Spatial patterns in the cover and composition of macroalgal assemblages on fringing and nearshore coral reefs
K. Webber A B * , M. Srinivasan A C , A. G. Coppock
A B * , M. Srinivasan A C , A. G. Coppock  A B and G. P. Jones A B
A B and G. P. Jones A B
A Marine Biology and Aquaculture, College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia.
B Australian Research Council Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, Qld 4811, Australia.
C Centre for Tropical Water and Aquatic Ecosystem Research, James Cook University, Townsville, Qld 4811, Australia.
Marine and Freshwater Research 73(11) 1310-1322 https://doi.org/10.1071/MF21349
Submitted: 13 December 2021 Accepted: 19 July 2022 Published: 8 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Although increases in macroalgal cover on coral reefs are often reported alongside declines in coral, the composition of algal assemblages and their spatial dynamics are not commonly investigated.
Aims: To quantify changes in macroalgal assemblage composition over two spatial environmental gradients, depth and distance from shore, within a nearshore reef system in Kimbe Bay, Papua New Guinea, where coral cover has declined.
Methods: Benthic cover was quantified at three depths (reef flat, 10 and 15 m) on the windward reef slopes of six reefs located three distances from shore (fringing reefs, and platform reefs 100–200 m and 0.7−1 km offshore).
Key results: Macroalgal cover was highest on the reef flat, and assemblage composition varied among depths and distances from shore. Macroalgal cover was not correlated with coral cover except where macroalgal cover was greater than 20%, where a negative correlation occurred. There was no correlation between macroalgal cover and turf algal cover. All three benthic groups were negatively correlated with the combined total cover of sand and gravel.
Conclusions: These results indicated a fine-scale spatial structure of macroalgal assemblages on coral reefs over a narrow depth range and short distance from shore and highlighted the importance of a solid substratum.
Implications: It is likely that the ecological interactions between corals and macroalgae vary considerably over narrow spatial gradients.
Keywords: benthic community, depth distribution, macroalgal diversity, marine ecology, Padina, Sargassum, terrestrial runoff, Turbinaria.
Introduction
Coral reef ecosystems are faced with a host of anthropogenic threats that are on the rise, including climate change and associated thermal bleaching events (Hoegh-Guldberg 1999; Hughes et al. 2018; Babcock et al. 2021) and the severity of storms (Bender et al. 2010), as well as more local impacts such as terrestrial runoff (Rogers 1990; Fabricius 2005) and overharvesting (Hughes 1994; Hughes et al. 2007). As a consequence, there is evidence of declining coral cover and increasing cover of algae, often their primary competitors, on coral reefs around the globe (e.g. Hughes 1994; Graham et al. 2015; Souter et al. 2021). In extreme cases, coral reef ecosystems have undergone phase-shifts from coral dominant reefs to reefs dominated by macroalgae (Hughes 1994; Mumby 2009) or non-scleractinian invertebrates (Norström et al. 2009). These alternative stable states can be reinforced through feedback mechanisms, such as reductions in coral recruitment, that make returning to coral dominance unlikely (Mumby 2009; Dell et al. 2016; Johns et al. 2018). The switch from a coral-dominated to a macroalgal state can have adverse effects on reef ecosystems, including reductions in reef stability (Done 1992) and shelter availability (Pratchett et al. 2008), declines in the abundance of coral-dependent species (Jones et al. 2004; Munday 2004; Baker et al. 2008), changes in fish community structure (Bellwood et al. 2006, 2012) and declines in fish diversity (Jones et al. 2004; Chong-Seng et al. 2012).
The community structure of organisms on coral reefs typically varies along spatial gradients, such as depth, reef zone and distance from shore. This is due to a number of physical and biological variables that also vary along these gradients, including light intensity, water quality, wave action, and herbivory, each of which can affect macroalgal abundance and composition. Light penetration rapidly decreases with depth, with this gradient being more pronounced on high-turbidity, poor water-quality reefs (Morgan et al. 2020) or reefs close to mainland shores (Fabricius 2005; De’ath and Fabricius 2010). Wave energy also decreases with depth (Gourlay 1994), and because coral reefs are effective dissipators of wave energy (Ferrario et al. 2014), reef habitats closer to shore or behind the reef crest tend to be more protected than are their more exposed offshore counterparts. Wave energy can also interact with water quality, as sheltered conditions may exacerbate the deposition of suspended sediments and the concentration of nutrients (Wolanski et al. 2005; Ceccarelli et al. 2020). Herbivorous fish feeding activity also varies spatially in reef systems and is typically highest in shallow habitats on offshore reefs (Russ 1984; Brokovich et al. 2010; Cheal et al. 2013). The influence of each of these variables on macroalgae depends on morphological and taxonomic differences in requirements for light (Markager and Sand-Jensen 1992; Leukart and Lüning 1994; Gómez and Huovinen 2011) and water quality (McCook et al. 1997; Schaffelke and Klumpp 1998; Umar et al. 1998), as well as susceptibility to wave dislodgement (Dudgeon and Johnson 1992; Starko et al. 2015) and herbivory (Hay 1981a; Marques et al. 2006; Mantyka and Bellwood 2007).
Few studies have quantified patterns in the abundance and assemblage composition of macroalgae across spatial gradients on coral reefs. On the Great Barrier Reef (GBR), the brown alga Sargassum is most abundant on inner shelf reefs, whereas it is absent on outer shelf reefs, where macroalgae are less abundant in general and dominated by red and green algae (Done 1982; McCook 1996; McCook et al. 1997). Increasing dominance of red algae with increasing distance from the coast has also been observed in the Pilbara Coast, Western Australia (Olsen et al. 2018). In Panama, during the 1980s, the shallow feeding activity of herbivorous fishes was inferred to be responsible for the restriction of some macroalgal taxa to the sand plain below the reef slope, despite it otherwise being a suboptimal environment owing to a lack of stable substratum for holdfast attachment (Hay 1981a). Both depth and coastline distance were found to be important drivers of macroalgal assemblage composition in French Polynesia (Adjeroud 1997). Such spatial patterns in macroalgal composition and abundance are important to consider, because they influence the likelihood, magnitude and ecological effects of macroalgal overgrowth on degrading reef communities.
Given the high spatial variability and taxonomic specificity of macroalgal abundance, it is clear that the prevalence of macroalga–coral interactions will also be spatially variable (Brown et al. 2018). In addition, macroalgae may compete for space with other forms of algae, such as algal turfs (Haas et al. 2010; Khalil et al. 2017), mixed assemblages of small, filamentous and fleshy algae, and early stages of macroalgae (Scott and Russ 1987). It is important to examine the inter-relationships among macroalgal, coral and turf cover, so as to assess whether and where these organisms are interacting (Barott et al. 2009; Haas et al. 2010; O’Brien and Scheibling 2018). Macroalgae, corals and turf algae may also respond in different ways to physical gradients in turbidity and sediment deposition (Airoldi 1998; Fabricius 2005; McCook 2001); however, these relationships have not commonly been quantified.
In the past three decades, the coastal reefs of Kimbe Bay, Papua New Guinea, have experienced multiple disturbances, including coral bleaching, increasing sedimentation from terrestrial runoff and crown of thorns starfish outbreaks, which have led to declines in coral cover (Brodie and Turak 2004; Jones et al. 2004; Chin et al. 2008; Souter et al. 2021). Effects appear to be worse in the coastal fringing reefs where land use is high (Brodie and Turak 2004). Despite recent increases in macroalgal cover in Kimbe Bay (G. P. Jones, pers. obs.), research into the algal assemblages present has been scarce, and has largely focused on fish–algae interactions within small, shallow areas defended by territorial damselfishes (e.g. Ceccarelli et al. 2005; Ceccarelli 2007; Eurich et al. 2018). In this study, we examined spatial patterns in the cover and assemblage composition of macroalgae on inshore reefs in Kimbe Bay. Across these spatial gradients, we also examined whether macroalgal cover was related to the cover of other benthic organisms, such as corals and turf algae, or sediments (such as sand or gravel), which may limit the availability of stable substratum for attachment (Hay 1981a). The following specific questions were addressed: (1) how does the cover and assemblage structure of macroalgae vary by depth, reef zone and distance from shore; and (2) are the spatial patterns in macroalgal cover related to those of corals, turf algae and sand–gravel?
Materials and methods
Study site and field methods
This research was conducted in Kimbe Bay, on the northern coast of West New Britain, Papua New Guinea, during November 2019. The sheltered inner bay consists of a dense network of platform and fringing reefs. The reefs investigated in this study are situated adjacent to the Mahonia Na Dari Research Centre on the western boundary of the bay (5°26′S, 150°5′E). Of these, two reefs were selected randomly from each of three distances from shore: fringing reefs, platform reefs between 0.1–0.2 km from shore (termed ‘mid’) and platform reefs 0.7–1 km from shore (termed ‘outer’; Fig. 1). Although the furthest distance is just ∼1 km from shore, we know from a sediment-monitoring project conducted in the area that there is a substantial reduction in suspended sediments over this distance (G. P. Jones, unpubl. data).
Macroalgae were surveyed at three depth isoclines at each reef, namely, the reef flat (∼5 m behind the crest), and depths of 10 and 15 m on the windward reef slope. These depths were chosen because preliminary observations showed that macroalgae would be detected at all three depths, but would be only very seldomly encountered on shallower parts of the reef slope. At each depth, five replicate 20-m transects were surveyed, each transect being separated by at least 5 m, with the starting position of the initial transect and the direction of swimming chosen haphazardly. Ten photo-quadrats of ∼1 m2 were taken at 2-m intervals along each transect, with a 50-cm ruler placed on the benthos in the centre of each photograph for scale. In total, 900 photographs were captured using a GoPro Hero 2018 camera by using the ‘wide’ frame setting.
Image analysis
Photo-quadrats were analysed to determine the percentage cover of macroalgae and other benthic groups. Cover was calculated by overlaying each photograph with 25 random points and identifying the benthic organism or substratum directly beneath each point. Macroalgae were identified to the lowest taxonomic level possible. All live hard corals were pooled together, as were algal turfs (identified in photos as small filamentous and fleshy algae with no observable plant-like structure) and unconsolidated substrates (sand and gravel).
Data analysis
Mixed-effects, nested ANOVAs with three factors, namely depth, distance from shore (both fixed factors), and site nested within distance (random factor), were performed to examine spatial effects on the percentage cover of macroalgae, hard corals, algal turf and sand–gravel, by using the mean percentage cover per transect as input data. According to Quinn and Keough (2002), the following method was used to improve power because of the low sample sizes used: where P-values greater than 0.25 indicated that the random factor site or its interaction with depth did not differ from the residual, a ‘pooled error’ term was used to test the fixed factors depth and distance. This pooled error term was calculated by summing the sum of squares of the residual with the sum of squares of the random factors or interactions that did not differ from it. The same was performed for the degrees of freedom, which allowed calculation of a pooled mean square, which could be used in F- and P-value calculations (Quinn and Keough 2002). For macroalgal species that were absent from the reef flat, the reef-flat data were excluded from the ANOVA. For macroalgal species found only on the reef flat, the depth factor was dropped from the ANOVA and only reef-flat data were included. If a species was completely absent from a particular distance from shore, data from that distance were excluded from the ANOVA. Where the assumptions of normality and homogeneity of variance were not met, square-root or log(x + 1) transformations were made. Where assumptions were still not met after transformation, ANOVAs were performed using the transformation that achieved the highest Shapiro–Wilk’s test P-value, given that ANOVA is usually robust when the sampling design is balanced (Quinn and Keough 2002; Underwood 1997). The potential associations between coral cover, macroalgal cover, turf algal cover and their combined total, as well as those among these living substrata and sand–gravel cover were tested using Pearson’s product–moment correlations. Data were square-root or log(x + 1) transformed if necessary. Spatial patterns in macroalgal assemblage composition were examined using non-metric multi-dimensional scaling (NMDS) using a Bray–Curtis dissimilarity matrix of the Wisconsin double-standardised percentage cover of each depth within each site for all macroalgal taxa. Overplotting was avoided by rounding the NMDS scores to two decimal places and applying random horizontal and vertical variation, with a maximum of 15% of the resolution of the rounded data. All analyses were performed using the software R (ver. 3.6.1, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/) and the ‘vegan’ package (ver. 2.4-1, Oksanen et al., see https://CRAN.R-project.org/package=vegan, accessed 19 May 2021) was used to conduct NMDS analysis.
Ethical approval
This work was conducted in compliance with the James Cook University Animal Ethics Committee regulations (ethics approval number A2659).
Results
Cover of all macroalgae, algal turf, live coral and sand–gravel
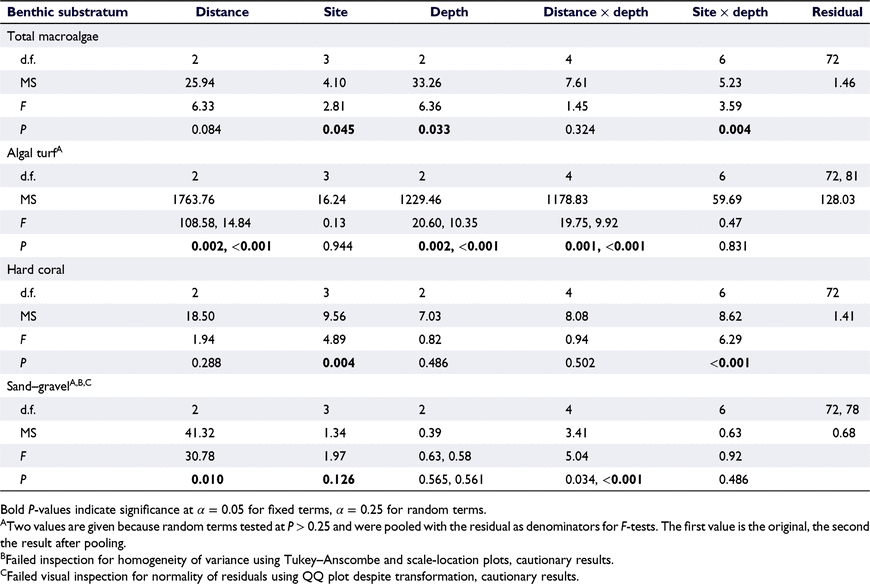
The percentage cover of all macroalgae combined varied significantly among depth strata (Table 1), with cover on the reef flat being ∼2.5 times higher, on average, than on the reef slope. Distance from shore did not have a significant effect on the cover of all macroalgae and there was no significant interaction between depth and distance from shore. There were significant site effects: the site with the lowest macroalgal cover was Walindi fringing reef and the site with the highest cover was Gava Gava, one of the ‘mid’ reefs (Fig. 2). There was a 4.5-fold difference in macroalgal cover between the reef flats of these two reefs. There was a significant interaction between site and depth (Table 1), with depth-related patterns in macroalgal cover clearly not consistent among sites. For instance, macroalgal cover was generally highest on the reef flat and declined with an increasing depth, but this was not seen at the ‘outer’ reef Luba Luba (Fig. 2).
|
|
|
The percentage cover of algal turf was generally higher than both coral and macroalgal cover. Turf cover differed significantly among distances from shore and among depths, and there was a significant interaction between distance from shore and depth, but there were no significant differences among sites and no interaction between site and depth (Table 1). The percentage cover of algal turf was highest on the ‘outer’ reef flats, with 62% cover, and lowest at 15 m on the fringing reefs, with 18% cover (Fig. 2). Elsewhere, turf cover was consistently high, ranging between 40 and 50% (Fig. 2).
Live coral cover did not differ significantly among depths or distances from shore, but there were significant differences among sites and a significant interaction between site and depth (Table 1). Coral cover was highly variable among sites and site-related patterns were not consistent among depths. On the reef flat, coral cover ranged from almost complete absence at the Mahonia fringing reef to 30% at the Walindi fringing reef (Fig. 2). At 10 m, whereas Gava Gava had 28% coral cover, the other sites had between 15 and 22%, and, at 15 m, the coral cover ranged from 3 to 4% at the two fringing reefs to 34% at Matane Huva. Coral cover was higher than macroalgal cover at most sites and depths, except for the reef flats of Mahonia, Gava Gava and Matane Huva, and the reef slope at 15 m at Luba Luba.
Sand–gravel cover differed significantly among distances from shore, but not among depths, and there was a significant interaction between distance from shore and depth (Table 1). The ‘mid’ and ‘outer’ reefs had similar sand–gravel cover across all depths, whereas on the fringing reefs, sand–gravel cover was greater at 15 m than it was in the shallower depths (Fig. 2). Sand–gravel cover was also higher, on average, at the fringing reefs (Table 1, Fig. 2).
Correlations among macroalgae, turf algae, coral and sand–gravel
When all data were included, there were no significant correlations between macroalgal cover and coral cover (r = −0.04, P = 0.712, d.f. = 88), turf cover and macroalgal cover (r = 0.09, P = 0.394, d.f. = 88), and turf cover and coral cover (r = 0.07, P = 0.543, d.f. = 88). The cover of macroalgae and turf algae combined was also not correlated with coral cover (r = −0.05, P = 0.662, d.f. = 88). However, on transects where macroalgae was above 20% (n = 17), there was a significant negative correlation between macroalgal and coral cover (r = −0.53, P = 0.027, d.f. = 15).
Macroalgal cover, coral cover and turf cover were each negatively correlated with the cover of sand–gravel (macroalgae: r = −0.38, P < 0.001, d.f. = 88, coral: r = −0.57, P < 0.001, d.f. = 88, turf: r = −0.40, P < 0.001, d.f. = 88; Fig. 3).
|
|
Species-specific patterns in macroalgal cover
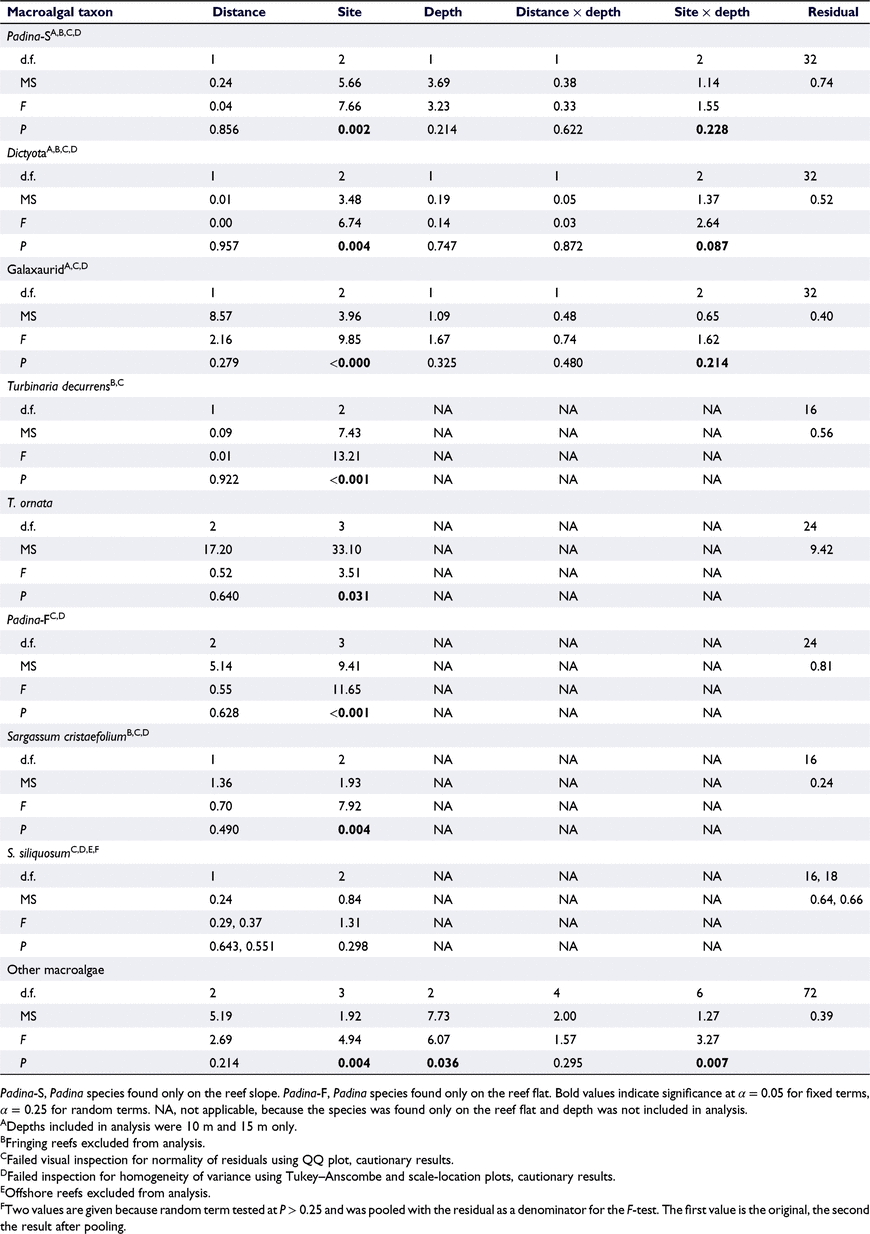
Most of the of the commonly encountered macroalgal taxa were not present at one or two depths and at some distances from shore. A Dictyota species, a Padina species and a galaxaurid species (Family Galaxauraceae) were found only at 10 and 15 m (Fig. 4) and were not found on the fringing reefs. For these three species, there were no significant differences in percentage cover between 10- and 15-m depths and between ‘mid’ and ‘outer’ distances from shore, but there were significant differences among sites (Table 2). Two species of Turbinaria (T. ornata and T. decurrens), two species of Sargassum (S. siliquosum and S. cristaefolium) and one Padina species, which was morphologically distinct from Padina on the reef slope, were found only on the reef flat (Fig. 4). Two of these species, namely T. decurrens and S. cristaefolium, were not found on the fringing reefs, and one of them, S. siliquosum, was absent from the ‘outer’ reefs. Aside from these complete absences, there were no significant differences in cover among distances from shore for any macroalgal species (Table 2). There were significant differences among sites for all species except S. siliquosum (Table 2).
|
Macroalgal assemblage structure
The separation in macroalgal assemblage composition between the reef flat and the slope was strong, whereas the two reef-slope depths were comparatively similar (Fig. 5a). Reef-flat assemblages were loosely grouped by distance from shore, with similarities in reef-flat macroalgal assemblages between the two ‘mid’ reefs and between the two ‘outer’ reefs. However, the two fringing reef flats did not group together (Fig. 5b), owing to Padina having high cover at the Mahonia fringing reef flat, and low cover at the Walindi fringing reef flat. On the reef slope, the fringing reefs grouped together (Fig. 5), because of their macroalgal assemblages being predominantly composed of macroalgae that were not able to be identified (included in ‘other macroalgae’).
Discussion
Macroalgae were a prominent feature of the substratum on the inshore reefs of Kimbe Bay, with cover exceeding that of coral at some sites and depths. Overall cover of macroalgae was comparable to levels found on inshore fringing reefs of the GBR (Ceccarelli et al. 2020). Macroalgal cover varied among depths, being consistent with findings from other studies conducted in the Indo-Pacific (e.g. Adjeroud 1997; Fabricius 2005), with highest cover being on reef flats, reaching a maximum of 60% on some transects. Unlike studies conducted over broader spatial scales (e.g. Adjeroud 1997; Schaffelke et al. 2005; Olsen et al. 2018), overall macroalgal cover did not vary with distance from shore, although there was considerable variation among sites at this spatial scale. Macroalgal assemblage composition differed both among depths and with distance from shore, which is similar to the findings of several other studies (e.g. Done 1982; Adjeroud 1997), with a unique assemblage being dominated by Turbinaria, Sargassum and occasional large stands of Padina on the reef flat. Several species were completely absent from the fringing reefs and one species was absent at the furthest distance from shore. Contrary to other studies (e.g. Ceccarelli et al. 2020), negative correlations between macroalgal cover and coral cover, turf cover and coral cover, or macroalgal cover and turf cover were not observed when all data were included. However, macroalgal and coral cover were negatively correlated where macroalgal cover exceeded 20%, a threshold observed by Ceccarelli et al. (2020), above which such correlations were strong on the inshore GBR. In addition, all three benthic categories were negatively correlated with the cover of sand–gravel, suggesting, as others have (e.g. Hay 1981a; Brown et al. 2018), that the availability of consolidated substratum may be an important driver of spatial patterns of algae and other coral reef organisms attached to hard substrate.
Macroalgal depth distribution
Depth played an important role in determining spatial patterns in macroalgal cover and structuring macroalgal assemblages on inner reefs in Kimbe Bay. Depth often plays a strong role in structuring tropical benthic assemblages because of factors such as decreasing light and turbulence with increasing depth (Veron 2000; Jacobucci et al. 2011), as well as changes in herbivory (Costa et al. 2002). The reef flat, an environment of high light intensity and wave action, typically had the highest macroalgal cover, with the brown algal family Sargassaceae, particularly two species of Turbinaria, consistently being found there and sometimes reaching high densities.
Macroalgal species of the reef flat were typically strongly attached by holdfast, whereas the three dominant macroalgae on the reef slope were loosely attached and more delicate. Whereas the majority of macroalgae found on the reef flat were non-calcifying, calcifying taxa occurred at greater proportions on deeper transects. These included a galaxaurid and a lightly calcifying Padina species, both found at some platform reef sites in large, loose mats, which were completely absent on the reef flat. Benthic communities at isolated coral reefs in the Pacific, despite having much lower overall macroalgal cover than those in Kimbe Bay, have also demonstrated decreasing fleshy macroalgal cover and increasing calcified macroalgae, particularly Halimeda, with depth (Williams et al. 2013). Vulnerability to dislodgement by waves may be the shared characteristic between Halimeda in that study (Williams et al. 2013) and the Padina and galaxaurid species observed in this study, which explains their preference for deeper environments. In contrast, the flexible yet strongly attached nature of the species found on the reef flat (e.g. Turbinaria spp.) allows them to withstand moderate wave action (Stewart 2008). In addition, their exposure to waves and the high buoyancy of their blades and stipes allow transportation to and colonisation of new shallow areas after intense wave action (Stiger and Payri 1999; Stewart 2008).
The drivers of these depth distributions require further investigation. Herbivore biomass and grazing rates often vary among different habitats and depths within coral reefs (e.g. Russ 1984; Lewis and Wainwright 1985; Fox and Bellwood 2007), and experimental studies have shown that this may have important consequences on the distribution of macroalgae (Hay 1981a; Lewis 1986). Some researchers have documented a peak in grazing below the first 1–2 m, where wave motion ceases to interfere with feeding by fishes, and a decline with depth (Hay 1981b; Vergés et al. 2009; Brokovich et al. 2010). Others have found a peak in grazing at the crest, and declines both towards the back reef and with increasing depth (Fox and Bellwood 2007), although habitat preferences for grazing differ somewhat among herbivore families (Russ 1984). Although light is potentially limiting for algal species living in deeper environments, there is likely to be some depth at which macroalgae are afforded a refuge from predation (Hay 1981a). This may be the reason that reef-slope macroalgal patches were present at depths of 10 m and greater but were largely absent from the shallower parts of the reef slope. Quantitative assessments of herbivory on these reefs are required, to determine whether this process has played a role in shaping the depth distributions of macroalgae.
Macroalgae and distance from shore
Overall, macroalgal cover did not vary with distance from shore. Macroalgae in other regions, such as the GBR, have been shown to increase in cover with proximity to nutrient-rich, high-turbidity coastal areas (e.g. Done 1982; Schaffelke et al. 2005; McClure et al. 2019), and also to increase in species richness (De’ath and Fabricius 2010). This is likely to be related to the large differences in scale between the present study and previous work. For example, cross-shelf gradients on the GBR occur at scales of tens to hundreds of kilometres (e.g. Schaffelke et al. 2005), which is multiple orders of magnitude greater than in the present study. Even so, there were assemblage-composition changes along this gradient, driven mainly by the complete absences of several macroalgal taxa on the fringing reefs. Additionally, Sargassum siliquosum was absent from outer reefs 0.7–1 km from shore. Macroalgae such as Sargassum can undergo substantial temporal changes to their biomass (Martin-Smith 1993; Fulton et al. 2014) and may have been detected more readily with a longer sampling period. However, as Kimbe Bay is equatorial, there is very little variation in temperature, and the major seasonal changes are in rainfall (Brodie and Turak 2004). The temporal dynamics of macroalgae have yet to be examined in this area; however, they are likely to differ, for example, from those on the nearby GBR, given the mild seasonality and non-dominance of Sargassum observed here.
Correlations among major substratum types
Our study found no significant overall correlation between macroalgal cover and coral cover, but the presence of such a correlation in other studies has been shown to depend on the level of macroalgae involved. For instance, Ceccarelli et al. (2020) found significant negative correlations between macroalgae and coral at several, but not all, sites on the GBR, and that the correlations were strongest where macroalgal cover was higher than 20%. In Kimbe Bay, in the present study, macroalgal cover was typically lower than this value (site averages ranged from 5.8 to 20.1%, overall average was 11.5%). However, where macroalgal cover did exceed 20% cover, a negative correlation between macroalgal and coral cover was observed. Beyond certain thresholds of macroalgal cover, coral cover and herbivory, effects by macroalgae on corals such as shading, abrasion (McCook et al. 2001), allelopathy and inhibition of recruitment (Johns et al. 2018; Evensen et al. 2019), and on fishes such as suppression of herbivory (Dell et al. 2016), may reach levels where feedback loops are strong enough to prevent coral recovery and maintain macroalgal dominance (Mumby 2009). On the basis of the correlation between coral and macroalgal cover where macroalgal cover was higher than 20%, such a threshold may be exceeded in some areas of Kimbe Bay. However, further investigation into coral–algal dynamics in the Bay is required before the cause of this relationship can be identified.
As in this study, algal turfs are often a major component of reef benthic assemblages, in both tropical and temperate regions (Connell et al. 2014), occupying more space than does coral even on many isolated Pacific reefs with no direct human impact (Vroom et al. 2005, 2006). Turfs were found in almost all surveys in an examination of algal distributions across the GBR (McCook et al. 1997). Like other benthic organisms, algal turfs may be heavily influenced by water-quality gradients and may increase in inshore environments with higher levels of nutrients and sediments (Fabricius 2005). However, a cross-shelf comparison of epilithic algal assemblages on the GBR showed that algal tissue production may be higher at offshore v. inshore sites (Russ and McCook 1999). In this case, cover, rather than production, followed a similar pattern, albeit on a much smaller spatial scale; the highest cover of turf algae occurred on the reef flat of outer reef sites, and the lowest cover occurred at 15 m on the fringing reefs. This may indicate that terrestrial influences, such as sediments, are above turf tolerance thresholds (Tebbett et al. 2018) at the innermost sites surveyed.
The cover of turf algae was not found to be correlated with that of macroalgae in this study, and in contrast to several previous studies, turf algal cover was also not correlated with coral cover (Fabricius 2005; Teichberg et al. 2018). Algal and coral interactions do not always favour algae (McCook et al. 2001; Swierts and Vermeij 2016), even on reefs with high terrestrial influence (McCook 2001). It appears in this case that effects of algae on coral and vice versa are relatively weak compared with other drivers of their abundance.
The role of sediment
Limited availability of stable substrata may be responsible for some of the benthic distribution patterns observed with distance, because there were significant negative correlations between macroalgal, turf algal and coral cover and sand–gravel cover. Cover of benthic organisms was highly variable at low sand–gravel cover, but macroalgal, turf, and coral cover were always low where sand–gravel cover was at its highest. Sand–gravel cover on the fringing reef slopes was very high relative to that on the platform reefs and the macroalgal taxa that characterised the reef slopes elsewhere were absent. This was especially true at 15 m, where sand cover reached very high levels on the fringing reefs, and macroalgae, coral and turf were minimal in cover. Unlike seagrasses, which have true root systems, algae are generally unable to anchor in soft sediments (Diaz-Pulido and McCook 2008). Even the loosely attached algal mats of Padina, the galaxaurid and Dictyota found on the slopes of some platform reefs were found only on harder, more complex substrates, which may prevent them from drifting away in currents (Alfaro et al. 2009). Additionally, it is likely that the higher level of turbidity found on the fringing reefs (K. Webber, pers. obs.) caused light availability to decrease more rapidly with depth (Fabricius 2005; Morgan et al. 2020) and contributed to the declines in macroalgae, turf and coral on the fringing reef slopes. Therefore, examining these organisms at the spatial scale of the present study highlighted patterns of terrestrial influence that contrasted with those observed at larger spatial scales. Namely, limited availability of solid substrata and reduced light limited the abundance of each of these groups at the innermost reefs of this study. Turbidity and the deposition of sediments are likely to vary greatly with seasonal rainfall cycles at this location (Brodie and Turak 2004), and further investigation into the temporal dynamics of sediments and their relationship with benthic organisms may be necessary for Kimbe Bay.
Conclusions
These results highlighted that macroalgae on coral reefs can be highly spatially dynamic. This dynamism can occur on fine scales with depth, distance from shore, and with the availability of suitable substrata. Furthermore, both abundance and assemblage composition of macroalgae can change dramatically along these gradients, indicating that assessing this diverse group purely with metrics such as ‘total macroalgal cover’ may ignore potentially important patterns. Finally, despite higher levels of macroalgae in Kimbe Bay than in the recent past (G. P. Jones, pers. obs.), a lack of an overall correlation between macroalgal and coral cover appears to indicate that macroalgae are not directly jeopardising coral reef development at most sites within this location. However, at some sites, macroalgal cover did exceed a level where a negative correlation with coral cover became apparent. This implies that, in Kimbe Bay, adverse effects on corals by macroalgae are likely to be small in comparison to the effects of global and local anthropogenic stressors, although macroalgae may add further stress at some, high macroalgae sites.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was supported by funding allocation to G. P. Jones from the ARC Centre of Excellence for Coral Reef Studies (CE0561435), James Cook University.
Acknowledgements
We acknowledge and extend a heartfelt thank you to the traditional owners for allowing access to the Tamare-Kilu reefs in Kimbe Bay. We also thank Marta Panero and Nisha Goldsworthy for providing assistance in the field. A sincere thank you also goes to the staff at Mahonia Na Dari Research and Conservation Centre and the staff at Walindi Plantation Resort for logistical support, and especially to our boat driver, Jerry Sikatua. Thanks also go to Kai Pacey for assistance with statistical analysis, Guillermo Diaz-Pulido for assisting with macroalgal identification, and two anonymous reviewers who helped improve this paper.
References
Adjeroud, M (1997). Factors influencing spatial patterns on coral reefs around Moorea, French Polynesia. Marine Ecology Progress Series 159, 105–119.| Factors influencing spatial patterns on coral reefs around Moorea, French Polynesia.Crossref | GoogleScholarGoogle Scholar |
Airoldi, L (1998). Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 79, 2759–2770.
| Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf.Crossref | GoogleScholarGoogle Scholar |
Alfaro, AC, Zemke-White, WL, and Nainoca, W (2009). Faunal composition within algal mats and adjacent habitats on Likuri Island, Fiji Islands. Journal of the Marine Biological Association of the United Kingdom 89, 295–302.
| Faunal composition within algal mats and adjacent habitats on Likuri Island, Fiji Islands.Crossref | GoogleScholarGoogle Scholar |
Babcock, RC, Thomson, DP, Haywood, MDE, Vanderklift, MA, Pillans, R, Rochester, WA, Miller, M, Speed, CW, Shedrawi, G, Field, S, Evans, R, Stoddart, J, Hurley, TJ, Thompson, A, Gilmour, J, and Depczynski, M (2021). Recurrent coral bleaching in north-western Australia and associated declines in coral cover. Marine and Freshwater Research 72, 620–632.
| Recurrent coral bleaching in north-western Australia and associated declines in coral cover.Crossref | GoogleScholarGoogle Scholar |
Baker, AC, Glynn, PW, and Riegl, B (2008). Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuarine, Coastal and Shelf Science 80, 435–471.
| Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook.Crossref | GoogleScholarGoogle Scholar |
Barott, K, Smith, J, Dinsdale, E, Hatay, M, Sandin, S, and Rohwer, F (2009). Hyperspectral and physiological analyses of coral–algal interactions. PLoS ONE 4, e8043.
| Hyperspectral and physiological analyses of coral–algal interactions.Crossref | GoogleScholarGoogle Scholar |
Bellwood, DR, Hoey, AS, Ackerman, JL, and Depczynski, M (2006). Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Global Change Biology 12, 1587–1594.
| Coral bleaching, reef fish community phase shifts and the resilience of coral reefs.Crossref | GoogleScholarGoogle Scholar |
Bellwood, DR, Baird, AH, Depczynski, M, González-Cabello, A, Hoey, AS, Lefèvre, CD, and Tanner, JK (2012). Coral recovery may not herald the return of fishes on damaged coral reefs. Oecologia 170, 567–573.
| Coral recovery may not herald the return of fishes on damaged coral reefs.Crossref | GoogleScholarGoogle Scholar |
Bender, MA, Knutson, TR, Tuleya, RE, Sirutis, JJ, Vecchi, GA, Garner, ST, and Held, IM (2010). Modeled impact of anthropogenic warming on the frequency of intense Atlantic hurricanes. Science 327, 454–458.
| Modeled impact of anthropogenic warming on the frequency of intense Atlantic hurricanes.Crossref | GoogleScholarGoogle Scholar |
Brodie J, Turak E (2004) Land use practices in the Stettin Bay catchment area and their relation to the status of the Coral Reefs in Kimbe Bay. Australian Centre for Tropical Freshwater Research Report number 04/01. Australian Centre for Tropical Freshwater Research, Townsville, Qld, Australia.
Brokovich, E, Ayalon, I, Einbinder, S, Segev, N, Shaked, Y, Genin, A, Kark, S, and Kiflawi, M (2010). Grazing pressure on coral reefs decreases across a wide depth gradient in the Gulf of Aqaba, Red Sea. Marine Ecology Progress Series 399, 69–80.
| Grazing pressure on coral reefs decreases across a wide depth gradient in the Gulf of Aqaba, Red Sea.Crossref | GoogleScholarGoogle Scholar |
Brown, KT, Bender-Champ, D, Kubicek, A, Van Der Zande, R, Achlatis, M, Hoegh-Guldberg, O, and Dove, SG (2018). The dynamics of coral–algal interactions in space and time on the southern Great Barrier Reef. Frontiers in Marine Science 5, 181.
| The dynamics of coral–algal interactions in space and time on the southern Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Ceccarelli, DM (2007). Modification of benthic communities by territorial damselfish: a multi-species comparison. Coral Reefs 26, 853–866.
| Modification of benthic communities by territorial damselfish: a multi-species comparison.Crossref | GoogleScholarGoogle Scholar |
Ceccarelli, DM, Jones, GP, and McCook, LJ (2005). Foragers versus farmers: contrasting effects of two behavioural groups of herbivores on coral reefs. Oecologia 145, 445–453.
| Foragers versus farmers: contrasting effects of two behavioural groups of herbivores on coral reefs.Crossref | GoogleScholarGoogle Scholar |
Ceccarelli, DM, Evans, RD, Logan, M, Mantel, P, Puotinen, M, Petus, C, Russ, GR, and Williamson, DH (2020). Long-term dynamics and drivers of coral and macroalgal cover on inshore reefs of the Great Barrier Reef Marine Park. Ecological Applications 30, e02008.
| Long-term dynamics and drivers of coral and macroalgal cover on inshore reefs of the Great Barrier Reef Marine Park.Crossref | GoogleScholarGoogle Scholar |
Cheal, AJ, Emslie, MJ, Macneil, MA, Miller, I, and Sweatman, H (2013). Spatial variation in the functional characteristics of herbivorous fish communities and the resilience of coral reefs. Ecological Applications 23, 174–188.
| Spatial variation in the functional characteristics of herbivorous fish communities and the resilience of coral reefs.Crossref | GoogleScholarGoogle Scholar |
Chin A, Sweatman H, Forbes S, Perks H, Walker R, Jones GP, Williamson D, Evans R, Hartley F, Armstrong S, Malcolm H, Edgar G (2008) Status of coral reefs in Australia and Papua New Guinea. In ‘Status of the coral reefs of the world: 2008’. (Ed C Wilkinson) pp. 159–176. (Global Coral Reef Monitoring Network, and Reef and Rainforest Research Centre: Townsville, Qld, Australia)
Chong-Seng, KM, Mannering, TD, Pratchett, MS, Bellwood, DR, and Graham, NAJ (2012). The influence of coral reef benthic condition on associated fish assemblages. PLoS ONE 7, e42167.
| The influence of coral reef benthic condition on associated fish assemblages.Crossref | GoogleScholarGoogle Scholar |
Connell, SD, Foster, MS, and Airoldi, L (2014). What are algal turfs? Towards a better description of turfs. Marine Ecology Progress Series 495, 299–307.
| What are algal turfs? Towards a better description of turfs.Crossref | GoogleScholarGoogle Scholar |
Costa, OS, Attrill, MJ, Pedrini, AG, and De-Paula, JC (2002). Spatial and seasonal distribution of seaweeds on coral reefs from southern Bahia, Brazil. Botanica Marina 45, 346–355.
| Spatial and seasonal distribution of seaweeds on coral reefs from southern Bahia, Brazil.Crossref | GoogleScholarGoogle Scholar |
De’ath, G, and Fabricius, K (2010). Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecological Applications 20, 840–850.
| Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Dell, CLA, Longo, GO, and Hay, ME (2016). Positive feedbacks enhance macroalgal resilience on degraded coral reefs. PLoS ONE 11, e0155049.
| Positive feedbacks enhance macroalgal resilience on degraded coral reefs.Crossref | GoogleScholarGoogle Scholar |
Diaz-Pulido G, McCook LJ (2008) Macroalgae (Seaweeds). In ‘The state of the Great Barrier Reef on-line’. (Ed. A Chin) pp. 1–19. (Great Barrier Reef Marine Park Authority: Townsville, Qld, Australia)
Done, TJ (1982). Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1, 95–107.
| Patterns in the distribution of coral communities across the central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Done, TJ (1992). Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247, 121–132.
| Phase shifts in coral reef communities and their ecological significance.Crossref | GoogleScholarGoogle Scholar |
Dudgeon, SR, and Johnson, AS (1992). Thick vs thin: thallus morphology and tissue mechanics influence differential drag and dislodgement of two co-dominant seaweeds. Journal of Experimental Marine Biology and Ecology 165, 23–43.
| Thick vs thin: thallus morphology and tissue mechanics influence differential drag and dislodgement of two co-dominant seaweeds.Crossref | GoogleScholarGoogle Scholar |
Eurich, JG, Shomaker, SM, McCormick, MI, and Jones, GP (2018). Experimental evaluation of the effect of a territorial damselfish on foraging behaviour of roving herbivores on coral reefs. Journal of Experimental Marine Biology and Ecology 506, 155–162.
| Experimental evaluation of the effect of a territorial damselfish on foraging behaviour of roving herbivores on coral reefs.Crossref | GoogleScholarGoogle Scholar |
Evensen, NR, Doropoulos, C, Morrow, KM, Motti, CA, and Mumby, PJ (2019). Inhibition of coral settlement at multiple spatial scales by a pervasive algal competitor. Marine Ecology Progress Series 612, 29–42.
| Inhibition of coral settlement at multiple spatial scales by a pervasive algal competitor.Crossref | GoogleScholarGoogle Scholar |
Fabricius, KE (2005). Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Marine Pollution Bulletin 50, 125–146.
| Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis.Crossref | GoogleScholarGoogle Scholar |
Ferrario, F, Beck, MW, Storlazzi, CD, Micheli, F, Shepard, CC, and Airoldi, L (2014). The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nature Communications 5, 3794.
| The effectiveness of coral reefs for coastal hazard risk reduction and adaptation.Crossref | GoogleScholarGoogle Scholar |
Fox, RJ, and Bellwood, DR (2007). Quantifying herbivory across a coral reef depth gradient. Marine Ecology Progress Series 339, 49–59.
| Quantifying herbivory across a coral reef depth gradient.Crossref | GoogleScholarGoogle Scholar |
Fulton, CJ, Depczynski, M, Holmes, TH, Noble, MM, Radford, B, Wernberg, T, and Wilson, SK (2014). Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnology and Oceanography 59, 156–166.
| Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem.Crossref | GoogleScholarGoogle Scholar |
Gómez, I, and Huovinen, P (2011). Morpho-functional patterns and zonation of South Chilean seaweeds: the importance of photosynthetic and bio-optical traits. Marine Ecology Progress Series 422, 77–91.
| Morpho-functional patterns and zonation of South Chilean seaweeds: the importance of photosynthetic and bio-optical traits.Crossref | GoogleScholarGoogle Scholar |
Gourlay, MR (1994). Wave transformation on a coral-reef. Coastal Engineering 23, 17–42.
| Wave transformation on a coral-reef.Crossref | GoogleScholarGoogle Scholar |
Graham, NAJ, Jennings, S, MacNeil, MA, Mouillot, D, and Wilson, SK (2015). Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97.
| Predicting climate-driven regime shifts versus rebound potential in coral reefs.Crossref | GoogleScholarGoogle Scholar |
Haas, A, el-Zibdah, M, and Wild, C (2010). Seasonal monitoring of coral–algae interactions in fringing reefs of the Gulf of Aqaba, northern Red Sea. Coral Reefs 29, 93–103.
| Seasonal monitoring of coral–algae interactions in fringing reefs of the Gulf of Aqaba, northern Red Sea.Crossref | GoogleScholarGoogle Scholar |
Hay, ME (1981a). Herbivory, algal distribution, and the maintenance of between-habitat diversity on a tropical fringing reef. The American Naturalist 118, 520–540.
| Herbivory, algal distribution, and the maintenance of between-habitat diversity on a tropical fringing reef.Crossref | GoogleScholarGoogle Scholar |
Hay, ME (1981b). Spatial patterns of agrazing intensity on a Caribbean barrier reef: herbivory and algal distribution. Aquatic Botany 11, 97–109.
| Spatial patterns of agrazing intensity on a Caribbean barrier reef: herbivory and algal distribution.Crossref | GoogleScholarGoogle Scholar |
Hoegh-Guldberg, O (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research 50, 839–866.
| Climate change, coral bleaching and the future of the world’s coral reefs.Crossref | GoogleScholarGoogle Scholar |
Hughes, TP (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551.
| Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef.Crossref | GoogleScholarGoogle Scholar |
Hughes, TP, Rodrigues, MJ, Bellwood, DR, Ceccarelli, D, Hoegh-Guldberg, O, McCook, L, Moltschaniwskyj, N, Pratchett, MS, Steneck, RS, and Willis, B (2007). Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biology 17, 360–365.
| Phase shifts, herbivory, and the resilience of coral reefs to climate change.Crossref | GoogleScholarGoogle Scholar |
Hughes, TP, Kerry, JT, and Simpson, T (2018). Large-scale bleaching of corals on the Great Barrier Reef. Ecology 99, 501.
| Large-scale bleaching of corals on the Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Jacobucci, GB, Güth, AZ, Turra, A, and Leite, FPP (2011). Influence of a narrow depth gradient and season on the morphology, phenology, and epibiosis of the brown alga Sargassum cymosum. Journal of the Marine Biological Association of the United Kingdom 91, 761–770.
| Influence of a narrow depth gradient and season on the morphology, phenology, and epibiosis of the brown alga Sargassum cymosum.Crossref | GoogleScholarGoogle Scholar |
Johns, KA, Emslie, MJ, Hoey, AS, Osborne, K, Jonker, MJ, and Cheal, AJ (2018). Macroalgal feedbacks and substrate properties maintain a coral reef regime shift. Ecosphere 9, e02349.
| Macroalgal feedbacks and substrate properties maintain a coral reef regime shift.Crossref | GoogleScholarGoogle Scholar |
Jones, GP, McCormick, MI, Srinivasan, M, and Eagle, JV (2004). Coral decline threatens fish biodiversity in marine reserves. Proceedings of the National Academy of Sciences of the United States of America 101, 8251–8253.
| Coral decline threatens fish biodiversity in marine reserves.Crossref | GoogleScholarGoogle Scholar |
Khalil, MT, Bouwmeester, J, and Berumen, ML (2017). Spatial variation in coral reef fish and benthic communities in the central Saudi Arabian Red Sea. PeerJ 5, e3410.
| Spatial variation in coral reef fish and benthic communities in the central Saudi Arabian Red Sea.Crossref | GoogleScholarGoogle Scholar |
Leukart, P, and Lüning, K (1994). Minimum spectral light requirements and maximum light levels for long-term germling growth of several red algae from different water depths and a green-alga. European Journal of Phycology 29, 103–112.
| Minimum spectral light requirements and maximum light levels for long-term germling growth of several red algae from different water depths and a green-alga.Crossref | GoogleScholarGoogle Scholar |
Lewis, SM (1986). The role of herbivorous fishes in the organization of a Caribbean reef community. Ecological Monographs 56, 183–200.
| The role of herbivorous fishes in the organization of a Caribbean reef community.Crossref | GoogleScholarGoogle Scholar |
Lewis, SM, and Wainwright, PC (1985). Herbivore abundance and grazing intensity on a Caribbean coral reef. Journal of Experimental Marine Biology and Ecology 87, 215–228.
| Herbivore abundance and grazing intensity on a Caribbean coral reef.Crossref | GoogleScholarGoogle Scholar |
Mantyka, CS, and Bellwood, DR (2007). Direct evaluation of macroalgal removal by herbivorous coral reef fishes. Coral Reefs 26, 435–442.
| Direct evaluation of macroalgal removal by herbivorous coral reef fishes.Crossref | GoogleScholarGoogle Scholar |
Markager, S, and Sand-Jensen, K (1992). Light requirements and depth zonation of marine macroalgae. Marine Ecology Progress Series 88, 83–92.
| Light requirements and depth zonation of marine macroalgae.Crossref | GoogleScholarGoogle Scholar |
Marques, LV, Villaca, R, and Pereira, RC (2006). Susceptibility of macroalgae to herbivorous fishes at Rocas Atoll, Brazil. Botanica Marina 49, 379–385.
| Susceptibility of macroalgae to herbivorous fishes at Rocas Atoll, Brazil.Crossref | GoogleScholarGoogle Scholar |
Martin-Smith, KM (1993). Abundance of mobile epifauna: the role of habitat complexity and predation by fishes. Journal of Experimental Marine Biology and Ecology 174, 243–260.
| Abundance of mobile epifauna: the role of habitat complexity and predation by fishes.Crossref | GoogleScholarGoogle Scholar |
McClure, E, Richardson, L, Graba-Landry, A, Loffler, Z, Russ, G, and Hoey, A (2019). Cross-shelf differences in the response of herbivorous fish assemblages to severe environmental disturbances. Diversity 11, 23.
| Cross-shelf differences in the response of herbivorous fish assemblages to severe environmental disturbances.Crossref | GoogleScholarGoogle Scholar |
McCook, LJ (1996). Effects of herbivores and water quality on Sargassum distribution on the central Great Barrier Reef: cross-shelf transplants. Marine Ecology Progress Series 139, 179–192.
| Effects of herbivores and water quality on Sargassum distribution on the central Great Barrier Reef: cross-shelf transplants.Crossref | GoogleScholarGoogle Scholar |
McCook, LJ (2001). Competition between corals and algal turfs along a gradient of terrestrial influence in the nearshore central Great Barrier Reef. Coral Reefs 19, 419–425.
| Competition between corals and algal turfs along a gradient of terrestrial influence in the nearshore central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
McCook LJ, Price IR, Klumpp DW (1997) Macroalgae on the GBR: causes or consequences, indicators or models of reef degradation? In ‘8th International Coral Reef Symposium’, 24–29 June 1996, Panama City, Panama. (Eds H Lessios, IG Macintyre) pp. 1851–1855. (Smithsonian Tropical Research Institute)
McCook, LJ, Jompa, J, and Diaz-Pulido, G (2001). Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19, 400–417.
| Competition between corals and algae on coral reefs: a review of evidence and mechanisms.Crossref | GoogleScholarGoogle Scholar |
Morgan, KM, Moynihan, MA, Sanwlani, N, and Switzer, AD (2020). Light limitation and depth-variable sedimentation drives vertical reef compression on turbid coral reefs. Frontiers in Marine Science 7, 571256.
| Light limitation and depth-variable sedimentation drives vertical reef compression on turbid coral reefs.Crossref | GoogleScholarGoogle Scholar |
Mumby, PJ (2009). Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28, 761–773.
| Phase shifts and the stability of macroalgal communities on Caribbean coral reefs.Crossref | GoogleScholarGoogle Scholar |
Munday, PL (2004). Habitat loss, resource specialization, and extinction on coral reefs. Global Change Biology 10, 1642–1647.
| Habitat loss, resource specialization, and extinction on coral reefs.Crossref | GoogleScholarGoogle Scholar |
Norström, AV, Nyström, M, Lokrantz, J, and Folke, C (2009). Alternative states on coral reefs: beyond coral–macroalgal phase shifts. Marine Ecology Progress Series 376, 295–306.
| Alternative states on coral reefs: beyond coral–macroalgal phase shifts.Crossref | GoogleScholarGoogle Scholar |
O’Brien, JM, and Scheibling, RE (2018). Turf wars: competition between foundation and turf-forming species on temperate and tropical reefs and its role in regime shifts. Marine Ecology Progress Series 590, 1–17.
| Turf wars: competition between foundation and turf-forming species on temperate and tropical reefs and its role in regime shifts.Crossref | GoogleScholarGoogle Scholar |
Olsen, YS, Mattio, L, Perez, AZ, Babcock, RC, Thompson, D, Haywood, MDE, Keesing, J, and Kendrick, GA (2018). Drivers of species richness and abundance of marine macrophytes on shallow tropical reefs of north-western Australia. Journal of Biogeography 46, 170–184.
| Drivers of species richness and abundance of marine macrophytes on shallow tropical reefs of north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Pratchett MS, Munday PL, Wilson SK, Graham NAJ, Cinner JE, Bellwood DR, Jones GP, Polunin NVC, McClanahan TR (2008) Effects of climate-induced coral bleaching on coral-reef fishes – ecological and economic consequences. In ‘Oceanography and marine biology: an annual review’. (Eds RN Gibson, RJA Atkinson, JDM Gordon) Vol. 46, pp. 251–296. (CRC Press)
| Crossref |
Quinn GP, Keough MJ (2002) ‘Experimental design and data analysis for biologists.’ (Cambridge University Press: New York, NY, USA)
Rogers, CS (1990). Responses of coral reefs and reef organisms to sedimentation. Marine Ecology Progress Series 62, 185–202.
| Responses of coral reefs and reef organisms to sedimentation.Crossref | GoogleScholarGoogle Scholar |
Russ, GR (1984). Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. II. Patterns of zonation of mid-shelf and outershelf reefs. Marine Ecology Progress Series 20, 35–44.
| Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. II. Patterns of zonation of mid-shelf and outershelf reefs.Crossref | GoogleScholarGoogle Scholar |
Russ, GR, and Mccook, LJ (1999). Potential effects of a cyclone on benthic algal production and yield to grazers on coral reefs across the central Great Barrier Reef. Journal of Experimental Marine Biology and Ecology 235, 237–254.
| Potential effects of a cyclone on benthic algal production and yield to grazers on coral reefs across the central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Schaffelke, B, and Klumpp, DW (1998). Nutrient-limited growth of the coral reef macroalga Sargassum baccularia and experimental growth enhancement by nutrient addition in continuous flow culture. Marine Ecology Progress Series 164, 199–211.
| Nutrient-limited growth of the coral reef macroalga Sargassum baccularia and experimental growth enhancement by nutrient addition in continuous flow culture.Crossref | GoogleScholarGoogle Scholar |
Schaffelke, B, Mellors, J, and Duke, NC (2005). Water quality in the Great Barrier Reef region: responses of mangrove, seagrass and macroalgal communities. Marine Pollution Bulletin 51, 279–296.
| Water quality in the Great Barrier Reef region: responses of mangrove, seagrass and macroalgal communities.Crossref | GoogleScholarGoogle Scholar |
Scott, FJ, and Russ, GR (1987). Effects of grazing on species composition of the epilithic algal community on coral reefs of the central Great Barrier Reef. Marine Ecology Progress Series 39, 293–304.
| Effects of grazing on species composition of the epilithic algal community on coral reefs of the central Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Souter D, Planes S, Wicquart J, Logan L, Obura D, Staub F (Eds) (2021) Chapter 9. Status and trends of coral reefs of the Pacific region. In ‘Status of Coral Reefs of the World: 2020’. (Global Coral Reef Monitoring Network)
Starko, S, Claman, BZ, and Martone, PT (2015). Biomechanical consequences of branching in flexible wave-swept macroalgae. New Phytologist 206, 133–140.
| Biomechanical consequences of branching in flexible wave-swept macroalgae.Crossref | GoogleScholarGoogle Scholar |
Stewart, HL (2008). The role of spatial and ontogenetic morphological variation in the expansion of the geographic range of the tropical brown alga, Turbinaria ornata. Integrative and Comparative Biology 48, 713–719.
| The role of spatial and ontogenetic morphological variation in the expansion of the geographic range of the tropical brown alga, Turbinaria ornata.Crossref | GoogleScholarGoogle Scholar |
Stiger, V, and Payri, CE (1999). Spatial and temporal patterns of settlement of the brown macroalgae Turbinaria ornata and Sargassum mangarevense in a coral reef on Tahiti. Marine Ecology Progress Series 191, 91–100.
| Spatial and temporal patterns of settlement of the brown macroalgae Turbinaria ornata and Sargassum mangarevense in a coral reef on Tahiti.Crossref | GoogleScholarGoogle Scholar |
Swierts, T, and Vermeij, MJA (2016). Competitive interactions between corals and turf algae depend on coral colony form. PeerJ 4, e1984.
| Competitive interactions between corals and turf algae depend on coral colony form.Crossref | GoogleScholarGoogle Scholar |
Tebbett, SB, Bellwood, DR, and Purcell, SW (2018). Sediment addition drives declines in algal turf yield to herbivorous coral reef fishes: implications for reefs and reef fisheries. Coral Reefs 37, 929–937.
| Sediment addition drives declines in algal turf yield to herbivorous coral reef fishes: implications for reefs and reef fisheries.Crossref | GoogleScholarGoogle Scholar |
Teichberg, M, Wild, C, Bednarz, VN, Kegler, HF, Lukman, M, Gärdes, AA, Heiden, JP, Weiand, L, Abu, N, Nasir, A, Miñarro, S, Ferse, SCA, Reuter, H, and Plass-Johnson, JG (2018). Spatio-temporal patterns in coral reef communities of the Spermonde Archipelago, 2012–2014, I: comprehensive reef monitoring of water and benthic indicators reflect changes in reef health. Frontiers in Marine Science 5, 33.
| Spatio-temporal patterns in coral reef communities of the Spermonde Archipelago, 2012–2014, I: comprehensive reef monitoring of water and benthic indicators reflect changes in reef health.Crossref | GoogleScholarGoogle Scholar |
Umar, MJ, Mccook, LJ, and Price, IR (1998). Effects of sediment deposition on the seaweed Sargassum on a fringing coral reef. Coral Reefs 17, 169–177.
| Effects of sediment deposition on the seaweed Sargassum on a fringing coral reef.Crossref | GoogleScholarGoogle Scholar |
Underwood AJ (1997) ‘Experiments in ecology: logical design and interpretation using analysis of variance.’ (Cambridge University Press: New York, NY, USA)
Vergés, A, Alcoverro, T, and Ballesteros, E (2009). Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Marine Ecology Progress Series 375, 1–11.
| Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea.Crossref | GoogleScholarGoogle Scholar |
Veron JEN (2000) ‘Corals of the world.’ (Australian Institute of Marine Science: Townsville, Qld, Australia)
Vroom, PS, Page, KN, Peyton, KA, and Kukea-Shultz, JK (2005). Spatial heterogeneity of benthic community assemblages with an emphasis on reef algae at French Frigate Shoals, Northwestern Hawai‘ian Islands. Coral Reefs 24, 574–581.
| Spatial heterogeneity of benthic community assemblages with an emphasis on reef algae at French Frigate Shoals, Northwestern Hawai‘ian Islands.Crossref | GoogleScholarGoogle Scholar |
Vroom, PS, Page, KN, Kenyon, JC, and Brainard, RE (2006). Algae-dominated reefs: numerous reports suggest that reefs must be dominated by coral to be healthy, but many thriving reefs depend more on algae. American Scientist 94, 430–437.
Williams, GJ, Smith, JE, Conklin, EJ, Gove, JM, Sala, E, and Sandin, SA (2013). Benthic communities at two remote Pacific coral reefs: effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ 1, e81.
| Benthic communities at two remote Pacific coral reefs: effects of reef habitat, depth, and wave energy gradients on spatial patterns.Crossref | GoogleScholarGoogle Scholar |
Wolanski, E, Fabricius, K, Spagnol, S, and Brinkman, R (2005). Fine sediment budget on an inner-shelf coral-fringed island, Great Barrier Reef of Australia. Estuarine, Coastal and Shelf Science 65, 153–158.
| Fine sediment budget on an inner-shelf coral-fringed island, Great Barrier Reef of Australia.Crossref | GoogleScholarGoogle Scholar |


