in September and November varying between 3.6 and 5.0 m over the past 40 years (Fig. 3). Four main rivers/streams flow into the lake: the Goodga River and Black Cat Creek come from the north, a swamp stream that originates north of Two Peoples Bay Road comes from the north-west, and a stream flowing through Black Cat Creek Farm comes from the west (Fig. 1).
Kaiupgidjukgidj/Moates Lake has an area of about 144 ha with 124 ha of open water. Total length of the lake, from east to west, is 2.75 km with a maximum north–south measurement of 0.7 km. Elevation is ~8 m a.s.l. The lake is situated in a depression between a low scarp of sandy laterite to the north and a 300 ha calcareous dune dating from the Holocene to the south. Much of the southern shoreline is a sandy beach, but apart from a few small laterite and sand beaches the northern shoreline is sedge-dominated. Salinity in September and November has varied from 0.1 to 0.8 g/L, with a median of 0.5 g/L. pH has varied from 4.5 to 8.9, with a median of 7.4 (Fig. 3). The major species of sedge in the water are Machaerina articulata in depths up to 1 m and B. juncea close to the shore. Triglochin procera grows in sheltered areas. The marginal sedgeland includes E. aristata, L. scariosus, L. coangustatus, and Anarthria scabra. Behind the sedges are low trees of Taxandria juniperina and A. linearifolia (Halse et al. 1993).
The 2.5 km section of the Goodga system between Kaiupgidjukgidj/Moates and Tyiurrtmiirity/Gardner Lakes is referred to locally as Juniperina Creek. It has a seasonal flow that meanders between lagoons and swamps and through a 1.5 m deep channel to the delta marsh at the western end of Tyiurrtmiirity/Gardner Lake (Fig. 1). There is a fall of 6–7 m in surface level between the two lakes; the gradient varies with fluctuating lake levels. The watercourse and the five or six lagoons between the dunes are locally referred to as the South-East Lagoons. The Machaerina/Juncus sedgelands in the seasonally wet, interdunal swales and along the creek-line are fringed by Taxandria juniperina and Banksia littoralis.
The slightly stained, brackish Tyiurrtmiirity/Lake Gardner receives water mainly from upstream parts of the Goodga drainage system via Juniperina Creek. It may also receive some tidal flow from Gardner Creek, when this is open to the sea, because the lakebed is only ~1.5 m a.s.l. Tyiurrtmiirity/Gardner Lake contains about 186 ha of open water when full but in winter an area of ~350 ha is flooded, with almost half the area densely vegetated. The lake area measures 1.8 km north–south and 1.6 km east–west. Available information, mostly from the 1980s, shows September and November depths of Tyiurrtmiirity/Lake Gardner vary from 2.0 to 2.4 m deep, with salinity ranging from 0.5 to 2.4 g/L and pH from 7.8 to 8.6 (Fig. 3; also see Lane et al. 2015).
Much of the substrate in Tyiurrtmiirity/Gardner Lake is silt over sand and laterite. The emergent sedges around the lake are mainly Machaerina articulata, Juncus krausii, Schoenus brevifolius, and Restio sp. (Fig. 5). In places along the eastern shore they extend 100 m into the water when the lake is full. The seasonally inundated margins support Gahnia trifida, Lepidosperma gladiatum and various shrubs, including Taxandria juniperina, Homalospermum firmum, Hakea sp., Lepyrodia sp., Thysanotis gracilis, and Centella asiatica (Halse et al. 1993).
Tyiurrtmiirity/Gardner Lake looking south from road and (a) fringing sedges (Credit: S. Halse).

The channel between Tyiurrtmiirity/Gardner Lake and the main beach at Two Peoples Bay is referred to as Gardner Creek. This creek is closed to the sea each summer by a sandbar. In early winter, water in the creek backs up on the landward side of the sandbar, which is opened by mechanical means, usually by the middle of June, to prevent flooding of the small road bridge over Gardner Creek.
As the 2.8 km long Gardner Creek leaves the eastern shore of Tyiurrtmiirity/Gardner Lake, it follows a north-easterly course through a grove of overhanging Melaleuca with little understorey (Fig. 6). Melaleuca cuticularis, M. raphiophylla, M. preissiana, and an undescribed species grow in this seasonally inundated area. The creek then turns south along the landward side of the dune line. Near its mouth, at a break in the dunes adjacent to the Reserve Office, the creek flows past a dense thicket of Phebalium anceps over Lepidosperma gladiatum, with Typha domingensis in a disturbed backwater.
The Angove drainage system, which is 14 km long, includes the Angove River, Miialyiin/Angove Lake and Angove Drain. The latter joins the Goodga drainage system 1 km inland from the dunes of the main Two Peoples Bay beach. Angove River arises ~90 m a.s.l. in small swamps north of the uncleared Water Catchment Reserve No. 13802 (Fig. 1). The river flows for 8 km through a gently sloping valley into a pipe-head dam that was constructed in 1912–13 to provide water for the town of Albany (Fig. 7). Soils consist of sand over gravels and clay, with mallee-woodland/heath growing on the valley sides and thickets of jarrah Eucalyptus marginata and Allocasuarina on swampy land in the valley (Churchward et al. 1988). Below the dam the stream flows over a sloping granite fault line, with a marked gradient of ~15 m over 1.5 km to a V-notch gauging weir (Fig. 4). Jarrah/Allocasuarina woodland grows on the valley slopes through this section.
After leaving the catchment reserve, the river flows more slowly through a stream reserve that is dominated by Melaleuca cuticularis with an understorey that includes the shrubs Pultenaea reticulata, Boronia crenulata, B. gracilipes, and Hypocalymma cordifolium Thresk. Its catchment area and volume are increased by the contribution of four tributaries: two from the north, one flowing from Reservoir Hill in the west across cleared farmland, and a short fast-flowing stream from Boulder Hill in the east (Fig. 1).
Miialyiin/Angove Lake, which is included in a separate ~89 ha northern section of the Nature Reserve, has an area of 22 ha, of which 15.6 ha is open water (Fig. 7). It is complemented by two lagoons to the west (measuring 3 ha and 4 ha respectively), and a third lagoon of 10 ha to the north-east, inland from the dunes at the northern end of Two Peoples Bay (Fig. 7). The total area of swamp and winter-wet land around Miialyiin/Angove Lake is ~100 ha. September and November depths at Angove Lake, mostly from the 1980s, have ranged from 1.8 to 2.8 m. with salinity between 0.3 and 0.9 g/L and pH between 7.8 and 8.6 (Fig. 3; see also Lane et al. 2015).
When settled, water in Miialyiin/Angove Lake is clear but the substrate of black silt is readily suspended by disturbance and settles slowly. Lake margins support extensive stands of Machaerina articulata in deeper water, with B. preisii, Juncus pallidus, J. capitatus, Isolepis prolifera, Triglochin procera, and Cotula coronopifolia in shallow water and on shore. Behind the sedgeland, on the northern side of the lake, the shrubs Taxandria juniperina and Kunzea aff. ericifolia grow, beyond which is a dense thicket of Melaleuca thymoides. Miialyiin/Angove Lake has been invaded by a number of weeds, including Rumex acetosella, Holcus lanatus, Psoralea pinnata, and Vellereophyton dealbatum.
There is a 3 km drain from Miialyiin/Angove Lake to Gardner Creek that is ~1.5 m deep and 10 m wide. It was originally part of a drainage system dug by hand in the late 1920s to allow summer vegetable crops to be grown on the seasonally inundated swamps of the floodplain south of Miialyiin/Angove Lake. The drain was enlarged and deepened to its present size by dragline in the late 1970s.
Where the drain leaves the lake, it contains dense stands of Homalospermum firmum and, for much of its course through farmland, the drain is fringed by a species of Melaleuca, Taxandria linearifolia, Taxandria juniperina, and Kunzea vestita. Close to Gardner Creek the drain follows a natural watercourse, fringed by Hibbertia furfuracea and sedges.
There is a different aquatic system on the Maardjitup Gurlin/Mt Gardner headland, which is drained by 12 small streams that are well-shaded and comparatively cool. The densely vegetated gullies, through which these streams flow, served as a refuge for the noisy scrub-bird Atrichornis clamosus when the species was locally extinct elsewhere (Danks et al. 1996). Streams on the ocean side of the headland rise at ~200 m a.s.l. and flow to the sea with a mean gradient of about 1:7 over their 1.3–1.5 km courses. The four major streams flowing to the ocean – Gardner, Robinson, Webster’s, and West Gullies – hold water permanently, although they recede to pools in dry years.
Robinson Gully is a clear upland stream with a sloping marshland of Schoenus grandiflorus in its middle reaches. Webster’s Gully consists of a series of gravel-bottomed, clearwater pools in its lower reaches with a 10 m drop over granite boulders onto Naarup/Waterfall Beach. The vegetation of this gully is dense eucalypt/Melaleuca forest with a varied understorey that includes Lepidospermum sp. and Anarthria scabra along the stream. West Gully is a rapidly flowing stream with few pools and a dense canopy of low eucalypts along its lower reaches.
Streams on the inland side of the headland arise at ~180 m a.s.l. and descend to the isthmus area where they seep into the dunes and swales. These streams only flow in their upper reaches, where they are seasonal, and are more akin to dry drainage lines for most of their ~2 km courses.
The fauna
Forty-two species of waterbird were recorded from the three lakes of Two Peoples Bay Nature Reserve between 1973 and 1990 during surveys by the Birdlife Australia and Department of Conservation and Land Management staff (Table 3, see Jaensch et al. 1988 for details). This represents comparatively high species richness for wetlands of south-western Australia: Jaensch et al. (1988) surveyed 197 nature reserves in the south-west during the same time period and ranked Two Peoples Bay 25th in terms of number of species. The individual lakes showed considerable differences in waterbird richness, with Miialyiin/Angove supporting fewer species (17) than Kaiupgidjukgidj/Moates (29) and Tyiurrtmiirity/Gardner (35) (Table 3), although Miialyiin/Angove was surveyed much less often and the lower number of species may be partly attributed to lower survey effort (Jaensch et al. 1988).
| Scientific name | Common name | Angove | Gardner | Moates | |
|---|---|---|---|---|---|
| Podicipedidae | Grebes | ||||
| Podiceps cristatus | Great crested grebe | 6 | 1 | ||
| Poliocephalus poliocephalus | Hoary-headed grebe | 40 | 4 | ||
| Tachybaptus novaehollandiae | Australasian grebe | 6 | 7 | ||
| Pelecanidae | Pelicans | ||||
| Pelecanus conspicillatus | Australian pelican | 2 | |||
| Anhingidae | Darters | ||||
| Anhinga melanogaster | Darter | 2* | |||
| Phalacrocoracidae | Cormorants | ||||
| Phalacrocorax carbo | Great cormorant | 1 | 10 | ||
| Phalacrocorax varius | Pied cormorant | 10 | 2 | ||
| Phalacrocorax sulcirostris | Little black cormorant | 1 | 6 | 8 | |
| Phalacrocorax melanoleucos | Little pied cormorant | 3 | 9 | 9* | |
| Ardeidae | Herons and bitterns | ||||
| Ardea novaehollandiae | White-faced heron | 11 | 17 | 4 | |
| Ardea alba | Great egret | 2 | 3 | ||
| Ixobyychus minutus | Little bittern | 1 | 1 | ||
| Botaurus poiciloptilus | Australasian bittern | 1 | 1 | 1 | |
| Threskornithidae | Ibises and spoonbills | ||||
| Threskiornis aethiopica | Sacred ibis | 18 | 1 | ||
| Threskiornis spinicollis | Straw-necked ibis | 4 | 1 | ||
| Platalea regis | Royal spoonbill | 3 | |||
| Platalea flavipes | Yellow-billed spoonbill | 3 | |||
| Anatidae | Ducks, geese and swans | ||||
| Cygnus atratus | Black swan | 2 | 45 | 10* | |
| Tadoma tadomoides | Australian shelduck | 2 | 54 | 10 | |
| Anas superciliosa | Pacific black duck | 65 | 164* | 13 | |
| Anas gibberifrons | Grey teal | 18 | 2 | ||
| Anas rhynchotis | Australasian shoveler | 2 | |||
| Chenonetta jubatam | Maned duck | 2 | 2 | ||
| Oxyura australis | Blue-billed duck | 53 | 1 | ||
| Biziura lobata | Musk duck | 3* | 50* | 4* | |
| Accipitridae | Hawks and harriers | ||||
| Circus aeruginosus | Marsh harrier | 1 | 1 | 2 | |
| Rallidae | Rails, coots and gallinules | ||||
| Porzana tabuensis | Spotless crake | 2 | 1* | 3 | |
| Porphyrio porphyrio | Purple swamphen | 3 | 7 | 3* | |
| Fulica atra | Eurasian coot | 216 | |||
| Charadriidae | Plovers and dotterels | ||||
| Pluvialus squatarola | Grey plover | 1 | |||
| Erythrogonys cinctus | Red-kneed dotterel | 1 | |||
| Charadrius ruficapillus | Red-capped plover | 54 | |||
| Charadrius melanops | Black-fronted plover | 2 | 5 | 6 | |
| Scolopacidae | Snipe and sandpipers | ||||
| Tringa hypoleucos | Common sandpiper | 1 | |||
| Tringa nebularia | Greenshank | 6 | 2 | ||
| Calidris ruficollis | Red-necked stint | 60 | |||
| Laridae | Gulls and terns | ||||
| Larus novaehollandiae | Silver gull | 23 | |||
| Chlidonias hybrida | Whiskered tern | 2 | |||
| Hydroprogne caspia | Caspian tern | 2 | |||
| Sterna bergii | Crested tern | 1 | |||
| Muscicapidae: Sylviinae | Old world warblers | ||||
| Acrocephalus stentoreus | Clamorous reed warbler | 3 | |||
| Megalurus gramineus | Little grassbird | ||||
| Number of surveys | 5 | 24 | 30 | ||
| No. of species | 14 | 25 | 24 | ||
| No. of breeding species | 1 | 3 | 5 | ||
| Highest counts totalled | 109 | 420 | 41 | ||
The highest number of birds recorded on a single occasion is shown for each species. Breeding species are indicated by an asterisk.
Waterbird abundance was low in all three lakes. Tyiurrtmiirity/Gardner was ranked 83 in abundance out of the 285 wetlands in the reserves surveyed by Jaensch et al. (1988). Kaiupgidjukgidj/Moates and Miialyiin/Angove support even lower abundance (Table 3). The waterbird conservation value of the lakes lies chiefly in the diversity and composition of species.
Species occurring in highest numbers are Eurasian coot, Pacific black duck, Australian shelduck, blue-billed duck, musk duck, red-necked stint, and red-capped plover (scientific names are given in Table 3). The occurrence of most of these species is sporadic, however. Those most frequently observed in Tyiurrtmiirity/Gardner are musk duck (85% of surveys), black swan (85%), and white-faced heron (70%). The most frequently observed species in Kaiupgidjukgidj/Moates are little pied cormorant (70%), little black cormorant (63%), and spotless crake (53%). There were too few surveys of Miialyiin/Angove for this analysis.
Musk duck, darter, little pied cormorant, Pacific black duck, spotless crake, and purple swamphen have been recorded breeding in the Reserve; musk ducks have bred at all three lakes (Table 3). Several other species may breed around the lakes (Jaensch et al. 1988), including Australasian bittern, which is listed under State and Commonwealth legislation as endangered. Kaiupgidjukgidj/Moates Lake area, in particular, is considered to be important habitat for this comparatively rare species (Department of Biodiversity, Conservation and Attractions 2018).
The freshwater and estuarine fish populations of Two Peoples Bay Nature Reserve have been surveyed on several occasions (current authors in 1990; Morgan et al. 1998, 2006; Morgan and Beatty 2006; N. Coy, unpubl. data), with fish collected from both the Angove and Goodga drainage systems. No fish have been recorded from the Maardjitup Gurlin/Mt Gardner Headland systems. The above surveys, combined with Western Australian Museum records and anecdotal records from several local people with a long-term interest in the area, suggest nine Indigenous freshwater species occur (Table 4).
| Scientific name | Common name | GR | ML | GL | GR | AS | |
|---|---|---|---|---|---|---|---|
| Galaxiidae | |||||||
| Galaxias maculatus (Jenyns) | Common jollytail | X | X | X | X | X | |
| Galaxias truttaceus (Valenciennes) | Trout minnow | X | X | ||||
| Galaxias occidentalis (OgilbyA ) | Western minnow | X | X | X | |||
| Percichthyidae | |||||||
| Nannoperca vittata (Castelnau) | Western pygmy perch | X | X | ||||
| Nannatherina balstoni (Regan) | Balston’s pygmy perch | X | |||||
| Atherinidae | |||||||
| Leptatherina wallacei (Prince Ivanstoff & Potter) | Western hardyhead | X | X | X | |||
| Mugilidae | |||||||
| Aldrichetta forsteri (Valenciennes) | Yellow-eye mullet | X | X | X | |||
| Gobiidae | |||||||
| Pseudogobius olorum (Sauvage) | Blue-spot goby | X | X | X | X | X | |
| Sparidae | |||||||
| Acanthopagrus butcheri (Munro) | Black bream | X | X | X | |||
| No. of species | 6 | 3 | 6 | 5 | 7 | ||
GR, Goodga River; ML, Kaiupgidjukgidj/Moates Lake; GL, Tyiurrtmiirity/Gardner Lake; AS, Angove system.
Although not unique, the fish assemblage of the area is noteworthy for supporting common south-west Western Australian species towards the eastern extent of their range, as well as supporting several galaxiids that are restricted to south coast catchments. Pseudogobius olorum (Swan River goby) and Galaxias maculatus (common jollytail) are the most widely distributed species in the Reserve. Morgan et al. (1998) reported Galaxias occidentalis (western minnow) from six locations in the system, however, a subsequent study on galaxiid passage on the Goodga River (Morgan and Beatty 2006) failed to mention this species and so the previous records may have been an error.
The coastal sections of both river systems appear to be nurseries for the euryhaline Leptatherina wallacei (hardyhead), Aldrichetta forsteri (yellow-eye mullet), and Acanthopagrus butcheri (black bream) (Table 4). Prior to 1980, a local family (the Wilsons) netted 40–60 cm long yellow-eye mullet and a variety of small, unidentified ‘bait fish’ (likely G. maculatus) from Tyiurrtmiirity/Gardner Lake several times each year. Acanthopagrus butcheri has been caught in Tyiurrtmiirity/Gardner Lake and Gardner Creek. Although it has not been collected, it is likely that Geotria australis (pouched lamprey) occurs in the Angove and Goodga systems (Miller et al. 2021). Large spawning runs of this species usually occur each winter in nearby King Creek, Waychinicup River, and the Kalgan River.
The presence of Galaxias truttaceus (trout minnow) and Galaxias maculatus in a relatively landlocked situation in the Goodga and Angove rivers is notable. Studies in the eastern states of Australia on G. truttaceus (Humphries 1989) and G. maculatus (Pollard 1971) have shown the former is usually anadromous (going upstream from the sea to spawn) and the latter catadromous (going downstream to the estuary to spawn), although both species can adapt to landlocked situations.
Galaxias maculatus was formerly known only from rivers and lakes between the Goodga River and the Daley River (50 km east of Esperance), with additional records from the Boat Harbour Lakes (Kent River). An intensive survey extended its distribution east by 50 km (Thomas River), west by approximately 40 km (Walpole River), with outlier records from the Harvey River (400 km north) and the Canning River (a further 100 km north; Western Australian Museum record) (Morgan et al. 2006).
The trout minnow, Galaxias truttaceus Valenciennes, 1846, is known from Western Australia, South Australia, Tasmania, and Victoria, and King, Flinders, and Clarke islands in Bass Strait (Allen et al. 2002). Although Western Australian populations of the species were originally described as a discrete taxon (Galaxias truttaceus hesperius Whitely, 1944), more recent studies synonymise G. truttaceus hesperius with G. truttaceus. Notwithstanding this, G. truttaceus hesperius is listed federally as ‘critically endangered’ under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act), and ‘endangered’ by the Western Australian Government under the Biodiversity Conservation Act 2016 (BC Act). The critically endangered Western Australian population of Galaxias truttaceus has been categorised by Allen (1982) as ‘our rarest species of native minnow’, and is known currently from the Goodga, Angove and King rivers in the south-west, with outlying, more western occurrence in the Kent River catchment (Colman 2010). Additional populations occur in south-eastern Australia but genetic and ecological investigations (Morgan et al. 2016) indicated there is limited contemporary gene flow between the eastern and western populations and concluded that the south-west population should be considered an evolutionary significant unit. In contrast to many eastern populations that are diadromous, all western populations are potamodromous. Adults live and spawn in riverine habitats and larvae drift downstream to coastal lakes, where they spend several months, before undertaking a distinct upstream recruitment migration as juveniles to colonise riverine habitats (Morgan and Beatty 2006; Morgan et al. 2016).
Goodga River also harbours the scarce Nannatherina balstoni (Balston’s pygmy perch) (Allen 1982, 1989), listed as ‘vulnerable’ under both the BC and EPBC Acts. This is another species that has experienced range contraction. Christensen (1982) collected it at only four of 120 sampling sites along the south coast hinterland between Augusta and Albany.
There are no confirmed records, but there are anecdotal reports of both Oncorhynchus mykiss (rainbow trout) and Perca fluviatilis (redfin perch) occasionally breaking the surface of Kaiupgidjukgidj/Moates Lake. Rainbow trout were first introduced to the Two Peoples Bay area in 1913 and were sporadically released in the Angove and Goodga systems by the Acclimatisation Society of Western Australia and the Albany, Denmark and Plantagenet Acclimatisation Society until after the Reserve was gazetted in 1967. Both Oncorhynchus mykiss and Salmo trutta (brown trout) maintain self-sustaining populations in nearby King Creek and O. mykiss also occurs in the Kalgan and Waychinicup Rivers (Lenanton 1974).
Both the Angove and Goodga rivers have relatively high V-notch weirs in their upper reaches (Fig. 4), which are barriers to upstream fish passage, with no fish recorded upstream of either the Angove (A. Storey, unpubl. data) or Goodga weirs (Morgan and Beatty 2006) in early surveys. A vertical slot fishway, designed to function during low flows to coincide with the upstream spawning migration of Galaxias truttaceus, was constructed on the Goodga Weir in 2003. Subsequent monitoring showed upstream movement of G. truttaceus and G. maculatus through the fishway and into the upper reaches of the Goodga, providing additional valuable habitat to both species (Morgan and Beatty 2006).
Samples of aquatic invertebrates were collected from the major flowing (Angove and Goodga rivers, West and Websters gullies) and standing (Miialyiin/Angove, Tyiurrtmiirity/Gardner, and Kaiupgidjukgidj/Moates lakes) waterbodies in the Two Peoples Bay area in June 1990 and February 1991 (Fig. 1). Additional samples of Cladocera were collected from the three lakes in September 1991 (see Storey et al. 1993 for details of sampling). Standard sampling techniques were used with 250 μm mesh nets in streams and both 50 μm and 250 μm mesh nets in lakes.
At least 248 species of aquatic invertebrate were collected (Appendix 1, Fig. 8). The lakes contained more species (171) than streams, with 72 taxa occurring in Tyiurrtmiirity/Gardner Lake, 97 in Miialyiin/Angove and 85, in Kaiupgidjukgidi/Moates Lake (Table 5). Twelve per cent of the invertebrate fauna was common to all three lakes.
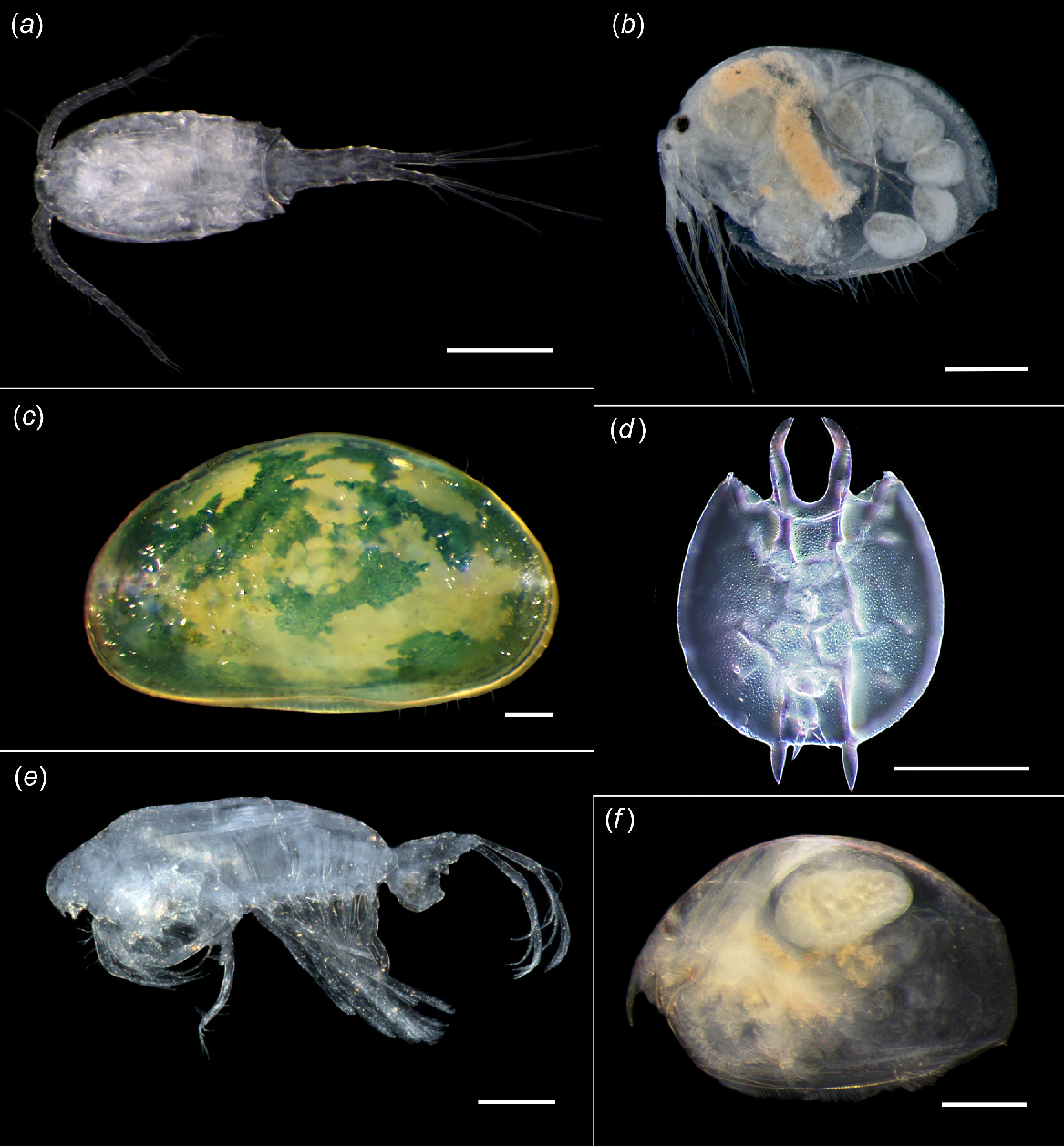
Microinvertebrates collected at Two Peoples Bay Nature Reserve. (a) Copepod Paracyclops chiltoni, (b) cladoceran Macrothrix cf. breviseta, (c) ostracod Alboa worooa, (d) rotifer Platyais quadricornis, (e) copepod Gladioferans imparipes, (f) cladoceran Dunhevedia crassa. Scale bars = 0.1 mm. (Credit: J. McRae).
| By site | For survey overall | ||||||
|---|---|---|---|---|---|---|---|
| Total | R + MC | MC | Total | R + MC | MC | ||
| This study | 84 (72–96) | 53 (49–59) | 33 (29–39) | 165 | 57 | 32 | |
| Edward et al (1994) | 39 (22–57) | – | 21 (8–43) | 211 | – | 15 | |
| Davis et al. (1993) | 53 (18–95) | – | 28 (18–45) | 253 | – | 29 | |
| Pinder et al. (2004) | 39 (0–107) | 33 (0–70) | 27 (0–67) | 993 | 48 | 29 | |
| Halse et al. (2000) | 492 | 47 | 33 | ||||
| Pinder et al. (2010) | 1011 | 38 | 18 | ||||
| Timms (1993) | 106 | 33 | 29 | ||||
| Timms (1998) | 33 | 42 | 36 | ||||
Total, mean (plus range) of number of species collected; R + MC, mean percentage (plus range) of rotifers and microcrustaceans; MC, mean percentage (plus range) of microcrustaceans.
Sampling was sufficiently standardised to show that Tyiurrtmiirity/Gardner Lake contained the greatest biomass of invertebrates (despite lower species richness) and Kaiupgidjukgidi/Moates Lake the least, although biomass in all three lakes was low compared with many wetlands in agricultural parts of the Western Australian Wheatbelt (Pinder et al. 2004) and Swan Coastal Plain (Storey et al. 1993). None of the lakes contained many benthic animals. Chironomids and ostracods, both of which are abundant in most nutrient-enriched lakes, occurred in low numbers. Several species with marine affinities (e.g. Gladioferans imparipes and Schizopera clandestina) occurred in Tyiurrtmiirity/Gardner Lake, reflecting potential seasonal connection to the sea. The occurrence of the harpacticoid copepod S. clandestina is of interest but is perhaps a problematic record; it is the first time this west European species has been recorded in the southern hemisphere. More significantly, the cladoceran Gondwanothrix halsei collected from Miialyiin/Angove Lake represents a new family related to Macrothricidae (Van Damme et al. 2007) and highlights, together with the work of Frey (1998), that endemism in the cladoceran fauna of south-western Australia is much greater than suggested by Smirnov and Timms (1983). Similarly, more recent examination of the rotifers more widely in south-western Australia in the light of the richness of the Reserve’s lakes has shown that what was considered to be a cosmopolitan group has relatively high endemism in the south-west (Segers and Shiel 2003; Suatoni et al. 2006).
There was substantial focus on microinvertebrates when identifying the animals in Reserve’s lake samples and 57% of the fauna identified were rotifers or microcrustaceans (Table 5). Analysis of the results of other surveys in south-western and eastern Australia suggests microinvertebrates nearly always contribute substantially to invertebrate richness in lentic waterbodies and that surveys targeting only macroinvertebrates usually provide a very incomplete picture of inland aquatic biodiversity. For example, sampling in south-western Australia by Pinder et al. (2004) in the Avon Wheatbelt region and Halse et al. (2000) in a semi-arid area north of the Geraldton Sandplain, collected only slightly fewer than 50% microinvertebrates (Table 5). This suggests that the 40 Swan Coastal Plain lakes sampled by Davis et al. (1993), which on average yielded 53 species, would likely have shown similar or greater richness than the Reserve’s lakes if rotifers and harpacticoid copepods had been identified in that study. Similarly, the omission by Edward et al. (1994) of rotifer identifications and lumping of identification of cyclopoid and harpacticoid copepods to order level in the 23 wetlands sampled along the coast in the Warren region would have significantly reduced reported richness in that study. It is likely that the true richness of these lakes is similar to the Reserve’s lakes. Eastern Australian lakes also appear to have substantial proportions of microinvertebrates, based on sampling of saline lakes in southern Queensland at Lake Wyara (Timms 1998) and the Paroo (Timms 1993) (Table 5). Shiel et al. (1998) reported a very large assemblage (252 species) of rotifers from pools on the Murray River floodplain, though most individual sites had relatively low numbers of species and the proportion of rotifers and other microinvertebrates in the pool communities could not be calculated.
Overall, 110 species were collected from the river and creek sites. Eighty-three species were found in the two ‘lowland’ rivers and 56 in the two headland streams. Fifty-three species were collected from Angove River, with 55, 40, and 43 species taken from Goodga River, West and Webster’s Gullies, respectively. Eleven per cent of the invertebrate fauna was common to all four sites, 23% were recorded only from the two headland streams and 49% was restricted to the two lowland rivers.
All the known major components of the aquatic invertebrate fauna of south-western Australian flowing waters were represented in the rivers and streams of Two Peoples Bay Nature Reserve (Bunn et al. 1986; Storey and Edward 1989; Storey et al. 1990; Growns and Davis 1991, 1994). The absence of decapod crustaceans from the headland streams may reflect an absence of suitable habitats, since these streams were fast flowing, possessed few large pools, had small accumulations of organic matter, and a coarse substratum. The lowland rivers were slow flowing, consisted of large deep pools, exhibited large accumulations of fine organic matter, and supported large populations of decapods.
Values for species richness in winter at each flowing water site (23–36 species) were mostly higher than reported by Bunn et al. (1986) for winter samples from 10 perennial ‘stream’ sites in the Wungong and North Dandalup catchments south-east of Perth (18–26 taxa). The higher values may be the result of different sampling methods, particularly since Bunn et al. (1986) sampled only riffle zones, whereas the present study sampled all major habitats, but also reflect the richness of the south coast aquatic fauna.
According to Morrissy (1978), marron Cherax cainii are not indigenous to the drainage systems of Two Peoples Bay Nature Reserve. He reports Fisheries Inspector Goodlad stating in 1939 ‘There are places just outside Albany where marron have been put in by private persons. Angove Creek for instance’ and was told of another translocation from waters west of Albany into Kaiupgidjukgidi/Moates Lake in about 1940. Although marron are now abundant in Goodga River and Kaiupgidjukgidi/Moates Lake, the average size caught by marroners decreased during the 1980s, most likely because of fishing pressure (N. Morrissy, pers. comm.).
The introduced yabby Cherax destructor was not evident in the waters of the Reserve in the early 1990s, but had reputedly been released into nearby farm dams (D. Wilson, pers. comm.). In the long-term this species, with its greater fecundity and environmental tolerance, could replace the Indigenous freshwater crayfishes of the Reserve (Lake and Sokol 1986). According to Morrissy (pers. comm.) most farmers in the region are unaware of the animals’ introduced status and misname it as the endemic koonac (C. plebejus or C. preissii).
Conclusion
The aquatic ecosystems of Two Peoples Bay Nature Reserve are important features. All systems are relatively undisturbed, with the catchments of the streams on Maardjitup Gurlin/Mt Gardner headland being entirely within the Reserve. Long-term collection of data on water chemistry and the aquatic fauna of the rivers, creeks and lakes in the Reserve can provide benchmark information with which to assess impacts of disturbances such as land clearing on aquatic systems elsewhere on the south coast of Western Australia.
Miialyiin/Angove, TyiurrtmiirityGardner, and Kaiupgidjukgidi/Moates Lakes support moderate diversity, albeit low abundance of waterbirds, and provide habitat for the endangered Australasian bittern. The Goodga and Angove systems also provide most of the known habitat for the genetically-distinct Western Australian population of the critically endangered Galaxias truttaceus. The systems contain high numbers of aquatic invertebrate species with high diversity of rotifers and cladocerans, including occurrence of the unusual Gondwanotrichidae family of cladocerans. The collection of a rich microinvertebrate community in the Reserve’s lakes in 1990–1991 has had a major impact on the design of many subsequent aquatic invertebrate surveys in Western Australia (Halse et al. 2000, 2014; Pinder et al. 2004, 2010). Their taxonomic focus was broadened beyond macroinvertebrates to routinely include rotifers, cladocerans, ostracods and copepods and to undertake species level identifications.
The conservation values of the Reserve’s inland aquatic systems are not limited to invertebrates, however, and there is a need for more information about fish as well as microinvertebrates to fully understand conservation values and plan management. The distributions and ecology of Galaxias truttaceus and Nannatherina balstoni should be a focus for fish surveys, and further surveys to complete the list of species in rivers, creeks, and lakes are important for aquatic invertebrates.
Generally in south-western Australia, the greatest threat to the invertebrate, fish, and waterbird faunas of the aquatic systems is a reduction in water quality or quantity (Halse et al. 2003; Atkinson et al. 2021). The headland streams appear to be at no risk from external factors other than fire and changing climate. The likely extent of impacts from future climate change are unclear. Based on data from the Bureau of Meteorology annual rainfall at Albany has shown no significant change since 1877, although there appears to be a slight downward trend (data from Bureau of Meteorology, station 9500). Water quality in Angove and Goodga Rivers and the lakes appears to have been little affected by agricultural clearing. However, further land clearing or intensified agricultural activities in the catchments may impact on water quality. Agricultural development has increased salinity levels in the adjacent Kalgan River, Waychinicup River, and King Creek catchments (Schofield et al. 1988). Another possible impact on the Angove system is reduction of flow as a result of water extractions from the pipe-head dam on the Angove River for Albany’s water supply. This has not been investigated in detail but, while at current levels, probably has at most a small effect (McKay and King 2006; Mbaka and Mwaniki 2015).
Data availability
The data that support this study are available in the article and accompanying Appendix.
Declaration of funding
This research would not have been possible without the assistance of salaries and operating budgets paid for by the Western Australian Government.
Acknowledgements
We thank Russell Shiel and the late Neil Coy for their very substantial contributions to this paper, Adrian Pinder for providing physico-chemical data about the lakes, Jane McRae for invertebrate photographs and Alan Danks for gentlemanly guidance around Two Peoples Bay Nature Reserve and sharing of his knowledge.
References
Atkinson ST, Cale D, Pinder A, Chambers JM, Halse SA, Robson BJ (2021) Substantial long-term loss of alpha and gamma diversity of lake invertebrates in a landscape exposed to a drying climate. Global Change Biology 27(23), 6263-6279.
| Crossref | Google Scholar | PubMed |
Bayly IAE, Halse SA, Timms BV (2011) Aquatic invertebrates of rockholes in the south-east of Western Australia. Journal of the Royal Society of Western Australia 94(4), 549-555.
| Google Scholar |
Beatty SJ, Morgan DL, Rashnavadi M, Lymbery AJ (2011) Salinity tolerances of endemic freshwater fishes of south-western Australia: implications for conservation in a biodiversity hotspot. Marine and Freshwater Research 62(1), 91-100.
| Crossref | Google Scholar |
Bunn SE (1988) Life histories of some benthic invetebrates form streams of the Northern Jarrah forest, Western Australia. Australian Journal of Marine and Freshwater Research 39(6), 785-804.
| Crossref | Google Scholar |
Bunn SE, Davies PM (1990) Why is the stream fauna of south-western Australia so impoverished? Hydrobiologia 194, 169-176.
| Crossref | Google Scholar |
Bunn SE, Edward DH, Loneragan NR (1986) Spatial and temporal variation in the macroinvertebrate fauna of streams of the northern jarrah forest, Western Australia: community structure. Freshwater Biology 16(1), 67-91.
| Crossref | Google Scholar |
Chatfield GR, Saunders DA (2024) History and establishment of Two Peoples Bay Nature Reserve. Pacific Conservation Biology 30, PC24004.
| Crossref | Google Scholar |
Christensen P (1982) The distribution of Lepidogalaxias salamandroides and other small fresh-water fishes in the lower south-west of Western Australia. Journal of the Royal Society of Western Australia 65(4), 131-141.
| Google Scholar |
Colman JG (2010) New records of Galaxias truttaceus (Galaxiidae) in the Kent River catchment, southwestern Australia. Journal of the Royal Society of Western Australia 93(4), 189-193.
| Google Scholar |
Cranston PS (2019) Identification guide to genera of aquatic larval Chironomidae (Diptera) of Australia and New Zealand. Zootaxa 4706(1), 71-102.
| Crossref | Google Scholar |
Davies PM, Stewart BA (2013) Aquatic biodiversity in the Mediterranean climate rivers of southwestern Australia. Hydrobiologia 719, 215-235.
| Crossref | Google Scholar |
De Deckker P (1983) Notes on the ecology and distribution of non-marine ostracods in Australia. Hydrobiologia 106, 223-234.
| Crossref | Google Scholar |
Edward D, Gazey P, Davies P (1994) Invertebrate community structure related to physico-chemical parameters of permanent lakes of the south coast of Western Australia. Journal of the Royal Society of Western Australia 77, 51-63.
| Google Scholar |
Frey DG (1998) Expanded description of Leberis aenigmatosa Smirnov (Anomopoda: Chydoridae): a further indication of the biological isolation between western and eastern Australia. Hydrobiologia 367, 31-42.
| Crossref | Google Scholar |
Geddes M (1981) Revison of Australian species of Branchinella (Crustacea: Anostraca). Australian Journal of Marine and Freshwater Research 32(2), 253-295.
| Crossref | Google Scholar |
Growns IO, Davis JA (1991) Comparison of the macroinvertebrate communities in streams in logged and undisturbed catchments 8 years after harvesting. Australian Journal of Marine and Freshwater Research 42(6), 689-706.
| Crossref | Google Scholar |
Growns IO, Davis JA (1994) Effects of forestry activities (clearfelling) on stream macroinvertebrate fauna in south-western Australia. Australian Journal of Marine and Freshwater Research 45(6), 963-975.
| Crossref | Google Scholar |
Halse SA, Pearson GB, Vervest RM, Yung FH (1995) Annual waterfowl counts in south-west Western Australia - 1991/92. CALMScience 2(1), 1-24.
| Google Scholar |
Halse SA, Shiel RJ, Storey AW, Edward DHD, Lansbury I, Cale DJ, Harvey MS (2000) Aquatic invertebrates and waterbirds of wetlands and rivers of the southern Carnarvon Basin, Western Australia. Records of the Western Australian Museum, Supplement 61, 217-263.
| Crossref | Google Scholar |
Halse SA, Ruprecht JK, Pinder AM (2003) Salinisation and prospects for biodiversity in rivers and wetlands of south-west Western Australia. Australian Journal of Botany 51(6), 673-688.
| Crossref | Google Scholar |
Halse SA, Scanlon MD, Cocking JS, Barron HJ, Richardson JB, Eberhard SM (2014) Pilbara stygofauna: deep groundwater of an arid landscape contains globally significant radiation of biodiversity. Records of the Western Australian Museum, Supplement 78, 443-483.
| Crossref | Google Scholar |
Hopkins AJM, Smith GT, Saunders DA (2024) Introduction to the special issue of the natural history of Two Peoples Bay Nature Reserve, Western Australia. Pacific Conservation Biology 30, PC24023.
| Crossref | Google Scholar |
Horwitz P (1997) Comparative endemism and richness of the aquatic invertebrate fauna in peatlands and shrublands of far southwestern Australia. Memoirs of Museum Victoria 56(2), 313-321.
| Crossref | Google Scholar |
Horwitz P, Rogan R, Halse S, Davis J, Sommer B (2009) Wetland invertebrate richness and endemism on the Swan Coastal Plain, Western Australia. Marine and Freshwater Research 60(10), 1006-1020.
| Crossref | Google Scholar |
Humphries P (1989) Variation in the life history of diadromous and landlocked populations of the spotted galaxias, Galaxias truttaceus Valenciennes, in Tasmania. Australian Journal of Marine and Freshwater Research 40, 501-18.
| Google Scholar |
Knapp L, Cummings D, Cummings S, Fielder PL, Hopper SD (2024) A Merningar Bardok family’s Noongar oral history of Two Peoples Bay Nature Reserve and surrounds. Pacific Conservation Biology 30, PC24018.
| Crossref | Google Scholar |
Lymbery AJ, Hassan M, Morgan DL, Beatty SJ, Doupé RG (2010) Parasites of native and exotic freshwater fishes in south-western Australia. Journal of Fish Biology 76(7), 1770-1785.
| Crossref | Google Scholar | PubMed |
Mbaka JG, Mwaniki MW (2015) A global review of the downstream effects of small impoundments on stream habitat conditions and macroinvertebrates. Environmental Reviews 23(3), 257-262.
| Crossref | Google Scholar |
McKay SF, King AJ (2006) Potential ecological effects of water extraction in small, unregulated streams. River Research and Applications 22(9), 1023-1037.
| Crossref | Google Scholar |
Miller AK, Baker C, Kitson JC, Yick JL, Manquel PEI, Alexander A, Gemmell NJ (2021) The Southern Hemisphere lampreys (Geotriidae and Mordaciidae). Reviews in Fish Biology and Fisheries 31, 201-232.
| Crossref | Google Scholar |
Morgan DL, Gill HS (2000) Fish associations within the different inland habitats of lower south-western Australia. Records of the Western Australian Museum 20, 31-37.
| Google Scholar |
Morgan DL, Beatty SJ (2006) Use of a vertical-slot fishway by galaxiids in Western Australia. Ecology of Freshwater Fish 15(4), 500-509.
| Crossref | Google Scholar |
Morgan DL, Gill HS, Potter IC (1998) Distribution, identification and biology of freshwater fishes in south-western Australia. Records of the Western Australian Museum, Supplement 56, 1-52.
| Google Scholar |
Morgan DL, Chapman A, Beatty SJ, Gill HS (2006) Distribution of the spotted minnow (Galaxias maculatus (Jenyns, 1842)) (Teleostei: Galaxiidae) in Western Australia including range extensions and sympatric species. Records of the Western Australian Museum 23, 7-11.
| Crossref | Google Scholar |
Morgan DL, Beatty SJ, Adams M (2013) Nannoperca pygmaea, a new species of pygmy perch (Teleostei: Percichthyidae) from Western Australia. Zootaxa 3637(4), 401-411.
| Crossref | Google Scholar | PubMed |
Morgan DA, Unmack PJ, Beatty SJ, Ebner BC, Allen MG, Donaldson JA, Murphy J (2014) An overview of the ‘freshwater fishes’ of Western Australia. Journal of the Royal Society of Western Australia 97(2), 263-278.
| Google Scholar |
Morgan DL, Beatty SJ, Close PG, Allen MG, Unmack PJ, Hammer MP, Adams M (2016) Resolving the taxonomy, range and ecology of biogeographically isolated and critically endangered populations of an Australian freshwater galaxiid, Galaxias truttaceus. Pacific Conservation Biology 22(4), 350-359.
| Crossref | Google Scholar |
Pen LJ, Potter IC (1991) Biology of the western minnow, Galaxias occidentalis Ogilby (Teleostei: Galaxiidae), in a south-western Australian river. Hydrobiologia 211, 89-100.
| Crossref | Google Scholar |
Pennifold MG, Williams KJ, Pinder AM, Harwood TD, Manion G, Ferrier S (2017) Whole-landscape modelling of compositional turnover in aquatic invertebrates informs conservation gap analysis: an example from south-western Australia. Freshwater Biology 62(8), 1359-1376.
| Crossref | Google Scholar |
Pinder AM, Halse SA, Shiel RJ, McRae JM (2000) Granite outcrop pools in south-western Australia: foci of diversification and refugia for aquatic invertebrates. Journal of the Royal Society of Western Australia 83, 117-129.
| Google Scholar |
Pinder AM, Halse SA, McRae JM, Shiel RJ (2004) Aquatic invertebrate assemblages of wetlands and rivers in the wheatbelt region of Western Australia. Records of the Western Australian Museum, Supplement 67(1), 7-37.
| Crossref | Google Scholar |
Pinder AM, Halse SA, Shiel RJ, McRae JM (2010) An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia. Records of the Western Australian Museum, Supplement 78, 205-246.
| Crossref | Google Scholar |
Pollard DA (1971) The biology of a landlocked form of the normally catadromous salmoniform fish Galaxias maculatus (Jenyns). I. Life cycle and origin. Australian Journal of Marine & Freshwater Research 22, 91-123.
| Google Scholar |
Segers H, Shiel R (2003) Microfaunal diversity in a biodiversity hotspot: new rotifers from southwestern Australia. Zoological Studies 42(4), 516-521.
| Google Scholar |
Shiel RJ, Green JD, Nielsen DL (1998) Floodplain biodiversity: why are there so many species? Hydrobiologia 387, 39-46.
| Crossref | Google Scholar |
Smirnov NN, Timms BV (1983) A revision of the Australian Cladocera (Crustacea). Records of the Australian Museum, Supplement 1, 1-132.
| Crossref | Google Scholar |
Smith MJ, Kay WR, Edward DHD, Papas PJ, Richardson KStJ, Simpson JC, Pinder AM, Cale DJ, Horwitz PHJ, Davis JA, Norris RH, Halse SA (1999) AusRivAS: using macroinvertebrates to assess ecological condition of rivers in Western Australia. Freshwater Biology 41(2), 269-282.
| Crossref | Google Scholar |
Stewart BA (2009) Two aquatic bioregions proposed for the South Coast Region, Western Australia. Journal of the Royal Society of Western Australia 92(3), 277-287.
| Google Scholar |
Stewart BA (2011) Assessing the ecological values of rivers: an application of a multi-criteria approach to rivers of the South Coast Region, Western Australia. Biodiversity and Conservation 20, 3165-3188.
| Crossref | Google Scholar |
Storey AW, Edward DH (1989) Longitudinal variation in community structure of Chironomidae (Diptera) in two southwestern Australian river systems. Acta Biologica Debrecina Oecologica Hungarica 3, 315-328.
| Google Scholar |
Storey AW, Bunn SE, Davies PM, Edward DH (1990) Classification of the macroinvertebrate fauna of two river systems in Southwestern Australia in relation to physical and chemical parameters. Regulated Rivers: Research & Management 5(3), 217-232.
| Crossref | Google Scholar |
Storey AW, Halse SA, Shiel RJ (1993) Aquatic invertebrate fauna of the Two Peoples Bay area, south-western Australia. Journal of the Royal Society of Western Australia 76, 25-32.
| Google Scholar |
Suatoni E, Vicario S, Rice S, Snell T, Caccone A (2006) An analysis of species boundaries and biogeographic patterns in a cryptic species complex: the rotifer—Brachionus plicatilis. Molecular Phylogenetics and Evolution 41(1), 86-98.
| Crossref | Google Scholar | PubMed |
Tickner D, Opperman JJ, Abell R, Acreman M, Arthington AH, Bunn SE, Cooke SJ, Dalton J, Darwall W, Edwards G, Harrison I, Hughes K, Jones T, Leclère D, Lynch AJ, Leonard P, McVlain ME, Muruven D, Olden JD, Ormerod SJ, Robinson J, Tharme RE, Thieme M, Tockner K, Wight M, Young L (2020) Bending the curve of global freshwater biodiversity loss: an emergency recovery plan. BioScience 70(4), 330-342.
| Crossref | Google Scholar | PubMed |
Timms BV (1993) Saline lakes of the Paroo, inland New South Wales, Australia. Hydrobiologia 267, 269-289.
| Crossref | Google Scholar |
Timms BV (1998) A study of Lake Wyara, an episodically filled saline lake in southwest Queensland, Australia. International Journal of Salt Lake Research 7(2), 113-132.
| Google Scholar |
Timms BV (2008) Further studies on the fairy shrimp genus Branchinella (Crustacea, Anostraca, Thamnocephalidae) in Western Australia, with descriptions of new species. Records of the Western Australian Museum 24, 289-306.
| Crossref | Google Scholar |
Timms BV (2012) An identification guide to the brine shrimps (Crustacea: Anostraca: Artemiina) of Australia. Museum Victoria Science Reports 16, 1-36.
| Crossref | Google Scholar |
Trayler KM, Davis JA (1998) Forestry impacts and the vertical distribution of stream invertebrates in south-western Australia. Freshwater Biology 40(2), 331-342.
| Crossref | Google Scholar |
Trayler KM, Davis JA, Horwitz P, Morgan D (1996) Aquatic fauna of the Warren bioregion, south-west Western Australia: does reservation guarantee preservation? Journal of the Royal Society of Western Australia 79(4), 281-291.
| Google Scholar |
Van Damme K, Shiel RJ, Dumont HJ (2007) Notothrix halsei gen. n., sp. n., representative of a new family of freshwater cladocerans (Branchiopoda, Anomopoda) from SW Australia, with a discussion of ancestral traits and a preliminary molecular phylogeny of the order. Zoologica Scripta 36(5), 465-487.
| Crossref | Google Scholar |
Watts CHS (1978) A revision of the Australian Dytiscidae (Coleoptera). Australian Journal of Zoology Supplementary Series 26(57), 1-166.
| Crossref | Google Scholar |
Appendix 1.Aquatic invertebrate species of the Two Peoples Bay area
Species collected from rivers, creeks, and lakes of the Two Peoples Bay area in 1990 and 1991 (see Storey et al. 1993).
| AR | GR | WG | WbG | AL | GL | ML | ||
|---|---|---|---|---|---|---|---|---|
| PROTOZOA | ||||||||
| Arcella sp. A | * | |||||||
| Centropyxis sp. A | * | |||||||
| +Difflugia acuminata (Ehrenberg) | * | |||||||
| Difflugia sp. A | * | |||||||
| Euglypha sp. A | * | |||||||
| +Lesquereusia spiralis (Ehrenberg) | * | * | ||||||
| ROTIFERA | ||||||||
| Bdelloida sp. A | * | |||||||
| Brachionus cf. angularis bidens (Plate) | * | * | ||||||
| Brachionus quadridentatus (Hermann) | * | |||||||
| +Dipleuchalnis propatula (Gosse) | * | * | ||||||
| Euchlanis dilatata (Ehrenberg) | * | |||||||
| +Filinia cf. australiensis (Koste) | * | |||||||
| +Filinia cf. pejleri (Hutchinson) | * | |||||||
| +Filinia sp. A | * | |||||||
| +Heterolepadella ehrenbergi (Perty) | * | |||||||
| Keratella javana (Hauer) | * | |||||||
| Keratella procurva (Thorpe) | * | |||||||
| Keratella sp. A | * | |||||||
| +Lecane noobjupi (Segers & Shiel) | * | |||||||
| +Lecane imbricata (Carlin) | * | * | ||||||
| +Lecane cf. ohioensis (Herrick) | * | |||||||
| +Lecane signifera (Jennings) | * | |||||||
| +Lepadella rottenburgi (Lucks) | * | |||||||
| +Lepadella triptera (Ehrenberg) | * | |||||||
| Lepadella sp. A | * | |||||||
| +Macrochaetus collinsi (Gosse) | * | |||||||
| Monommata sp. A | * | |||||||
| Monostyla bulla (Gosse) | * | * | * | |||||
| Monostyla hamata (Stokes) | * | * | ||||||
| Monostyla lunaris (Ehrenberg) | * | |||||||
| +Monostyla crenata (Harring) | * | * | ||||||
| +Monostyla furcata (Murray) | * | * | ||||||
| +Monostyla quadridentate (Ehrenberg) | * | |||||||
| +Monostyla rhophalura (Harring & Myers) | * | |||||||
| +Monostyla sp. nov. A | * | |||||||
| +Notommata sp. nov. A | * | |||||||
| Platyias quadricornis (Ehrenberg) | * | |||||||
| +Testudinella amphora (Hauer) | * | |||||||
| Testudinella insinuata (Hauer) | * | |||||||
| Testudinella nr. patina (Hermann) | * | |||||||
| +Testudinella tasmaniensis (Koste & Shiel) | * | |||||||
| Trichocerca elongata (Gosse) | * | |||||||
| Trichocerca pusilla (Jennings) | * | |||||||
| +Trichocerca rattus carinata (Ehrenberg) | * | |||||||
| +Trichocerca rattus cristata (Harring) | * | |||||||
| Trichocerca sp. A1 | * | * | ||||||
| Trichocerca sp. A2 | * | |||||||
| Trichotria tetractis (Ehrenberg) | * | * | ||||||
| NEMATODA | ||||||||
| Nematoda spp. | S | S | B | B | B | |||
| MOLLUSCA | ||||||||
| GASTROPODA | ||||||||
| Ancylidae | ||||||||
| Ferrissia petterdi (Johnston) | W | B | B | W | ||||
| Planorbidae | ||||||||
| Physastra sp. A | B | |||||||
| ANNELIDA | ||||||||
| Oligochaeta spp. | B | B | B | W | B | B | B | |
| ARTHROPODA | ||||||||
| ORIBATIDA | ||||||||
| Oribatida spp. | W | B | B | |||||
| HYDRACARINA | ||||||||
| Arrenuridae | ||||||||
| Arrenurus sp. A | S | |||||||
| Arrenurus sp. B | S | |||||||
| Oxidae | ||||||||
| Flabellifrontipoda sp.A | W | |||||||
| Limnochares australica (Lundblad) | S | |||||||
| Oxus sp. A | W | B | B | |||||
| Frontipoda sp. A | S | |||||||
| Unionicolidae | ||||||||
| Koenikea sp. A | B | |||||||
| Newmania sp. A | S | |||||||
| Hygrobatidae | ||||||||
| Corticarus sp. A | W | |||||||
| Gretacarus sp. A | S | S | ||||||
| Coaustraliobates sp. A | S | |||||||
| Halacaridae | ||||||||
| Halacaridae sp. A | W | |||||||
| Soldanellonyx sp. A | S | |||||||
| CRUSTACEA | ||||||||
| CLADOCERA | ||||||||
| Sididae | ||||||||
| Latonopsis cf. brehmi (Petkovski) | S | |||||||
| Chydoridae | ||||||||
| Alonella cf. clathratula (Sars) | S | |||||||
| Biapertura cf. affinis (Leydig) | W | |||||||
| Biapertura cf. rigidicaudis (Smirnov) | W | |||||||
| +Biapertura cf. setigera (Brehm) | S | S | ||||||
| +Biapertura sp. M1 | B | |||||||
| Camptocercus cf. australis (Sars) | S | |||||||
| +Chydorus sp. A | B | W | S | |||||
| Dunhevedia crassa (King) | W | |||||||
| Ephemeroporus barroisi (s. l. Frey) | S | |||||||
| +Euryalona cf. orientalis (Daday) | W | |||||||
| Graptoleberis cf. testudinaria (Fischer) | W | |||||||
| Monope reticulata (Henry) | S | |||||||
| +cf. Pleuroxus sp. A | W | W | ||||||
| +Rhynchochydorus cf. australiensis (Smirnov & Timms) | S | |||||||
| +Chydoridae sp. A1 | B | |||||||
| +Chydoriae sp. M2 | S | |||||||
| Macrothricidae | ||||||||
| Echinisca sp. A | W | |||||||
| Ilyocryptus sp. A | W | |||||||
| Macrothrix cf. breviseta (Smirnov & Timms) | B | |||||||
| Neothrix armata (Gurney) | B | |||||||
| +Gondwanotrichidae | ||||||||
| +Gondwanothrix halsei (Van Damme, Shiel & Dumont) | S | |||||||
| Daphniidae | ||||||||
| Scapholeberis cf. kingi (Sars) | S | S | ||||||
| Simocephalus exspinosus australiensis (Dana) | S | |||||||
| Simocephalus sp. A | W | |||||||
| Bosminidae | ||||||||
| Bosmina meridionalis (Sars) | W | B | ||||||
| Bosmina sp. A | W | |||||||
| OSTRACODA | ||||||||
| Ostracoda sp. 287 | S | |||||||
| Cyprididae | ||||||||
| Cypretta baylyi (McKenzie) | B | W | ||||||
| +Ilyodromus sp. 255 | B | S | ||||||
| Kennethia cristata (De Deckker) | B | W | W | |||||
| Alboa worooa (De Deckker) | S | W | ||||||
| Mesocypris aff. tasmaniensis (De Deckker) | S | |||||||
| Darwinulidae | ||||||||
| Darwinulidae sp. | W | S | ||||||
| Limnocytheridae | ||||||||
| Gomphodella maia (De Deckker) | B | B | ||||||
| Limnocythere mowbrayensis (Chapman) | W | |||||||
| +Paralimnocythere sp. nov. | B | S | ||||||
| Candonidae | ||||||||
| Candonopsis tenuis (Brady) | W | W | S | |||||
| COPEPODA | ||||||||
| Centropagidae | ||||||||
| Calamoecia attenuata (Fairbridge) | B | B | ||||||
| Calamoecia tasmanica (s. l. Smith) | S | |||||||
| Calamoecia tasmanica subattenuata (Fairbridge) | B | W | B | |||||
| Gladioferens imparipes (Thomson) | S | B | ||||||
| Cyclopidae | ||||||||
| Eucyclops australiensis (Morton) | S | S | S | S | ||||
| Halicyclops sp. A | S | |||||||
| Microcyclops sp. A | W | W | W | |||||
| Thermocyclops sp. A | B | W | W | |||||
| Macrocyclops albidus (Jurine) | S | W | W | B | ||||
| Paracyclops chiltoni (Thomson) | S | S | ||||||
| Paracyclops sp. A | W | S | ||||||
| Canthocamptidae | ||||||||
| +Canthocamptidae sp. A | W | W | ||||||
| Canthocamptidae sp. 15 | S | S | ||||||
| Canthocamptidae sp. 16 | S | |||||||
| Ameiridae | ||||||||
| Leptomesochra sp. A | S | |||||||
| Nitocra sp. A | S | B | ||||||
| Laophontidae | ||||||||
| Onychocamptus bengalensis (Sewell) | S | |||||||
| +Onychocamptus chathamensis | W | W | ||||||
| Diosaccidae | ||||||||
| +Schizopera clandestina (Klie) | W | |||||||
| DECAPODA | ||||||||
| Parastacidae | ||||||||
| Cherax plebejus (Hess) | B | W | W | |||||
| Cherax quinquecarinatus (Gray) | B | S | W | S | ||||
| Cherax tenuimanus (Smith) | W | B | W | S | ||||
| Palaemonidae | ||||||||
| Palaemonetes australis (Dakin) | B | B | B | B | ||||
| AMPHIPODA | ||||||||
| Ceinidae | ||||||||
| Austrochiltonia subtenuis (Hurley) | W | B | B | |||||
| Gammaridae | ||||||||
| Perthia branchialis (Nicholls) | B | S | B | S | ||||
| Perthia acutitelson (Straskraba) | W | |||||||
| Uroctena setosa (Nicholls) | B | |||||||
| Talitroidea | ||||||||
| Agilestia sp. A | S | S | ||||||
| Austrotroides pectinalis (Friend) | S | |||||||
| ISOPODA | ||||||||
| Philosciidae | ||||||||
| ?Laevophiloscia sp. A | S | |||||||
| ?Plymophiloscia sp. A | S | W | ||||||
| Styloniscidae | ||||||||
| Styloniscus australiensis australiensis (Vandel) | S | W | ||||||
| INSECTA | ||||||||
| MEGALOPTERA | ||||||||
| Chauliodidae | ||||||||
| Archichauliodes cervulus (Theischinger) | B | |||||||
| LEPIDOPTERA | ||||||||
| Lepidoptera sp. B | S | |||||||
| DIPTERA | ||||||||
| Simuliidae | ||||||||
| Cnephia tonnoiri tonnoiri (Drummond) | B | W | W | W | ||||
| Austrosimulium furiosum (Skuse) | B | W | ||||||
| Culicidae | ||||||||
| Aedes sp. A | W | |||||||
| Anopheles annulipes (Walker) | S | |||||||
| Culex australicus (Dobrotworsky & Drummond) | S | S | ||||||
| Culex globocoxitus (Dobrotworsky) | W | |||||||
| Chironomidae | ||||||||
| Tanypodinae | ||||||||
| Coelopynia pruinosa (Freeman) | S | |||||||
| Paramerina levidensis (Skuse) | S | S | B | B | S | W | B | |
| Macropelopia dalyupensis (Freeman) | W | S | ||||||
| Macropelopia sp. V9 | W | |||||||
| ?Ablabesmyia sp. V10 | B | |||||||
| Tanypodinae sp. V20 | S | |||||||
| Orthocladiinae | ||||||||
| Corynoneura ?scutellata (Winnertz) | W | |||||||
| Cricotopus annuliventris (Skuse) | B | B | W | B | ||||
| Stictocladius uniserialis (Freeman) | W | |||||||
| Nanocladius sp. VCD7 | W | |||||||
| Thienemanniella sp. V19 | B | W | W | W | ||||
| Limnophyes pullulus (Skuse) | S | B | W | W | B | |||
| ?Limnophyes sp. V31 | W | S | ||||||
| Orthocladiinae sp. V11 | B | B | W | |||||
| Orthocladiinae sp. VTPB1 | W | W | ||||||
| Orthocladiinae sp. VTPB2 | S | |||||||
| Orthocladiinae sp. VTPB3 | S | |||||||
| Orthocladiinae sp. V59 | B | |||||||
| Orthocladiinae sp. A | W | W | ||||||
| Chironominae | ||||||||
| Cladopelma curtivalva (Kieffer) | S | S | S | |||||
| ?Harnischia sp. VTPB4 | S | S | ||||||
| Polypedilum sp. V3 | B | S | B | W | W | B | ||
| Polypedilum sp. V33 | B | B | ||||||
| Polypedilum sp. A | S | |||||||
| Procladius paludicola (Skuse) | S | S | S | S | ||||
| Riethia sp. V4 | B | B | S | S | ||||
| Riethia sp. V5 | B | B | S | S | ||||
| Cladotanytarsus ?mancus (Walker) | S | S | ||||||
| Tanytarsus sp. V6 | B | B | W | B | W | B | ||
| Tanytarsus sp. A | S | S | ||||||
| Tanytarsus sp. B | S | |||||||
| Stempellina ?australiensis (Freeman) | B | S | B | S | ||||
| ?Paratendipes sp. V12 | W | |||||||
| Rheotanytarsus sp. V18 | S | W | S | |||||
| Cryptochironomus griseidorsum (Kieffer) | B | S | W | |||||
| Stenochironomus sp. V27 | B | |||||||
| Chironomus aff. alternans (Walker) | S | S | ||||||
| Tanytarsini sp. A | W | W | ||||||
| Chironomini sp. V21 | S | |||||||
| Dicrotendipes sp. V47 | S | |||||||
| Tipulidae | ||||||||
| Limoniinae sp. A | S | B | B | W | ||||
| Limoniinae sp. B | B | W | ||||||
| ?Pedicia sp. A | W | W | ||||||
| Tipulinae sp. A | S | B | S | |||||
| Ceratopogonidae | ||||||||
| Ceratopogonidae sp. A | W | W | B | |||||
| Ceratopogonidae sp. B | B | S | B | W | B | W | ||
| Ceratopogonidae sp. C | W | |||||||
| Ceratopogonidae sp. D | W | |||||||
| Ceratopogonidae sp. F | S | |||||||
| Ceratopogonidae sp. G | S | |||||||
| Ceratoogonidae sp. K | S | |||||||
| Ceratopogonidae sp. O | S | |||||||
| Stratiomyidae | ||||||||
| Stratiomyidae sp. A | W | |||||||
| Stratiomyidae sp. B | S | |||||||
| Dolichopodidae | ||||||||
| Dolichopodidae sp. A | W | |||||||
| Emphididae | ||||||||
| Empididae sp. A | S | S | ||||||
| Empididae sp. B | S | S | S | |||||
| ZYGOPTERA | ||||||||
| Coenagrionidae | ||||||||
| Ischnura sp. A | W | W | ||||||
| Ischnura aurora (Brauer) | S | |||||||
| ANISOPTERA | ||||||||
| Aeschnidae | ||||||||
| Austroaeschna anacantha (Tillyard) | B | B | B | B | S | |||
| Corduliidae | ||||||||
| Lathrocordulia metallica (Tillyard) | S | |||||||
| Corduliidae sp A (immature) | W | |||||||
| Hemicordulia tau (Selys) | S | |||||||
| Gomphidae | ||||||||
| Austrogomphus collaris (Hagen) | B | |||||||
| Libellulidae | ||||||||
| Diplacodes haematodes (Burmeister) | W | |||||||
| Synthemidae | ||||||||
| Synthemis macrostigma occidentalis (Tillyard) | W | |||||||
| Synthemis cyanitincta (Tillyard) | S | |||||||
| HEMIPTERA | ||||||||
| Veliidae | ||||||||
| Veliidae sp. A | W | |||||||
| Corixidae | ||||||||
| Agraptocorixa sp. A | S | |||||||
| Micronecta robusta (Hale) | W | |||||||
| EPHEMEROPTERA | ||||||||
| Leptophlebiidae | ||||||||
| Nyungara bunni (Dean) | B | |||||||
| Bibulmena kadjina (Dean) | W | B | B | |||||
| Neboissophlebia occidentalis (Dean) | S | S | ||||||
| Caenidae | ||||||||
| Tasmanocoenis tillyardi (Lestage) | B | W | ||||||
| PLECOPTERA | ||||||||
| Gripopterygidae | ||||||||
| Newmanoperla exigua (Kimmins) | W | |||||||
| Gripopterygidae sp. A (immature) | W | |||||||
| TRICHOPTERA | ||||||||
| Hydropsychidae | ||||||||
| Smicrophylax australis (Ulmer) | B | B | ||||||
| Ecnomidae | ||||||||
| Ecnomina scindens/trulla/merga group | B | |||||||
| Ecnomus pansus/turgidus complex | B | W | S | |||||
| Leptoceridae | ||||||||
| Condocerus aptus (Neboiss) | B | B | ||||||
| Lectrides parilis (Neboiss) | B | S | B | B | ||||
| Oecetis spp. | S | S | S | S | ||||
| Triplectides sp. A | W | |||||||
| Triplectides australis (Navas) | W | W | B | |||||
| Notoperata tenax (Neboiss) | S | |||||||
| Hydroptilidae | ||||||||
| Hellyethira malleoforma (Wells) | S | S | S | S | ||||
| Oxyethira retracta (Wells) | W | S | ||||||
| Maydenoptila ?rupina (Neboiss) | W | |||||||
| Hydroptila losida (Mosely) | S | |||||||
| Hydrobiosidae | ||||||||
| Taschorema pallescens (Banks) | B | B | B | B | ||||
| Polycentropodidae | ||||||||
| Plectrocnemia ?exima (Neboiss) | S | |||||||
| COLEOPTERA | ||||||||
| Dytiscidae | ||||||||
| Antiporus femoralis (Boheman) | S | |||||||
| Lancetes lanceolatus (Clark) | W | W | ||||||
| Liodessus dispar (Sharp) | W | |||||||
| Megaporus howitti (Clark) | W | |||||||
| Megaporus solidus (Sharp) | S | |||||||
| Necterosoma darwini (Babington) | B | |||||||
| Necterosoma sp. B (larva) | S | S | ||||||
| Rhantus suturalis (MacLeay) | S | W | ||||||
| Sternopriscus browni (Sharp) | W | |||||||
| Sternopriscus sp. A | S | |||||||
| Platynetes decempunctatus (Fabricius) | S | |||||||
| Helodidae | ||||||||
| Helodidae sp. A | S | S | B | B | W | |||
| Hydrophilidae | ||||||||
| Hydrophilidae sp. I | S | |||||||
| No. of species | 53 | 55 | 40 | 43 | 96 | 72 | 85 | |
S, summer; W, winter; B, both seasons. *sampled only in summer. First records for Western Australia marked with +. Voucher specimen names are used for some undescribed species (e.g. Biapertura sp. M1). AR, Angove River; GR, Goodga River; WG, West Gully; WbG, Webster’s Gully; AL, Miialyiin/Angove Lake; GL, Tyiurrtmiirity/Gardner Lake; ML, Kaiupgidjukgidj/Moates Lake.




