Traditional Ecological Knowledge and practices associated with the Vanate (Raggiana Bird of Paradise, Paradisaea raggiana) along the Kokoda Track in Central Province, Papua New Guinea
Challis Pulotu A * , Eric Katovai
A * , Eric Katovai  B and Patrick Pikacha A
B and Patrick Pikacha A
A
B
Abstract
Traditional Ecological Knowledge (TEK) is lost due to the increase of urbanisation, and the lack of transfer of this knowledge to younger generations.
The aim of this study was to examine the TEK of the Koiari Indigenous People of Central Province, Papua New Guinea (PNG) concerning the Vanate (Paradisaea raggiana), which is the national bird of PNG.
We used the mixed methods research methodology, specifically the convergent parallel mixed method design with a questionnaire. Forty eight interviews were conducted with respondents aged 17–83 years of age, from the villages of Ioribaiwa, Agulogo, Manari, and Efogi along the Kokoda Track. We collected information on the birds’ language name, habitat, abundance, and conservation status.
There was high linguistic diversity in names used for Birds of Paradise (BOPs). The common generic name for ‘bird’ is Ugu, and for the Paradisaea raggiana, it is the Vanate. Ten species of BOPs were reported by respondents to occur in the area. Respondents identified lowland wet and lower montane wet forests as habitats for the Vanate. In terms of conservation status, most locals (52%) said the Vanate was hunted opportunistically. Most respondents (40%) stated that the Vanate was increasing in abundance due to a reduction in disturbance, and less hunting.
The Akaike Information Criterion (AIC) value showed that the most parsimonious model for Vanate abundance was locals who hunt and type of weapons (traditional) are the most parsimonious predictors of predicted abundance of the Vanate.
Keywords: abundance, biocultural conservation, biocultural knowledge, culturally significant species, decolonise, human rights, Koiari Indigenous People, Traditional Environmental Knowledge.
Introduction
Traditional Ecological Knowledge (TEK), or indigenous science (Whyte et al. 2016), is defined as the accrued volume of knowledge, practice, and belief, advanced by adaptive means and passed down through generations by cultural conveyance (Martin et al. 2010; Gómez-Baggethun et al. 2013; Michalos 2014). TEK has shaped people and their environment. This includes forming belief systems, customs, and has connected people to their environment (Moller et al. 2004; Martin et al. 2010; Si 2016). TEK can strengthen community resilience by responding to stressors of environmental change, and TEK should always be considered when planning for sustainable resource management (Martin et al. 2010). With cultural and biological diversity closely linked (Pretty et al. 2009), and the strength of that link fast disappearing as a result of habitat loss and rapid development (Shearman and Bryan 2011; Baker et al. 2014), it is imperative that TEK be preserved.
Along with land ownership as an inherent right based on association by genealogy, TEK is passed down through generations. This has given Indigenous People rights to conserve their land, biological resources (i.e. animals and plants), traditions, and cultures. Indigenous People occupy 22% of the world’s land surface (usually in the tropics where biodiversity thrives) (Garnett et al. 2018), and plays a substantial role in species conservation and management. Indigenous People constitute about 5% of the world’s population, account for 15% of the world’s poor (Maffi and Woodley 2010), and protect 22% of the Earth’s surface with 80% of planetary biodiversity (International Labour Office 2017).
With the ongoing loss of TEK, there is consensus amongst some scientists for a standard definition of threats to TEK, and conservation actions that prevent TEK degradation and the consequent effects on biodiversity conservation planning (Tang and Gavin 2016). There are six defined threats to TEK that are applicable in this context and to varying degrees: (1) loss of pathways of TEK transmission; (2) reduction in traditional livelihood practices; (3) weakening of traditional religion and beliefs; (4) loss of environment and natural resources; (5) diminished traditional rights; and (6) displacement of traditional institutions (Tang and Gavin 2016).
In areas where Indigenous People hold ownership to customary land, which is widespread in PNG (Weiner and Glaskin 2007) and Melanesia (McDonnell et al. 2017), rarely do the successes of conservation management depend solely upon the efforts of biologists or environmental organisations alone. Community-based conservation initiatives led by locals with their intimate knowledge of their environment (Hunn 1993; Hunn 2002), have resulted in successful conservation outcomes. For the use of natural resources to be sustainable, the inclusion of Indigenous People throughout the whole conservation management process and allowing them to tell their story is vital to understand their values and perceptions about biodiversity (Fernández-Llamazares and Cabeza 2018).
In PNG, TEK is seen in the local context as leading to a sustainable way of life through exercising the values of respect, responsibility and reciprocity for nature’s continuity (Tiu 2016). Half of PNG’s population has fewer than 6 years of primary education (UNDP 2014). Three quarters or more of the nation’s population live in rural areas, 83% lack access to electricity, and 80% are engaged in subsistence horticulture and/or fishing (Hunn 1993; Department of National Planning and Monitoring 2020). Because human environmental linkages remain strong in rural, traditional communities that lack access to urban and developmental advantages (Gómez-Baggethun et al. 2010), it is likely that a rich wealth of TEK is still present amongst its Indigenous Peoples.
The Vanate (Paradisaea raggiana) (Fig. 1) belongs to the family Paradisaeidae (Beehler and Pratt 2016), and is endemic to New Guinea (West Papua – Indonesia and PNG). Feathers from male birds have been historically traded as prized fashion attire due to its stunning plumes. Between 1905 and 1920, between 30,000 and 80,000 Bird of Paradise (BOP) skins were exported yearly from New Guinea to feather auctions in Europe, particularly London, Paris, and Amsterdam, which brought about the plume bloom of 1908 (Swadling et al. 1996; Kirsch 2013). The affluent populations of Europe, China, and the East Indies used plumes of the Vanate as attractive ornaments on turbans, helmets, and horses (Kirsch 2013; Andaya 2017). To the Papuans and some of the eastern Indonesian people, the feathers of the Vanate are believed to give invulnerability to warriors who wore them to battle (Andaya 2017). This is true for some Papua New Guinean cultures such as those of the Central Province. However, it is unsure as to when this belief died out but is still believed in some cultures today. While for the Maring tribe of Simbu Province in the Highlands of PNG, men decorate themselves with BOP plumes to show a man’s health and vitality (Healey 1993). In PNG, feathers of the Blue BOP (a related species) are worn at funerals and for tribal fighting (Van Den Bergh et al. 2013). These rare feathers and plumes exhibit high status and are linked to the Papuan (Central) people of PNG’s concept of fertility – important to wedding settlement (Andaya 2017).
The Vanate is protected by law through the Fauna Act of 1966–73, and is listed in the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) Schedule 1 of Wildlife Protection Act (CITES 2016); commercial sale of any Vanate (living or dead) is therefore prohibited (CITES n.d.). Limited trade in the Vanate plumes is legitimately allowed only by PNG citizens and only for customary purposes (Supuma 2018). Killing of the Vanate by any instrument other than traditional means is also prohibited (Sekhran and Miller 1994; Van Den Bergh et al. 2013).
Since 2004, the International Union for Conservation of Nature and Natural Resources (IUCN) Red list has designated the Vanate as of Least Concern (BirdLife International 2018; CITES n.d.). This due to its large range, stable population trend, and population size not quantified (BirdLife International 2018). The trade and subsistent use of birds is significant in the culture and livelihood of Papua New Guineans (Supuma 2018). The collection of birds in culture takes various forms including as a source of protein (Dwyer 1974), for trade, aesthetics in cultural adornment, and symbolism of beauty and power (Supuma 2018). With this scope, the Vanate is rightly positioned for us to explore the connection between culture and ecology (Supuma 2018), and the interplay between Indigenous People and their TEK of this historically and contemporary culturally notable bird, the Vanate.
The aim of this study was to explore the TEK of the Koiari Indigenous People of the mountains of Central Province, PNG, toward the Vanate. We assessed the TEK, attitudes, perceptions, and practices associated with the Vanate.
Materials and methods
Study sites
PNG is the third largest island country in the South-west Pacific. Inclusive of its outlying islands, it is located between 0–12°S and 140–160°E, and is composed of 600 small islands and four archipelagos (Beehler and Laman 2020), extending over 2800 km east-west by 750 km north-south (Beehler and Pratt 2016). PNG has a total land area of 461,739 km2 (Bryan and Shearman 2014; Beehler and Laman 2020).
We interviewed Koiari Indigenous People from four villages within the Koiari rural Local-level Government (LLG) areas: (1) Ioribaiwa (9°18′50″S, 147°33′00″E); (2) Agulogo (9°14′00″S, 147°36′56″E); (3) Manari (9°11′16″S, 147°37′09″E); and (4) Efogi (9°09′39″S, 147°39′46″E). These villages are located along the Kokoda Track within Central Province, PNG (Fig. 2).
Location of Papua New Guinea (a), and study sites along the Kokoda track, Central Province (b). (Adapted from Burton 2018).
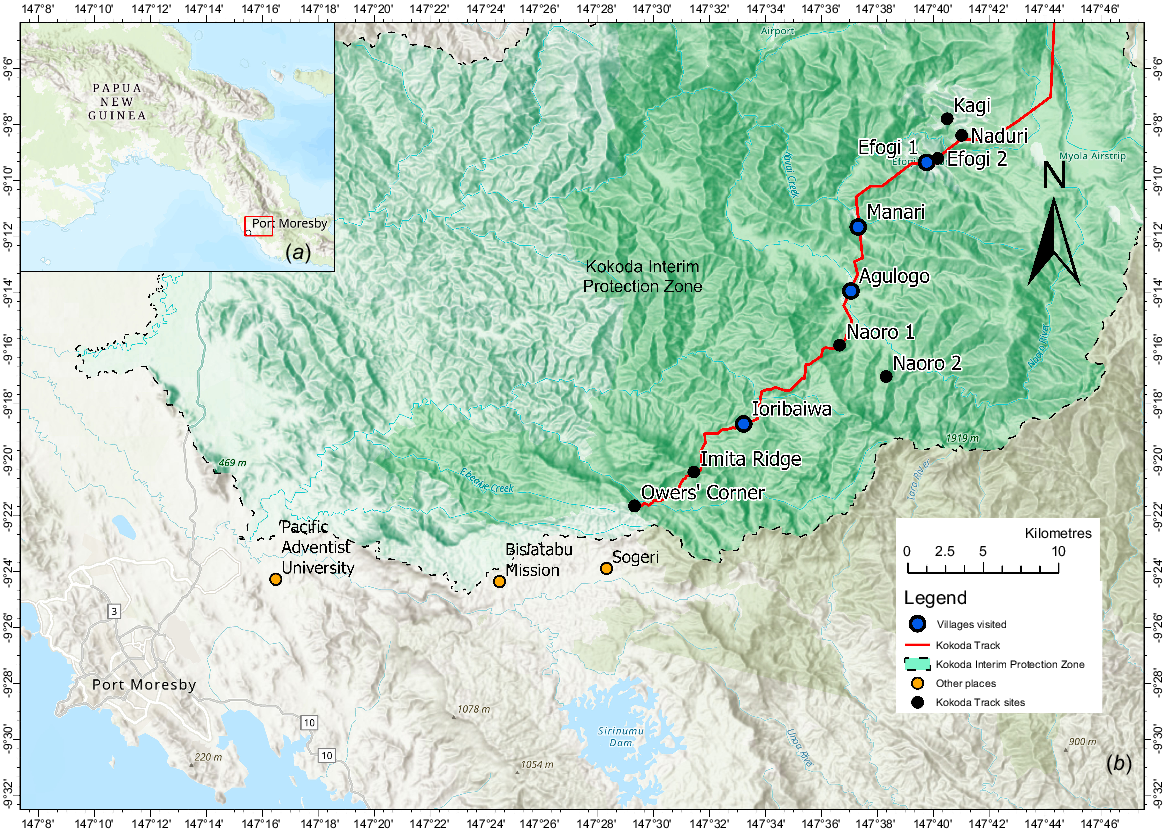
The wet season in Central Province has an average rainfall of 272.4 mm (PNG National Weather Service 2020). The Koiari LLG has an estimated adult population of 15,922 residents (National Statistial Office of Papua New Guinea 2011) of which 954 residents live in the four villages visited. Ioribaiwa has a total population of 65, Agulogo 37, Manari 272, and Efogi 580 residents. The Koiari or Mountain Koiari people includes the southern Central Province coastal people and northward towards Orokaiva and Kokoda territories to what is known as the Oro coastal areas. Their ancestors are known to have originated in the Kumusi-Emo River area of the Owen Stanley Range in Oro Province (Burton et al. 2015). Owers’ Corner to Efogi is approximately 49.75 km by foot, on a track that meanders through a rough terrain of mountainous hills and valleys. Ioribaiwa is situated at an elevation of 650 m, Agulogo 740 m, Manari 850 m, and Efogi 1220 m above sea level (ASL). Although Efogi is situated at an elevation of 1220 m ASL, its range in New Guinea and PNG is from 0 to 1400 m and presumably, it can be accessed at lower elevations.
Survey questionnaires were designed for individuals from a village setting in both English and Tok Pisin. These interviews were carried out to record Koiari Indigenous People’s perceptions, knowledge on Vanate abundance, conservation, hunting, traditional value, conservation, uses, and knowledge transfer systems practiced between adults and children (see Supplementary material). Questionnaires were used to gather information regarding the Vanate and TEK. Responses were translated by the authors into the most relevant scientific taxa and assigned to use categories. A pilot study trialling the questionnaire with local Koiari landowners and people was conducted in May 2019 and November 2019, and a modified questionnaire used in January 2020 and February 2020.
Forty eight semi-structured interviews or questionnaires were used to gauge information from Koiari Indigenous People regarding BOPs with Focus Group Discussions (FGD) at Ioribaiwa and Manari (five participants) using the same questionnaires. The total number of respondents from each village was as follows: four respondents at Ioribaiwa; four from Agulogo; 12 from Manari; and 28 from Efogi. A larger sample size of respondents from the first two villages was not possible as very few people were present at Ioribaiwa and Agulogo. Most of the questionnaires were spoken in Tok Pisin. Respondents that spoke only Koiari and Motu languages conversed through a translator.
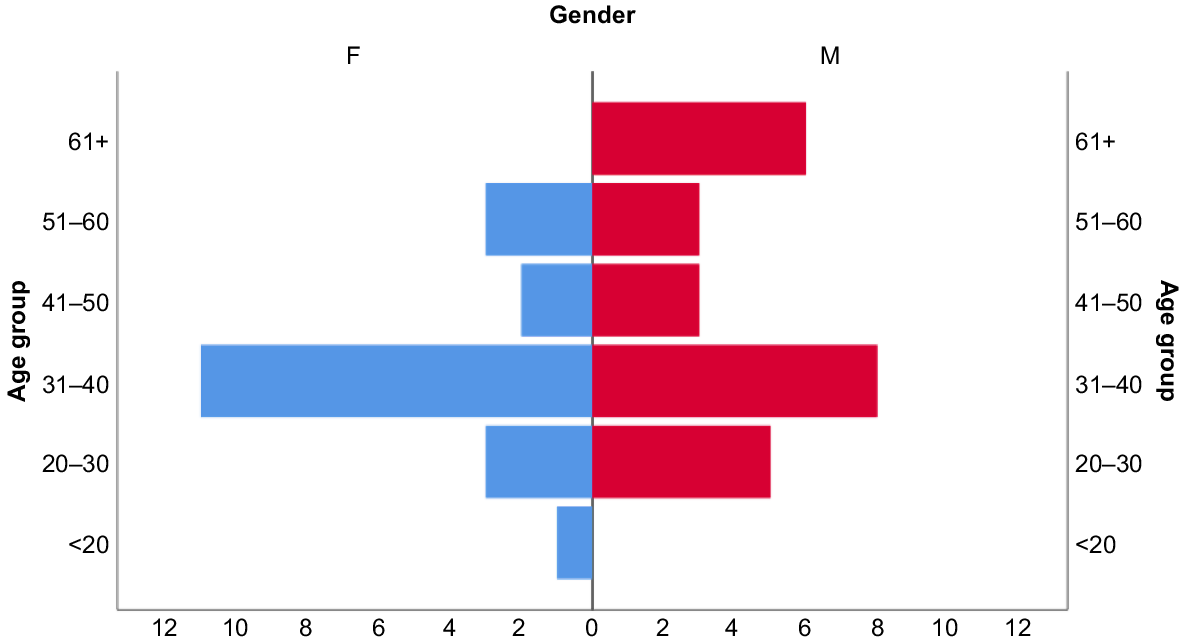
Six questionnaires were conducted with individuals over the age of 61 years, 32 questionnaires with individuals between the ages of 31 and 60 years, and 10 questionnaires with individuals under the age of 30 years (Fig. 3).
Each questionnaire took approximately 45 min per person (or FGD) to complete. A gender ratio of 20 females to 25 males was imposed. Interviewees were selected based on their availability and willingness to participate. BOPs likely to be encountered in the interviews were identified beforehand (by the authors) based on presence and distribution records of BOPs along the Kokoda Track. We used the method of Pollard et al. (2015) and Mittermeier et al. (2018) where interviewees were asked to describe a BOP species. This was followed by confirmation of the species from the ornithology book Birds of New Guinea, second edition (Pollard et al. 2015; Pratt and Beehler 2015; Mittermeier et al. 2018). While questionnaires covered issues other than those reported in this chapter, they included specific questions relating to the TEK of local BOP species, primarily the Vanate. All the language names for birds including the Vanate were recorded in the Koiari language. These local names were gauged as TEK looks at the knowledge of animals, plants, food resources, and climate change through lived experiences (Setalaphruk and Price 2007; Lefale 2010; Hosen et al. 2020). By looking at the local context of culture, society, economy, and bio-physical environment, they help to know the knowledge people gain (Setalaphruk and Price 2007).
We classified forest types in the study sites according to forest types in PNG (Shearman et al. 2008; Shearman and Bryan 2011; Bryan and Shearman 2015) (Table 1). The literature shows the Vanate to occur mostly in lower montane forest, which accounts for 27% of total forest cover in PNG (Bryan and Shearman 2015). The Vanate is known to occur from sea-level to 1600 m in Lowland Tropical and Lower Montane Forests (Beehler and Pratt 2016).
| Forest type | Description | % of total forest cover | |
|---|---|---|---|
| Sub-alpine forest | Occurs on mountain tops above 3200 m to the tree line around 3900 m. Low in species diversity and generally with a dwarf canopy height of 8–12 m. | 2% | |
| Upper-montane forest | Occurs between approximately 2500 m and 3200 m and primarily composed of gymnospermous canopy tree species (e.g. low canopied and structurally simple) | ||
| Mid-montane forest | Occurs ca. 2000–2500 m and is typically permanently wet (per humid), often with broken and uneven canopies. | 27% | |
| Lower montane forest | Lying ca. 1000–2000 m elevation. Can be split into Lower Montane Wet Forest which receives more than 3.5 m of rain and lower Montane Humid forests which receives less than 3.5 m of rain. Variable in structure and canopy height. | ||
| Lowland tropical rainforest | Below 1000 m and receiving >2.5 m of rain per year. Can be split into forests below 500 m that receive more than 3.5 m of rain (Lowland Wet Forest) and those below 1000 m receive between 2.5 and 3.5 m of rainfall (Lowland Humid Forest). Canopy is usually closed and high (35–45 m). | 57% | |
| Monsoon forest or dry evergreen forest | Occurs near sea level in areas <2.5 m of rain per year and prolonged dry season. Relatively open canopy, 20–30 m high. | 2% | |
| Lowland broadleaf swamp forest | Inundated for part or much of the year but it highly variable in structure and species composition. | 10% | |
| Mangrove forest | Typically inundated by salt or brackish water. | 2% |
Data analysis
We used a mixed method approach to analyse the data (Brewer and Hunter 1989; Bernard 1994; Tashakori and Teddlie 1998; Creswell and Clark 2011), using social, environmental, and ecological research designs (Glassford and Barger-Elliot 2011; Creswell and Creswell 2018; Kumar 2019). We applied the convergent parallel mixed methods design (Creswell and Creswell 2018) using questionnaires. Both quantitative and qualitative data were collected with the use of the sound recorder application on a Samsung Galaxy J2 Core mobile phone, model number SM-J260F. This was played back on VLC media player to capture qualitative information relating to TEK of the Vanate. Throughout all the interviews, we managed interviews to avoid leading the interviewee.
Statistical analysis
The non-parametric Mann–Whitney U test was used to test whether distance of villages along the Kokoda Track from urban Port Moresby, influenced the respondents’ responses to the question of habitat (lowland tropical rainforest or lower montane forest) of BOP. A Chi-square test was used to assess the response similarity between male and female respondents to the question of habitat of the Vanate. These were computed using the statistics package IBM SPSS ver. 27 (IBM Corporation 2020). We then tested our results for predicted abundance by modelling them using the AIC and Mallow’s Cp test (Razak et al. 2020). Abundance was selected as the dependent variable of the Vanate and classified into two categories: 1 = common, which was defined by the regular spotting of the Vanate (e.g. seeing the bird during the day in gardens, forests, or trails); and 2 = uncommon, which referred to the infrequent or rare spotting of the Vanate.
Prior to statistical testing, we selected variables. The independent variables tested were: (1) if the Vanate was hunted; (2) who hunted it; (3) habitat; (4) price of a Vanate sold; (5) weapons used either traditional or modern; (6) hunting season either wet or dry; (7) cultural importance of the Vanate; (8) importance of the Vanate to locals; and (9) if locals practiced bird conservation. Regression was used to identify the relationship between Vanate abundance and selected independent variables. We conducted best-fit modelling using R ver. 4.1.1 (R Core Team 2021). With abundance set as the dependent variable, multiple variables were examined simultaneously using linear regression. To select the best regression model, we used Akaike’s Information Criterion (AIC) (Akaike 1987; Bozdogan 1987; Aho et al. 2014; Cavanaugh and Neath 2019). The AIC value is sufficient as it provides quantified information on what model is best and is not dependent on a significant P-value (Halsey 2019).
Results
Nine species of BOP are known to occur in the forests of the Owen Stanley Range along the Kokoda Track. Except for one species, all the BOP are listed as ‘Least Concern’ on the IUCN Red list. The Blue BOP is recorded as ‘Vulnerable’. Respondents were able to identify six species of BOP from interviews.
Local names
Interviewees were asked to identify the local names of ‘bird’ and the Paradisaea raggiana. Interview responses are in ascending order in Table 2.
Habitat
There was no significant difference (Mann–Whitney U, P = 0.131) between respondents from a village closer to Port Moresby (Ioribaiwa), and those further along the track or more remote (Agulogo, Manari, and Efogi) in relation to the habitat of the Vanate. However, the responses of males and females regarding the habitat of the Vanate differed significantly (χ2 = 12.17, P < 0.01). Both sexes nominated lowland wet and lower montane wet forests as ideal habitats for the Vanate. All respondents stated lowland wet forests (16 of 43 respondents; 37%), lowland humid forests (nine of 43 respondents; 21%), and lower montane wet forests (16 of 43 respondents, 37%), compared to lower montane humid forest (two of 43 respondents, 5%) were habitat for the Vanate (Fig. 4). Lowland wet forests were identified by respondents as habitats characterised by certain species of trees, and domains of warm damp climate. The respondents also stated that mareta or pandanus (Pandanus conoideus), pigs (Sus scrofa), muruk or cassowary (Casuarius casuarius), pisin (Gallicolumba sp. and Ptilinopus sp.), kapul or cuscus (Phalanger sp.), Megapode birds (Megapodidae), and flying foxes (Pteropus sp.) are harvested or hunted in this forest type. The Vanate was said to be also found in lowland humid forest where the same animals listed above are hunted in lowland wet forest, with the addition of doves (Columbidae). This is where yam (Dioscorea) gardens are located on the slopes and ridges near Efogi village.
Habitats of the Vanate and the forest corridor of habitat important to the Vanate according to respondents. The trendline shows lowland wet and lowland montane wet forests as the expected habitat of the Vanate.
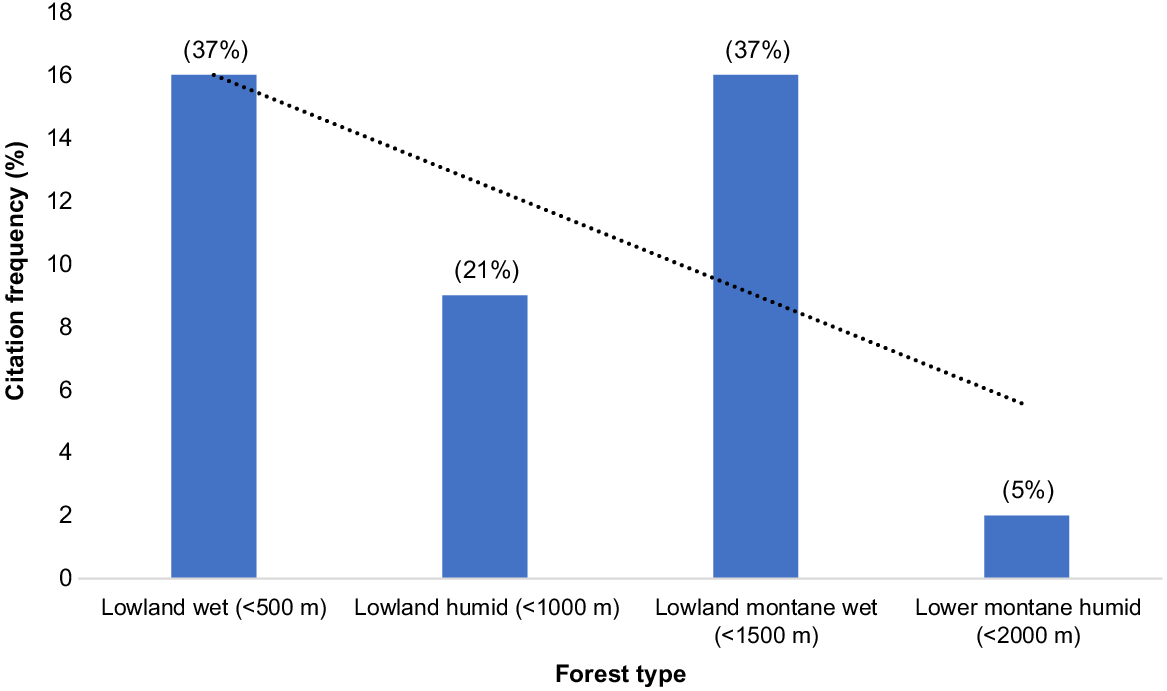
Lower montane wet forests are characterised by large trees and where native pandanus trees grow. These are forests adjacent to villages on ridges, or forests located midway up hills or mountains. Lower montane humid forest were sites where wallaby, kapul or cuscus are hunted. The results showed that the forest corridor between lowland wet forests and lower montane wet forests were the main habitat of the Vanate (Fig. 4). There was no sacred forest area where the Vanate are protected by tradition (tambu or taboo).
Conservation status
Most of the respondents stated that the Vanate are present within their traditional lands (Table 2). A total of 43 respondents (90%) said that the Vanate was common during the fruiting season, which is between June and December. During mornings and evenings, they were heard singing and dancing, and in the dry season they were easier to hunt (Table 3). Four respondents (8%) said that the Vanate is not present and commented that the bird is not always present but was occasionally found singing in the morning and afternoon, and especially in December during the wet season. Only one respondent (2%) was unsure but stated that the Vanate was hunted in the month of February, perhaps referring to an isolated occurrence.
| Response | Citation frequency n (%) | Comments | |
|---|---|---|---|
| No | 4 (8) | Morning and afternoon. December. Wet season. | |
| Unsure | 1 (2) | February. | |
| Yes | 43 (90) | When pandanus (mareta) (Pandanus conoideus) is ripe. Morning and afternoon the bird was heard singing and seen dancing. During the dry season (April–September) it is easier to hunt Vanate. | |
| Total | 48 (100) |
Including respondents’ comments to fruiting season of the pandanus (Pandanus conoideus).
When asked if the Vanate were hunted, 90% of respondents answered ‘yes’, and 8% said ‘no’ (Table 3). Of those that stated ‘yes’, these elaborated by stating that: they opportunistically hunt the bird; the Vanate were killed for their feathers; and the meat is not as palatable compared to other birds (such as the pigeons, Gallicolumba sp.). Several said that the Vanate was hunted more frequently before Christianity was established. The respondents that said ‘no hunting of the Vanate occurred’ also stated that since Christianity was introduced, they stopped killing it. Some considered that it was a ‘special’ bird, hence its protection by national laws; and that hunting of the species only happened in the past (Table 4). Most respondents were Seventh-Day Adventists whose religious beliefs forbade certain foods, including the consumption of the Vanate.
| Response | Citation frequency n (%) | Comments | |
|---|---|---|---|
| No | 22 (46) | Vanate is not seen, but when it is the young people sometimes kill them and are reprimanded by the elders of the village. They were hunted before the missionary’s influence. Traditionally used for ‘singsing’ (dancing). There is new food introduced so the bird is not hunted. People are busy with school and working their gardens today. Vanate is considered a ‘special’ bird. The Conservation Environmental Protection Authority (CEPA) raised awareness of the protected status of the bird. | |
| Unsure | 1 (2) | Unsure. | |
| Yes | 25 (52) | Hunters will opportunistically kill the bird for feathers. Meat is strong and not tasty compared to other birds. Before the Seventh-Day Adventist church arrived, the birds were frequently hunted. Killed for traditional purpose. | |
| Total | 48 (100) |
Abundance
We categorised respondents’ responses to the question of abundance trend, into four variables: (1) increasing; (2) decreasing; (3) no change; and (4) unsure (Table 5). Most of the respondents (44%) stated that the Vanate populations have increased naturally as the bird is conserved by it not being killed and by law enforcement by villagers. Fourteen respondents (29%) were unsure, stating that they were also unsure about reasons for any change in abundance of the Vanate (Fig. 5). Ten respondents (21%) said there was no change due to variation of weather because they do not hunt the bird to sell, and that because the bird is not hunted, and that it remains undisturbed. Some noted no change but were unsure why. Three (6%) respondents said the bird was decreasing in number.
| Response | Citation frequency n (%) | Comment | |
|---|---|---|---|
| Increased | 21 (44) | Populations have increased due to natural increase, no disturbance, less hunting, no weapons or guns used, no shooting or killing of this bird. Due to law enforcement by villagers. | |
| Decreased | 3 (6) | Migration of Vanate to lower forests. | |
| No change | 10 (21) | Birds’ adaptability to climate change, they don’t hunt them to sell, no killing, mostly undisturbed, and some unsure. | |
| Unsure | 14 (29) | Those unsure about change in abundance were also unsure of reasons for change. |
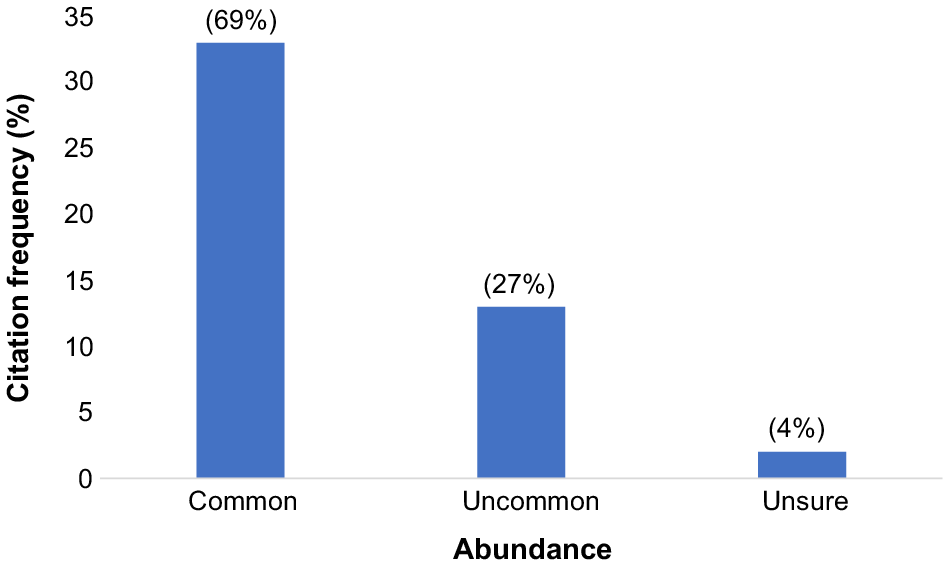
Our interviews relating to abundance showed that 33 of 48 respondents (69%) from across all villages stated that the Vanate was common in forests, 13 (27%) said it was uncommon, and two (4%) respondents said they were unsure of the Vanate’s abundance (Fig. 5). Of those respondents who said it was common; 16 of 33 (49%) were from Efogi, nine (27%) were from Manari, four (12%) were from Agulogo, and the remaining four of 33 (12%) were from Ioribaiwa. The 10 of 13 (77%) who said the Vanate was uncommon were from Efogi with the remaining (three of 13) (23%) from Manari. Only two (4%) respondents from Efogi said they were unsure (two of two).
Most of the respondents said the Vanate was common. Of these, four of 30 (13%) were <30 years of age, 14 (47%) were aged 31–40 years, five (17%) were aged 41–50 years, and seven (23%) were 51+ years of age. Interestingly, most respondents (67%) who said that the Vanate was common were male compared to female respondents (33%). While for those respondents who claimed that the Vanate was uncommon seven of 13 (54%) were female, compared to six of 13 (46%) males. More men than women hunted birds. Although this does present marked bias because Koiari men are primarily the bird hunters, this does not mean that by including Koiari women it would affect the results drastically.
The best-fit model showed that abundance was regulated by Koiari Indigenous People who (locals, non-locals, others) hunted the Vanate and what weapons were used (Table 6). When hunted only by locals, the model showed an increase in the abundance of the Vanate. Local hunting was irregular and opportunistic as 43 (90%) respondents said ‘locals’ hunted the Vanate, four (8%) respondents said ‘others’ hunted, and only one (2%) respondent said both ‘locals and others’ hunted the Vanate.
| Model number | Model components | K | R 2 (%) | Adj. R 2 (%) | Mallow’s Cp | AIC | Delta score (ΔAIC) | F-value | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abundance = −15.37 + 16.81 Who hunts – 0.24 Weapons | 2 | 31 | 87 | −0.30 | 56.4 | 10.1 | 0.01 | |
| 2 | Abundance = −15.691 + 16.84 Who hunts + 0.078 Weapons – 0.16 Vanate Importance + 0.16 Bird Conservation | 4 | 31 | 87 | 0.88 | 62.2 | 4.33 | 0.01 |
Variables in the models were: who hunted (locals or outsiders); weapons used (traditional or modern); Vanate importance (important or not important); and bird conservation (if locals practiced bird conservation or not).
P-values were not included as there were no significant differences.
The criteria for weapons were traditional (stones, slingshots (catapel or sangai), bow and arrows, traditional traps, use of breadfruit sap, or a net) or modern (guns). When only traditional weapons were used, the model showed a decrease in abundance of the Vanate. Half of respondents (n = 25, 52%) preferred traditional weapons over modern weapons, 21 (44%) respondents said ‘both traditional and modern weapons’ are used to hunt, whereas only two (4%) respondents said only ‘modern weapons’ are used to hunt the Vanate.
Discussion
Limitations
A limitation of this study was that hunters would interact more with the birds and therefore observe more birds and as a result have higher TEK. However, it was assumed that the information the Koiari Indigenous People (both hunters and outsiders) gave us was true and valid. Highlighting the importance of indigenous-led approaches and how collaboration between Indigenous and Western knowledge systems is crucial and needed for the Vanate’s abundance and habitat with conservation efforts by Koiari Indigenous People. This is similar to research on culturally significant species co-designed and co-managed by Aboriginal People from Australia (Campbell et al. 2024).
Local names
The Koiari Indigenous People make up 5.9% of people in Central Province, PNG (Papua New Guinea National Statistical Office 2011). Most of them speak the national lingua franca, Tok Pisin, and Motu, and the Koiari language known as Gado. When locals were asked the name of the Vanate in Gado, they responded by giving seven different names for the male Vanate, demonstrating the linguistic diversity that exists in the Koiari area and along the Kokoda Track, with the southern region where the Koiari Indigenous People inhabit known as one of the most linguistically diverse in PNG (Evans et al. 2017). The Kokoda Track runs through both Central and Oro Provinces, with Central Province having 31 languages and Oro Province having 29 languages (SIL International 2015). A total of 60 distinct languages through both provinces combined with some that are shared.
Interestingly, a previous study found that elderly women from Koiari recall their ancestry better than the elderly men. Men are assumed to be custodians of traditional knowledge because culturally the Koiari are patrilineal. Other reports reported the difficulty of getting women on the Kokoda Track to speak (IWDA 2014). This could relate to TEK being gender biased towards men, which seems to be the case in most of Melanesia (McCarter and Gavin 2014; Aswani et al. 2018). However, all the women that we interviewed were willing and responsive, unless interviewed in FGD’s where the men or chiefs generally spoke.
Habitat
Both male and female respondents reported that the forest corridor from lowland wet and lowland montane wet forest is critical habitat of the Vanate. The Vanate is found in the Koiari area in lowland forests near Ioribaiwa to Manari. This was voiced by local respondents and evidenced when we heard the Vanate call or krai in the forests along the Kokoda Track. Previous sightings of the Vanate were reported in the Maguli Range in hill forests (Gillard 1951). This is consistent with Beehler and Pratt (2016) who state that the Vanate is found in the lowland and hill forests, or the elevational range between 500 and 1000 m (Beehler and Pruett-Jones 1983; Pratt and Beehler 2015).
The respondents also reported that the Vanate occupied habitats where there was high canopy, and forests characterised by large trees. Beehler and Pruett-Jones (1983) reported that the Vanate congregate in lek during the mating season (Beehler 1989; Kirkpatrick and Ryan 1991). In a cluster of exposed limbs and vines within the canopy of a sole tree in the lek, Vanate males assemble, sing, display, and mate with females. The display territories of the Vanate include the limbs on which they frequently perch (Beehler and Pruett-Jones 1983). Whilst displaying on open limbs, BOP are vulnerable, and it is here that individuals are shot for their feathers (Van Den Bergh et al. 2013; Supuma 2018). In PNG, hunting is an integral part of customary practice and survival for Indigenous People (Dwyer 1982; Japan International Cooperation Agency 2013).
Conservation status
Our study shows that the Vanate is opportunistically hunted in Koiari forests. Recent studies assert that hunting is the main driver of species decline in tropical forests compared to habitat loss (Harrison et al. 2016; Benítez-López et al. 2017). Together with habitat loss, climate change, and landscape modification (Van Den Bergh et al. 2013; Tracewski et al. 2016; BirdLife International 2018; Supuma 2018), hunting is the main reason for the declining Vanate population.
The Vanate is currently (since 2018) listed as of Least Concern due to its very large range (BirdLife International 2018). While the usual response is that rural communities such as the Koiari should refrain from opportunistically hunting the Vanate, Indigenous People or locals in PNG may not traditionally know or practice conservation according to the western paradigm. Hunting is seen as a necessity for resource-dependent communities (Kwapena 1984). Within this paradigm, thorough knowledge of hunting grounds, prey, hunting styles, and traditional stories are passed on (Kwapena 1984; Thomas 1999). In an oral society, through these hunting experiences the transfer of TEK is made by Indigenous People showing how they survive in forests (Reo and Whyte 2012; Lam et al. 2020). In West Papua for instance, selecting BOP species to hunt may vary depending on cultural reasons and religious symbolism of birds passed down through generations (Pattiselanno et al. 2016). Though the Koiari said they did not have sacred forests where the BOPs live, they are assumed to hunt sustainably because hunters rely on traditional hunting weapons such as a slingshot or sangai and rarely used guns. Hence, the Koiari people state that Vanate numbers have increased.
Most Koiari Indigenous People are Seventh-Day Adventists. Their tenets of faith mean that they do not partake of certain fauna due to biblical prohibitions on food and diet (Jacka 2010; Hayes and Hayes 2016). This helps reduce their ecological footprint (Fraser 2003; General Conference of Seventh-day Adventists 2015), and may aid in conservation of the Vanate. This may seem advantageous, but the Koiari Indigenous People (and many other Indigenous People in PNG) still hunt and kill the Vanate.
Abundance
The Koiari Indigenous People indicated that the Vanate is increasing in abundance. In comparison, the IUCN reports that the population of the Vanate is trending downwards due to habitat loss (Tracewski et al. 2016; BirdLife International 2018). Additionally, the polygynous behaviour of the Vanate, where males mate with as many females as possible during the breeding season, may support its population increase. This increases the survival of this species in the wild (Beehler 1989).
In relation to abundance of the Vanate, most respondents said that they were common in their forests. However, more males (n, 28) reported the Vanate as common than females (n, 20); indeed, the hunters in the Koiari villages are all male. This is consistent with the social responsibilities in traditional PNG society where males are the hunters (Gibbs 2016), and females spend more time in the gardens (Koian 2010). TEK for the Koiari is helpful in complementing Scientific Ecological Knowledge (SEK) within socio-ecological systems (Reo and Whyte 2012). It also varies by gender, social position, and age, and is usually shared through oral transmission (Houde 2007). Similar features are common in other tribal or indigenous communities in Papua (Indonesian New Guinea) and among Native Americans that practice subsistence hunting (Reo and Whyte 2012; Sheil et al. 2015).
It is uncertain what the genetic consequences are on the hunting of the Vanate. However, the impacts of hunting on vertebrates include the altering of gene flow, loss of genetic variation (Allendorf et al. 2008), and reducing fitness by intentionally culling individuals with certain traits (e.g. colourful bright feathers) (Harris et al. 2002). To support productiveness of harvested populations, it is imperative to include genetic considerations in a species conservation and management (Allendorf et al. 2008).
The AIC model shows that there is an abundance of the Vanate when locals hunted using mainly traditional weapons (Table 6). This is consistent with research showing that hunters from Indonesian New Guinea who were reliant on locally-made or traditional hunting weapons collected limited prey using traditional weapons (Pattiselanno et al. 2016). By using traditional weapons and traditional hunting practices, this can limit the number of Vanate killed, with consideration that Vanate are located nearby and in abundance (Petriello and Stronza 2020; Ingram et al. 2021). In contrast, non-locals and outsiders using modern weapons increases more indiscriminate hunting and decreases bird abundance. No P-values were used in our results as none were significant. Recent findings contend that the AIC value can replace the P-value as it provides quantified information on what model is best (Halsey 2019). According to the IUCN, the Vanate is listed as of Least Concern but with a decreasing population trend (BirdLife International 2018).
Conclusion
Our findings show that local names of the Vanate vary, its habitat is within lowland forested areas, and the Koiari Indigenous People do not promote the conservation of wildlife. Our results show that there is relevant TEK concerning the Vanate within Koiari communities along the Kokoda Track. Using the AIC value, abundance of the Vanate in Koiari forests would increase if locals used traditional weapons and traditional hunting methods.
Recommendations
By protecting the Koiari forests, we can preserve TEK that exists and with it, the historical, cultural, artistic, religious values, practices, and innovations. This should help Indigenous People in PNG embrace their culture by capturing, recording, and storing proper TEK of the Koiari people and other Indigenous People in PNG; thus, encouraging locals to share their TEK or knowledge of the environment with one another, especially the elderly. To ensure an increase in Vanate abundance, we suggest that Koiari Indigenous People learn their traditional hunting methods using traditional weapons, as well as encouraging Koiari children to learn and speak their local language or vernacular to better facilitate the transfer of TEK between generations.
TEK is being lost at a rapid pace, and introducing TEK content into primary and high school curriculums on the conservation and protection of the Vanate is critical.
By supporting initiatives from the Conservation Environmental Protection Authority (CEPA), and the Kokoda Track Authority (KTA), we are supporting the education of Indigenous People. Ideally, we would do well to include Indigenous People in natural resource management decisions. By enacting policies that recognise the rights and TEK of Indigenous People in PNG in relation to the conservation of the Vanate and its environment, we can prevent the loss of this species.
Data availability
The data supporting this study on TEK of the Vanate is managed in accordance with the ethical guidelines of Pacific Adventist University (PAU) and the PAU Research Ethics Committee. Access to the data is governed by protocols that respect the cultural, intellectual, and moral rights of the Koiari Indigenous People. Due to the sensitive nature of TEK, access is restricted and subject to community consent and approval. Researchers seeking access should contact the corresponding author and demonstrate compliance with the PAU Research Ethics Code and any agreements established with the Koiari Indigenous People, their communities and the author. Where appropriate and permissible, de-identified or aggregated data may be shared under these conditions.
Declaration of funding
This research was funded in part by Pacific Adventist University research funds with ethical approval EC2619. Also funding from Ela Medical Centre (EMC) grant 137 for research fieldwork and flights.
Acknowledgements
We thank the Koiari Indigenous People from Ioribaiwa, Agulogo, Manari, and Efogi villages along the Kokoda Track who consented and participated in this research. We especially thank the following and their families who helped immensely along the Kokoda Track: Hollen Mado; Landy (KTA ranger); Wopa; and field research assistants; Vuiaboto Moide (General Practitioner (GP); and Ronald Haihavu. We also thank Deborah Kakis for statistical help in ‘R’ software, Anne Pulotu for translation of questionnaires from English to Tok Pisin, and Professor David Blair for helpful edits.
References
Aho K, Derryberry DW, Peterson T (2014) Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95(3), 631-636.
| Crossref | Google Scholar | PubMed |
Akaike H (1987) Factor analysis and AIC. Psychometrika 52(3), 317-332.
| Crossref | Google Scholar |
Allendorf FW, England PR, Luikart G, Ritchie PA, Ryman N (2008) Genetic effects of harvest on wild animal populations. Trends in Ecology & Evolution 23(6), 327-337.
| Crossref | Google Scholar | PubMed |
Andaya LY (2017) Flights of fancy: the bird of paradise and its cultural impact. Journal of Southeast Asian Studies 48(3), 372-389.
| Crossref | Google Scholar |
Aswani S, Lemahieu A, Sauer WH (2018) Global trends of local ecological knowledge and future implications. PLoS ONE 13(4), e0195440.
| Crossref | Google Scholar | PubMed |
Baker LR, Olubode OS, Tanimola AA, Garshelis DL (2014) Role of local culture, religion, and human attitudes in the conservation of sacred populations of a threatened ‘pest’ species. Biodiversity and Conservation 23, 1895-1909.
| Crossref | Google Scholar |
Beehler B (1989) The birds of paradise. Scientific American Magazine 261(6), 116-123.
| Crossref | Google Scholar |
Beehler B, Pruett-Jones SG (1983) Display dispersion and diet of birds of paradise: a comparison of nine species. Behavioral Ecology and Sociobiology 13, 229-238.
| Crossref | Google Scholar |
Benítez-López A, Alkemade R, Schipper AM, Ingram DJ, Verweij PA, Eikelboom JAJ, Huijbregts MAJ (2017) The impact of hunting on tropical mammal and bird populations. Science 356(6334), 180-183.
| Crossref | Google Scholar | PubMed |
BirdLife International (2018) Species factsheet: Raggiana Bird-of-paradise Paradisaea raggiana. pp. 1–7. Available at https://datazone.birdlife.org/species/factsheet/raggiana-bird-of-paradise-paradisaea-raggiana
Bozdogan H (1987) Model selection and akaike’s information criterion (AIC): the general theory and its analytical extensions. Psychometrika 52(3), 345-370.
| Crossref | Google Scholar |
Burton J (2018) Life expectancy of Kokoda Track Authority communities in Central Province, Papua New Guinea. Contemporary PNG Studies 29, 34-50 https://search.informit.org/doi/10.3316/informit.636004912772727.
| Google Scholar |
Campbell B, Russell S, Brennan G, Condon B, Gumana Y, Morphy F, Ens E (2024) Prioritising animals for Yirralka Ranger management and research collaborations in the Laynhapuy Indigenous Protected Area, northern Australia. Wildlife Research 51, WR24071.
| Crossref | Google Scholar |
Cavanaugh JE, Neath AA (2019) The Akaike information criterion: background, derivation, properties, application, interpretation, and refinements. WIREs Computational Statistics 11(3), e1460.
| Crossref | Google Scholar |
CITES (n.d.) CITES-database. Available at cites.org [accessed November 2020]
Dwyer PD (1974) The price of protein: five hundred hours of hunting in the new guinea highlands. Oceania 44(4), 278-293.
| Crossref | Google Scholar |
Dwyer PD (1982) Wildlife conservation and tradition in the highlands of Papua New Guinea. Traditional Conservation in Papua New Guinea: Implications for Today. Proc. Conference 1980 173-189 IASER, Papua New Guinea, Monograph 16.
| Google Scholar |
Evans N, Arka W, Carroll M, Choi YJ, Döhler C, Gast V, Kashima E, Mittag E, Olsson B, Quinn K, Schokkin D, Tama P, van Tongeren C, Siegel J (2017) The languages of southern New Guinea. In ‘The languages and linguistics of the New Guinea area: a comprehensive guide’. (Ed. B Palmer) pp. 641–744. (De Gruyter Mouton)
Fernández-Llamazares Á, Cabeza M (2018) Rediscovering the potential of indigenous storytelling for conservation practice. Conservation Letters 11(3), e12398.
| Crossref | Google Scholar |
Garnett ST, Burgess ND, Fa JE, Fernández-Llamazares Á, Molnár Z, Robinson CJ, Watson JEM, Zander KK, Austin B, Brondizio ES, Collier NF, Duncan T, Ellis E, Geyle H, Jackson MV, Jonas H, Malmer P, McGowan B, Sivongxay A, Leiper I (2018) A spatial overview of the global importance of indigenous lands for conservation. Nature Sustainability 1, 369-374.
| Crossref | Google Scholar |
General Conference of Seventh-day Adventists (2015) 28 Fundamental Beliefs. Available at www.adventist.org
Gibbs P (2016) Men’s matters: changing masculine identities in Papua New Guinea. In ‘Gender violence & human rights: seeking justice in Fiji, Papua New Guinea and Vanuatu. Vol. 1.’, 1st edn. (Ed. ABMJAMM) pp. 127–158. (ANU Press) 10.22459/GVHR.12.2016.03
Glassford D, Barger-Elliot L (2011) Toward intergenerational ministry in a Post-Christian Era. Christian Education Journal: Research on Educational Ministry 8(2), 364-378.
| Crossref | Google Scholar |
Gómez-Baggethun E, Minigorría S, Reyes-García V, Calvet L, Montes C (2010) Traditional ecological knowledge trends in the transition to a market economy: empirical study in the doñana natural areas. Conservation Biology: The Journal of the Society for Conservation Biology 24(3), 721-729.
| Crossref | Google Scholar | PubMed |
Gómez-Baggethun E, Corbera E, Reyes-García V (2013) Traditional ecological knowledge and global environmental change: research findings and policy implications. Ecology and Society 18(4), 72.
| Crossref | Google Scholar |
Halsey LG (2019) The reign of the p-value is over: what alternative analyses could we employ to fill the power vacuum? Biology Letters 15, 20190174.
| Crossref | Google Scholar |
Harris RB, Wall WA, Allendorf FW (2002) Genetic consequences of hunting: what do we know and what should we do? Wildlife Society Bulletin 30(2), 634-643.
| Google Scholar |
Harrison RD, Sreekar R, Brodie JF, Brook S, Luskin M, O’Kelly H, Rao M, Scheffers B, Velho N (2016) Impacts of hunting on tropical forests in Southeast Asia. Conservation Biology 30(5), 972-981.
| Crossref | Google Scholar | PubMed |
Healey C (1993) Folk taxonomy and mythology of birds of paradise in the new guinea highlands. Ethnology 32(1), 19-34.
| Crossref | Google Scholar |
Hosen N, Nakamura H, Hamzah A (2020) Adaptation to climate change: does traditional ecological knowledge hold the key? Sustainability 12(2), 676.
| Crossref | Google Scholar |
Houde N (2007) The six faces of traditional ecological knowledge: challenges and opportunities for Canadian co-management arrangements. Ecology and Society 12(2), 34 Available at http://www.ecologyandsociety.org/vol12/iss2/art34/.
| Google Scholar |
Hunn E (1993) Traditional ecological knowledge: wisdom for sustainable development. In ‘Traditional ecological knowledge: wisdom for sustainable development’. (Eds NM Williams, G Baines) pp. 13–30. (Centre for Resource and Environmental Studies, Australian National University (ANU)) 10.1017/9781108552998
Ingram DJ, Coad L, Milner-Gulland EJ, Parry L, Wilkie D, Bakarr MI, Benítez-López A, Bennett EL, Bodmer R, Cowlishaw G, El Bizri HR, Eves HE, Fa JE, Golden CD, Iponga DM, Minh NV, Morcatty TQ, Mwinyihali R, Nasi R, Nijman V, Ntiamoa-Baidu Y, Pattiselanno F, Peres CA, Rao M, Robinson JG, Rowcliffe JM, Stafford C, Supuma M, Tarla FN, van Vliet N, Wieland M, Abernethy K (2021) Wild meat is still on the menu: progress in wild meat research, policy, and practice from 2002 to 2020. Annual Review of Environment and Resources 46, 221-254.
| Crossref | Google Scholar |
Jacka JK (2010) The spirits of conservation: ecology, christianity, and resource management in highlands Papua New Guinea. Journal for the Study of Religion, Nature and Culture 4(1), 24-47.
| Crossref | Google Scholar |
Kirkpatrick M, Ryan MJ (1991) The evolution of mating preferences and the paradox of the Lek. Nature 350, 33-38.
| Crossref | Google Scholar |
Kirsch S (2013) History and the birds of paradise: surprising connections from New Guinea. Expeditions 48(1), 15-21.
| Google Scholar |
Kwapena N (1984) Traditional conservation and utilization of wildlife in Papua New Guinea. Environmentalist 4, 22-26.
| Crossref | Google Scholar |
Lam DPM, Hinz E, Lang DJ, Tengö M, von Wehrden H, Martín-López B (2020) Indigenous and local knowledge in sustainability transformations research: a literature review. Ecology and Society 25(1), 3.
| Crossref | Google Scholar |
Lefale PF (2010) Ua ‘afa le Aso Stormy weather today: traditional ecological knowledge of weather and climate. The Samoa experience. Climatic Change 100, 317-335.
| Crossref | Google Scholar |
Martin JF, Roy ED, Diemont SAW, Ferguson BG (2010) Traditional ecological knowledge (TEK): ideas, inspiration, and designs for ecological engineering. Ecological Engineering 36(7), 839-849.
| Crossref | Google Scholar |
McCarter J, Gavin MC (2014) Local perceptions of changes in traditional ecological knowledge: a case study from Malekula Island, Vanuatu. Ambio 43, 288-296.
| Crossref | Google Scholar | PubMed |
McDonnell S, Allen MG, Filer C (2017) Kastom, property and ideology: land transformations in Melanesia. The Journal of Pacific History 52(3), 425-426.
| Crossref | Google Scholar |
Michalos A (2014) Traditional ecological knowledge. In ‘Encyclopedia of quality of life and well-being research’. p. 6705. (Springer, Dordrecht). 10.1007/978-94-007-0753-5_104248
Mittermeier JC, Dutson G, James RE, Davies TE, Tako R, Uy JAC (2018) The avifauna of Makira (San Cristobal), Solomon Islands. The Wilson Journal of Ornithology 130(1), 235-255.
| Crossref | Google Scholar |
Moller H, Berkes F, Lyver PO, Kislalioglu M (2004) Combining science and traditional ecological knowledge: monitoring populations for co-management. Ecology and Society 9(3), 2.
| Crossref | Google Scholar |
National Statistial Office of Papua New Guinea (2011) 2011 National population & housing census ward population profile. National Statistical Office, Port Moresby, PNG. Available at http://www.nso.gov.pg/
Papua New Guinea National Statistical Office (2011) Final figures: national population and housing census of Papua New Guinea 2011. Available at www.nso.gov.pg
Pattiselanno F, Koibur JF, Yohanes CH (2016) Traditional ecological knowledge (Tek) in hunting: from culture to nature. KnE Social Sciences 1(1), 66-70.
| Crossref | Google Scholar |
Petriello MA, Stronza AL (2020) Campesino hunting and conservation in Latin America. Conservation Biology 34(2), 338-353.
| Crossref | Google Scholar | PubMed |
PNG National Weather Service (2020) PNG government national weather service. Available at http://www.pngmet.gov.pg/Climate_Division/
Pollard EJM, Thaman R, Brodie G, Morrison C (2015) Threatened biodiversity and traditonal ecological knowledge: associated beliefs, customs, and uses of herpetofauna among the ’Are’Are on Malaita Island, Solomon Islands. Ethnobiology Letters 6, 99-110.
| Crossref | Google Scholar |
Pretty J, Adams B, Berkes F, De Athayde SF, Dudley N, Hunn E, Maffi L, Milton K, Rapport D, Robbins P, Sterling E, Stolton S, Tsing A, Vintinnerk E, Pilgrim S (2009) The intersections of biological diversity and cultural diversity: towards integration. Conservation and Society 7, 100-112.
| Crossref | Google Scholar |
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Available at https://www.r-project.org/
Razak SA, Saadun N, Azhar B, Lindenmayer DB (2020) Smallholdings with high oil palm yield also support high bird species richness and diverse feeding guilds. Environmental Research Letters 15(9), 094031.
| Crossref | Google Scholar |
Reo NJ, Whyte KP (2012) Hunting and morality as elements of traditional ecological knowledge. Human Ecology 40, 15-27.
| Crossref | Google Scholar |
Setalaphruk C, Price LL (2007) Children’s traditional ecological knowledge of wild food resources: a case study in a rural village in Northeast Thailand. Journal of Ethnobiology and Ethnomedicine 3, 33.
| Crossref | Google Scholar |
Shearman P, Bryan J (2011) A bioregional analysis of the distribution of rainforest cover, deforestation and degradation in Papua New Guinea. Austral Ecology 36(1), 9-24.
| Crossref | Google Scholar |
Shearman P, Bryan J, Ash J, Hunnam P, Mackey B, Lokes B (2008) The state of the forests of Papua New Guinea. Mapping the extent and condition of forest cover and measuring the drivers of forest change in the period 1972-2002. Available at http://www.scienceinpublic.com.au/blog/wp-content/uploads/State-of-Forests-of-PNG_Concise.pdf
Sheil D, Boissière M, Beaudoin G (2015) Unseen sentinels: local monitoring and control in conservation’s blind spots. Ecology and Society 20(2), 39.
| Crossref | Google Scholar |
SIL International (2015) Language distribution maps. SIL International. Available at https://pnglanguages.sil.org/resources/language_map
Tang R, Gavin MC (2016) A classification of threats to traditional ecological knowledge and conservation responses. Conservation and Society 14(1), 57-70.
| Crossref | Google Scholar |
Tracewski Ł, Butchart SHM, Di Marco M, Ficetola GF, Rondinini C, Symes A, Wheatley H, Beresford AE, Buchanan GM (2016) Toward quantification of the impact of 21st-century deforestation on the extinction risk of terrestrial vertebrates. Conservation Biology 30(5), 1070-1079.
| Crossref | Google Scholar | PubMed |
Van Den Bergh MOL, Kusters K, Dietz AJT (2013) Destructive attraction: factors that influence hunting pressure on the Blue Bird-of-paradise Paradisaea rudolphi. Bird Conservation International 23(2), 221-231.
| Crossref | Google Scholar |
Whyte KP, Brewer JP, Johnson JT (2016) Weaving indigenous science, protocols and sustainability science. Sustainability Science 11, 25-32.
| Crossref | Google Scholar |