Climate extreme triggers cold-water community rescue
B. C. Ebner A B * , J. Lobegeiger C , J. Coe D , S. Balcombe E , D. Latimer F , G. Pickering C and J. C. Marshall C F
A B * , J. Lobegeiger C , J. Coe D , S. Balcombe E , D. Latimer F , G. Pickering C and J. C. Marshall C F
A
B
C
D
E
F
Abstract
Mountain-top associated instream fauna with restricted ranges and limited dispersal capability are especially vulnerable to extinction under global warming and climate extremes.
Rescue and housing of multiple cold-water taxa on short timelines in reaction to extreme drought.
We undertook multi-species rescue (fishes: Gadopsis marmorata, n = 50; Galaxias olidus, n = 150; and a crayfish Euastacus sulcatus, n = 50) from the headwaters of the Condamine–Balonne catchment, temporarily holding animals in small (200 L) and large (1000 L) aquaria in single- and mixed-species contexts, at below 23°C.
Galaxias olidus was successfully kept in one of the small aquaria, but did not survive in mixed-species aquaria, partly being due to predation by other species. Euastacus sulcatus showed decreased survivorship at moulting (predation). Large Gadopsis marmorata (>100-mm total length, TL) was aggressive, whereas maintaining smaller individuals and using large aquaria served to dampen overall aggression levels.
Holding mixed taxa following field rescues requires attention to detail, including developmental-stage combinations within and across species, enclosure volume and availability of structure.
The simultaneous rescue of multiple co-occurring endemic taxa represents a promising aspect of research and adaptive management in the era of global climate change.
Keywords: bureaucracy, community rescue, drought, Euastacus, Gadopsis, Galaxias, moulting, predation, threatened species.
Introduction
Past climate has shaped the global distributions of endemic species (Jansson 2003). More recently, the effects of climate change, including extended droughts, mega-bushfires, increasing temperatures and heatwaves, are having unprecedented impacts on ecosystems and their constituent biodiversity (Covich et al. 1997; O’Reilly et al. 2003; Lindenmayer and Taylor 2020; Ward et al. 2020). Endemic freshwater species with highly localised distributions and poor dispersal capability are especially vulnerable to climate-change impacts including drought, riparian clearing and human use of surface and groundwater (Covich et al. 1997; Bogan et al. 2015; Vinarski et al. 2022; Hossack et al. 2023). Furthermore, those species that have evolved in isolated cold-climate habitats (e.g. on mountain ranges or inland away from ocean buffering) can be further constrained by the narrowing thermal niches arising from global warming (e.g. Costion et al. 2015; Kovach et al. 2017; Vinarski et al. 2022).
Rescuing animals, including threatened species from locations that are becoming uninhabitable to species as a function of climate change, is an ethical and, in some contexts, a legal matter (Palmer 2021). However, rescuing animals during extreme events is typically ad hoc and under-resourced (Hammer et al. 2013; Palmer 2021). Furthermore, temporary housing of rescued aquatic taxa is prohibitively costly. Discrete waterways and infrastructure requirements are not always readily available to house aquatic rescue animals, and potential receiving waterways require checking for suitability at short notice (Hammer et al. 2013). Immediate funding and labour resources are not always forthcoming. Rescues can create tension with and within institutions partly because rescues are perceived as unusual events or circumstances, are an inconvenience, and have an immediacy that creates challenges for society. Animal rescues have associated ethical dilemmas that encompass disparate perspectives, and proactive action for threatened species is contingent on individuals, institutions and cultural values (Gregory et al. 2021; Palmer 2021; Ladle et al. 2023). Climate-change interaction with a growing list of formally and informally recognised threatened species provides testing circumstances for conservationists and society. Nevertheless, we contend that developing systems and facilities that cater for these circumstances, including rescuing and housing cold-water specialist fauna, is worthy of serious attention. In this regard, bolstering natural aquatic habitats (e.g. Turunen et al. 2021), translocating populations to human water storages (e.g. fire-fighting water reservoirs, farm dams, town water reservoirs; Beatty et al. 2017) or establishing captive populations (e.g. threatened species hatcheries) are some of the key proactive strategies available. Here, we explore the idea of recreating aquatic assemblages or communities that are co-housed as multiple taxa rather than housed only in monocultures.
The housing of multiple taxa in aquaria or ponds is traditionally the domain of public and private aquarists more so than it is in aquaculture, although this is shifting with the development of ecological aquaculture and the ecosystem approach to aquaculture (Costa-Pierce 2021) and recent progress in conservation aquaculture (Froehlich et al. 2017). There are several logistical and ethical challenges in housing multiple taxa together, in the long term or even temporarily (Monreal-Pawlowsky et al. 2021; Palmer 2021). Often a comprehensive knowledge base for informing this is lacking and adaptive management is required by carers (e.g. Monreal-Pawlowsky et al. 2021; Krol et al. 2023; Smith 2023), which is set against a backdrop where ethical considerations of animal-assisted response to climate change are still in their infancy (Palmer 2021). Despite many ecological publications that concentrate on understanding climate-change effects on upland streams and thermal refuges, the focus is often entirely or predominantly on field studies or desk-top modelling of single species or only the thermal aspects of the ecology of multiple species (e.g. McCullough et al. 2009; Whitney et al. 2015; Kovach et al. 2017; Turschwell et al. 2017; Richardson 2019; Hossack et al. 2023). A subset of studies has focused on species richness patterns in relation to thermal gradients or stream altitude (e.g. Negus et al. 2020; Gebert et al. 2022; Kirk and Rahel 2022). However, in-depth ecological knowledge of species interactions (e.g. competition, predation, parasite exchange) is rarely available to inform captive community housing or breeding, especially regarding small-bodied, non-ornamental species.
Aquatic species vulnerable to localised or complete extinction have in some cases been the focus of translocations or ark populations in Australia (Bond et al. 2008; Koehn et al. 2013; Todd and Lintermans 2015). This has been achieved for several large-bodied fishes because of their societal value in aquaculture, fisheries (catch-and-release or food resources) and conservation, most notably in south-eastern Australia and especially involving Murray–Darling Basin (MDB) fishes (Lintermans et al. 2005, 2015; Koehn et al. 2013). There have also been efforts applied to the conservation of small-bodied fishes and other taxa, although these efforts are often short term, ad hoc in nature or are under-resourced relative to those of the large-bodied angling fish species (Raadik et al. 2010; Hammer et al. 2013; Lintermans et al. 2015; Moy and Unmack 2017; Stoessel et al. 2020).
The Spring Creek catchment is set in one of the coldest parts of Queensland (Fig. 1). As a function of the dominance of groundwater input to its streamflow (Hatlow 2022), and dense catchment shading provided by mesophilic vegetation, the stream maintains water temperatures that are exceptionally cool for Queensland. For example, during extreme heatwave conditions in the Austral summer of 2019–2020, water temperature in the forested reach of Spring Creek peaked at only 23°C, despite streamside air temperature reaching maxima of 34°C. Further downstream, in the de-vegetated reaches of the creek, water temperature reached 36°C, being comparable to the air temperature of 37°C (Queensland Government, unpubl. data). Downstream further in the Condamine River, of which Spring Creek is a tributary, although experiencing some failures, loggers recorded water temperatures up to 31, 32 and 34°C at stream gauging stations 422394A, 422310C and 422316A respectively (Queensland Government Water Monitoring Information Portal, see https://water-monitoring.information.qld.gov.au/).
Locations where northern river blackfish, mountain galaxias and spiny crayfish were collected in February 2020 (yellow circles) and the more northerly site (Adjinbilly Creek; green circle) where northern river blackfish were released in July of that year.
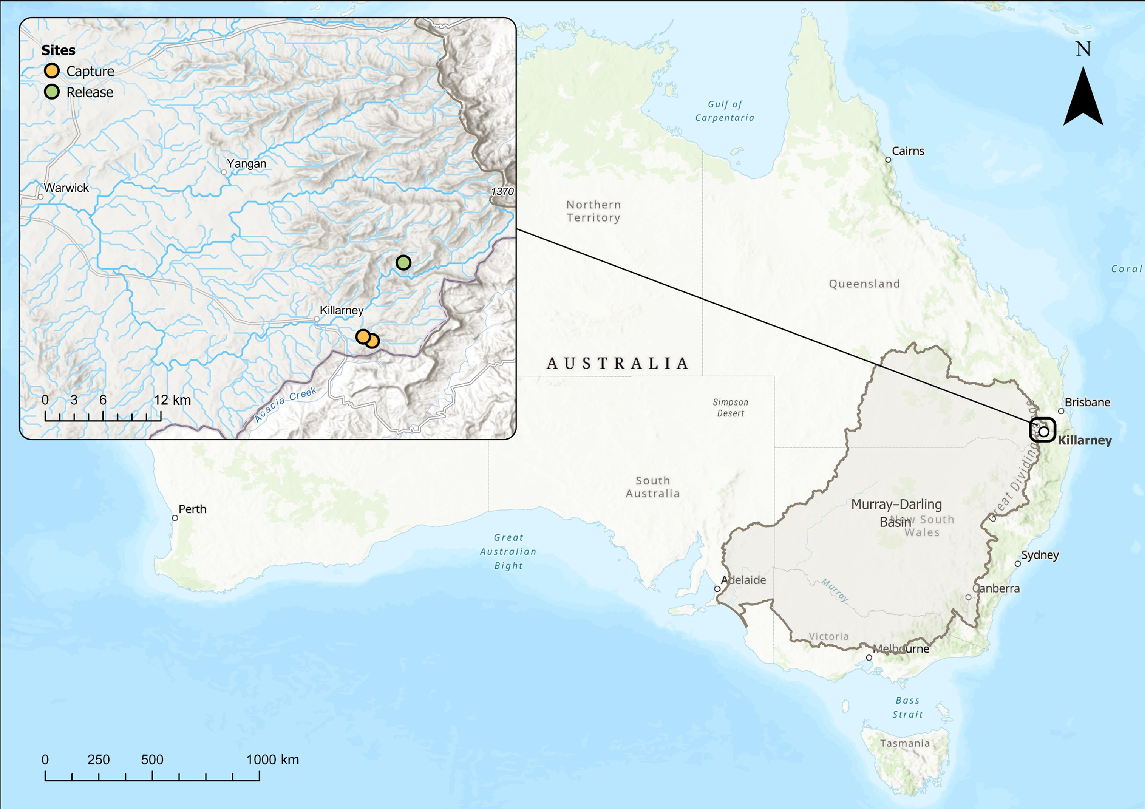
Because of the unique habitat and ecosystem, the area is identified as a key asset of Basin significance in the Basin-wide Environmental Watering Strategy, a component of the Murray–Darling Basin plan that prioritises expected environmental improvements (Murray–Darling Basin Authority 2019). In the lead up to salvaging aquatic fauna from Spring Creek (this study), eastern Australia was experiencing the worst drought on record, including highest recorded temperatures in south-eastern Australia, which encompasses the northern Murray–Darling Basin (Lindenmayer and Taylor 2020; Ward et al. 2020; World Meteorological Organization 2020). Stream flow had almost ceased at the stream gauge on Spring Creek (Note 422321B Spring Creek at Killarney, is a recently decommissioned gauge) and water temperature was approaching thermal limits for northern river blackfish (Gadopsis marmorata Ricardson, 1848) and spiny crayfish (Euastacus sulcatus Riek, 1951; references provided above; Supplementary Fig. S1). Sedimentation was affecting the availability of interstitial spaces. These microhabitats (and burrows) are important for northern river blackfish and spiny crayfish survival, including during parental care of young (Furse and Wild 2002; Khan et al. 2004; Furse 2010; Coughran 2013; Lintermans 2023).
We performed a rapid rescue of one of the last remaining remnant populations of river blackfish in the northern Murray–Darling Basin, with the aim of temporarily housing animals for reintroduction post-drought. Specifically, we collected animals in case of total extinction of these remnant populations (especially in terms of northern river blackfish). Our subaims were to (a) determine whether this species could be held temporarily with its own kind and individuals of different body sizes in aquaria with sufficient structure and extent, (b) determine whether these fish could be temporarily co-located with other rescued cold-water species, namely a smaller upland fish, the mountain galaxias (Galaxias olidus Gűnther, 1866) and an endemic spiny crayfish (E. sulcatus) and (c) outline interdisciplinary issues encountered in this salvage attempt. This rescue operation was considered important as an immediate response to severe climate impacts in a catchment that has seen long-term retraction of the range of cold-water, aquatic species, and northern river blackfish especially (Turschwell et al. 2016; Huey et al. 2017). It was never envisaged that these animals would be held indoors in aquaria long term, and it was hoped that a proposed second stage of the project based on large outdoor ponds would provide a longer-term ark solution.
Study species
The northern river blackfish (Gadopsis marmorata; Fig. 2a) is a medium-sized species typically attaining 200–250-mm total length (TL). It is endemic to the Murray–Darling Basin (Hammer et al. 2014; Unmack et al. 2017), with the small northern populations comprising discrete genetic subunits (Huey et al. 2017). The biogeography of the species is a product of a life history that includes nest guarding, a heavily site-attached existence within small home-ranges and a low likelihood of large-scale dispersal or recolonisation of distant streams (Khan et al. 2004; Koster and Crook 2008; Crook et al. 2010; Huey et al. 2017; Lintermans 2023). The northern river blackfish is carnivorous, primarily feeding on benthic invertebrates (Jackson 1978; Rees et al. 2020; Lintermans 2023), and is known to associate with complex structures including undercut banks (Khan et al. 2004). There has been substantial research into the thermal physiology and biology of this species, demonstrating that it requires water temperatures lower than ~28°C (Dobson and Baldwin 1982a, 1982b; Turschwell et al. 2016, 2017, 2018, 2020). Because of the small and isolated nature of populations of northern river blackfish in the northern Basin, range extension or establishment of additional populations has been identified as a priority in the Basin Wide Environmental Watering Strategy (Murray–Darling Basin Authority 2019).
The two focal fish species of the rescue: (a) northern river blackfish and (b) mountain galaxias, and the focal crustacean (c) spiny crayfish.

Similarly, the Lamington spiny crayfish (Euastacus sulcatus; referred to herafter as spiny crayfish; Fig. 2c) is an upland species occupying a cold-water niche in forested upland streams in south-eastern Queensland and north-eastern New South Wales (Coughran 2008, 2013; Bone et al. 2014). Experiments have shown this species to become agitated as water temperatures increase to 23°C and incapacitated when they reach 27°C (Lowe et al. 2010; Bone et al. 2014). The northern river blackfish and the spiny crayfish are protected as ‘no-take species’ under the Queensland Fisheries Act 1994.
The mountain galaxias (Galaxias olidus; Fig. 2b) is a widespread species associated with upland streams in the eastern and southern Murray–Darling Basin but has a small range within Queensland (the species is also found outside of the Basin in coastal catchments; Marshall 1989; Hammer et al. 2014; Lintermans 2023). Local mountain galaxias does not tolerate water temperature higher than 29°C in captivity (Marshall 1989). The species attains a small size (usually <100 mm TL), is short-lived and feeds on benthic invertebrates and terrestrial insects (Lintermans 2023).
Traditional owners
The Githabul People, represented by the Githabul Nation Aboriginal Corporation, have a strong connection to Spring Creek, Browns Falls and the surrounding landscape. This connection extends to all the local plants and animals, including the cold-water dependent northern river blackfish, spiny crayfish and mountain galaxias. The Githabul People have invested strongly in caring for Country with development of their own Aboriginal Rangers Program. They have also focused on building capacity to care for and share traditional ecological knowledge and science.
Our team contacted local Githabul Aboriginal Rangers prior to undertaking the Killarney rescue operation to discuss their views and invite their participation in the rescue operation. Githabul representatives also accompanied members of the project team during the release of captive northern river blackfish to Adjinbilly Creek. These were important opportunities for the project team and Githabul representatives to share their interest in the project outcomes and discuss opportunities to highlight Githabul cultural and traditional ecological knowledge and understanding. The project team members were able to explain in more detail the reasons for the rescue operation and how it would be undertaken and monitored. Opportunities for more extensive involvement and collaboration with the Githabul People were limited during 2020 because of COVID-19 restrictions and extreme weather.
Although no formal agreements were entered into with the Githabul Nation Aboriginal Corporation, there is considerable scope for future involvement and collaboration with the Githabul People in relation to Spring Creek and upper Condamine rehabilitation and other activities under the Murray–Darling Native Fish Management and Recovery Strategy, as well as other natural resource management activities in this part of the Queensland Murray–Darling Basin (Murray–Darling Basin Authority 2020).
Direct observation of wild fauna
At a few locations, the primary author was able to make direct observations of the daytime habits of the focal species to inform later interpretations of mixed-species captive care for this aquatic community. This included 15 min of snorkelling with weight belt, camera and dive torches in each of two permanent pools: a large plunge pool immediately below Browns Falls (<1-m visibility) and further downstream in Spring Creek in an upland bedrock-dominated pool with leaf litter and some fine woody debris (visibility >2 m). Effort was made to minimise disturbance to instream fauna, with torches being used sparingly for photography and filming or inspecting areas with poor visibility (e.g. under hangs, deeper parts of the plunge pool).
The plunge pool had small spiny crayfish present around the edges and were clearly using rock interstices for refuge. The other pool (further downstream) contained multiple individuals of river blackfish and spiny crayfish. There, adult and juvenile northern river blackfish were coexisting with small juveniles loosely grouped, frequently away from structure and not in conflict with one another. Large adult blackfish were associated with structure, and were solitary with respect to other adults, but were clearly tolerating the presence of small nearby juveniles. Spiny crayfish of a range of sizes (juveniles and adults) were observed at burrow entrances, in interstices and moving over open benthos. Active spiny crayfish were primarily feeding on and within leaf-litter deposits (as has been reported elsewhere, e.g. Starrs et al. 2015) alongside juvenile northern river blackfish. From above water, solitary, large spiny crayfish were also commonly seen out foraging, including sifting through sediment and handling leaf litter in various pools and runs.
By contrast, galaxiids were mainly observed from above water and were always present in tight schools (10–50 individuals). This included in small (<5 m2) and very shallow (<0.1-m depth) drying bedrock pools, in larger (~50 m2, at least 0.5-m maximum depth), more complex, shallow pools (where river blackfish and spiny crayfish were present), and in shallow (<0.3-m depth) bedrock runs. Close-up video showed that galaxiids were feeding from algal-covered, hard substratum (mainly bedrock) in flowing water.
Field work was conducted under Queensland General Fisheries Permits 205834, 198642, 207792; James Cook University Animal Ethics Permit A2674 and Griffith University Animal Ethics Permit ENV/1720/AEC; and Queensland Government Environment and Heritage Protection Ethics Approval AEC SA2013/02/413.
Stream status, collection and transport of taxa
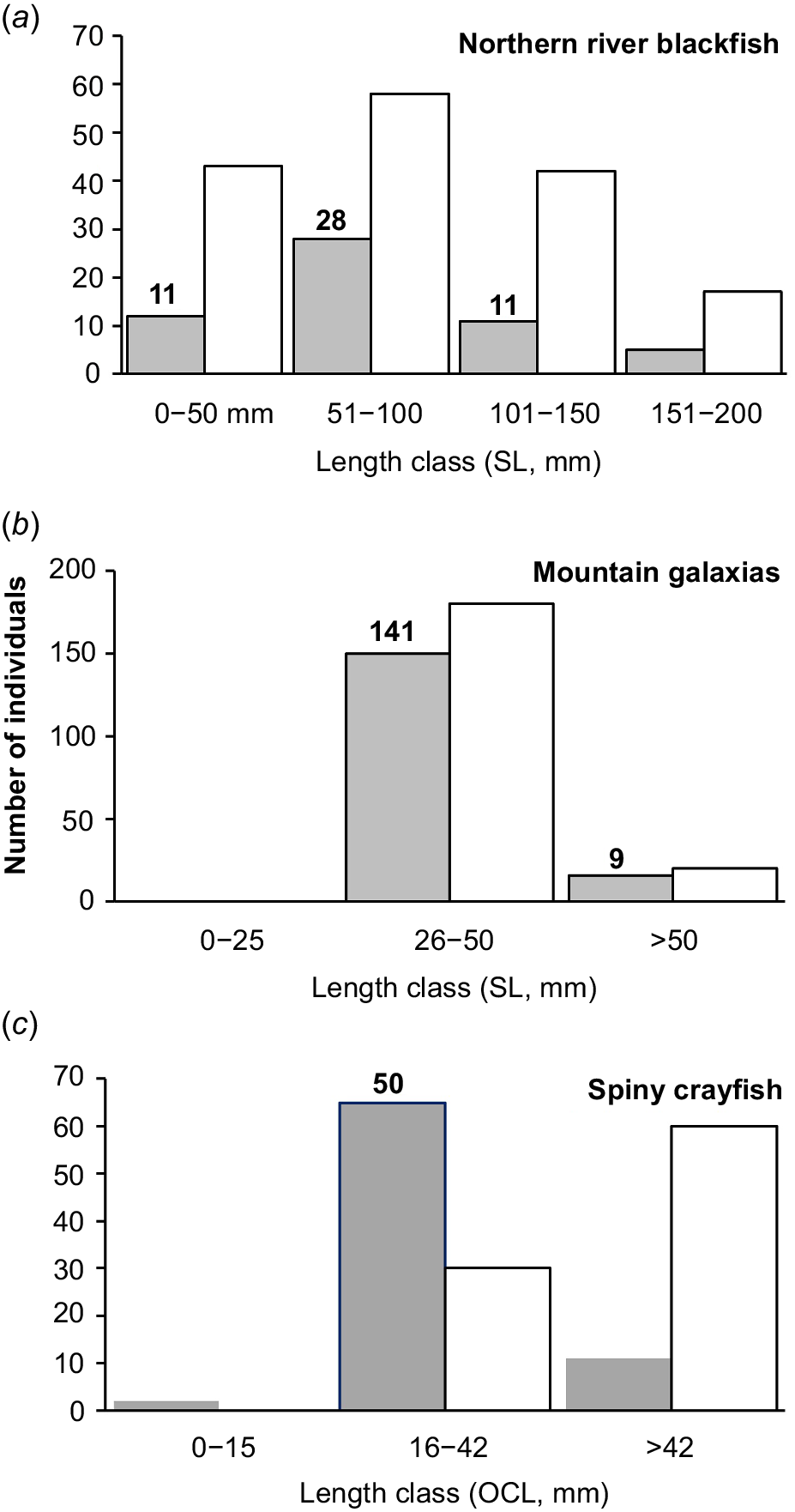
Over a 2-day period over 3–4 February 2020, 150 mountain galaxias and 50 of each of the northern river blackfish and spiny crayfish were collected and taken into captivity. Northern river blackfish were collected by back-pack electrofishing (Fig. 3a) as were small proportions of the spiny crayfish and mountain galaxias encountered. Most spiny crayfish were collected in dip-nets in shallow pools and runs. Baits (lamb bones) were used in deep water (>1 m) and primarily in the plunge pool below Browns Falls to lure crayfish to the water edge for dip-netting. Most mountain galaxias were simply collected in dip-nets. Fish were measured for standard length (SL, mm) and crayfish were measured for occipital carapace length (OCL, mm). We primarily retained the abundant smaller juvenile crayfish (<40 mm OCL; cf. Coughran 2008, 2013) and juvenile blackfish (Turschwell et al. 2020 considered adults >115 mm TL) to leave adults to breed and maintain wild populations (noting these species are long-lived and somewhat slow to mature) if they survived the harsh and worsening conditions at the time (Fig. 4). We retained mostly small galaxiids (141 individuals, 28–50 mm SL) and some larger fish (9 individuals, 52–72 mm SL) as per availability (Fig. 4). We did not retain large adult river blackfish because of their known aggression in small confines.
In February 2020, collection of northern river blackfish from Spring Creek was achieved by (a) backpack electrofishing, and (b) mountain galaxias were collected in shallow bedrock pools, whereas (c) some parts of the upper reaches were nearly dry, and (d) the lower reaches were heavily silted with minimal riparian tree cover.

The size range of each focal species, (a) northern river blackfish, (b) mountain galaxias, (c) spiny crayfish, with white bars representing captures, grey bars representing observed, and bold numbers are the number of individuals retained for captivity. Note that we endeavoured to keep small northern river blackfish and spiny crayfish.
The surface water of Spring Creek had ceased to flow at some parts along the reach at the time of the salvage effort (e.g. Fig. 3c), although there was isolated flow at some specific micro-locations (Fig. 3a, b, d) and heavy siltation was observed in bedrock-dominated pools and channels (Fig. 3a). Data loggers showed that the water temperature in the cleared sections exceeded the critical thermal maxima (27–28°C) of the cold-water species (Bone et al. 2014; Turschwell et al. 2020) in the month prior to our collecting, whereas in the forested sections, the water temperature was cooler and remained below species tolerance limits (Fig. S1). Unsurprisingly, the initial collection of fauna showed that the target species were mostly in the forested part of the creek. The northern river blackfish were essentially confined to the pools, the spiny crayfish were observed in the pools and runs but not the shallow riffles, and the galaxiids were schooling mainly in slow flowing, shallow water (<20-cm depth) over bedrock (Fig. 3b). The entrances to juvenile crayfish burrows were easily observed (localised clean sediment) and were numerous. Juvenile crayfish were conspicuous in rocky runs and cobble-boulder sections of pools.
Lower Spring Creek catchment (450 m downstream of Browns Falls) gives way sharply to farming land and has negligible intact forest and limited riparian shading. Eroded banks and in-channel siltation were a dominant feature of the unforested part of the stream (Fig. 3d). We collected several common yabbies, Cherax destructor (Clark, 1936), but very few northern river blackfish in association with isolated structure there. No alien fish or alien crustacean species were detected during the collection.
Fishes were transported by vehicle in a 600-L, 80-mm-thick, fully insulated transport container. Fishes were gradually exposed to the transport-container water by adding this water into the buckets where fishes were temporarily held for transfer from the stream to vehicle. It was filled with 400 L of chilled water (maximum 20°C) and aerated with pure oxygen at a rate of 0.5 L min−1. Small fishes (all mountain galaxias, and northern river blackfish <80 mm TL) were separated from larger northern river blackfish, with the former being held in fine-mesh bait-trap compartments (45 × 25 × 25 cm). Spiny crayfish were transported in the airconditioned cabin of the vehicle, within foam boxes; individually contained within plastic jars with breather holes in the lids and wet tissue paper on the floor to provide a moist environment and maintain humidity. Fishes and crayfish were transported to the captive environment in Brisbane (~2.5-h drive from collection site) without mortality. A visual check on animals was provided ~1 h into the commute. Fish were collected with soft mesh nets and placed in buckets with one-third water from the transport container, then topped up gradually with water from the aquaria flow through system over a period of an hour, before being placed in specific aquaria. Crayfish were added last to multi-species aquaria and simply transferred directly without gradual acclimation to the new aquaria water.
Captivity
Animals were held in indoor aquaria in two parallel banks at Jardini Pty Ltd, a privately owned aquaculture facility used for ornamental aquarium fish and invertebrates in Brisbane. Each bank comprised five 200-L aquaria (600 × 600 × 600 mm) on a top shelf, a 1000-L aquarium (2400 × 600 × 600 mm) on the mid-level and a trickle filter and sump on the lower level and all aquaria had glass lids to prevent loss of animals (Fig. 5a). This recirculating system incorporated two chiller units (Teco TK-2000) ensuring water temperature remained below 23°C and contained mains water supply (Brisbane) following dechlorination. A sand filter was used with water returns at the water surface and additional aeration was provided by air lifts (neutral pH similar to that at Spring Creek). Aquaria Bank-A had been maintained for 6 weeks, whereas Aquaria Bank-B was plumbed in only 2 weeks before arrival of animals. However, half the sump filter media (i.e. bioballs) was transferred from Bank-A to Bank-B and topped up with new media and similarly water was transferred across banks and topped up with town water supply. Water temperature was monitored daily, and pH, nitrite, nitrate and ammonia tested weekly at a minimum. Day–night cycle was maintained with 12 h on-time of LED lighting. Aeration was also used in aquaria. Structure was provided in the form of polyvinyl–chloride (PVC) pipes (25-mm length, 50-mm diameter) and vertical shade cloth weighted by cobble (Fig. 5b). Substantial open water space in the two larger aquaria was intended to provide mountain galaxias with some opportunity to coexist with the other species, keeping in mind that constructing more elaborate riffle-run-pool habitat at an extent of tens of metres was beyond the scope for the resourcing of this exercise.
Captive holding of aquatic fauna made use of banks of aquaria, as shown here by (a) Bank-A, with multiple small (200-L) aquaria on top level, large 1000-L aquaria on middle level and trickle filter below; (b) aquaria contained two types of purpose-built structure namely tube stacks comprised of 5- × 150-mm length of (25-mm diameter) and (50-mm diameter) polyvinyl-chloride (PVC) secured by cable ties to a 200- × 100- × 8-mm tile, and vertical mesh ladders composed of rock-weighted, fine, garden-mesh; (c) the remains of a recently moulted spiny crayfish immediately after multiple northern river blackfish had eaten much of the individual.

Animals were allocated by placing an individual of a similar size in the opposing replicate aquarium such that one replicate (individual) was assigned to Bank-A aquaria and the other to Bank-B. Both banks received the same treatments. The treatments allocated to small aquaria were (a) 3 northern river blackfish only, (b) 45 mountain galaxias only, (c) 3 spiny crayfish only, and (d) community comprising 3 northern river blackfish, 5 mountain galaxias and 3 spiny crayfish. The large aquaria each contained a community of 15 northern river blackfish, 15 spiny crayfish and 25 mountain galaxias. The remaining two small aquaria were used to house the eight remaining northern river blackfish that were substantially larger than the other northern river blackfish in our sample, to minimise conflict in the treatments. Captive animals were observed daily for general condition and fed to satiation at least 3 days per week (and typically 4–5 days per week).
We suspected a water-quality problem early in the trial because mortality of mountain galaxias was observed in Bank-B aquaria. Nitrite, nitrate, and ammonia were undetectable, and we suspect the problem was related to a PVC glue recently used in plumbing a filter. An immediate water change improved the situation; however, most mountain galaxias in Bank-B had already suffered irreversible damage and the condition of the base of the tail (caudal peduncle) of several northern river blackfish (Bank-B) had rapidly deteriorated. This compromised comparison across the two banks of aquaria. The following interpretations come largely from Bank-A aquaria and, therefore, should be viewed with caution because of a lack of replication (Fig. 6).
Progression of survivorship of animals in the two large aquaria: (a) Bank-A, which was unaffected by water-quality issue, and (b) Bank-B, in which water quality was affected (mountain galaxias. black; spiny crayfish, dark grey; northern river blackfish, light grey). Note: there was rapid ‘natural’ predation of mountain galaxias in Bank-A and even more rapid decline of mountain galaxias in the water quality-affected aquarium. There was also a delay in mortality of several northern river blackfish associated with the original poor water-quality incident in Bank-B. Reduced spiny crayfish survivorship was consistently a function of moulting, and notably later in the trial.
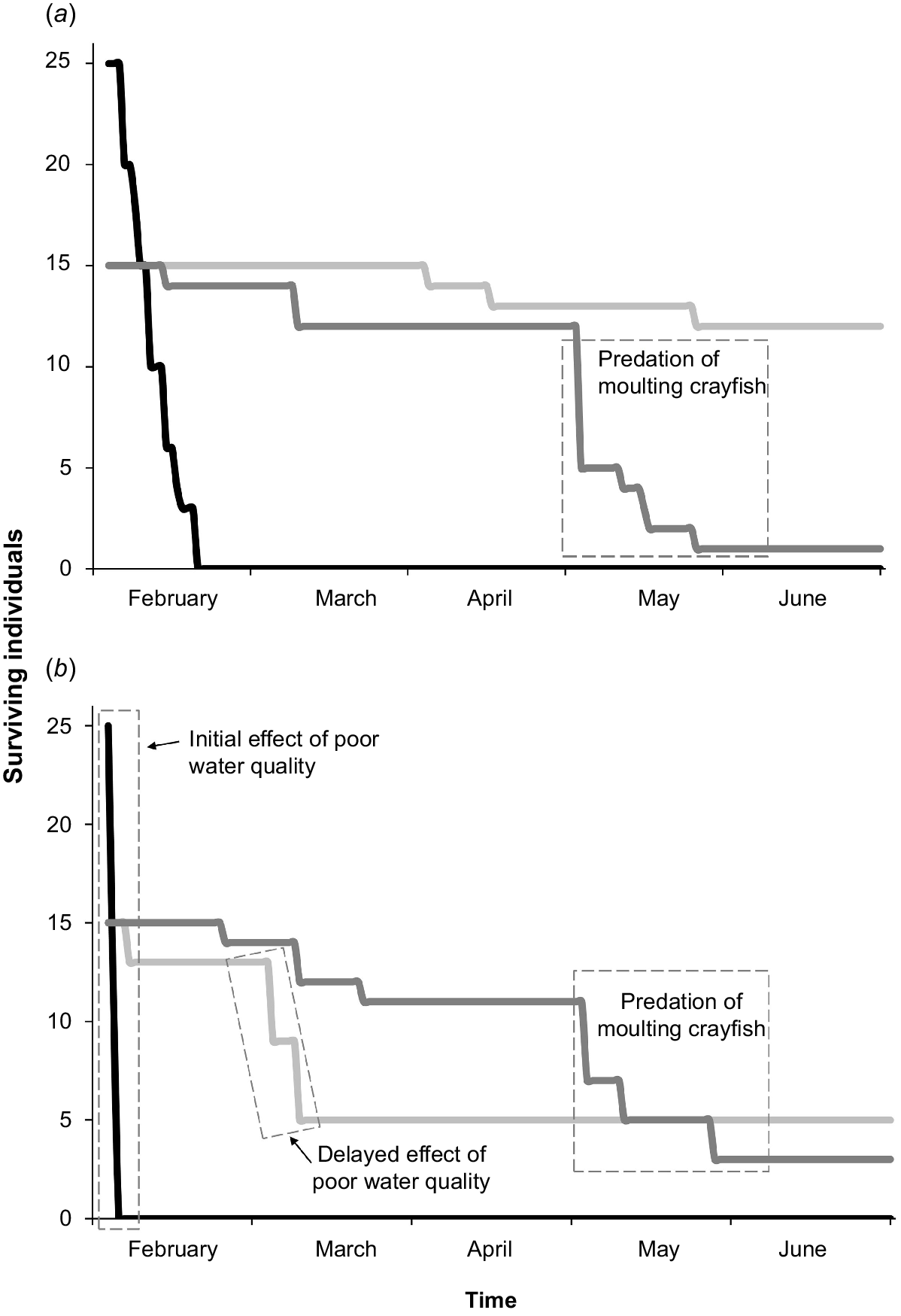
Mountain galaxias fed, grew, and showed high survivorship (89%) in the galaxias-only Bank-A aquarium. This simply reinforced what was known before the experiment, namely, that this species can be kept in groups in small aquaria as long as upper temperature thresholds are not approached (e.g. Marshall 1989). Mountain galaxias did not fare well in small or large aquaria in mixed-species treatments owing to harassment by northern river blackfish and, ultimately, predation by northern river blackfish and spiny crayfish. If this species is to be maintained in longer-term community arks such as in outdoor ponds for years, it would be useful to determine at which scale habitat heterogeneity enables this small, short-lived fish to persist in the presence of other upland macrofauna. Specifically, we recommend experimenting with favourable habitats such as shallow or flowing-water habitat patches akin to what was observed of this species in their spatial isolation from spiny crayfish and northern river blackfish in Spring Creek. Furthermore, it is worth noting that in June 2020, mountain galaxias in Spring Creek were found only in the same shallow, flowing, bedrock habitats as previously observed, whereas they also occupied deeper pool habitat in Adjinbilly Creek in the absence of northern river blackfish.
Equally, an understanding of unfavourable habitats for northern river blackfish and spiny crayfish can be factored into testing appropriate habitat complexity in ponds. For instance, a lack of interstices and structure in substantial areas of pond bed would be likely to reduce negative interactions for the mountain galaxias in relation to the other two species. It may be worth testing engineering solutions such as coarse-mesh fencing to enhance segregation of mountain galaxias from other species.
Spiny crayfish had an overall survivorship of 25% across the entire study. Predation, including cannibalism, was the main reason for this poor survivorship. Specifically, much of the poor survivorship occurred late in the captive trial when individuals moulted and became exposed to predators, including by cannibalism (Fig. 5c, 6). Similarly, other studies have found that juvenile crayfish survivorship is a function of moulting frequency and habitat complexity (Stein 1977; Taugbøl and Skurdal 1992; Olsson and Nyström 2009). The moulting period temporarily removed the protection afforded by a hard exoskeleton, rendering individuals vulnerable to cannibalism and predation by northern river blackfish. Notably, in aquaria, northern river blackfish at times acted in packs to prey on individual crayfish that were moulting or had recently moulted (Fig. 5c). The relevance of this cooperative feeding behaviour under field conditions is unknown and it may be an artefact of captive conditions. In fact, there is little direct field-based evidence of Gadopsis spp. feeding on crayfish, with dietary studies indicating a reliance on insect larvae (Jackson 1978; Lintermans 1998; Rees et al. 2020). The shade-cloth curtains had been added to the aquaria specifically to mediate crayfish moulting-related predation (including cannibalism). Although the probability of natural survivorship of juvenile spiny crayfish is unknown, clearly, more complex refuge habitat, and possibly reducing crayfish density (relative to levels used here), warrant investigation in community ark development involving this species (or other spiny crayfish).
The juvenile northern river blackfish coexisted in small and large aquaria, exhibiting infrequent intraspecific aggression. Juveniles were also seen coexisting and not being aggressive in the field prior to collection. In comparison, individuals of the two-spined blackfish, Gadopsis bispinosus, can be aggressive to one another even as early juveniles (~50 mm TL, Ebner et al. 2009). The current study demonstrated that juvenile northern river blackfish can be maintained temporarily with a high survivorship (for months), but adults are far more problematic in confined space. This is in line with previous findings of subadult and adult Gadopsis being hostile in captivity (e.g. Lintermans 1998; Westergaard and Ye 2010). Therefore, if the intention is to only temporarily maintain a captive sample from a population before returning them to the wild once suitable conditions occur at the point of capture (Hammer et al. 2009), logistically, juvenile-phase Gadopsis makes an attractive prospect relative to keeping large adults. Specifically, juveniles can coexist. We recommend further investigation into coexistence of river blackfish of different developmental stages, with a focus on habitat complexity, and the extent of enclosure.
Fast-growing or large individuals, and especially adult northern river blackfish, provide a different scenario. Territoriality becomes a driving factor for adults within small aquaria, with dominant individuals attacking conspecifics and preventing them from accessing food or maintaining sheltering positions. Intraspecific aggression of large juvenile and subadult northern river blackfish was observed in large aquaria but was mediated to an extent by the greater space available for subordinates to find refuge away from dominant individuals. The trial demonstrated that juvenile northern river blackfish can cohabit in confined areas for months, whereas, adults should be kept in solitary confines if insufficient space is available for multiple territories to be established. This aligns with suspected aggression reported of adults held in captivity by Westergaard and Ye (2010). In the wild, juveniles in Spring Creek were coexisting within the territories of larger adults. This aspect of life-history stage coexistence warrants further investigation in wild and captive environments, especially regarding scales that incorporate multiple adjacent adult territories.
The short-term captive trial demonstrated that in small enclosures, aggression and predation are important considerations when housing multiple individuals of conspecific and multiple species. Rapid growth of northern river blackfish such that they approach maturity and become increasingly territorial and aggressive (Westergaard and Ye 2010), and the moulting of crayfish (Furse and Wild 2004), are key (ontogenetic) processes to consider. The observation of O’Connor and Zampatti (2006) also serves as a reminder that Euastacus predation on eggs of Gadopsis may be an important process in mixed-species arks, further reinforcing the need for the provision of adequate interstices. Collectively, these observations indicate that short-term holding of the spiny crayfish and northern river blackfish should be conducted with solitary individual-dedicated aquaria, and longer-term housing should be investigated in large systems (e.g. ponds) with adequate structure to mediate aggression and predation, facilitate territory establishment and potentially enable breeding and coexistence of multiple life stages and species.
Release challenges and options
The COVID-19 pandemic provided several complications for the project and among these were safety and legal requirements for staff to travel and operate in society. Access to field sites, including Spring Creek, was not permitted by government during March–July 2020. This inhibited necessary surveys to determine the status of Spring Creek hydrology and of wild populations of focal species, and to facilitate and inform potential reintroduction or translocation site assessment. Fortunately, the project team was granted an extension for holding species in captivity from the Department of Agriculture and Fisheries (DAF) and the Department of Environment and Science (DES) was able to resource another month of captive housing of fauna, and Jardini Pty Ltd was able to consolidate aquaria for use.
Among the options canvassed were finding resources to continue housing the animals in DES custody, finding public display (e.g. public aquaria), or non-profit institutional facilities (e.g. universities) to house the animals, releasing the animals into Spring Creek, releasing the animals elsewhere or euthanasing animals. The project team made several enquiries in this regard and the options were uncertain and shifting rapidly in the COVID-19 environment. For instance, public aquaria were not open for business and were certainly not looking to expand their number of exhibits and quantity of fauna to house and feed under those circumstances.
In June 2020, a field survey was deemed COVID-19 safe and legal, and this confirmed that Spring Creek was in a slightly improved hydrological state relative to during the previous summer. Both fish species were persisting (Supplementary Table S1), and individuals captured by electrofishing appeared healthy. This meant there were no major population level benefits in returning animals to their source location and possibly we could have collected animals prematurely (although if extinction had occurred the narrative would be radically different). Adjinbilly Creek, a nearby location, had historically supported a northern river blackfish population but they were thought to have been extirpated by upstream land use practices a decade or more previously. A survey of the stream confirmed it no longer contained a functioning northern river blackfish population (Table S1). The recent survey also found Adjinbilly Creek to harbour a healthy population of mountain galaxias, as it did historically (Table S1). Euastacus (crayfish) hibernates in burrows over winter, so spiny crayfish presence could not be confirmed by this survey. However, there was an assumption made that if the galaxiids survived the hot summer and were healthy, the crayfish would be too. Local property owners confirmed that spiny crayfish occupy Adjinbilly Creek. Thus, the Adjinbilly Creek site was identified as the candidate release site for the remaining captive northern river blackfish (Fig. 1).
Reintroduction of all three species to Spring Creek was ruled out, as per the stipulated conditions of the DAF General Fisheries Permit of the project, which precluded reintroduction into extant, healthy populations to mitigate the risk of introducing disease. The animals had initially been collected primarily to guard against a mass extinction event throughout Spring Creek. Additionally, there was little perceived gain of returning the individuals (primarily juveniles) into established populations, given this would be likely to disrupt the social hierarchies of territorial northern river blackfish and spiny crayfish. Similarly, there was no possibility of returning spiny crayfish or mountain galaxias to the new Adjinbilly Creek location. A preferable solution to euthanasia (the default last resort on the salvage permit if no relocation was possible) was found and custody of the non-release species was transferred to research groups for experimental work. The spiny crayfish were transferred by permit to the University of the Sunshine Coast and the mountain galaxias to the University of Queensland. The latter research has measured the thermal and swimming ability of mountain galaxias. Each of these live animal transfers occurred with the approval of DAF.
On 29 July 2020, the remaining 26 northern river blackfish were individually bagged in oxygenated water and transported in cooler boxes (Esky) to Adjinbilly Creek. On site, fish were acclimatised to ambient creek water temperature by floating the bags for 30 min, and to ambient water chemistry with several partial water exchanges during this period. They were then released into two large, deep pools in the stream, and all appeared healthy on release. Environmental DNA (eDNA) water samples of Adjinbilly Creek were taken before and after release, as well as from the transport bags and Spring Creek capture site. This confirmed the absence of river blackfish before release in Adjinbilly Creek and the presence of river blackfish in both streams after the release (subsequently, eDNA monitoring showed short-term persistence at the Adjinbilly Creek site). Prior trials had confirmed the suitability of existing primers for northern river blackfish that had been developed from Victorian river blackfish specimens (EnviroDNA, Melbourne, Vic., Australia).
Discussion
The current study serves as a reminder that rapid rescue, housing of cold-water specialist taxa, and repatriation are not straightforward processes. Below we discuss the significance of our findings for future adaptive management of cold-water specialist stream taxa, noting that pending extinction of these fauna is a global problem (McCullough et al. 2009; Whitney et al. 2015; Kovach et al. 2017; Turschwell et al. 2017; Richardson 2019; Hossack et al. 2023).
Rescue
The activity reported on here involved logistical, legal and ethical considerations in performing animal rescues. Complications included legally collecting, transporting and holding threatened species at short notice, as has been reported elsewhere (Hammer et al. 2013; Gregory et al. 2021; Palmer 2021; Ladle et al. 2023). Responding to threatened species population needs during environmental disasters creates a challenging and tenuous environment in which to take action and in some cases can be achieved outside of government (e.g. Hammer et al. 2013) or in cooperation between government and private entities (this study). Government agencies and politicians are becoming increasingly familiar with climate-change impacts and natural disaster response to achieve utilitarian objectives. The rhetoric associated with response and preparedness for climate change to aid humans can be extended to convey the requirements of threatened fauna in countering climate change. For instance, evacuation centres serve as locations for temporary human occupation during natural disasters (e.g. cyclones, floods, bushfires) and similarly government and non-government facilities may serve this function for temporary protection of fauna. We reiterate the suggestions of others in strategically using natural and constructed wetland refuges (e.g. Beatty et al. 2017), or custom facilities (e.g. Johnson and Jensen 1991), for securing high-value aquatic species, both temporarily and on longer-term bases. Given the observable and projected escalation in the frequency and severity of extreme events, there is a strong and growing need for rescue procedures and options to be planned strategically in advance of the next disaster, rather than these being reactive and ad hoc, as was the case here.
An added consideration for rescuing cold-water specialist taxa during drought is minimising stress to already stressed animals. In our case, this involved using large transport containers to buffer against heating of water during transport of aquatic species (i.e. fishes) from mountain regions to the captive environment, which is set in a lowland, subtropical climate (in chilled tanks) and segregating potentially aggressive individuals (i.e. each crayfish) prior to transport. When natural disasters occur rapidly (e.g. cyclones, heatwaves, bushfires), there is little scope for choosing when to perform rescue of wild populations of animals, whereas during gradual climate extremes (e.g. drought), there is some scope for choosing seasons or even days that minimise thermal stress during collection and transport.
Housing multiple taxa
Temporary housing and husbandry of multiple species is a logistical challenge, particularly if holding facilities must pivot from maintaining other taxa (e.g. warm-water species, ornamental species, recreational angling species) and staff must act within the scope of their existing workloads and tasks.
In captivity we observed aggressive encounters within and among species and notably among adult northern river blackfish in close confines, and among crayfish. Intraspecific aggression is recognised as an important behaviour in juvenile and adult Gadopsis spp. and Euastacus spp. (Hazlett et al. 2007; Ebner et al. 2009; Hammer et al. 2013; Lopez et al. 2019; O’Hea Miller et al. 2022). Severe aggression (including injury or mortality) is obviously less than ideal in ark populations where a central aim is to maintain small numbers of species as either brood animals or for direct translocation or reseeding wild populations. In aquaculture, grading fish and crayfish by size has long been used to minimise loss of individuals owing to intraspecific aggression and predation, and physical isolation of individuals is at the extreme end of strategies used to ensure survival. In the current study, we observed that juvenile river blackfish can coexist, given sufficient extent and structure. However, ensuring that non-lethal levels of aggression are maintained in housing multiple individuals and species is a challenge for community rescues. We contend that this should be attainable, given coexistence of species and their multiple life stages in small streams and confined pools under field conditions.
In the case of Spring Creek, juvenile and adult northern river blackfish and a range of size classes of spiny crayfish coexist within small pools and run sequences. This includes multiple adult river blackfish and adult spiny crayfish sheltering in proximity (within metres). A priority for future investigation is understanding how to house multiple adults of each and both species in captivity. Both Gadopsis spp. and Euastacus spp. have small home ranges and even smaller home-shelter or territorial spaces (nested with the home range; Khan et al. 2004; Koster and Crook 2008; Ryan et al. 2008; Broadhurst et al. 2012). Central to maintaining multiple adult river blackfish and spiny crayfish is likely to be provision of adequate proportions of structure and spatial extent. Importantly, structure has been the key focus in experiments aimed at understanding aggression of spiny crayfish especially, and this frequently involves quantifying behaviour of combatants during short-term contests lasting minutes (Hazlett et al. 2007; Lopez et al. 2019; O’Hea Miller et al. 2022) for ethical and logistical reasons.
Conversely, for the purposes of keeping multiple individuals, species and life stages, we argue that specific focus on spatial extent (water volume, benthic surface area) and habitat heterogeneity warrants detailed investigation. This may be best achieved in large aquaria (e.g. ~1000 L), above-ground enclosures or earthern ponds. Locating or engineering these enclosures to maintain an adequate thermal regime is one challenge, but maintaining capability to visually monitor and quantify inter- and intraspecific interactions is also essential for progress. For instance, we have negligible quantitative or qualitative behavioural understanding of coexistence of juvenile and adult river blackfish within adult territories, let alone interactions among multiple species that coexist with river blackfish (e.g. spiny crayfish, galaxiids) and their different life stages. Much of our understanding is confined to single-species studies (Khan et al. 2004; Starrs et al. 2015; Turschwell et al. 2017, 2018, 2020). Transition from glass aquaria to larger systems will likely aid coexistence of multiple taxa but will require indirect surveillance techniques (e.g. remote video) or other forms of direct observation (e.g. snorkelling, viewing windows or underwater observatories). This is vastly different from the minimum requirements for direct observation that occur in traditional monoculture-based aquaculture, including conventional threatened species husbandry based on broodfish ponds and dedicated grow-out ponds for progeny for which interspecies interactions are not relevant.
From observations made in Spring Creek, and based on our captive housing, G. olidus, represents a more significant challenge in the multi-species housing context (single-species success with captive galaxiids was acknowledged in the Introduction). The species schools and is often associated with shallow running water. Drought conditions and extended periods without patches of lotic habitats (from micro- to macro-scales) may be problematic for this species, at least in community contexts involving predatory macrofauna such as northern river blackfish and crayfish, and certainly alien trout (Lintermans 2000). Future experimentation involving multi-species housing that includes galaxiids should investigate shallow- and running-water microhabitats as well as larger mesohabitats or refugia, because there is currently a rudimentary appreciation for how galaxiids persist in multi-macrofauna contexts involving predators and intraguild competition in upland systems.
Bureaucracy and climate-change capacity
Hammer et al. (2013) spoke of the challenges of working to rescue and recover small-bodied threatened fishes in South Australia during drought conditions in the lower Murray River section of the southern MDB. They included mention of an initial ad hoc phase of action requiring permitting, a lack of government resourcing, and an overall absence of key technical information regarding focal species, including habitat requirements and captive care, which posed impediments to protecting populations facing immediate extinction. They concluded with a call for preparedness in drought-prone regions. From the experiences detailed in the current paper, we echo similar rhetoric from our case study of cold-water-specialists in the northern MDB. It also leads us to recommend that the northern MDB requires increased resourcing for aquatic fauna rescue in response to climate change and human-exacerbated problems. The cold-water specialists represent a unique subset within the wider aquatic fauna of the region and dryland regions more generally, and a faunal group that requires identified and pre-event-established holding capabilities.
Riparian zone rehabilitation to buffer stream overheating is one field-based method of dampening the effect of climate change (e.g. Turunen et al. 2021) and one that might be considered in parallel with temporary ark populations.
The absence of a dedicated state government hatchery for housing cold-water species in Queensland is of concern because projected climate shifts and increasing demand for human water resources (Ndehedehe et al. 2023) will inevitably place further pressure on isolated freshwater fauna assemblages, including cold-water specialists (e.g. Kovach et al. 2017; Vinarski et al. 2022). The absence of a government hatchery or dedicated facility is unsurprising, given how few cold-water specialist fishes are recognised in Queensland (on account of it being almost entirely comprised of tropical, subtropical, arid and semi-arid ecosystems and a plethora of recreational fishing species that are stocked into reservoirs). However, the precariously small distribution of the 19 described and likely many undescribed spiny crayfish species that are endemic to the state and associate with mountain peaks (e.g. McCormack 2012) highlights the need for an equipped cold-water facility.
Some success with single-species, captive breeding of temperate galaxiids provides a level of optimism for their captive care (e.g. Mitchell 1989; Stoessel et al. 2015, 2020). In this regard, there is scope for interstate collaboration for conserving fauna from the northern MDB uplands. In turn, this might pave the way for permanent ponds or enclosures featuring climate-resilience attributes that inform wider societal protection of natural waterways and farm dams, noting that human protection of surface water is likely to be one of the best chances that threatened aquatic species have to persist (Beatty et al. 2017).
We have discussed several significant challenges of holding mixed taxa following rescues of cold-water specialist stream fauna. There is also some merit in developing philosophy around the potential benefits of maintaining assemblages or communities and not simply maintaining monocultures. One of these potential benefits is minimising domestication (see Hagen et al. 2019) and maintaining populations of species that are prepared for being rewilded (Corlett 2016). We argue that this preparation may be better achieved by creating near to real scenarios (with regard to competitors, predator–prey and dominance hierarchies, habitat conditions) rather than simply providing simulations of single processes (e.g. scaring fish or exposing them to the odour of a predator; cf. Corlett 2016). In the process, there is inevitably going to be variable levels of survivorship of animals, including real and perceived failure, and yet this should not hinder transparency, learning and reporting (e.g. Cochran-Biederman et al. 2015).
Claussen and Philipp (2023) presented an argument for diverting investment away from hatcheries and restocking toward reserve-based systems and partial alleviation of field-based threats to fish biodiversity. Although we largely align with this thinking, we also contend that there is some merit in captive care of select species, assemblages or communities, under a subset of circumstances. From a conservation perspective, those circumstances include where highly localised endemic species or remnant communities exist in the riverscape and when these remnants are vulnerable to stochastic events including natural disasters (see also Caughley 1994; Caughley and Gunn 1996). An additional advantage of recreating venues (e.g. farm dams, aquaculture ponds) for housing multiple freshwater species derived from rescues, which we express here, is using them as venues for intensive learning as to how assemblages and communities function. If cold-water-specialist taxa that exist in isolated upland assemblages are to be better understood in captivity, this will also require a rethink as to the habitat structure that mediates ecological interactions and is typically found in wild systems (see, for instance, Hutchison et al. 2020). Importantly, these bedrock-dominated systems differ substantially from the earthen ponds and farm dams commonly used to house captive freshwater fishes.
Rescue triggers
Stream gauging stations provide an important means of identifying when stream temperature or discharge is approaching harmful thresholds for priority populations or aquatic communities (Crook et al. 2010). Unfortunately, the recent decommissioning of the relevant stream gauge at Spring Creek during the life of our project illustrates a resourcing problem and an environmental prioritisation issue for the Queensland state government. Temporary rescues are then one of the main options for ensuring that important remnant populations or even species are retrievable from extinction events (Lintermans et al. 2015). Farm dam populations sensu ‘natural hatcheries’ (Hammer et al. 2009; Lintermans et al. 2015) or other human water storages in the landscape (Beatty et al. 2017) can serve as important refuges for threatened aquatic fauna. Here, we contend that it may be worth championing more sophisticated mixed-species communities that mimic real systems. The trigger for which should be considered prior to the climatic events that activate specific rescues, so that infrastructure and fauna keeping problems can be progressed a priori. Effectively, this might take the form of long-term/multigenerational housing of taxa in non-intensive ponds. As was discussed earlier, for cold-water specialist species, this will involve careful site selection and thermal buffering features.
Conclusions
Holding mixed taxa following field rescues requires attention to detail, including knowledge and monitoring of the developmental-stage combinations that are interacting within and across species, enclosure volume and availability of structure. Cold-water specialist organisms present an added challenge for institutions situated in predominantly warm-water regions, where expertise, capability and facilities typically serve warm-water fauna. The simultaneous rescue of multiple co-occurring endemic taxa has received minimal scientific attention compared with single-species rescues, but represents a promising aspect of research and adaptive management in the era of global climate change. Short-term multiple species care and longer-term multiple species care, including multi-generational population refuges, warrant consideration and planning for countering the effects of climate-change processes and natural disasters. Given the observed and projected changes to climate and weather extremes, such actions are becoming increasingly critical if we are to prevent the increasingly likely extinctions of many cold-water specialist freshwater species.
Declaration of funding
The project was funded through the Emergency Response Plan under the Native Fish Recovery Strategy.
Acknowledgements
We acknowledge the Githabul, the traditional people of the lands in which this project was conducted. The project was funded through the Emergency Response Plan under the Native Fish Recovery Strategy. This was achieved through the Native Fish Recovery Strategy Steering Group as informed by the Queensland Interdepartmental Fish Death Response Committee. Thanks go to Stuart Little (MDBA), Mal Smith, Thomas Hart (DAF), David Wiskar (DNRME), Paul Lawrence. Glenn McGregor (DESI), Natasha Chan (DESI) and Karin Vanderheijde (DNRME) for assistance. Greg Ringwood provided feedback on an earlier draft of the work and Mark Lintermans and Paul Ryan (NSW DPI) provided useful suggestions regarding relevant literature. Two external reviewers provided input to improve the document.
References
Beatty S, Allen M, Lymbery A, Jordaan MS, Morgan D, Impson D, Marr S, Ebner B, Weyl OLF (2017) Rethinking refuges: implications of climate change for dam busting. Biological Conservation 209, 188-195.
| Crossref | Google Scholar |
Bogan MT, Boersma KS, Lytle DA (2015) Resistance and resilience of invertebrate communities to seasonal and supraseasonal drought in arid-land headwater streams. Freshwater Biology 60(12), 2547-2558.
| Crossref | Google Scholar |
Bond NR, Lake PS, Arthington AH (2008) The impacts of drought on freshwater ecosystems: an Australian perspective. Hydrobiologia 600(1), 3-16.
| Crossref | Google Scholar |
Bone JWP, Wild CH, Furse JM (2014) Thermal limit of Euastacus sulcatus (Decapoda: Parastacidae), a freshwater crayfish from the highlands of central eastern Australia. Marine and Freshwater Research 65(7), 645-651.
| Crossref | Google Scholar |
Broadhurst BT, Lintermans M, Thiem JD, Ebner BC, Wright DW, Clear RC (2012) Spatial ecology and habitat use of two-spined blackfish Gadopsis bispinosus in an upland reservoir. Aquatic Ecology 46, 297-309.
| Crossref | Google Scholar |
Caughley G (1994) Directions in conservation biology. Journal of Animal Ecology 63, 215-244.
| Crossref | Google Scholar |
Claussen JE, Philipp DP (2023) Assessing the role of supplementation stocking: a perspective. Fisheries Management and Ecology 30(6), 583-591.
| Crossref | Google Scholar |
Cochran-Biederman JL, Wyman KE, French WE, Loppnow GL (2015) Identifying correlates of success and failure of native freshwater fish reintroductions. Conservation Biology 29(1), 175-186.
| Crossref | Google Scholar | PubMed |
Corlett RT (2016) Restoration, reintroduction, and rewilding in a changing world. Trends in Ecology & Evolution 31(6), 453-462.
| Crossref | Google Scholar | PubMed |
Costa-Pierce BA (2021) The principles and practices of ecological aquaculture and the ecosystem approach to aquaculture. World Aquaculture 52(1), 25-31.
| Google Scholar |
Costion CM, Simpson L, Pert PL, Carlsen MM, John Kress W, Crayn D (2015) Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biological Conservation 191, 322-330.
| Crossref | Google Scholar |
Coughran J (2008) Distinct groups in the genus Euastacus. Freshwater Crayfish 16, 123-130.
| Google Scholar |
Coughran J (2013) Biology of the Mountain Crayfish Euastacus sulcatus Riek, 1951 (Crustacea: Parastacidae), in New South Wales, Australia. Journal of Threatened Taxa 5(14), 4840-4853.
| Crossref | Google Scholar |
Covich AP, Fritz SC, Lamb PJ, Marzolf RD, Matthews WJ, Poiani KA, Prepas EE, Richman MB, Winter TC (1997) Potential effects of climate change on aquatic ecosystems of the Great Plains of North America. Hydrological Processes 11(8), 993-1021.
| Crossref | Google Scholar |
Crook DA, Reich P, Bond NR, McMaster D, Koehn JD, Lake PS (2010) Using biological information to support proactive strategies for managing freshwater fish during drought. Marine and Freshwater Research 61(3), 379-387.
| Crossref | Google Scholar |
Dobson GP, Baldwin J (1982a) Regulation of blood oxygen affinity in the Australian Blackfish Gadopsis marmorata: I. Correlations between oxygen-binding properties, habitat and swimming behaviour. Journal of Experimental Biology 99(1), 223-243.
| Crossref | Google Scholar |
Dobson GP, Baldwin J (1982b) Regulation of blood oxygen affinity in the Australian blackfish Gadopsis marmorata: II. Thermal acclimation. Journal of Experimental Biology 99(1), 245-254.
| Crossref | Google Scholar |
Ebner B, Clear R, Godschalx S, Beitzel M (2009) In-stream behaviour of threatened fishes and their food organisms based on remote video monitoring. Aquatic Ecology 43, 569-576.
| Crossref | Google Scholar |
Froehlich HE, Gentry RR, Halpern BS (2017) Conservation aquaculture: shifting the narrative and paradigm of aquaculture’s role in resource management. Biological Conservation 215, 162-168.
| Crossref | Google Scholar |
Furse JM, Wild CH (2002) Prediction of crayfish density from environmental factors for Euastacus sulcatus (Crustacea: Decapoda: Parastacidae). Freshwater Crayfish 13, 316-329.
| Google Scholar |
Furse JM, Wild CH (2004) Laboratory moult increment, frequency, and growth in Euastacus sulcatus. Freshwater Crayfish 14, 205-211.
| Google Scholar |
Gebert F, Obrist MK, Siber R, Altermatt F, Bollmann K, Schuwirth N (2022) Recent trends in stream macroinvertebrates: warm-adapted and pesticide-tolerant taxa increase in richness. Biology Letters 18(3), 20210513.
| Crossref | Google Scholar | PubMed |
Gregory R, Kozak R, Peterson St-Laurent G, Nawaz S, Satterfield T, Hagerman S (2021) Under pressure: conservation choices and the threat of species extinction. Climatic Change 166, 2.
| Crossref | Google Scholar |
Hagen IJ, Jensen AJ, Bolstad GH, Diserud OH, Hindar K, Lo H, Karlsson S (2019) Supplementary stocking selects for domesticated genotypes. Nature Communications 10(1), 199.
| Crossref | Google Scholar |
Hammer MP, Bice CM, Hall A, Frears A, Watt A, Whiterod NS, Beheregaray LB, Harris JO, Zampatti BP (2013) Freshwater fish conservation in the face of critical water shortages in the southern Murray–Darling Basin, Australia. Marine and Freshwater Research 64(9), 807-821.
| Crossref | Google Scholar |
Hammer MP, Unmack PJ, Adams M, Raadik TA, Johnson JB (2014) A multigene molecular assessment of cryptic biodiversity in the iconic freshwater blackfishes (Teleostei: Percichthyidae: Gadopsis) of south-eastern Australia. Biological Journal of the Linnean Society 111(3), 521-540.
| Crossref | Google Scholar |
Hazlett BA, Lawler S, Edney G (2007) Agonistic behavior of the crayfish Euastacus armatus and Cherax destructor. Marine and Freshwater Behaviour and Physiology 40(4), 257-266.
| Crossref | Google Scholar |
Hossack BR, LeMoine MT, Oja EB, Eby LA (2023) Cryptic declines of small, cold-water specialists highlight potential vulnerabilities of headwater streams as climate refugia. Biological Conservation 277, 109868.
| Crossref | Google Scholar |
Huey JA, Balcombe SR, Real KM, Sternberg D, Hughes JM (2017) Genetic structure and effective population size of the most northern population of the Australian river blackfish, Gadopsis marmoratus (Richardson 1848): implications for long-term population viability. Freshwater Science 36, 113-123.
| Crossref | Google Scholar |
Hutchison M, Norris A, Nixon D (2020) Habitat preferences and habitat restoration options for small-bodied and juvenile fish species in the northern Murray–Darling Basin. Ecological Management & Restoration 21(1), 51-57.
| Crossref | Google Scholar |
Jackson PD (1978) Benthic invertebrate fauna and feeding relationships of brown trout, Salmo trutta Linnaeus, and river blackfish, Gadopsis marmoratus Richardson, in the Aberfeldy River, Victoria. Marine and Freshwater Research 29(6), 725-742.
| Crossref | Google Scholar |
Jansson R (2003) Global patterns in endemism explained by past climatic change. Proceedings of the Royal Society of London. Series B: Biological Sciences 270(1515), 583-590.
| Crossref | Google Scholar |
Khan MT, Khan TA, Wilson ME (2004) Habitat use and movement of river blackfish (Gadopsis marmoratus R.) in a highly modified Victorian stream, Australia. Ecology of Freshwater Fish 13(4), 285-293.
| Crossref | Google Scholar |
Kirk MA, Rahel FJ (2022) Air temperatures over-predict changes to stream fish assemblages with climate warming compared with water temperatures. Ecological Applications 32(1), e02465.
| Crossref | Google Scholar |
Koehn JD, Lintermans M, Lyon JP, Ingram BA, Gilligan DM, Todd CR, Douglas JW (2013) Recovery of the endangered trout cod, Maccullochella macquariensis: what have we achieved in more than 25 years? Marine and Freshwater Research 64, 822-837.
| Crossref | Google Scholar |
Koster WM, Crook DA (2008) Diurnal and nocturnal movements of river blackfish (Gadopsis marmoratus) in a south-eastern Australian upland stream. Ecology of Freshwater Fish 17(1), 146-154.
| Crossref | Google Scholar |
Kovach RP, Al-Chokhachy R, Whited DC, Schmetterling DA, Dux AM, Muhlfeld CC (2017) Climate, invasive species and land use drive population dynamics of a cold-water specialist. Journal of Applied Ecology 54(2), 638-647.
| Crossref | Google Scholar |
Krol L, Melton B, Delbeek JC, Dunker FH, Shepherd B (2023) The iconic philippine coral reef at steinhart aquarium: the husbandry, welfare, behavior, and veterinary care considerations of a large multi-taxa living coral reef system. Journal of Zoological and Botanical Gardens 4(4), 738-750.
| Crossref | Google Scholar |
Ladle RJ, Alves-Martins F, Malhado ACM, Reyes-García V, Courchamp F, Di Minin E, Roll U, Jarić I, Correia RA (2023) Biocultural aspects of species extinctions. Cambridge Prisms: Extinction 1, e22.
| Crossref | Google Scholar |
Lindenmayer DB, Taylor C (2020) New spatial analyses of Australian wildfires highlight the need for new fire, resource, and conservation policies. Proceedings of the National Academy of Sciences 117(22), 12481-12485.
| Crossref | Google Scholar |
Lintermans M (2000) Recolonization by the mountain galaxias Galaxias olidus of a montane stream after the eradication of rainbow trout Oncorhynchus mykiss. Marine and Freshwater Research 51(8), 799-804.
| Crossref | Google Scholar |
Lintermans M, Rowland S, Koehn J, Butler G, Simpson B, Wooden I (2005) The status, threats and management of freshwater cod species Maccullochella spp. in Australia. In ‘Management of Murray Cod in the MDB: Statement, Recommendations, and Supporting Papers’, 3–4 June 2004, Canberra, ACT, Australia. (Eds M Lintermans, B Phillips) pp. 15–29. (Murray–Darling Basin Commission: Canberra, ACT, Australia) Available at https://fish.gov.au/Archived-Reports/2014/Documents/2014_refs/MDBC%202005%20Management%20of%20Murray%20cod%20in%20the%20Murray-Darling%20Basin%20statement,%20recommendations%20and%20supporting%20papers.pdf
Lintermans M, Lyon JP, Hammer MP, Ellis I, Ebner BC (2015) Underwater, out of sight: lessons from threatened freshwater fish translocations in Australia. In ‘Advances in reintroduction biology of Australian and New Zealand fauna’. (Eds DP Armstrong, MW Hayward, D Moro, PJ Seddon) pp. 237–253. (CSIRO Publishing: Melbourne, Vic., Australia)
Lopez LK, Hendry K, Wong MYL, Davis AR (2019) Insight into invasion: interactions between a critically endangered and invasive crayfish. Austral Ecology 44(1), 78-85.
| Crossref | Google Scholar |
Lowe K, FitzGibbon S, Seebacher F, Wilson RS (2010) Physiological and behavioural responses to seasonal changes in environmental temperature in the Australian spiny crayfish Euastacus sulcatus. Journal of Comparative Physiology B 180(5), 653-660.
| Crossref | Google Scholar |
McCullough DA, Bartholow JM, Jager HI, Beschta RL, Cheslak EF, Deas ML, Ebersole JL, Foott JS, Johnson SL, Marine KR, Mesa MG, Petersen JH, Souchon Y, Tiffan KF, Wurtsbaugh WA (2009) Research in thermal biology: burning questions for coldwater stream fishes. Reviews in Fisheries Science 17(1), 90-115.
| Crossref | Google Scholar |
Marshall J (1989) Galaxias olidus in southern Queensland. Fishes of Sahul 5(3), 223-225.
| Google Scholar |
Mitchell CP (1989) Laboratory culture of Galaxias maculatus and potential applications. New Zealand Journal of Marine and Freshwater Research 23(3), 325-336.
| Crossref | Google Scholar |
Monreal-Pawlowsky T, Vaicekauskaitė R, Palencia Membrive G, Delfour F, Manteca X (2021) Goal-oriented behavioural and environmental enrichment in aquarium species. Journal of Zoo and Aquarium Research 9(4), 273-280.
| Crossref | Google Scholar |
Moy KG, Unmack PJ (2017) Update on saving the Running River Rainbowfish: new populations established and going well!. Fishes of Sahul 31(4), 1189-1197.
| Google Scholar |
Ndehedehe CE, Ferreira VG, Adeyeri OE, Correa FM, Usman M, Oussou FE, Kalu I, Okwuashi O, Onojeghuo AO, Getirana A, Dewan A (2023) Global assessment of drought characteristics in the Anthropocene. Resources, Environment and Sustainability 12, 100105.
| Crossref | Google Scholar |
Negus PM, Marshall JC, Steward AL, Mcgregor GB, O’Connor RA (2020) Aquatic biota in hot water: thermal gradients in rheocrene hot spring discharges as analogues for the effects of climate warming. Knowledge & Management of Aquatic Ecosystems 421, 49.
| Crossref | Google Scholar |
Olsson K, Nyström P (2009) Non-interactive effects of habitat complexity and adult crayfish on survival and growth of juvenile crayfish (Pacifastacus leniusculus). Freshwater Biology 54(1), 35-46.
| Crossref | Google Scholar |
O’Connor JP, Zampatti BP (2006) Spawning season and site location of Gadopsis bispinosus Sanger (Pisces: Gadopsidae) in a montane stream of southeastern Australia. Transactions of the Royal Society of South Australia 130(2), 227-232.
| Google Scholar |
O’Hea Miller SB, Davis AR, Wong MYL (2022) Does habitat complexity and prior residency influence aggression between invasive and native freshwater crayfish? Ethology 128(5), 443-452.
| Crossref | Google Scholar |
O’Reilly CM, Alin SR, Plisnier P-D, Cohen AS, McKee BA (2003) Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa. Nature 424(6950), 766-768.
| Crossref | Google Scholar | PubMed |
Palmer C (2021) Assisting wild animals vulnerable to climate change: why ethical strategies diverge. Journal of Applied Philosophy 38(2), 179-195.
| Crossref | Google Scholar |
Rees GN, Shackleton ME, Watson GO, Dwyer GK, Stoffels RJ (2020) Metabarcoding demonstrates dietary niche partitioning in two coexisting blackfish species. Marine and Freshwater Research 71(4), 512-517.
| Crossref | Google Scholar |
Richardson JS (2019) Biological diversity in headwater streams. Water 11(2), 366.
| Crossref | Google Scholar |
Ryan KA, Ebner BC, Norris RH (2008) Radio-tracking interval effects on the accuracy of diel scale crayfish movement variables. Freshwater Crayfish 16, 87-92.
| Google Scholar |
Smith SA (2023) Fish welfare in public aquariums and zoological collections. Animals 13(16), 2548.
| Crossref | Google Scholar |
Starrs D, Ebner BC, Fulton CJ (2015) Ceasefire: minimal aggression among Murray River crayfish feeding upon patches of allochthonous material. Australian Journal of Zoology 63(2), 115-121.
| Crossref | Google Scholar |
Stein RA (1977) Selective predation, optimal foraging, and the predator-prey interaction between fish and crayfish. Ecology 58(6), 1237-1253.
| Crossref | Google Scholar |
Stoessel DJ, Raadik TA, Ayres RM (2015) Spawning of threatened barred galaxias, ‘Galaxias fuscus’. (Teleostei: Galaxiidae). Proceedings of the Linnean Society of New South Wales 137, 1-6.
| Google Scholar |
Stoessel DJ, Raadik TA, Nicol MD, Fairbrother PS, Campbell-Beschorner R (2020) Captive breeding of two rare non-migratory galaxiids (Teleostei: Galaxiidae) for species conservation. Proceedings of the Royal Society of Victoria 132(1), 42-48.
| Crossref | Google Scholar |
Taugbøl T, Skurdal J (1992) Growth, mortality and moulting rate of noble crayfish, Astacus astacus L., juveniles in aquaculture experiments. Aquaculture Research 23(4), 411-420.
| Crossref | Google Scholar |
Todd CR, Lintermans M (2015) Who do you move? A stochastic population model to guide translocation strategies for an endangered freshwater fish in south-eastern Australia. Ecological Modelling 311, 63-72.
| Crossref | Google Scholar |
Turschwell MP, Peterson EE, Balcombe SR, Sheldon F (2016) To aggregate or not? Capturing the spatio-temporal complexity of the thermal regime. Ecological Indicators 67, 39-48.
| Crossref | Google Scholar |
Turschwell MP, Balcombe SR, Steel EA, Sheldon F, Peterson EE (2017) Thermal habitat restricts patterns of occurrence in multiple life-stages of a headwater fish. Freshwater Science 36(2), 402-414.
| Crossref | Google Scholar |
Turschwell MP, Stewart-Koster B, Leigh C, Peterson EE, Sheldon F, Balcombe SR (2018) Riparian restoration offsets predicted population consequences of climate warming in a threatened headwater fish. Aquatic Conservation: Marine and Freshwater Ecosystems 28(3), 575-586.
| Crossref | Google Scholar |
Turschwell MP, Stewart-Koster B, Balcombe SR, Sheldon F, Peterson EE (2020) Multiscale relationships between stream temperature and juvenile recruitment in an imperilled freshwater fish. Marine and Freshwater Research 71(10), 1269-1280.
| Crossref | Google Scholar |
Turunen J, Elbrecht V, Steinke D, Aroviita J (2021) Riparian forests can mitigate warming and ecological degradation of agricultural headwater streams. Freshwater Biology 66(4), 785-798.
| Crossref | Google Scholar |
Unmack PJ, Sandoval-Castillo J, Hammer MP, Adams M, Raadik TA, Beheregaray LB (2017) Genome-wide SNPs resolve a key conflict between sequence and allozyme data to confirm another threatened candidate species of river blackfishes (Teleostei: Percichthyidae: Gadopsis). Molecular Phylogenetics and Evolution 109(2017), 415-420.
| Crossref | Google Scholar |
Vinarski MV, Von Oheimb PV, Aksenova OV, Gofarov MY, Kondakov AV, Nekhaev IO, Bolotov IN (2022) Trapped on the Roof of the World: taxonomic diversity and evolutionary patterns of Tibetan Plateau endemic freshwater snails (Gastropoda: Lymnaeidae: Tibetoradix). Integrative Zoology 17(5), 825-848.
| Crossref | Google Scholar | PubMed |
Ward M, Tulloch AIT, Radford JQ, Williams BA, Reside AE, Macdonald SL, Mayfield HJ, Maron M, Possingham HP, Vine SJ, O’Connor JL, Massingham EJ, Greenville AC, Woinarski JCZ, Garnett ST, Lintermans M, Scheele BC, Carwardine J, Nimmo DG, Lindenmayer DB, Kooyman RM, Simmonds JS, Sonter LJ, Watson JEM (2020) Impact of 2019–2020 mega-fires on Australian fauna habitat. Nature Ecology & Evolution 4(10), 1321-1326.
| Crossref | Google Scholar | PubMed |
Westergaard S, Ye Q (2010) A captive spawning and rearing trial of northern river blackfish (Gadopsis marmorata): efforts towards savings local genetic assets with recognised conservation significance from the South Australian Murray–Darling Basin. Report to the Department of Environment, Water and Natural Resources. SARDI Publication Number F2010/000183-1, SARDI Research Report Series Number 471. (South Australian Research and Development Institute: Adelaide, SA, Australia) Available at https://pir.sa.gov.au/__data/assets/pdf_file/0016/232063/No_471_A_captive_spawning_and_rearing_trial_of_river_blackfish_efforts_etc_conservation_significance_from_the_SA_Murray-Darling_Basin.pdf
Whitney JE, Gido KB, Pilger TJ, Propst DL, Turner TF (2015) Consecutive wildfires affect stream biota in cold- and warmwater dryland river networks. Freshwater Science 34(4), 1510-1526.
| Crossref | Google Scholar |
World Meteorological Organization (2020) WMO statement on the state of the global climate in 2019. WMO-No. 1248. (World Meteorological Organization: Geneva, Switzerland) Available at https://www.actu-environnement.com/media/pdf/news-35129-etat-mondial-du-climat-2019.pdf


