Using the prey captured by breeding Crested Terns to assess the availability of forage fish for other coastal meso-predators
J. Nic Dunlop A , Erin Clitheroe A B * and Donna Chapman A
A B * and Donna Chapman A
A
B
Abstract
The diets of seabirds are an effective indicator of changes in forage fish abundance and availability providing insight into how changing fish stocks impact the meso-predators that consume them. Non-invasive methods for monitoring seabird diets are a valuable tool in conservation.
We aimed to assess the availability of forage fish that were carried by Crested Terns for the threatened Little Penguins (Eudyptula minor) and other meso-predators on Penguin Island, Western Australia.
We used digital photography with 400–500 mm telephoto lenses to identify prey carried to Crested Tern (Thalasseus bergii) colonies on Penguin Island during the 2021, 2022 and 2023.
Crested Terns breeding on Penguin Island captured a wider range of prey (62 species) than recorded in other diet studies at other colonies in southern Australia and South Africa. Blue Sprat (Spratelloides robustus) and Sandy Sprat (Hyperlophus vittatus) dominated the forage fish taken by the terns in 2021 and 2022 breeding seasons with Sardines (Sardinops vagax) and Anchovies (Engraulis australis) becoming more common in 2023.
A recruitment event was recorded in Sandy Sprats in 2021 after a near record winter rainfall in the region. This recruitment event was significant as Sandy Sprats, a critical resource for Little Penguins breeding on Penguin Island, were thought to have been unavailable in local waters since a marine heatwave event in 2011.
Early indications were consistent with Crested Tern diet influencing Penguin breeding performance; however, this can only be confirmed with a longer time series. Ongoing monitoring of forage fish using bill-loading Crested Terns may have an important role in the future management of the Little Penguin colony on Penguin Island.
Keywords: citizen science, Crested Tern diet, digital telephoto photography, forage fish availability, Little Penguin conservation, monitoring, non-invasive sampling, Sandy Sprat.
Introduction
The diets of seabirds have been used successfully as indicators of fish populations (Velarde et al. 1994; McLeay et al. 2009a) including the cohort strength of forage fishes (e.g. Sardines, Sardinops vagax) prior to recruitment into a fishery (Velarde et al. 2013). Seabirds are also indicators of the impact of changing forage fish stocks on meso-predators, such as Crested Terns (Thalasseus bergii), that consume them (McLeay et al. 2009b).
Seabird diets can be characterised using a variety of methods including the analysis of gut contents, spontaneous regurgitates, emetic or water off-loaded regurgitates, hard-parts in regurgitated pellets or faeces and the DNA analysis of any of these sources. All these methods involve entering and disturbing breeding colonies, and some involve the relatively invasive capture and handling of the birds. Observing the marine animals carried to colonies, roosts, or clubs by bill-loading seabird species provides an alternative method of assessing prey selection.
Observational methods have been advanced by the emergence of high resolution, telephoto digital photography (Edney and Wood 2021). Images can be examined in forensic detail, stored, verified by taxonomic experts and catalogued into voucher collections to underpin ongoing monitoring. Digital photography also facilitates participation by the citizen scientist as it usually does not require all the licences and permits required for using the more invasive methods (Forys and Hevesh 2017; Owen et al. 2024) and generally reserved for ‘professionals’.
Many methods used in seabird dietary analysis have biases and limitations (Barrett et al. 2007; Owen et al. 2024). Stomach contents and pellets can overestimate the prey-species that leave undigested hard-parts. Regurgitates (and off-loaded stomach contents) tend to contain the larger, most recently consumed prey items. The number of individual prey items can generally not be obtained from DNA analysis and the method depends on a comprehensive library of prey-genomes, DNA analysis is currently too expensive to be used as an ongoing monitoring method. With the exception of regurgitated pellets, dietary sampling is generally restricted to colony areas during the breeding season. Bill-loading generally occurs when adults are feeding young or mates during the breeding season. The adults may select and consume different prey that will not be detected using digital photography.
The ability to non-invasively assess prey selection and availability offers a means to investigate the impacts of changes in prey availability on small and declining seabird populations where more invasive methods are difficult or undesirable. The Little Penguin (Eudyptula minor) colony on Penguin Island, Western Australia (Fig. 1) has seen a 94% reduction in population size since 2008 and is now less than 75 pairs (Cannell 2024). This decline has been partly attributed to a severe marine heatwave in 2011 and the apparent disappearance of a key forage fish, Sandy Sprat (Hyperlophus vittatus), from the colony’s prey-field south of the Island at that time and in the subsequent year (Cannell et al. 2024). In 2011 and 2012 Tropical Sardines (Sardinella lemuru) and Sardines (Sardinops vagas) were the main prey species (Cannell et al. 2024).
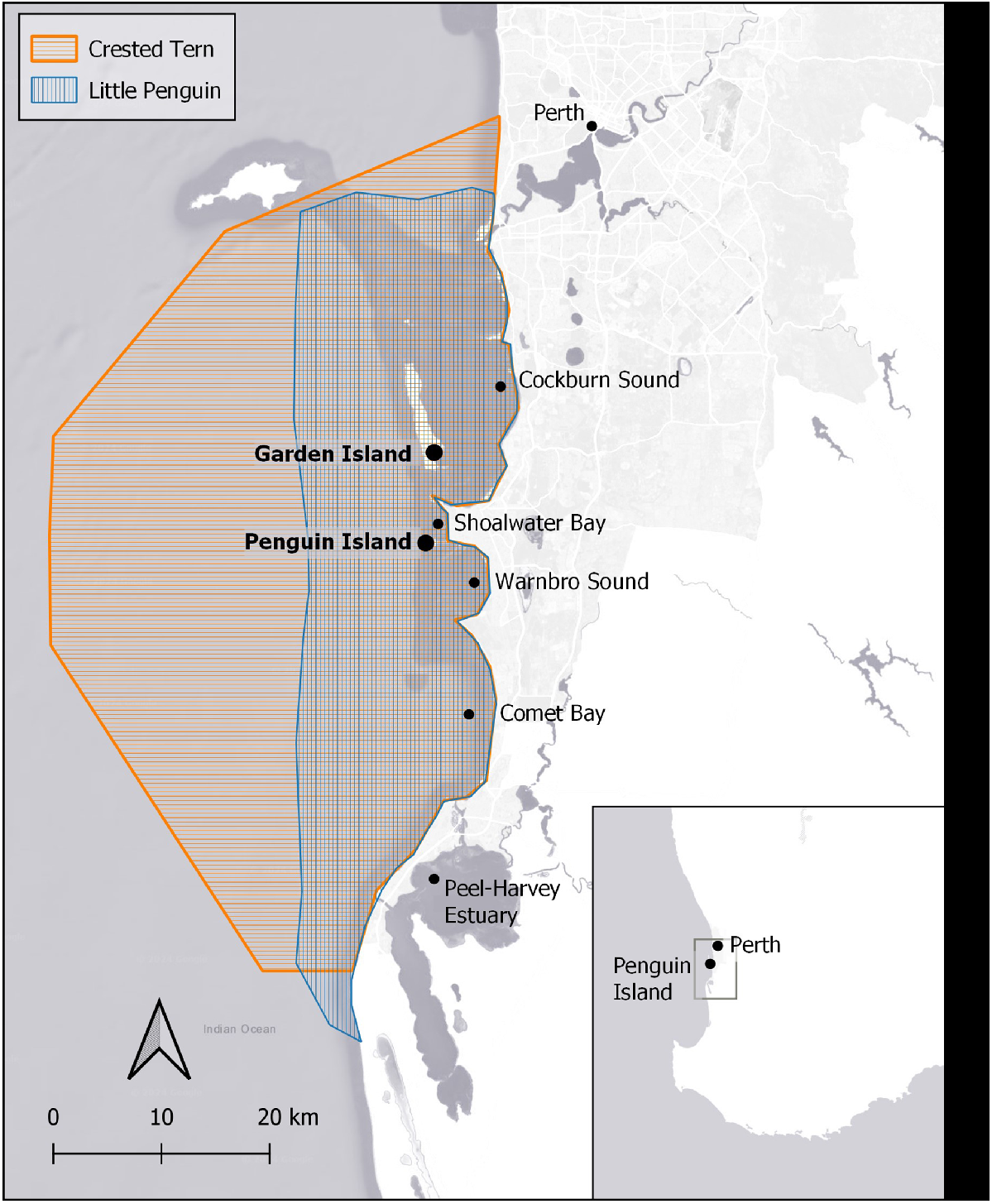
Study area showing location of Penguin Island and Garden Island and the expected local foraging range of Little Penguins (indicated by vertical blue lines; Cannell 2016, 2017, 2018; Sutton 2022; Cannell et al. 2024) and Crested Terns (indicated by horizontal orange lines; Mcleay et al. 2010). Note: Little Penguins are also known to forage in waters approximately 150 km south of Penguin Island (not depicted here) during breeding when local food abundance is reduced.
Previous intermittent diet studies have been conducted on the Little Penguins on Penguin Island. Initially these were based on water-offloading stomach contents and latterly on DNA analysis of faeces (Cannell et al. 2024). Due to a lack of resources no dietary sampling was undertaken after 2012 that might inform the prospects of the colony recovering and the merits of ongoing land-based conservation actions. Sandy sprats are considered a critical food source during the chick-rearing phase of Little Penguins breeding on Penguin Island (Cannell et al. 2024). In south-western Australia, Sandy Sprats are a significant component of the region’s commercial fishery, primarily supplying the bait market, with annual catches of 150–200 tonnes reported in the early 2000s (Rogers et al. 2006). Annual monitoring of catch rates revealed a collapse in the fishery following the 2011 marine heatwave prompting a reassessment of sustainability. As a result, previous catch allowances were deemed unsustainable, and commercial catches have been restricted to 25 tonnes per year (Newman et al. 2023). The fishery for Sandy Sprat operating in the Little Penguin prey field was closed following the collapse and there has been limited monitoring of forage fish abundance in the region to provide information on changes in prey availability.
The diet and foraging range of the Little Penguin colony breeding on Penguin Island are likely to overlap with other central-place foraging species in the region, such as the Crested Tern breeding in the Perth region (Fig. 1). Crested Terns are widely distributed across coastal subtropical and tropical waters of the Indian Ocean, as well as the Indo-Pacific. In Australia, they are the most widely distributed tern species, occurring along the entire continental coastline (Dunlop 1987). Crested Terns nest in highly synchronous, densely packed colonies on coastal islands within range of aggregations of forage fishes (Dunlop 1987). They are a plunge diving, bill-loading species that forage at short range from breeding colonies (generally within 40 km; Mcleay et al. 2010). The availability of appropriately sized and nutritious forage fishes is critical for egg formation (Dunlop 1987) and for provisioning small nestlings shortly after hatching (Surman and Wooller 2006; Gaglio et al. 2018; Quiring et al. 2021). During incubation, Crested Terns may exhibit a high degree of flexibility in prey selection and feed their larger running chicks on a wide range of non-forage fish species, squid and crustaceans (Chiaradia et al. 2002; Surman and Wooller 2006; Gaglio et al. 2018; Quiring et al. 2021). However, certain prey species may be more profitable. A collapse in Sardine abundance following a herpesvirus epidemic was shown to reduce chick survival rates, and lead to stunted adults (McLeay et al. 2009b).
Early investigations of prey selection in Crested Terns were based on spontaneous regurgitations of food items collected during banding operations (Chiaradia et al. 2002; Surman and Wooller 2006; Mcleay et al. 2009a, 2009b). More recently less invasive methods have been employed using the digital photography of fish carried into breeding colonies at various stages in the nesting cycle (Gaglio et al. 2017, 2018; Quiring et al. 2021).
This project pilots the efficacy of using digital photography to monitor the prey carried by Crested Terns to a colony on Penguin Island as a means of assessing local forage fish availability for the penguins and other meso-predators. The engagement of citizen-scientists in photo-sampling is anticipated to reduce the cost of monitoring prey-availability relative to other methods.
Materials and methods
Study area
This investigation was based on a Crested Tern colony on Penguin Island, Western Australia (Fig. 1). There are currently two Little Penguin colonies in the Perth region, one located on Penguin Island and another on the south-eastern corner of Garden Island. The prey field for Little Penguins foraging from the Garden Island colony occupies the southern end of Cockburn Sound (Cannell et al. 2024). The core foraging areas of Little Penguins breeding on Penguin Island include the waters around and south of the Island encompassing Warnbro Sound and Comet Bay, and north along the Sepia Depression and the western side of Garden Island (Cannell 2016, 2017, 2018; Sutton 2022; Cannell et al. 2024). During incubation, some also forage at the southern end of Cockburn Sound (Cannell et al. 2024). The expected foraging range of breeding Crested Terns (i.e. out to 40 km) encompasses the prey-fields of both Little Penguin colonies (Mcleay et al. 2010).
Sampling methods
Digital photography with 400–500 mm telephoto lenses was used to identify prey carried to Crested Tern colonies on Penguin Island during the 2021, 2022, and 2023 breeding seasons. Laying commenced in October each year and three photographic sampling sessions took place in November, December, and January allowing different stages in the nest cycle to be sampled capturing incubation, chicks (nest young), runners, and fledglings. Eight volunteer (citizen science) photographers contributed to the sampling with one to three photographers sampling at a time. Observers positioned themselves at vantage points that allowed normal behaviour, i.e. with no evidence of avoidance or flight intention movements. Depending on the nesting stage photographers could be stationed 10–30 m from their subjects.
Each sampling trip was conducted over 2–4 days, with sampling carried out on the following dates (and corresponding photographer hours): 17–19 November 2021 (21 h); 14–16 December 2021 (18 h); 4–6 January 2022 (6 h); 4–6 November 2022 (13.5 h); 1–4 December 2022 (12 h); 10–12 January 2023 (7.5 h); 22–24 November 2023 (19.5 h), 8–9 December 2023 (3 h); and 28–30 December 2024 (12 h). Sampling generally occurred in 2-h sessions, one in the morning starting at 1 h after sunrise and another in the late afternoon beginning at approximately 16:00 hours. The number of photographic stations (positions from which photos were taken) varied from one to three depending on the structure of sub-colonies and the stage of breeding. Nesting colonies with eggs and nest young occupied the sand-dunes on the western side of the island. Runners collected in creches on the western beach and fledglings were located on both the western and eastern beaches.
Crested Terns frequently delayed donating their prey to chicks (often to avoid gull kleptoparasitism) and the same fish could be photographed multiple times. Several characteristics were used to identify individual prey in the photographs including cap condition (condition of the black head plumage) in the fish-carrying Crested Tern, prey species, prey size, prey condition, angle and orientation in the bill, and marks and wounds inflicted by the bird. Prey were identified using available identification guides (Hutchins and Swainston 1995; Swainston 2020; Whisson and Hoschke 2021) and where necessary, assistance was provided by fish biologist Kurt Krispyn at Murdoch University. The best photographs of each prey species were maintained in a voucher collection. Prey length was estimated by comparing fish length with average Crested Tern bill length of 65 mm. All individual fish were identified and measured in a few multiple bill loads.
Chi-squared tests were used to compare the abundance of the forage fish species between years in the November (early season) samples and across the entire season. The average number across the 3 years was used for the expected values. Tests where P < 0.05 were considered significant. Data manipulation and plotting was carried out in Excel.
Results
Over the three Crested Tern breeding seasons, 1900 prey items, representing 62 species, were identified using 17,707 photographs. Of these species, 59 were prey species were fish, two were prawns; and one was squid. A complete list of prey species recorded in each of the three breeding seasons is in Table 1.
| Latin name | Common name | Percentage frequency | |||
|---|---|---|---|---|---|
| 2021 | 2022 | 2023 | |||
| Scolecenchelys australis | Shorthead Worm Eel | 0 | 0 | 0.17 | |
| Etrumeus jacksoniensis | Maray | 1.24 | 0.72 | 1.01 | |
| Hyperlophus vittatus | Sandy Sprat | 19.5 | 22.06 | 10.23 | |
| Nematalosa vlaminghi | Perth Herring | 0.23 | 0 | 0 | |
| Sardinella lemuru | Scaly Mackerel | 0.34 | 1.2 | 1.85 | |
| Sardinops sagax | Australian Sardine | 13.42 | 9.35 | 20.47 | |
| Spratelloides robustus | Blue Sprat | 28.75 | 22.78 | 6.88 | |
| Engraulis australis | Australian Anchovy | 11.95 | 5.28 | 17.79 | |
| Gonorynchus greyi | Beaked Salmon | 0.11 | 0 | 1.17 | |
| Cnidoglanis macrocephalus | Estuary Catfish | 0.11 | 0 | 0.5 | |
| Plotosus lineatus | Striped Catfish | 0 | 0 | 1.34 | |
| Metavelifer multiradiatus | Common Veilfin | 0 | 1.44 | 1.17 | |
| Dipulus caecus | Orange Eelpout | 0 | 0 | 1.01 | |
| Aldrichetta forsteri | Yellow-Eyed Mullet | 0 | 0.72 | 0.17 | |
| Mugil cephalus | Sea Mullet | 0.79 | 0.48 | 0.67 | |
| Atherinomorus vaigiensis | Common Hardeyhead | 1.58 | 2.64 | 1.34 | |
| Hemiramphus robustus | Robust Garfish | 0.11 | 0.96 | 1.34 | |
| Hyporhamphus melanochir | Southern Garfish | 0 | 0 | 0.17 | |
| Lissocampus sp. | Pipefish | 0.11 | 0 | 0 | |
| Centropogon latifrons | Western Fortesque | 0 | 0 | 0.17 | |
| Ostorhinchus rueppellii | Western Gobbleguts | 0.45 | 1.92 | 1.34 | |
| Ostorhinchus victoriae | Striped Cardinalfish | 0 | 0 | 0.17 | |
| Vincentia punctata | Orange Cardinalfish | 0 | 0.24 | 0.84 | |
| Sillaginodes punctatus | King George Whiting | 0.68 | 1.2 | 0.17 | |
| Sillago bassensis/vittata | School Whiting | 1.58 | 0.72 | 2.68 | |
| Sillargo burrus | Trumpeter Whiting | 0 | 0 | 0.67 | |
| Sillargo schomburgkii | Yellowfin Whiting | 0.56 | 0 | 0 | |
| Pomatomus saltatrix | Tailor | 0 | 0 | 0.17 | |
| Pseudocaranx georgianus | Silver Trevally | 0 | 0.24 | 0.34 | |
| Seriola hippos | Samsonfish | 0 | 0 | 0.67 | |
| Trachinotus botia | Common Dart | 0 | 0 | 0.17 | |
| Pentapodus vitta | Western Butterfish | 0.11 | 1.44 | 0.34 | |
| Gerres subfasciatus | Roach | 0 | 0 | 0.17 | |
| Parequula melbournensis | Silverbelly | 2.14 | 2.16 | 2.85 | |
| Upeneus australiae | Australian Goatfish | 0 | 0 | 0.17 | |
| Pempheris sp. | Bullseye | 0.23 | 0.48 | 0.34 | |
| Arripis georgianus | Australian Herring | 0.68 | 0 | 0 | |
| Girella tephroeops | Western Rock Blackfish | 0.11 | 0 | 0 | |
| Helotes octolineatus | Western Striped Grunter | 5.19 | 6.47 | 14.6 | |
| Haletta semifasciata | Blue Weed Whiting | 0 | 0.24 | 0 | |
| Heteroscarus acroptilus | Rainbow Cale | 0 | 0 | 0.17 | |
| Neoodax balteatus | Little Weed Whiting | 0 | 0.24 | 1.17 | |
| Notolabrus parilus | Brownspotted Wrasse | 1.58 | 3.84 | 3.36 | |
| Dotalabrus aurantiacus | Castelnau’s Wrasse | 0 | 0.24 | 0 | |
| Siphonognathus radiatus | Long-Rayed Weed Whiting | 0.68 | 0.72 | 0.67 | |
| Chironemus georgianus | Western Kelpfish | 0 | 0.24 | 0.67 | |
| Heteroclinus roseus | Rosy Weedfish | 0.68 | 0.48 | 2.35 | |
| Petroscirtes sp. | Sabretooth Blenny | 0.11 | 0.24 | 0 | |
| Aspidontus taeniatus | False Cleaner Fish | 0.11 | 0 | 0 | |
| Halcogramma decurrens | Blackthroat Threefin | 0 | 0.24 | 0 | |
| Pseudocalliurichthys goodladi | Goodlad’s Stinkfish | 0 | 0 | 0.17 | |
| Sphyaena novaehollandiae | Snook | 0 | 0.48 | 0 | |
| Scomber australasicus | Blue Mackerel | 3.16 | 0 | 0 | |
| Ammotretis sp. | Flounder | 0 | 0.34 | 0 | |
| Acanthaluteres spilomelanurus | Bridled Leatherjacket | 0 | 0 | 1.85 | |
| Scobinichthys granulatus | Rough Leatherjacket | 1.47 | 5.28 | 2.68 | |
| Torquigener pleurogramma | Common Blowfish | 0.45 | 3.36 | 2.18 | |
| Metapenaeus sp. | School Prawn | 0 | 0.96 | 0.17 | |
| Penaeus latisulcatus | King Prawn | 1.24 | 0.72 | 1.17 | |
| Sepioteuthis callamari | Southern Calamari Squid | 0.11 | 0 | 0 | |
Importance values are the percentage of total fish identified in each season. Species are ordered taxonomically according to Hutchins and Swainston (1995).
Overall, the dominant prey were clupeid forage fishes (64.17%), Blue Sprat (Spratelloides robustus) (21%), Sandy Sprat (Hyperlophus vittatus) (17%), Sardine (Sardinops vagax) (15%), Tropical Sardine (Sardinella lemuru) (1%), Anchovy (Engraulis australis) (9.16%), and Marray (Etrumeus jacksoniensis) (1.01%). Important secondary prey were mainly species inhabiting seagrass meadows including Brown-spotted Wrasse (Notolabrus parilus) (2.63%), Western Striped Grunter (Helotes octolineatus) (8.42%), Rough Leatherjacket (Scobinichthys granulatus) (2.68%), and Silverbelly (Parequula melbournensis) (2.37%).
The abundance of forage fish species varied over the three seasons (Fig. 2). Blue and Sandy Spats were the most abundant forage fishes in 2021 and 2022 but declined in 2023 as the numbers of Sardines and Anchovies carried by Crested Terns increased. In November during the peak in nesting activity, the abundance of Blue Sprat differed significantly between seasons (χ2 = 16.64, P < 0.001) as did the numbers of Sardines (χ2 = 10.9, P < 0.005). The differences in the number of Sandy Sprat and Anchovies recorded was not significant. At an overall seasonal level, there were significant differences in the numbers of Blue Sprat (χ2 = 13.12, P < 0.005), Sandy Sprat (χ2 = 4.88, P < 0.01), Sardines (χ2 = 4.39, P < 0.05), and Anchovies (χ2 = 6.73, P < 0.05).
Percentage frequency of forage fish species carried to Crested Tern colonies on Penguin Island in 2021, 2022, and 2023 breeding seasons.
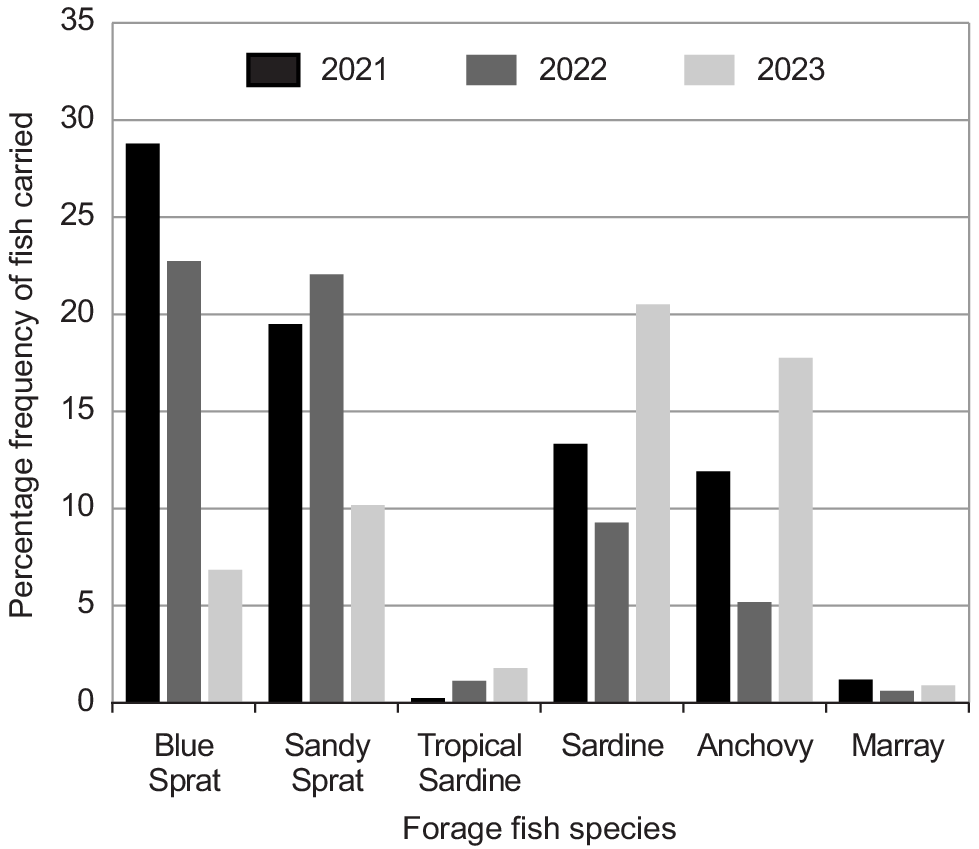
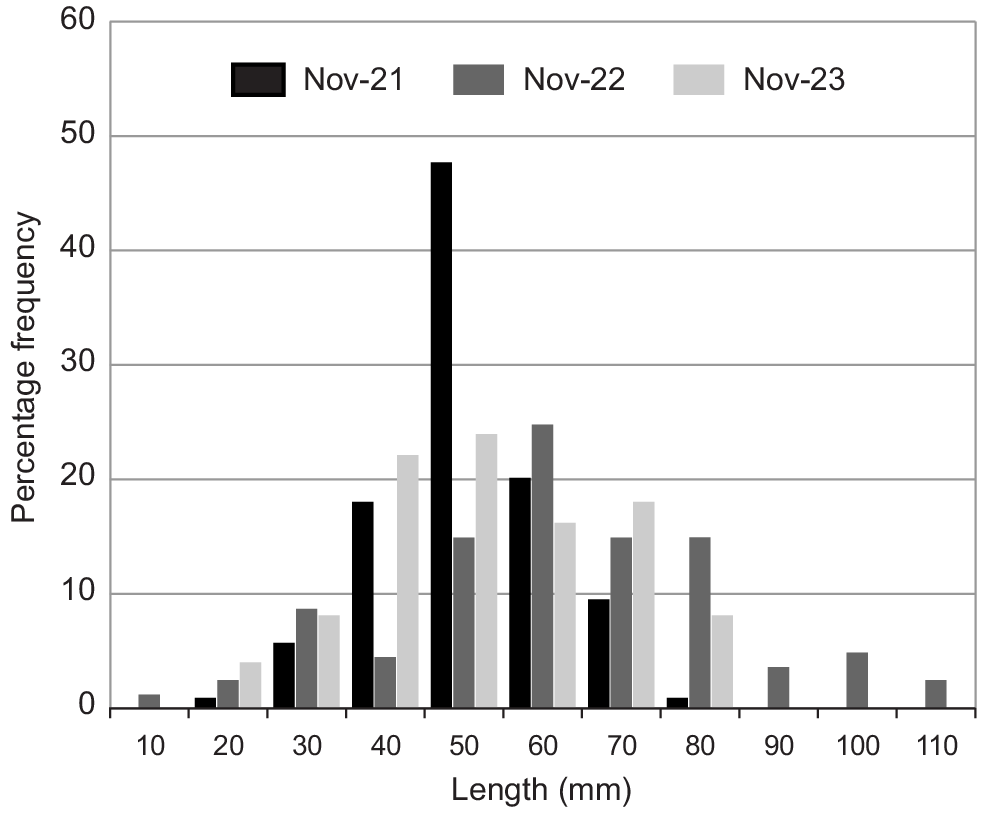
Fig. 3 shows the length classes of Sandy Sprats recorded in each Crested Tern breeding season. In 2021, Sandy Sprats were relatively abundant with all fish being small and mostly below the adult size of around 60 mm, indicating Sandy Sprat recruitment following autumn winter/spawning (Gaughan et al. 1996) in 2021. In 2022, recruitment continued with some of the younger fish having moved into the adult age classes. In 2023, the Sandy Spat population was spread across both immature fish and those of spawning age.
Discussion
Dietary studies from Crested Tern colonies in southern Australia and southern Africa have documented a diversity varying from 11 (Chiaradia et al. 2002) to 12 (Quiring et al. 2021), 36 (Mcleay et al. 2009b), and 53 (Gaglio et al. 2018) prey taxa. The 62 prey species taken from the Crested Tern colonies on Penguin Island was comparatively diverse. Of the 53 species captured at Robben Island in South Africa, 94% were classified as pelagic compared with 12.9% from the coastal waters around Penguin Island. The ‘non-pelagic’ taxa included species from shallow water habitats including seagrass meadows, sand flats and banks, macroalgae dominated limestone reefs, beach-wrack, and open water areas up to around 30 m in depth within Cockburn Sound, Shoalwater Bay, Warnbro Sound, and adjacent shelf waters. It is likely that this foraging habitat diversity contributed to the number of species recorded.
Generally, the diet of breeding Crested Terns is dominated by one or two forage fish species (at least in the prey fed to chicks). Anchovy has been an almost ubiquitous dominant forage fish in dietary investigations; e.g. making up 65% (Gaglio et al. 2018), 84.5% (Quiring et al. 2021), 63% (Chiaradia et al. 2002), and 36.3% (McLeay et al. 2009b) of the prey captured. Important (sometimes co-dominant) forage fish prey included Sardines and Jack Mackerel (Trachurus declivis). Anchovy and Sardine were the dominant prey off the western Cape of South Africa (Crawford 2003).
Between 1978 and 1987, Sardines were the dominant prey taken from Crested Tern colonies breeding in the Perth metropolitan region between April and June (Dunlop 1987; J. N. Dunlop pers. obs.). At Rottnest Island, the timing of the arrival of the Sardine shoals evidently influencing the onset of laying (Dunlop 1987). The autumn–winter breeding sub-season (Dunlop 1985) vanished from the local waters sometime after the Pilchard (Sardine) herpesvirus epidemics in 1995 and 1998 (Whittington et al. 2008) and has never re-established. Similar major shifts in colony persistence/location have been observed in southern Africa in response to changing forage fish (Sardine/Anchovy) distribution and abundance (Crawford 2003).
The diet of Crested Terns foraging from Penguin Island differs from all other colonies studied in that ‘sprats’, small coastal clupeids, tend to dominate the forage fish taken. This is also the case in comparison with Little Penguin colonies around the coastline of southern Australia (e.g. Chiaradia et al. 2010; Cannell et al. 2024). Sandy Sprats were a major contributor to the Crested Tern prey intake on Penguin Island in all three seasons. Blue Sprats were abundant in 2021 and early in the 2022 season but declined sharply in 2023. Coincidently, the later Crested Tern clusters (sub-colonies) abandoned their chicks soon after hatching in 2022 and this may have been related to the sudden disappearance of Blue Sprats at that time. Blue Sprats provide a prey-size that is suitable for newly hatched Crested Terns.
In 2021, Sandy Sprats were abundant in November with some Crested Terns returning to the Penguin Island colony with multiple bill loads (Fig. 4). In this season, the fish were mostly below 60 mm total length indicating the recruitment of new cohort (Fig. 3). The Year 0 fish were graduating into the adult (>60 mm) age classes in 2022 and in 2023, both Year 0 and older fish were represented indicating that the stock had become established. This suggests that Sandy Sprats, which are the most important prey type for breeding Little Penguins on Penguin Island, was available again.
The factors that contributed to the Sandy Sprat recovery are not known. However, 2021 was the second wettest year on record for the region (892 mm) in what is generally a drying climate (Bureau of Meteorology Report, 8 February 2022). The higher rainfall will have increased the outflow and the nutrient/organic matter plume from the Peel Harvey Estuary, elevating the productivity of the waters immediately south of Penguin Island within the Little Penguin foraging area. This in turn may have improved the growth and survival of Sandy Sprat larvae (Grimes and Kingsford 1996). The abundance of Sandy Sprat was demonstrated to be positively influenced by freshwater flows into the Murray River estuary (Bice et al. 2015).
Sandy Sprats disappeared from the Little Penguin diet on Penguin Island during and after the marine heatwave event in 2011 (Cannell et al. 2024). Tropical Sardine and Sardine dominated the penguin prey in 2011 and 2012 (Cannell et al. 2024). Between 2021 and 2023, tropical sardines made a negligible contribution to the prey taken by Crested Terns and the small number that were observed were in age/size classes above those preferred by the penguins (Cannell et al. 2024).
The potential return of Sprat within the foraging range of the Little Penguin may play a critical role in supporting the recovery of the Perth population, particularly the critically low Penguin Island colony. Further investigation into the relationship between Crested Tern diets and Little Penguin breeding productivity will be required to establish the value of this method in predicting periods of increased productivity. This could provide valuable guidance on management and indicate when intervention and conservation actions may be most beneficial.
Data availability
The data that support this study are available in the paper. Images from the voucher collection can be made available on request.
Acknowledgements
The authors thank the citizen science photographers that assisted in the sampling, in particular Brendan Kinsella, Claire Greenwell, and Raelene Smith. We gratefully acknowledge the assistance of Dr Kurt Krispyn of Murdoch University for identifying a number of tricky fish species. The Department of Biodiversity Conservation and Attractions (DBCA) granted permission to do the research on Penguin Island and provided access and accommodation in the research quarters.
References
Barrett RT, Camphuysen K, Anker-Nilssen T, Chardine JW, Furness RW, Garthe S, Huppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies in seabirds: a review and recommendations. ICES Journal of Marine Science 64, 1675-1691.
| Crossref | Google Scholar |
Bice CM, Furst D, Lamontagne S, Oliver RL, Zampatti BP, Revill A (2015) The influence of freshwater discharge on productivity, microbiota community structure and trophic dynamics in the Murray estuary: evidence of freshwater derived trophic subsidy in the sandy sprat. Goyder Institute for Water Research Technical Report No. 15/40, Adelaide, South Australia.
Cannell BL (2017) Understanding the toll of consecutive years of warm waters on Little Penguins and refining their capacity as bioindicators of the marine coastal ecosystem. Report 1 for the City of Rockingham and Fremantle Ports, Murdoch University. Available at https://www.fremantleports.com.au/docs/default-source/environment-documents/little-penguin-report-year-1-april-2017-(1).pdf?sfvrsn=364d82ff_2
Cannell BL (2018) Understanding the toll of consecutive years of warm waters on Little Penguins and refining their capacity as bioindicators of the marine coastal ecosystem. Report 2 for City of Rockingham and Fremantle Ports. Murdoch University. Available at https://www.fremantleports.com.au/docs/default-source/environment-documents/little-penguins-report-year2-april-2018.pdf?sfvrsn=5c8b9475_2
Cannell BL (2024) Population estimate of the Little Penguin colony on Penguin Island during September to November 2023. Report for the City of Rockingham, University of Western Australia. Available at https://rockingham.wa.gov.au/forms-and-publications/your-city/protecting-our-environment/population-estimate-of-the-little-penguin-colony-o
Cannell BL, Kendall WL, Tyne JA, Bunce M, Hetzel Y, Murray D, Radford B (2024) Marine heatwaves affect breeding, diet and population size but not body condition of a range-edge Little Penguin colony. Marine Ecology Progress Series 737, 193-213.
| Crossref | Google Scholar |
Chiaradia A, Dann P, Jessop R, Collins P (2002) The diet of Crested Tern (Sterna bergii) chicks on Phillip Island, Victoria, Australia. Emu - Austral Ornithology 102(4), 367-371.
| Crossref | Google Scholar |
Chiaradia A, Forero MG, Hobson KA, Cullen JM (2010) Changes in diet and trophic position of a top predator 10 years after a mass mortality of a key prey. ICES Journal of Marine Science 67(8), 1710-1720.
| Crossref | Google Scholar |
Crawford RJM (2003) Influence of food on numbers breeding, colony size and fidelity to localities of Swift Terns in South Africa’s Western Cape, 1987–2000. Waterbirds 26(1), 44-53.
| Crossref | Google Scholar |
Dunlop JN (1985) Reproductive periodicity in a population of Crested Terns, Sterna bergii Lichtenstein in south Australia. Australian Wildlife Research 12(1), 95-102.
| Crossref | Google Scholar |
Dunlop JN (1987) Social-behavior and colony formation in a population of Crested Terns, Sterna-Bergii, in Southwestern Australia. Wildlife Research 14(4), 529-540.
| Crossref | Google Scholar |
Edney AJ, Wood MJ (2021) Applications of digital imaging and analysis in seabird monitoring and research. IBIS 163(2), 317-337.
| Crossref | Google Scholar |
Forys EA, Hevesh AR (2017) Investigating black skimmer chick diets using citizen science and digital photography. Southeastern Naturalist 16(3), 317-325.
| Crossref | Google Scholar |
Gaglio D, Cook TR, Connan M, Ryan PG, Sherley RB (2017) Dietary studies in birds: testing a non-invasive method using digital photography in seabirds. Methods in Ecology and Evolution 8(2), 214-222.
| Crossref | Google Scholar |
Gaglio D, Cook TR, McInnes A, Sherley RB, Ryan PG (2018) Foraging plasticity in seabirds: a non-invasive study of the diet of greater crested terns breeding in the Benguela region. PLoS ONE 13(1), e0190444.
| Crossref | Google Scholar | PubMed |
Grimes CB, Kingsford MJ (1996) How do riverine plumes of different sizes influence fish larvae: do they enhance recruitment? Marine and Freshwater Research 47(2), 191-208.
| Crossref | Google Scholar |
McLeay LJ, Page B, Goldsworthy SD, Ward TM, Paton DC, Waterman M, Murray DM (2009a) Demographic and morphological responses to prey depletion in a crested tern (Sterna bergii) population: can fish mortality events highlight performance indicators for fisheries management? ICES Journal of Marine Science 66(2), 237-247.
| Crossref | Google Scholar |
McLeay LJ, Page B, Goldsworthy SD, Ward TM, Paton DC (2009b) Size matters: variation in the diet of chick and adult crested terns. Marine Biology 156, 1765-1780.
| Crossref | Google Scholar |
McLeay LJ, Page B, Goldsworthy SD, Paton DC, Teixeira C, Burch P, Ward T (2010) Foraging behaviour and habitat use of a short-ranging seabird, the crested tern. Marine Ecology Progress Series 411, 271-283.
| Crossref | Google Scholar |
Owen E, Haddon SN, Hughes RD, Barratt A, Barton JH, Bevan W, Broholm T, Cachia-Zammit C, Cleasby I, Dunkley F, Edney A, Fink A, Ford K, Henderson J, Horton K, Kosová E, Longmoor G, Morgan G, Prince O, Sheikh S, Snead H, West F, Tremlett CJ (2024) Spatial and within-season variation in the diet of a declining seabird described through digital photography and citizen science. Avian Conservation and Ecology 19(1), 17.
| Crossref | Google Scholar |
Quiring K, Carroll G, Champion C, Heymann EW, Harcourt R (2021) The diet of greater crested terns off southeast Australia varies with breeding stage and sea surface temperature. Marine Biology 168, 143.
| Crossref | Google Scholar |
Surman CA, Wooller RD (2006) Comparative foraging ecology of five sympatric terns at a sub-tropical island in the eastern Indian Ocean. Journal of Zoology 259(3), 219-230.
| Crossref | Google Scholar |
Velarde E, Tordesillas MdLS, Rocio Esquivel LV (1994) Seabirds as indicators of important fish populations in the Gulf of California. ColCOFI Rep 35, 137-143.
| Google Scholar |
Velarde E, Ezcurra E, Anderson DW (2013) Seabird diets provide early warning of sardine fishery declines in the Gulf of California. Scientific Reports 3, 1332.
| Crossref | Google Scholar | PubMed |
Whittington RJ, Crockford M, Jorden D, Jones B (2008) Herpesvirus that caused epizootic mortality in 1995 and 1998 in pilchard, Sardinops sagax neopilchardus (Steindachner), in Australia is now endemic. Journal of Fish Diseases 31(2), 97-105.
| Crossref | Google Scholar | PubMed |