Whale sharks as oceanic nurseries for Golden Trevally
M. Sheaves A B * , C. Mattone
A B * , C. Mattone  A B , A. Barnett A B , K. Abrantes A B , M. Bradley A B , A. Sheaves A B , J. Sheaves A B and N. J. Waltham A B C
A B , A. Barnett A B , K. Abrantes A B , M. Bradley A B , A. Sheaves A B , J. Sheaves A B and N. J. Waltham A B C
A College of Science and Engineering, James Cook University, Townsville, Qld, Australia.
B Marine Data Technology Hub, James Cook University, Townsville, Qld, Australia.
C TropWater, James Cook University, Townsville, Qld, Australia.
Abstract
The Golden Trevally, Gnathanodon speciosus, is a large predatory fish with an extremely broad tropical Indo-Pacific distribution that crosses many biogeographical boundaries. Both published information and freely available imagery suggest that small juvenile G. speciosus are often associated with whale sharks, Rhincodon typus; an association that could explain the unusually widespread distribution of G. speciosus, and suggests a novel nursery relationship. The possibility of such an association has the potential to reshape our understanding of the ecological roles played by long-range migrants such as R. typus and other megafauna, our understanding of the full extent of their conservation value, and how we manage both members of the relationship.
Keywords: commensalism, dispersal, Gnathanodon speciosus, Golden Trevally, Indo-Pacific, mobile nurseries, Rhincodon typus, whale shark.
Introduction
Golden Trevally, Gnathanodon speciosus, is a large predatory carangid fish with an extremely broad tropical Indo-Pacific distribution (Grandcourt et al. 2004) that spans substantial biogeographic barriers (Bellwood and Wainwright 2002). They are found from the east coast of Africa (Blaber and Cyrus 1983; Berkström et al. 2012) across the Indian and Pacific Oceans to the Galápagos (Todd and Grove 2010) and the west coast of Central and North America (Gunter 1979; Strand 1988), north to the Ryukyu Islands (Shibuno et al. 2008) and south to northern Australia (Liu et al. 1985; Blaber et al. 1995). Adult and later juvenile G. speciosus utilise a diversity of nearshore habitats including sand, rocky and coral reefs (Grandcourt et al. 2004; Gomelyuk 2009), and seagrass meadows (Kimani et al. 1996; Henderson et al. 2017), as well as being found in artificial salt concentrator ponds (Molony and Parry 2006), and around fish aggregating devices (Folpp and Lowry 2006), floating logs (Hampton and Bailey 1992) and oil platforms (Torquato et al. 2017). They occur from shallow intertidal areas to depths of greater than 180 m (Sileesh et al. 2018).
Gnathanodon speciosus are captured artisanally (Assan and Dorto 2009) and are sought-after sport fish (Smith et al. 2007; Ryan et al. 2015), as well as being important targets in the aquarium trade (Okemwa et al. 2016). Although G. speciosus is an important fisheries species in some areas (Grandcourt et al. 2008), it comprises only a minor component of catches over most of its range (e.g. Ramm et al. 1993; Blaber et al. 1994), but is marketable when captured in large enough numbers (Errity and Fish 2003).
The relatively low fisheries importance of G. speciosus despite high marketable quality suggests that, notwithstanding its widespread distribution, it occurs in relatively low numbers over much of its range. An unusually extensive range, coupled with relatively low densities, suggests that G. speciosus has an unusual life history and/or dispersal mechanism. One interesting possibility is that G. speciosus may utilise an unconventional oceanic nursery strategy, with extensive dispersal across biogeographic boundaries, facilitated by a commensal relationship with large mobile organisms such as whale sharks, Rhincodon typus. There is some evidence to support this. While investigating the nursery role of inshore waters in northern Australia, Blaber et al. (1995) noted that G. speciosus was one of a small group of species where smaller juveniles were found offshore, despite larger juveniles occurring in inshore or estuarine areas. Additionally, while studying R. typus, Gunn et al. (1999) noted that most R. typus were accompanied by juvenile G. speciosus ranging from 30 to 150 mm fork length.
Materials and methods
Exploring the possibility of a mobile R. typus nursery for G. speciosus is difficult because appropriate data are not available and the types of studies needed to investigate the question would be expensive given the need to collect data over a wide geographic range. As a first step toward determining if extensive research makes sense we searched the World Wide Web for imagery depicting associations between G. speciosus and other fauna using the terms ‘Gnathanodon speciosus’, ‘Gnathonodon speciosus’ (a common misspelling) and the common name ‘Golden Trevally’. We then assessed the imagery to quantify associations between G. speciosus and animals and structures in the marine environment.
Results and discussion
Despite issues of potential biases in using online image data (e.g. selective focus on photos of particular hosts), the freely available image data do provide a source of independent validation of the idea that R. typus provide a mobile nursery that is used extensively by G. speciosus juveniles.
Fifty-two images, showing clearly identifiable G. speciosus closely associated with a host, remained once images that appeared to be of the same G. speciosus/host pair were excluded. Colour patterns indicated that all the G. speciosus demonstrating a close association with a host were juveniles or sub-adults, with no indication of associations between adults and any host. Twenty-eight images showed associations between G. speciosus and a variety of sharks, fish, dugongs and turtles in shallow water situations, usually over reef or seagrass (Fig. 1). In line with previous studies (Blaber et al. 1995) most of the G. speciosus involved in shallow water interactions appeared to be larger juvenile or sub-adults. In contrast, R. typus were by far the most commonly depicted host (15 images) in apparent pelagic situations, and in many cases the G. speciosus were positioned close to the shark’s mouth, the location where individuals of 30–100 mm are usually found (Gunn et al. 1999). Additionally recent hydrodynamic models have shown that the front of R. typus seems to be where small fish benefit the most from reduced drag, thereby reducing their travel costs (Sumikawa and Miyoshi 2022).
Percentage of images depicting G. speciosus in close association with (a) pelagic host organisms or structures and (b) coastal/shallow water host organisms or structures.
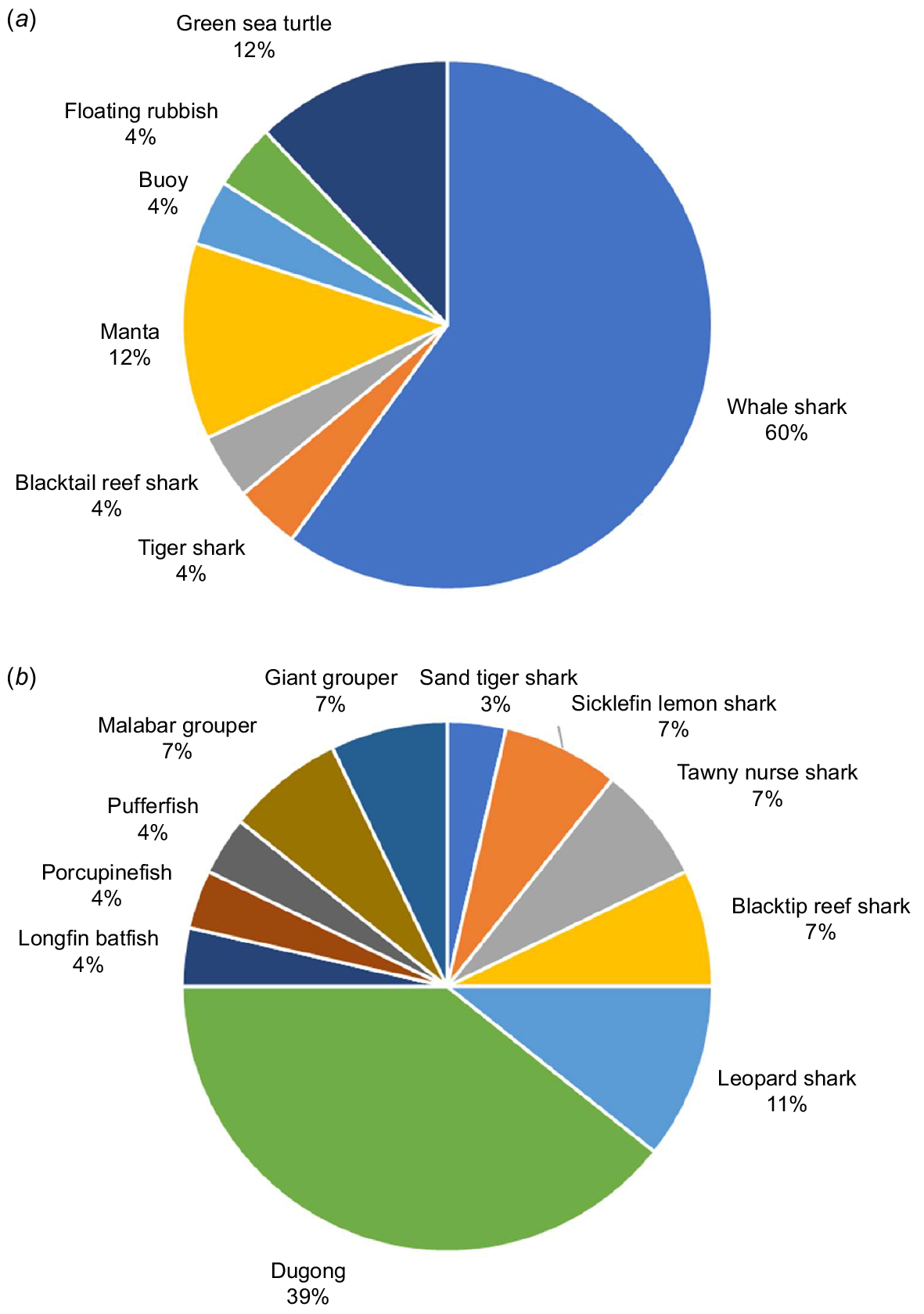
The two offshore images that did not include R. typus show juvenile G. speciosus associated with a buoy and with floating rubbish. Very small juveniles have been reported to live amongst the tentacles of jellyfish (Myers 1989; Lieske and Myers 1994). However, these reports lack defined citation tracks, and the available online images of juvenile carangids with yellow and black bars, that are associated with jellyfish are not juvenile G. speciosus but rather Carangoides ferdau juveniles, when images were clearly identifiable. Consequently, the extent to which hosts other than R. typus are used by small juvenile G. speciosus requires further investigation.
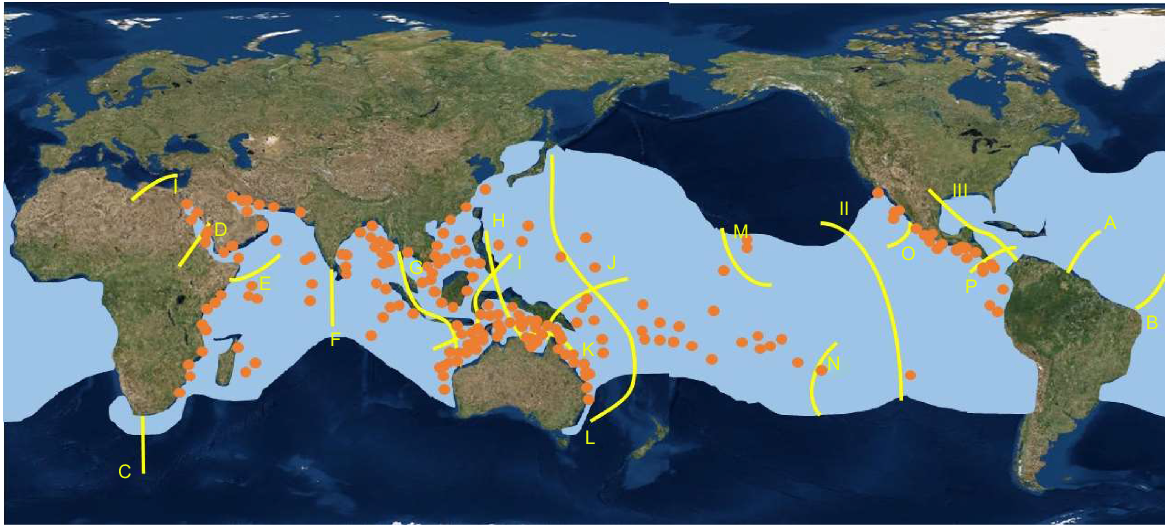
Although genetic studies are inconclusive about the extent of connectivity of R. typus populations across the Indo-Pacific (Vignaud et al. 2014), R. typus are known to undertake extremely long distance migrations over short periods of time, with one shark tagged in the tropical Eastern Pacific, travelling over 20 000 km to the western Indo-Pacific in 841 days (Guzman et al. 2018). This indicates that it could be possible for juvenile G. speciosus to make extensive cross-biogeographic boundary migrations utilising R. typus as vectors. If so, the use of this unusual nursery relationship could explain the broad distribution of G. speciosus, which appears insensitive to the biogeographic boundaries that constrain the distributions of most other species with reef/coastal associated adults (Bellwood and Wainwright 2002) (Fig. 2).
Comparison of the global distribution of G. speciosus (orange dots) (derived from FishBase), the range of R. typus (light blue shaded areas) (derived from Womersley et al. 2022). The yellow lines indicate major reef fish biogeographic boundaries (after Bellwood and Wainwright 2002).
More extensive studies are required to validate and extend on the idea of a mobile nursery association between G. speciosus and R. typus, and indeed to investigate relationships between other fish and animal vectors or structures. There is an opportunity for future studies to work in collaboration with tourism operators and wildlife photographers to better understand these relationships and minimise the biases associated with photographing charismatic megafauna.
However, one of the most definitive lines of evidence could come from stable isotope studies of small juveniles, with the prediction that juveniles should consistently show oceanic isotope signatures for a much longer period than similar fish that only use pelagic oceanic habitats during the larval stage. Stable isotopes could also provide information on oceanic nursery duration via an isotopic clock methodology (Guelinckx et al. 2008). Another valuable study would be a comparison of the genetics of G. speciosus, R. typus, and pilot fish, Naucrates ductor (an oceanic fish often found associated with megafauna) across the Indo-Pacific range of G. speciosus.
If this unique commensal relationship is valid, there are implications for the way we understand the complexity of the ecological roles played by long-range migrants such as R. typus and other megafauna, how we understand the full extent of their conservation values, and how we manage both members of the relationship. In a practical sense, a mobile nursery relationship for G. speciosus extends the concept of connectivity between spawning, nursery and adult habitats beyond the scope of local-scale management, into the more complex international realm. Not only does this greatly complicate any attempt to manage G. speciosus, but it implies that decisions about the management of R. typus need to extend beyond a focus on the conservation of an important megafauna species to include consideration of the needs of an exploited fisheries species. Additionally, the possible presence of this unusual nursery relationship brings into question the relevance of this or similar models for other species.
Data availability
All data are freely available online at https://www.dropbox.com/sh/yjpjewyryjqtsj9/AABX_RqiDAdsiKW_zfzx96Aaa?dl=0.
References
Assan CN, Dorto JL (2009) Seychelles artisanal fisheries statistics for 2008. Seychelles fishing authority technical report. Available at http://hdl.handle.net/1834/4974 [Accessed 7 May 2023]
Berkström C, Gullström M, Lindborg R, Mwandya AW, Yahya SAS, Kautsky N, Nyström M (2012) Exploring ‘knowns’ and ‘unknowns’ in tropical seascape connectivity with insights from East African coral reefs. Estuarine, Coastal and Shelf Science 107, 1-21.
| Crossref | Google Scholar |
Blaber SJM, Cyrus DP (1983) The biology of carangidae (teleostei) in natal estuaries. Journal of Fish Biology 22, 173-188.
| Crossref | Google Scholar |
Blaber SJM, Brewer DT, Harris AN (1994) Distribution, biomass and community structure of demersal fishes of the Gulf of Carpentaria, Australia. Marine and Freshwater Research 45, 375-396.
| Crossref | Google Scholar |
Blaber SJM, Brewer DT, Salini JP (1995) Fish communities and the nursery role of the shallow inshore waters of a tropical bay in the gulf of Carpentaria, Australia. Estuarine, Coastal and Shelf Science 40, 177-193.
| Crossref | Google Scholar |
Folpp H, Lowry M (2006) Factors affecting recreational catch rates associated with a fish aggregating device (fad) off the NSW coast, Australia. Bulletin of Marine Science 78, 185-193.
| Google Scholar |
Gomelyuk VE (2009) Fish assemblages composition and structure in three shallow habitats in north australian tropical bay, Garig Gunak Barlu National Park, Northern Territory, Australia. Journal of the Marine Biological Association of the United Kingdom 89, 449-460.
| Crossref | Google Scholar |
Grandcourt EM, Al Abdessalaam TZ, Francis F, Al Shamsi A (2004) Population biology and assessment of representatives of the family carangidae: Carangoides bajad and Gnathanodon speciosus (Forsskål, 1775), in the Southern Arabian Gulf. Fisheries Research 69, 331-341.
| Crossref | Google Scholar |
Guelinckx J, Maes J, Geysen B, Ollevier F (2008) Estuarine recruitment of a marine goby reconstructed with an isotopic clock. Oecologia 157, 41-52.
| Crossref | Google Scholar |
Gunn JS, Stevens JD, Davis TLO, Norman BM (1999) Observations on the short-term movements and behaviour of whale sharks (Rhincodon typus) at ningaloo reef, Western Australia. Marine Biology 135, 553-559.
| Crossref | Google Scholar |
Gunter G (1979) Marine fishes of Panama as related to the canal. Gulf Research Reports 6, 267-273.
| Crossref | Google Scholar |
Guzman HM, Gomez CG, Hearn A, Eckert SA (2018) Longest recorded trans-Pacific migration of a whale shark (Rhincodon typus). Marine Biodiversity Records 11, 8.
| Crossref | Google Scholar |
Hampton J, Bailey KM (1992) Fishing for tunas associated with floating objects: a review of the Western Pacific fishery. In ‘Proceedings of the international workshop on the ecology and fisheries for tunas associated with floating objects’. pp. 222–284. (Oceanic Fisheries Programme, Secretariat of the Pacific Community: Noumea, New Caledonia)
Henderson CJ, Olds AD, Lee SY, Gilby BL, Maxwell PS, Connolly RM, Stevens T (2017) Marine reserves and seascape context shape fish assemblages in seagrass ecosystems. Marine Ecology Progress Series 566, 135-144.
| Crossref | Google Scholar |
Kimani EN, Mwatha GK, Wakwabi EO, Ntiba JM, Okoth BK (1996) Fishes of a shallow tropical mangrove estuary, Gazi, Kenya. Marine and Freshwater Research 47, 857-868.
| Crossref | Google Scholar |
Liu H-C, Sainsbury KJ, Chiu T-S (1985) Trawl cod-end mesh selectivity for some fishes of North-Western Australia. Fisheries Research 3, 105-129.
| Crossref | Google Scholar |
Molony BW, Parry GO (2006) Predicting and managing the effects of hypersalinity on the fish community in solar salt fields in north-western Australia. Journal of Applied Ichthyology 22, 109-118.
| Crossref | Google Scholar |
Okemwa GM, Kaunda-Arara B, Kimani EN, Ogutu B (2016) Catch composition and sustainability of the marine aquarium fishery in Kenya. Fisheries Research 183, 19-31.
| Crossref | Google Scholar |
Ramm DC, Mounsey RP, Xiao Y, Poole SE (1993) Use of a semi-pelagic trawl in a tropical demersal trawl fishery. Fisheries Research 15, 301-313.
| Crossref | Google Scholar |
Shibuno T, Nakamura Y, Horinouchi M, Sano M (2008) Habitat use patterns of fishes across the mangrove-seagrass-coral reef seascape at Ishigaki Island, southern Japan. Ichthyological Research 55, 218-237.
| Crossref | Google Scholar |
Sileesh MS, Alphi K, Harish KC, Viji V (2018) Species assemblages and community structure of deep-sea demersal ichthyofauna of the South-eastern Arabian Sea (SEAS). Journal of the Marine Biological Association of the United Kingdom 98, 1775-1781.
| Crossref | Google Scholar |
Strand S (1988) Following behavior: interspecific foraging associations among Gulf of California reef fishes. Copeia 351-357.
| Crossref | Google Scholar |
Sumikawa H, Miyoshi T (2022) The pressure drag reduction effect of tandem swimming by Caranx sexfasciatus and Rhincodon typus. Ichthyological Research 69, 132-139.
| Crossref | Google Scholar |
Todd VLG, Grove JS (2010) First records of golden trevally (Gnathodon speciosus, carangidae), sharp-tail mola (Masturus lanceolatus, molidae) and evidence for white shark (Carcharodon carcharias, lamnidae) in the Galápagos Islands, Ecuador. Marine Biodiversity Records 3, E104.
| Crossref | Google Scholar |
Torquato F, Jensen HM, Range P, Bach SS, Ben-Hamadou R, Sigsgaard EE, Thomsen PF, Møller PR, Riera R (2017) Vertical zonation and functional diversity of fish assemblages revealed by ROV videos at oil platforms in The Gulf. Journal of Fish Biology 91, 947-967.
| Crossref | Google Scholar |
Vignaud TM, Maynard JA, Leblois R, Meekan MG, Vázquez-Juárez R, Ramírez-Macías D, Pierce SJ, Rowat D, Berumen ML, Beeravolu C, Baksay S, Planes S, et al. (2014) Genetic structure of populations of whale sharks among ocean basins and evidence for their historic rise and recent decline. Molecular Ecology 23, 2590-2601.
| Crossref | Google Scholar |
Womersley FC, Humphries NE, Queiroz N, Vedor M, da Costa I, Furtado M, Tyminski JP, Abrantes K, Araujo G, Bach SS (2022) Global collision-risk hotspots of marine traffic and the world’s largest fish, the whale shark. Proceedings of the National Academy of Sciences 119, e2117440119.
| Crossref | Google Scholar |


