Corymbia calophylla (Marri) (K. D. Hill & L. A. S. Johnson) (Myrtaceae) is a major resource for native bees in the southwest western Australian biodiversity hotspot
Kit S. Prendergast A B * and Nicole Willers C
A B * and Nicole Willers C
A
B
C
Abstract
A theoretical paradigm proposes that certain species can serve as ‘keystone species’ or ‘magnets’, being particularly important for biodiversity.
We present evidence that in the context of supporting Indigenous native bees, this is indeed the case for the tree Corymbia calophylla (Marri), a Myrtaceae endemic to southwest Western Australia.
To assess the role of C. calophylla as a resource for native bees, we collated the number of species recorded from surveys across 16 sites, and specimens lodged in the WA Museum. Its capacity to support wild bees was assessed from abundance of bees visiting this species (total and relative to other plants visited) from 24 sites.
Corymbia calophylla was visited by 81 species of native bees, and is often the main, or only, plant species visited. It blooms at a crucial time when most species have finished flowering at the end of summer.
We argue that C. calophylla represents a crucial landscape resource for native bees.
Protection and management of C. calophylla is likely to be important for preservation of native bee biodiversity.
Keywords: Australian native bees, bees, biodiversity, landscape resource, Marri, Myrtaceae, plant–pollinator interactions, pollinators.
Introduction
Native bees are a major component of biodiversity, with over 20,000 described species worldwide (Orr et al. 2021) and contribute to the maintenance and generation of biodiversity through pollination (Ollerton 2021). Australia is a megadiverse country (McDonald et al. 2015), and southwest Western Australia is a biodiversity hotspot (Myers et al. 2000; Laurie 2015; Lambers 2019). Although there is much taxonomic work to be done, there are presently 1661 described species of Australian native bees (Australian Fauna Directory 2023), and it is estimated there are at least 500 more undescribed species (Taxonomy Australia 2020). Although there are many gaps in our knowledge of the pollinator assemblages of a given flowering species, and likewise the host plants visited by a given native bee species, research to date has revealed Australia has many specialised plant–pollinator interactions, which have arisen over thousands of years of geographic isolation (Hopper and Gioia 2004; Hopper 2009; Hopper 2021). Although there has been little long-term population monitoring in Australia, it is evident that wild bees and their pollinator interactions are declining worldwide (Dicks et al. 2021).
A major threat to native bees is loss of plants that provide them with their nutritional needs (nectar and pollen), and so to conserve bees, a key strategy is protection, restoration and planting of host plants. Plant–pollinator interactions in Australia however are poorly known, especially at the taxonomic resolution required (Prendergast and Hogendoorn 2021). In the Northern Hemisphere, strategies to conserve pollinators or enhance their populations typically involve planting flower strips comprised of herbaceous native and exotic flowers (Decourtye et al. 2010; Hellwig et al. 2022; Image et al. 2022). In contrast, in Australia, a major component of flowers in the natural landscape is flowering trees, with ‘gumtrees’ (Myrtaceae) being dominant components of the vegetation (Hopper 2021).
Corymbia calophylla, commonly known as ‘Marri’ or ‘blood wood’, is a gumtree endemic to southwest Western Australia (Spooner 2004). Its distribution extends from north of Geraldton (28°S), south to Cape Riche (34°S), and inland beyond Narrogin (32°56′S 117°E) (Spooner 2004). It is often the dominant canopy species (Powell and Emberson 1990). It flowers en masse later than most flora in the southwest, starting from December, peaking in February and March, but extending until May (Spooner 2004). Flowering in not consistent, and rather goes through heavy and sparse cycles on approximately a 2 to 3 year cycle (Dowell 2018).
This paper describes the incredible diversity of bees known to visit C. calophylla.
Methods
Data on the species of native bees that forage on C. calophylla were collected from surveys conducted between 2018 and 2022 across a number of urban and remnant vegetation sites during the austral spring, summer and autumn (Fig. 1):
Lake Claremont (Mount Claremont) (Prendergast 2020b)
Herdsman Lake Regional Park (Herdsman) (Prendergast 2021e)
Araluen Botanic Gardens (Roleystone) (Prendergast 2022f)
Maida Vale Reserve (City of Kalamunda) (Prendergast 2021f)
Poison Gully Reserve (City of Kalamunda) (Prendergast 2021f)
Ledger Hill Reserve (City of Kalamunda) (Prendergast 2021f)
Arbour Park (City of Bayswater) (Prendergast 2021a)
Lightning Swamp (City of Bayswater) (Prendergast 2021a, 2022a)
Kooljerrenup Nature Reserve (Birchmont) (Prendergast 2021g)
Marrarup Nature Reserve (Waroona) (Prendergast 2021d)
Karnet Nature Reserve (Serpentine) (Prendergast 2021c)
Chandler Forest Block (Ashendon) (Prendergast 2021b)
Marrinup Forest Block (rehabilitation site, Marrinup) (Prendergast 2022c)
Pindalup Forest Block (Wurmaring) (Prendergast 2022d)
Howse Forest Block (Wurmaring) (Prendergast 2022b)
Urbrae Forest Block (rehabilitation site, Huntly) (Prendergast 2022e)
Map of the locations where native bees were collected foraging on C. calophylla by author KSP.
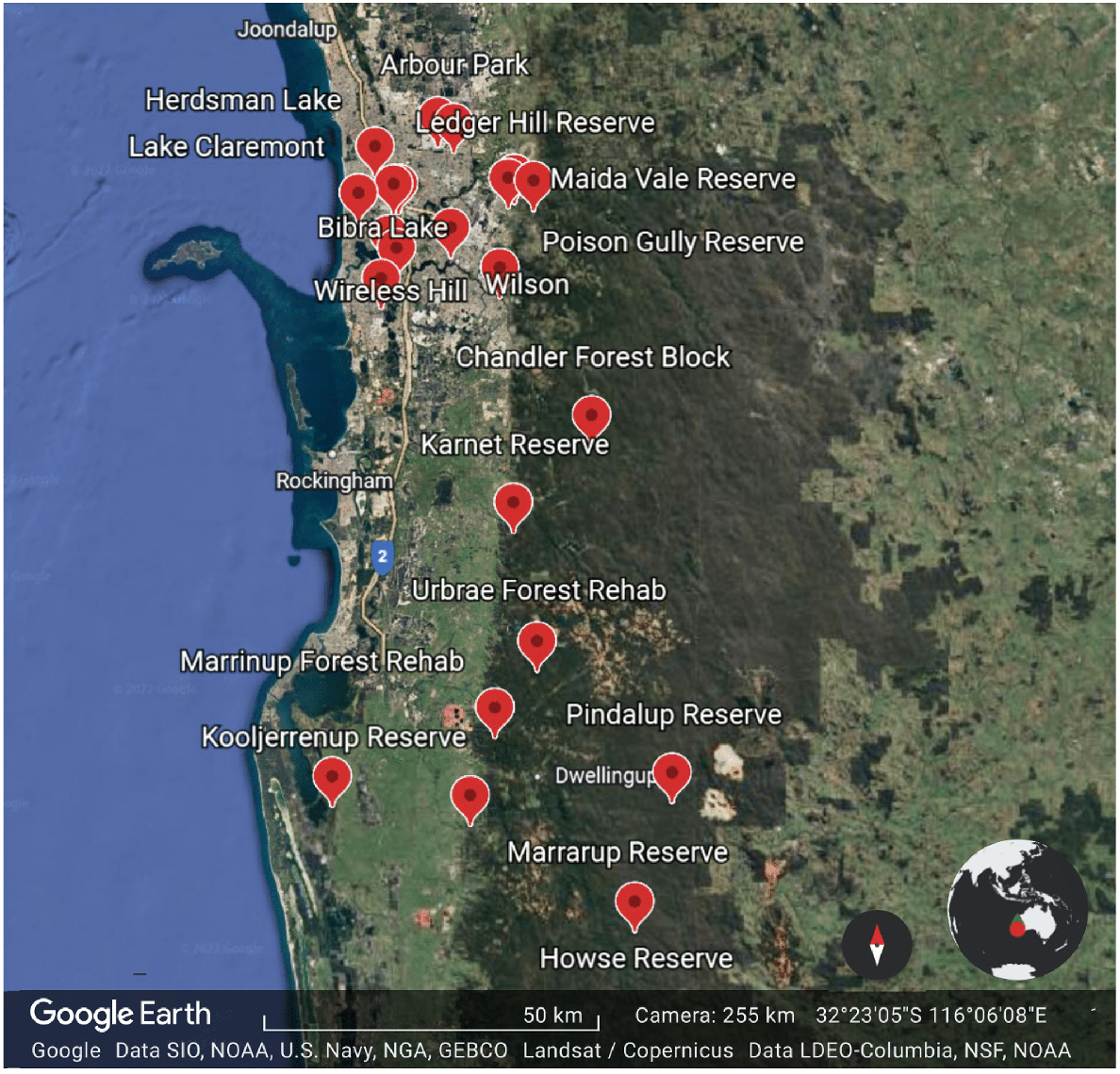
These sites were selected blind with respect to the flower composition, and represent a haphazard sample of sites ranging from urban bushland remnants, to mining rehabilitation sites, to state forest, to nature reserves, and were part of a collection of surveys conducted to assess the native bee biodiversity in the region. They were not selected on the basis of Marri populations.
Survey methods are described in more detail in the above citations associated with each site; in brief, a single experienced entomologist (author KSP) walked around each survey site stopping to collect native bees with an entomological sweepnet (Australian Entomological Supplies, 119 cm long handle, 38 cm diameter hoop) that were foraging on flowering vegetation. Approximately 2 min was spent observing patches of flowers and if no bees were observed, the next patch of flowers was observed. If bees were observed, up to 10 min were spent collecting bees. Plants where bees had been observed were revisited if no further bees were observed at other flowering plants within the survey area. Surveys per site lasted between 2 and 3 h.
Specimens were pinned and identified under a microscope using taxonomic keys where available, with reference to the PaDIL database (PaDIL 2024), and the WA Museum Entomology collection. All specimens were deposited in the WA Museum Entomology collection.
The number of native bee species recorded visiting C. calophylla was supplemented by species recorded as having been collected on C. calophylla in the Western Australian Museum Entomology collection (Houston 2000). As records at the WA Museum did not yield information on abundance, these records were only used to determine what other native bee species had been recorded on C. calophylla.
The average abundance of bees visiting C. calophylla was calculated from the number of native bees visiting this plant species from the above surveys and from data from surveys that were for the PhD project of KSP conducted across residential gardens and bushland remnants in the urbanised region of the Swan Coastal Plain, Perth metropolitan region (Prendergast 2020c). Fourteen sites were surveyed from Nov–Feb 2016/17 to Oct–March 2017/18 and of the 14 sites surveyed, 8 had C. calophylla flowering during 1 or more surveys (Fig. 1):
Over the 2 years, 21 surveys that involved bees visiting C. calophylla were undertaken at these sites (Prendergast 2020c). Here, visual observations supplemented sweep-netting, and surveys were confined to a 100 × 100 m area. For further details of the study sites and methods refer to Prendergast et al. (2022b). As surveys by KSP were not focused exclusively on C. calophylla, data included species and abundances that were recorded visiting other plant species. Therefore these datasets were used to calculate the proportion of species collected during each survey that visited C. calophylla out of all flowering plants visited, and the proportion of individual bees species collected during each survey that visited C. calophylla out of all flowering plants visited. Only months where C. calophylla was present and flowering at a site were included.
Overlap between the distribution of C. calophylla and the native bees collected on this species was assessed by looking at the geographic maps of records on the Atlas of Living Australia (ALA, Atlas of Living Australia 2021) for each described native bee species. We acknowledge that the ALA is incomplete in that many records of native bee species are not uploaded to this resource, and so distribution overlap is approximate.
Results
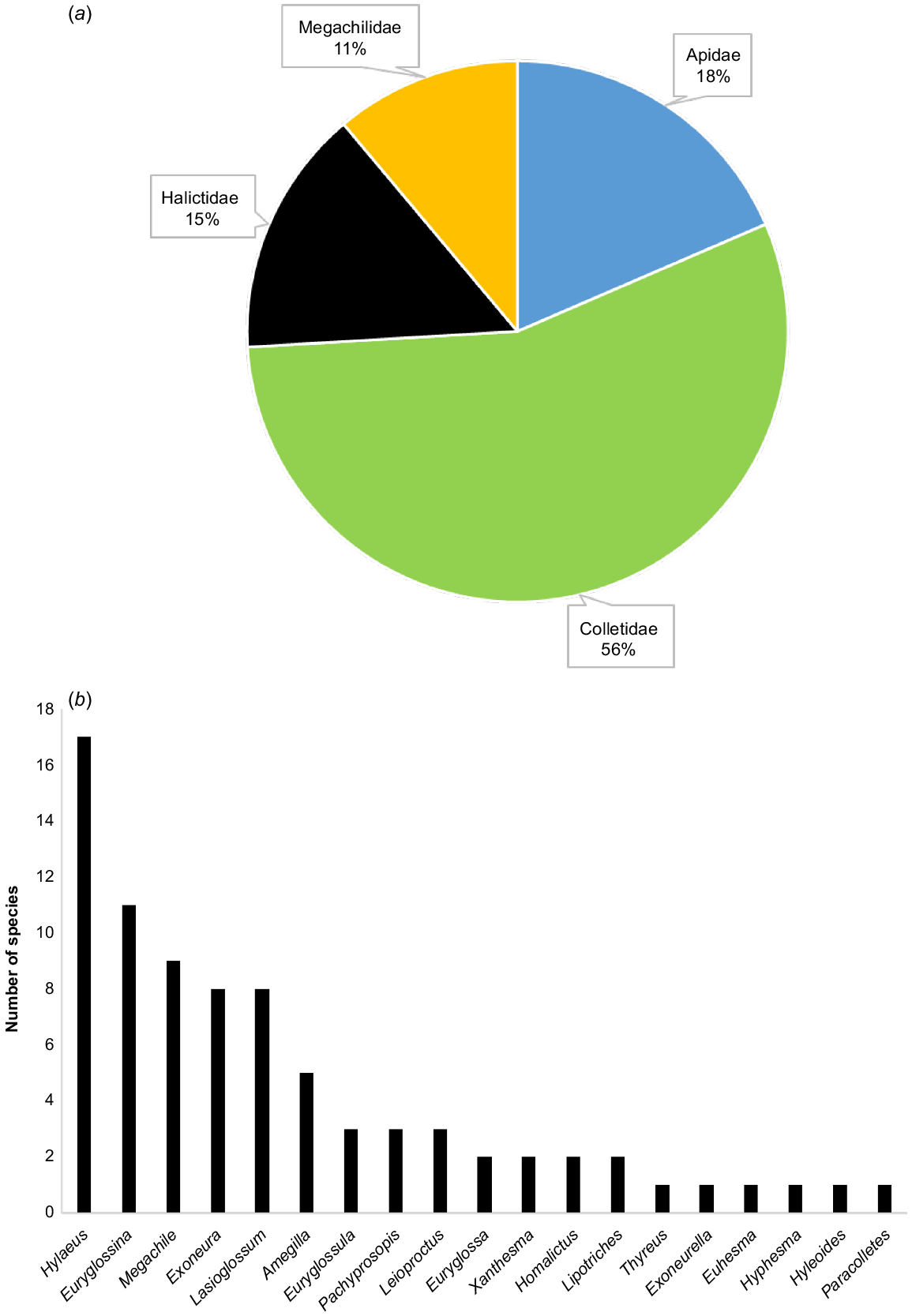
Eighty-one species of native bees are known to visit C. calophylla (Table 1). Of these, 75 were recorded during surveys across the 23 sites by KSP, with an additional six species documented in Houston (2000) (Table 1). Fifteen of these species (18.5%) were not able to be identified to currently described species in the taxonomic literature and likely represent undescribed species (Table 1). The higher taxonomic-level diversity was also high, with 19 genera from all 4 Australian bee families except the rare Stenotritidae recorded visiting C. calophylla (Table 1). Colletids comprised the most records in terms of number of species, with similar proportions for Apidae, Halictidae and Megachilidae (Fig. 2). The genera with the greatest number of species recorded to visit C. calophylla were Hylaeus and Euryglossina (Fig. 2).
| Taxa recorded by KSP | |
|---|---|
| Amegilla (Notomegilla) chlorocyanea | |
| Amegilla (Asaropoda) preissi | |
| Exoneura (Brevineura) gracilis | |
| Exoneura (Brevineura) ‘latte’A | |
| Exoneura (Brevineura) minutissima | |
| Exoneura (Exoneura) laeta | |
| Exoneura cf. pictifrons | |
| Exoneura sp. DBCAPerthHills_3A | |
| Exoneura sp. DBCAPerthHills_6A | |
| Exoneura sp. DBCAPerthHills_7A | |
| Exoneurella setosa | |
| Euhesma DBCAPerthHills_3A | |
| Euryglossa calaina | |
| Euryglossa rubricate | |
| Euryglossina (Euryglossina) argocephala | |
| Euryglossina (Euryglossina) narifera | |
| Euryglossina (Euryglossina) flaviventris | |
| Euryglossina (Euryglossina) micheneri | |
| Euryglossina (Euryglossina) kellyi | |
| Euryglossina (Euryglossina) lynettae | |
| Euryglossina (Euryglossina) perpusilla | |
| Euryglossinae 65[13?] Euryglossina ‘Spp. C’A | |
| Euryglossina (Euryglossina) hypochroma | |
| Euryglossina (Turnerella) atomaria | |
| Euryglossina (Microdontura) mellea | |
| Euryglossula carnarvonensis | |
| Euryglossinae 68 M Euryglossula sp.A | |
| Euryglossula fultoni | |
| Pachyprosopis (Parapachyprosopis) eucyrta | |
| Pachyprosopis (Pachyprosopula) purnongensis | |
| Pachyprosopis (Pachyprosopula) xanthodonta | |
| Hylaeus (Euprosopoides) obtusatus | |
| Hylaeus (Euprosopoides) ruficeps kalamundae | |
| Hylaeus (Euprosopis) elegans | |
| Hylaeus (Gnathoprosopis) amiculus | |
| Hylaeus (Gnathoprosopis) euxanthus | |
| Hylaeus (Hylaeorhiza) nubilosus | |
| Hylaeus (Prosopisteron) aralis | |
| Hylaeus (Prosopisteron) bicurvatus | |
| Hylaeus (Prosopisteron) breviscapatus | |
| Hylaeus (Prosopisteron) chlorosoma | |
| Hylaeus (Prosopisteron) latifacies | |
| Hylaeus (Rhodohylaeus) lateralis | |
| Hylaeus (Rhodohylaeus) proximus | |
| Hylaeus (Prosopisteron) quadratus | |
| Hylaeus (Prosopisteron) trimerops | |
| Hylaeus (Prosopisteron) vittatifrons | |
| Hyphesma atromicans | |
| Hylaues sp. ‘Marrinup’A | |
| Homalictus (Homalictus) dotatus | |
| Homalictus (Homalictus) urbanus | |
| Lasioglossum (Chilalictus) hemichalceum | |
| Lasioglossum (Chilalictus) sp. ‘sculum’A | |
| Lasioglossum (Chilalictus) mirandum | |
| Lasioglossum (Chilalictus) ebeneum | |
| Lasioglossum (Chilalictus) melanopterum | |
| Lasioglossum (Chilalictus) supralucens | |
| Lasioglossum (Chilalictus) ?cephalochilum | |
| Lasioglossum (Parasphecodes) sp. ‘PX1’A | |
| Leioproctus (Leioproctus) clarki | |
| Leioproctus sp. ‘Howse’A | |
| Lipotriches (Austronomia) australica | |
| Lipotriches (Austronomia) flavoviridis spp-group | |
| Megachile aurifrons | |
| Megachile (Eutricharaea) chrysopyga | |
| Megachile (Eutricharaea) obtusa | |
| Megachile apicata | |
| Megachile (Hackeriapis) oblonga | |
| Megachile (Hackeriapis) tosticauda species-group | |
| Megachile cf. clypeata | |
| Megachile cf. callura | |
| Paracolletes crassipes | |
| Xanthesma sp. ‘Marrinup’A | |
| Xanthesma (Xenohesma) brachycera | |
| Thyreus waroonensis |
| Extra records for the WA Museum in Houston (2000) | |
|---|---|
| Amegilla (Asarapoda) sp. F65A | |
| Amegilla (Asarapoda) sp. M85A | |
| Amegilla (Zonamegilla) pulchra | |
| Megachile rugosa | |
| Leioproctus (Leioproctus) sp. F281/M256A | |
| Hyleoides zonalis |
Taxonomic composition of native bee species recorded visiting Corymbia calophylla. (a) Breakdown of the representation of species by family; (b) number of species per genus.
From data collected in the remnant vegetation sites (bushland remnants or jarrah forest, n = 30 surveys), an average of 7.1 ± 0.93 species was collected from C. calophylla per survey, representing an average of 89.6% ± 4.1% of all species collected (Supplementary material Table S1). In terms of abundance, an average of 18.1 ± 3.47 bees was collected on C. calophylla representing 89.2% ± 4.5% of all bees (Table S1).
From data collected during the PhD surveys of KSP in the urbanised southwest WA biodiversity hotspot (8 sites, n = 21 surveys), which included recording bees observed foraging, not just those that could be collected with a sweepnet, a similar proportion of bees foraged on C. calophylla: 94.4% ± 36.0%. On average during these surveys, 75.0 ± 22.7 bees were recorded on C. calophylla (Table S1).
In addition to providing a nutritional resource to native bees (Fig. 3), C. calophylla also provides other non-food resources to native bees, including nesting resources (Fig. 3). Native Megachile bees were observed collecting the resin for use in their nests, and megachilids as well as other cavity-nesting colletids (most Hylaeinae, various genera of Euryglossinae) will nest in holes in the trees created by wood-boring beetles. Furthermore, fallen logs of C. calophylla can also be nested in, and Euryglossa rubricata has been observed nesting among the soil-caked roots of fallen trees (K. S. Prendergast 2022, pers. obs.) (Fig. 3).
Native bees utilising Corymbia calophylla. (a) Lipotriches (Austronomia) flavoviridis species-group foraging; (b) Exoneura sp. foraging; (c) Megachile oblonga collecting resin; (d) Euryglossa rubricata females (inset) nesting among the roots of a dead tree. See also Supplementary material video S1. All photos © Kit Prendergast.

Discussion
Corymbia calophylla represents an invaluable resource for native bee conservation, being visited by an extremely high and taxonomically diverse range of species including numerous oligolectic species. Furthermore, it blooms during a period when few other flowering resources are available (George 2002), and as our data have revealed, on average represents the dominant resource that native bees are visiting in a given area where it occurs.
There are few flowering plant species studies in Australia recording the diversity of native bee visitors, identified to species-level (the taxonomic impediment) (Prendergast and Hogendoorn 2021). However, it appears that the number of species recorded here far exceeds that for other plant species for which there are published records. For example, 17 native bees were recorded visiting Eucalyptus marginata in WA (limited to one site) (Yates et al. 2005), and 33 species to Eucalyptus globulus globulus in Tasmania (Hingston and Potts 1998). Thirty species of bees were collected from E. viminalis, which was the greatest number of bee species for any of 114 plant species surveyed (Hingston and Mc Quillan 2000). Together this data suggests that eucalypts (Corymbia and Eucalyptus) may be particularly important for bees.
The number of species presented visiting C. calophylla here is likely an underestimate as there is high turnover of native bees between sites in this region, even visiting the same plant species (e.g. Prendergast 2021a, 2021b, 2021c, 2021d, 2021f), and thus other C. calophylla trees in the southwest may support additional native bee species. Furthermore, the collection of bees was limited to the area of reach of the sweepnet – approximately from ground height to 2.5 m. However, mature C. calophylla trees can reach heights exceeding 40 m (Spooner 2004). There were also instances when C. calophylla was in flower, yet only flowering high in the canopy. As this was too high to observe or catch bees, no native bees were recorded, but this does not mean native bees were not present foraging on C. calophylla, only that bees foraging high in the canopy could not be determined; hence our data presented here are likely underestimates. Determining the foraging height preferences of native bees on C. calophylla should be a key research priority (Prendergast 2021b, 2021c, 2021d). This would have implications for management of C. calophylla stands e.g. if bees have a foraging height limit (Maclvor 2016), ensuring younger (shorter) trees are present would ensure adequate access to this flowering resource. There may also be different foraging height preferences among different native bee taxa (Levin and Kerster 1973; Roubik 1993; Roubik et al. 1995), or with introduced A. mellifera, which would provide a mechanism for niche partitioning to reduce competition (Nagamitsu and Inoue 1997). Foraging height preferences would also influence pollination (Waddington 1979; Donnelly et al. 1998), and outcrossing rates (Hingston and Potts 2005).
The assemblage visiting C. calophylla was most frequented by small colletids in the genera Hylaeus and Euryglossina. Both these genera swallow pollen, so although they are unlikely to represent important pollinators (Michener 2007), they contribute to a major component of Australian biodiversity. Of the 768 described species of Hylaeus worldwide (Ascher and Pickering 2020), nearly one-fifth (143 species) are found in Australia (Australian Fauna Directory 2022), and there are many more Australian species that have yet to be described (T. F. Houston, pers. comm; K. S. Prendergast, pers. obs.). All Euryglossina are endemic to Australia (Michener 2007). Many of these species are also endemic to the region in which C. calophylla is distributed (Supplementary material Table S2). These genera also are largely oligolectic on Myrtaceae (Michener 2007), and therefore C. calophylla would be a major, important resource for these taxa.
Future avenues for research
Corymbia calophylla has been introduced outside its endemic range in Western Australia into other states on the east coast such as Victoria, where it is planted as an urban park or street tree (City of Melbourne 2015; Prendergast 2020a). Future research should investigate what other bee species visit the plant in these introduced regions, which would no doubt extend the number of bee species known to visit this species, based on observations that ‘near native’ plants (sensuSalisbury et al. 2015) are often still highly attractive to native bees, including Australian oligoleges.
Wild type C. calophylla has cream flowers, and there is a rare pink flowered form, as well as hybrids (Australian Native Plants Society (Australia) 2022). Given that flower colour is known to influence bee foraging (Rowe et al. 2020), and that horticultural variety can impact flower preference among bees, it would be interesting to see if a different assemblage visits these other forms.
This research revealed the diversity of native bees that utilise C. calophylla for nutrition (nectar and/or pollen), and in doing so, may serve as pollinators. However, floral visitation does not necessarily result in pollination. To determine which bees serve as pollinators, this would involve studies looking at pollen loads, foraging behaviour, pollen tube germination and fertilisation, and DNA analyses e.g. (Munyuli 2014; Gorenflo et al. 2017; de Santiago-Hernández et al. 2019; Tommasi et al. 2023). It should be noted that other non-bee taxa can also serve as pollinators, with C. calophylla being visited by a diversity of organisms, including flies, beetles, wasps, birds and mammals (Churchill and Christensen 1970; Abbott et al. 2000; Evelegh et al. 2001; Mathieson et al. 2020; KSP, pers. obs.).
We have revealed how important C. calophylla is as a foraging resource to native bees (Fig. 3), and that C. calophylla also provides other non-food resources to native bees, including nesting resources such as resin (Chui et al. 2022), and substrates for cavity-nesting bees that nest in holes in the trees created by wood-boring beetles (Michener 2007). This further underscores the importance of this plant as a resource for the life-cycles and conservation of native bees.
Conservation implications
We have demonstrated how crucial C. calophylla is to the conservation of the native bee assemblages in the southwest Western Australian biodiversity hotspot, and therefore protecting this species from threatening processes should be a priority – these include illegal logging, extreme fire events, disease, and clearing for urban and industrial development and agriculture.
Apis mellifera, the European honeybee, is an introduced bee that has the potential to outcompete Australian native bees for nectar and/or pollen resources (Prendergast et al. 2022a). Previous research in this region has indicated that increases in honeybee abundance are significantly associated with fewer native bees (Prendergast and Wilson 2022), especially for native bee taxa that have high niche overlap with honeybees (Paini and Roberts 2005; Prendergast et al. 2021). Such situations arise for oligoleges, which cannot forage on alternative resources, and when there are high honeybee abundances in the landscape and few preferred alternative resources for them to forage on, C. calophylla is a major honey source and beekeepers will place hives close to C. calophylla for their honey business (e.g. White 2022). It is likely therefore that competitive interactions may occur, with honeybees outcompeting native bees for this crucial resource, especially under conditions of scarcity such as drought, poor flowering seasons or after major fires (Prendergast and Wilson 2022). In light of how important C. calophylla is to native bee biodiversity, it would be advisable that honeybee densities be carefully managed in relation to the flowering of C. calophylla so that resources do not become limiting to the detriment of native bee biodiversity, and that feral honeybee colonies are eradicated (Prendergast et al. 2022a).
In conclusion, C. calophylla represents an important resource for the native bee assemblages of Western Australia. This single species supports the foraging of over 80 species of native bees, and often represents the dominant foraging resource for native bees at a locality. Protection, management and restoration of these trees will be a vital strategy for ensuring the conservation of the biodiverse native bee assemblages they support.
Declarations of funding
We thank DBCA, ALCOA, City of Kalamunda, City of Bayswater, Araluen Botanic Gardens, Herdsman Lake Gould League, Friends of Lake Claremont, the Forrest Research Foundation, BeeDay Australia, and Zanthorrea Nursery for financially supporting this research.
Acknowledgements
We are grateful for the native bee records on C. calophylla collected by Terry Houston at the WA Museum. Thanks to Margaret Byrne for valuable feedback on the manuscript.
References
Abbott I, Wills A, Burbidge T, Heurck PV (2000) Arthropod faunas of crowns of jarrah (Eucalyptus marginata) and marri (Corymbia calophylla) in mediterranean-climate forest: a preliminary regional-scale comparison. Australian Forestry 63, 21-26.
| Crossref | Google Scholar |
Ascher J, Pickering J (2020) Discover Life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). Discover Life. Vol. 51. (GBIF). Available at https://www.discoverlife.org/mp/20q?guide=Apoidea_species [Verified 29 November 2022]
Atlas of Living Australia (2021) Atlas of Living Australia. (CSIRO: Australia). Available at https://www.ala.org.au/ [Verified 29 November 2022]
Australian Fauna Directory (2022) Australian Fauna Directory. (Commonwealth of Australia, Department of the Environment and Energy, Canberra). Available at https://biodiversity.org.au/afd/taxa/APIFORMES/statistics [Verified 29 November 2022]
Australian Native Plants Society (Australia) (2022) Corymbia calophylla. (Australian Native Plants Society (Australia)). Available at https://anpsa.org.au/plant_profiles/corymbia-calophylla/ [Verified 29 November 2022]
Chui SX, Keller A, Leonhardt SD (2022) Functional resin use in solitary bees. Ecological Entomology 47, 115-136.
| Crossref | Google Scholar |
Churchill DM, Christensen P (1970) Observations on pollen harvesting by brush-tongued lorikeets. Australian Journal of Zoology 18, 427-437.
| Crossref | Google Scholar |
de Santiago-Hernández MH, Martén-Rodríguez S, Lopezaraiza-Mikel M, Oyama K, González-Rodríguez A, Quesada M (2019) The role of pollination effectiveness on the attributes of interaction networks: from floral visitation to plant fitness. Ecology 100, e02803.
| Crossref | Google Scholar | PubMed |
Decourtye A, Mader E, Desneux N (2010) Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 41, 264-277.
| Crossref | Google Scholar |
Dicks LV, Breeze TD, Ngo HT, et al. (2021) A global-scale expert assessment of drivers and risks associated with pollinator decline. Nature Ecology & Evolution 5, 1453-1461.
| Crossref | Google Scholar |
Donnelly SE, Lortie CJ, Aarssen LW (1998) Pollination in Verbascum thapsus (Scrophulariaceae): the advantage of being tall. American Journal of Botany 85, 1618-1625.
| Crossref | Google Scholar | PubMed |
Evelegh NCP, Majer JD, Recher HF (2001) The effects of reducing bird predation on canopy arthropods of marri (Eucalyptus calophylla) saplings on the Swan Coastal Plain, Western Australia. Journal of the Royal Society of Western Australia 84, 13-21.
| Google Scholar |
George AS (2002) The south-western Australian flora in autumn: 2001 Presidential Address. Journal of the Royal Society of Western Australia 85, 1-15.
| Google Scholar |
Gorenflo A, Diekötter T, van Kleunen M, Wolters V, Jauker F (2017) Contrasting pollination efficiency and effectiveness among flower visitors of Malva sylvestris, Borago officinalis and Onobrychis viciifolia. Journal of Pollination Ecology 21, 62-70.
| Crossref | Google Scholar |
Hellwig N, Schubert LF, Kirmer A, Tischew S, Dieker P (2022) Effects of wildflower strips, landscape structure and agricultural practices on wild bee assemblages – A matter of data resolution and spatial scale? Agriculture, Ecosystems & Environment 326, 107764.
| Crossref | Google Scholar |
Hingston A, Potts B (1998) Floral visitors of Eucalyptus globulus subsp. globulus in eastern Tasmania. Tasforests 10, 125-139.
| Google Scholar |
Hingston AB, Potts BM (2005) Pollinator activity can explain variation in outcrossing rates within individual trees. Austral Ecology 30, 319-324.
| Crossref | Google Scholar |
Hingston AB, Mc Quillan PB (2000) Are pollination syndromes useful predictors of floral visitors in Tasmania? Austral Ecology 25(6), 600-609.
| Google Scholar |
Hopper SD (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil 322, 49-86.
| Crossref | Google Scholar |
Hopper SD (2021) Out of the OCBILs: new hypotheses for the evolution, ecology and conservation of the eucalypts. Biological Journal of the Linnean Society 133, 342-372.
| Crossref | Google Scholar |
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35, 623-650.
| Crossref | Google Scholar |
Image M, Gardner E, Clough Y, et al. (2022) Does agri-environment scheme participation in England increase pollinator populations and crop pollination services? Agriculture, Ecosystems & Environment 325, 107755.
| Crossref | Google Scholar |
Levin DA, Kerster HW (1973) Assortative pollination for stature in Lythrum salicaria. Evolution 144-152.
| Crossref | Google Scholar |
Maclvor JS (2016) Building height matters: nesting activity of bees and wasps on vegetated roofs. Israel Journal of Ecology and Evolution 62, 88-96.
| Crossref | Google Scholar |
Mathieson TJ, Close PG, Van Helden BE, Speldewinde PC, Comer SJ (2020) New evidence of unexpectedly high animal density and diet diversity will benefit the conservation of the critically endangered western ringtail possum. Austral Ecology 45, 596-608.
| Crossref | Google Scholar |
McDonald JA, Carwardine J, Joseph LN, Klein CJ, Rout TM, Watson JEM, Garnett ST, McCarthy MA, Possingham HP (2015) Improving policy efficiency and effectiveness to save more species: a case study of the megadiverse country Australia. Biological Conservation 182, 102-108.
| Crossref | Google Scholar |
Munyuli T (2014) Influence of functional traits on foraging behaviour and pollination efficiency of wild social and solitary bees visiting coffee (Coffea canephora) flowers in Uganda. Grana 53, 69-89.
| Crossref | Google Scholar |
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403, 853-858.
| Crossref | Google Scholar | PubMed |
Nagamitsu T, Inoue T (1997) Aggressive foraging of social bees as a mechanism of floral resource partitioning in an Asian tropical rainforest. Oecologia 110, 432-439.
| Crossref | Google Scholar | PubMed |
Orr MC, Hughes AC, Chesters D, Pickering J, Zhu C-D, Ascher JS (2021) Global patterns and drivers of bee distribution. Current Biology 31, 451-458.e4.
| Crossref | Google Scholar |
PaDIL (2024) PaDIL website Australian Pollinators. (Australian Government Department of Agriculture: Australia). Available at https://www.padil.gov.au/search/pollinators [Verified 18 Oct 2024]
Paini DR, Roberts JD (2005) Commercial honey bees (Apis mellifera) reduce the fecundity of an Australian native bee (Hylaeus alcyoneus). Biological Conservation 123(1), 103-112.
| Crossref | Google Scholar |
Prendergast KS (2020a) Critiquing the notion of a species natural range in an era of unprecedented change. Austral Ecology 45, 672-674.
| Crossref | Google Scholar |
Prendergast KS (2020c) Urban native bee assemblages and the impact of the introduced European honeybee on plant-pollinator networks in the southwest Australian biodiversity hotspot. PhD thesis. Curtin University of Technology. Available at https://espace.curtin.edu.au/handle/20.500.11937/84947
Prendergast K (2021a) City of Bayswater Native Bee Surveys November – February 2020/21. (City of Bayswater: WA, Australia). Available at https://www.bayswater.wa.gov.au/getattachment/3511fbd7-0d69-4cd5-8841-f59888917161/City_of_Bayswater_Native_Bee_Surveys_November_%E2%80%93_February_2020-21.pdf?lang=en-AU [Verified 29 November 2022]
Prendergast K (2021b) DBCA Perth Hills Native Bee Report: Chandler Forest Block, South of Wungong Dam post-mining rehabilitation areas. Report produced by Dr Kit Prendergast for the Western Australian Government’s Department of Biodiversity, Conservation and Attractions (DBCA). (DBCA: Kensington, WA, Australia)
Prendergast K (2021g) Kooljerrinup Nature Reserve Native Bee Report. Results of native bee surveys September – January 2020/21 with a focus on the Critically Endangered Neopasiphae simplicior. Report produced by Dr Kit Prendergast for the Western Australian Government’s Department of Biodiversity, Conservation and Attractions (DBCA). (DBCA: Kensington, WA, Australia)
Prendergast KS, Hogendoorn K (2021) FORUM: Methodological shortcomings and lack of taxonomic effort beleaguer Australian bee studies. Austral Ecology 46, 880-884.
| Crossref | Google Scholar |
Prendergast KS, Dixon KW, Bateman PW (2021) Interactions between the introduced European honey bee and native bees in urban areas varies by year, habitat type and native bee guild. Biological Journal of the Linnean Society 133, 725-743.
| Crossref | Google Scholar |
Prendergast KS, Dixon KW, Bateman PW (2022a) The evidence for and against competition between the European honeybee and Australian native bees. Pacific Conservation Biology 29, 89-109.
| Crossref | Google Scholar |
Prendergast KS, Tomlinson S, Dixon KW, Bateman PW, Menz MHM (2022b) Urban native vegetation remnants support more diverse native bee communities than residential gardens in Australia’s southwest biodiversity hotspot. Biological Conservation 265, 109408.
| Crossref | Google Scholar |
Roubik DW (1993) Tropical pollinators in the canopy and understory: field data and theory for stratum “preferences”. Journal of Insect Behavior 6, 659-673.
| Crossref | Google Scholar |
Roubik DW, Inoue T, Hamid AA (1995) Canopy foraging by two tropical honeybees: bee height fidelity and tree genetic neighborhoods. Tropics 5, 81-93.
| Crossref | Google Scholar |
Rowe L, Gibson D, Bahlai CA, Gibbs J, Landis DA, Isaacs R (2020) Flower traits associated with the visitation patterns of bees. Oecologia 193, 511-522.
| Crossref | Google Scholar | PubMed |
Salisbury A, Armitage J, Bostock H, Perry J, Tatchell M, Thompson K (2015) EDITOR’S CHOICE: Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): should we plant native or exotic species? Journal of Applied Ecology 52, 1156-1164.
| Crossref | Google Scholar |
Spooner A (2004) Corymbia calophylla (Lindl.) K.D.Hill & L.A.S.Johnson. In ‘FloraBase.’ (Ed. Western Australian Herbarium). (Department of Biodiversity, Conservation and Attractions: Western Australia). Available at https://florabase.dbca.wa.gov.au/browse/profile/17104 [Verified 18 Oct 2024]
Taxonomy Australia (2020) Discovering and documenting Australia’s native bees. (Taxonomy Australia). Available at https://www.taxonomyaustralia.org.au/discoverbees [Verified 29 November 2022]
Tommasi N, Biella P, Maggioni D, Fallati L, Agostinetto G, Labra M, Galli P, Galimberti A (2023) DNA metabarcoding unveils the effects of habitat fragmentation on pollinator diversity, plant-pollinator interactions, and pollination efficiency in Maldive islands. Molecular Biology 32(23), 6394-6404.
| Google Scholar |
Waddington KD (1979) Divergence in inflorescence height: an evolutionary response to pollinator fidelity. Oecologia 40, 43-50.
| Crossref | Google Scholar | PubMed |
White B (2022) The economic value of honeybee flora in Western Australia. Available at https://www.crchoneybeeproducts.com/wp-content/uploads/2022/12/Final-report-The-economic-value-of-honey-bee-flora-in-Western-Australia.pdf
Yates C, Hopper S, Taplin R (2005) Native insect flower visitor diversity and feral honeybees on jarrah (Eucalyptus marginata) in Kings Park, an urban bushland remnant. Journal of the Royal Society of Western Australia 88(4), 147-153.
| Google Scholar |


