No support for purported effects of salt-tolerant stream invertebrates on the salinity responses of salt-sensitive stream invertebrates
Bruce C. Chessman
Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, UNSW Sydney, NSW 2052, Australia. Email: brucechessman@gmail.com
Marine and Freshwater Research - https://doi.org/10.1071/MF20163
Submitted: 26 May 2020 Accepted: 23 July 2020 Published online: 26 August 2020
Journal Compilation © CSIRO 2020 Open Access CC BY
Abstract
Increases in salinity can severely affect freshwater ecosystems, and research on the salt tolerances of freshwater species, and factors that modify tolerance, can improve our understanding and prediction of the effects of salinity. In order to test the hypothesis that salt-tolerant freshwater invertebrates can alter the salinity responses of salt-sensitive freshwater invertebrates, publicly available data from a recent study of artificial mesocosms that claimed to confirm this hypothesis were analysed in the present study. No supporting evidence was found for the hypothesis, with apparent salinity responses of salt-sensitive invertebrates varying no more with greater or lesser exposure to salt-tolerant invertebrates than expected merely by chance. The original findings were apparently misguided through unrecognised confounding of the experimental design, inadequate statistical hypothesis testing and accepting ostensible effects without considering their biological and ecological plausibility.
Keywords: biotic interaction, experimental design, mesocosm, statistical confounding.
Introduction
Although the salinity of inland waters varies naturally from near zero to supersaturation, anthropogenic salinisation affects salt-sensitive aquatic organisms in many parts of the world (James et al. 2003; Cañedo-Argüelles et al. 2013). Internationally, a large body of research has sought to understand the salinity tolerances of freshwater species and how the effects of salinity may be modified by other factors (Cañedo-Argüelles Iglesias 2020). One question that arises in this sphere, but has received scant attention from researchers, is whether biotic interactions, such as predation and competition, may influence the outcome of community exposure to increased salinity. If the salinity responses of aquatic invertebrate species are highly modified by biotic interactions, extrapolation from single-species laboratory tests to predict the effects of salinity in natural environments is questionable.
A novel study by Bray et al. (2019) professed to demonstrate that salt-tolerant stream invertebrates can alter the responses of salt-sensitive invertebrates to salinity stress. Such modification may seem plausible, because many studies of freshwater invertebrates have reported an interplay between toxicant exposure and interspecific interactions. For example, toxicant-induced mortality of sensitive species may benefit more tolerant prey or competitors (Fairchild et al. 1992; Friberg-Jensen et al. 2003; Kesavaraju et al. 2010). However, on inspection, the analysis and interpretation in Bray et al. (2019) are problematic.
Bray et al. (2019) set out to test ‘whether salinity effects were modified by interspecific biotic interactions between salt-tolerant organisms, collected from a high salinity site, and a community expected to be more salt-sensitive, collected from a low salinity site’. Bray et al. (2019) conducted an experiment with 32 artificial mesocosms (1000-L troughs of dechlorinated town water, placed outdoors at the University of Canberra, Australia), each stocked with one of two types of stream invertebrate assemblage. The first assemblage type, which Bray et al. (2019) call ‘salt-sensitive communities’, comprised invertebrates obtained only from the Cotter River, a stream with low electrical conductivity (EC) that Bray et al. (2019) assumed to contain both salt-sensitive and salt-tolerant invertebrates. Each mesocosm stocked with this assemblage received colonisation trays of river gravels, pebbles and cobbles that had lain in the Cotter River for 44 days, associated 15-g leaf packs and two kick-net samples from ‘riffle habitat’ in the Cotter River. Hereafter these mesocosms are referred to as ‘Cotter’ mesocosms. The second assemblage type, which Bray et al. (2019) call ‘salt-tolerant and sensitive communities’, comprised a mixture of invertebrates from the Cotter River and Cunningham Creek, the latter being a stream of higher EC that Bray et al. (2019) assumed to contain only salt-tolerant invertebrates. Each mesocosm stocked with this assemblage received colonisation trays and leaf packs from the Cotter River, one kick-net sample from ‘riffle habitat’ in the Cotter River and one kick-net sample from ‘riffle habitat’ in Cunningham Creek. Hereafter, these mesocosms are referred to as ‘Cotter–Cunningham’ mesocosms. The addition of varying quantities of synthetic sea salt to the mesocosms resulted in each receiving one of five salinity treatments with mean ECs of ~20, 50, 100, 250 and 500 mS m–1. Three or four mesocosms of each assemblage type were exposed to each salinity treatment for 75 days, after which invertebrates were retrieved and identified to various taxonomic levels.
Bray et al. (2019) do not state how many trays were added to each mesocosm, but their fig. 2 shows three. In that case, and given their reported tray dimensions, the total area of trays per mesocosm would have been 0.3 m2, whereas a kick-net sample covered 3.3 m2 in the Cotter River and 1 m2 in Cunningham Creek. Thus, it seems likely that a substantial proportion of the fauna in the mesocosms came from the kick-net samples. Bray et al. (2019) also do not state whether their tray-plus-leaf-pack samples were obtained from the riffles where their Cotter River kick-net samples were obtained. However, even if they were, faunal composition would have differed between these two sample types because many studies have shown that different methods of sampling stream invertebrates from the same habitats, such as colonisation trays, kick sampling, Surber sampling and leaf packs, have different sampling efficiencies for particular taxa (e.g. Hughes 1975; Giri et al. 2010; Everall et al. 2017). Thus, the starting assemblage of salt-sensitive invertebrates in Cotter mesocosms, derived from three Cotter River tray-plus-leaf-pack samples and two Cotter River kick-net samples, would have differed in both density and composition from the starting assemblage of salt-sensitive invertebrates in the Cotter–Cunningham mesocosms, derived from three Cotter River tray-plus-leaf-pack samples and one Cotter River kick-net sample. Consequently, the experimental design confounded differences in the starting assemblage of salt-sensitive invertebrates with differences in their exposure to salt-tolerant invertebrates.
Bray et al. (2019) conducted two statistical analyses to compare the invertebrate samples from the end of the experiment between the two types of stocked assemblages and among salinity treatments. The dependent variables for these analyses were the abundances of purportedly salt-sensitive taxa, which were selected by Bray et al. (2019) by excluding nearly all taxa ‘known to occur from the Cunningham Creek’. The independent variables included abiotic variables (EC, alkalinity and water velocity) and biotic variables (assemblage type and the abundances of purportedly salt-tolerant taxa).
The first analysis, titled ‘community analysis’, was a multivariate analysis that related relative abundances of the purportedly salt-sensitive taxa at the end of the experiment to the independent variables. This analysis found that the composition of the salt-sensitive faunal component at the end of the experiment was significantly related to EC, velocity, the abundance of salt-tolerant invertebrates and ‘biotic treatment’ (i.e. Cotter v. Cotter–Cunningham mesocosms). Bray et al. (2019) interpreted this result as demonstrating ‘patterns that were driven by both conductivity … and the densities of tolerant taxa’. However, the difference in the salt-sensitive faunal component at the end of the experiment between the Cotter and Cotter–Cunningham mesocosms, and hence in relation to densities of salt-tolerant invertebrates, can be explained simply as an inevitable consequence of the difference in the salt-sensitive faunal component between the Cotter and Cotter–Cunningham mesocosms at the start of the experiment.
The second analysis, titled ‘single taxa and metric analysis’, fitted and graphed linear (or occasionally non-linear) relationships between EC and final densities (individuals m–2) of purportedly salt-sensitive taxa separately for the Cotter and Cotter–Cunningham mesocosms. This fitting was done for each of the 20 most common salt-sensitive taxa and for combined densities of all Ephemeroptera, all Plecoptera, all Trichoptera and all taxa. The fits are highly variable, with diverse intercepts and slopes, and the fits for Cotter and Cotter–Cunningham mesocosms for particular taxa variously converge, diverge or remain parallel as EC increases. Bray et al. (2019) interpret these disparate patterns as demonstrating a great variety of effects of both salinity and interactions between salt-sensitive and salt-tolerant invertebrates, but without explaining why the biology or ecology of individual taxa may make them respond in such diverse ways. In addition, the 95% credible intervals for the fits are wide and overlap extensively. Bray et al. (2019) provide no statistical hypothesis testing to demonstrate how the diversity of fits compares with that expected by chance, bearing in mind the characteristically high variability of replicate stream invertebrate samples (e.g. Downes et al. 1993; Heino et al. 2004; Brooks et al. 2005).
Thus, neither of the two analyses in Bray et al. (2019) statistically tests the hypothesis that salt-tolerant invertebrates alter the salinity responses of salt-sensitive invertebrates. Their first analysis is not a valid test of this hypothesis, and their second analysis lacks statistical hypothesis testing entirely. Thus, the present study conducted further statistical analysis of the publicly available raw data from the study of Bray et al. (2019) to independently test this hypothesis and see whether any support could be found for their conclusions.
Materials and methods
Study data were downloaded from the Dryad Digital Repository via the link provided in Bray et al. (2019; http://dx.doi.org/10.5061/dryad.n541d0t, accessed 21 May 2020). These data include densities of the purportedly salt-sensitive taxa in each mesocosm at the end of the experiment and associated EC values. To test the hypothesis that variation in the density of salt-tolerant invertebrates affected the salinity responses of salt-sensitive invertebrates, the apparent salinity response of each salt-sensitive taxon in each mesocosm type (Cotter or Cotter–Cunningham) was first quantified as the coefficient of Spearman rank correlation between EC and taxon density across the 16 mesocosms of that type. Thus, a negative correlation suggested a negative taxon response to higher salinity, whereas a positive correlation suggested a positive response. A non-parametric test was used because the density data were highly skewed with many zero values. Then, EC–density correlations were compared between the two mesocosm types. Statistical tests were done with XLSTAT (ver. 2020.1, Addinsoft, Long Island, NY, USA, see https://www.xlstat.com) and VassarStats (R. Lowry, see http://vassarstats.net/, accessed 24 May 2020).
Results and discussion
The Dryad data included 88 taxa at various taxonomic levels from order to species or voucher species. However, 46 of these taxa were not recorded from any of the mesocosms of one type, and therefore had to be excluded from the present analysis. For the remaining 42 taxa, the mean coefficient of correlation between EC and density was –0.13 for Cotter mesocosms and –0.15 for Cotter–Cunningham mesocosms, a statistically non-significant difference (paired t-test, P = 0.68). Of the 20 most common taxa modelled by Bray et al. (2019), two were not recorded from any of the mesocosms of one type, and, for the remaining 18, the mean coefficient of correlation between EC and density was –0.20 for Cotter mesocosms and –0.20 for Cotter–Cunningham mesocosms, also a statistically non-significant difference (paired t-test, P = 0.99). Thus, the average salinity response of these purportedly salt-sensitive taxa was negative, as expected, but was not significantly related to their level of exposure to salt-tolerant taxa.
For individual taxa, the coefficient of correlation between EC and density was sometimes positive for one mesocosm type and negative for the other mesocosm type (Fig. 1). The value of the correlation coefficient was higher in the Cotter mesocosms for 24 taxa and higher in the Cotter–Cunningham mesocosms for 18 taxa, a statistically non-significant difference (z-test, P = 0.44). Moreover, the difference in correlation coefficients between the two mesocosm types was statistically significant for only 2 of the 42 taxa (z-test, P < 0.05), 2 taxa also being the number expected by chance at α = 0.05. For both these taxa, the coefficient was negative for the Cotter mesocosms and positive for the Cotter–Cunningham mesocosms, notionally suggesting that greater exposure to salt-tolerant invertebrates somehow changed their salinity response from negative to positive, an implausible proposition. The two significant taxa were both taxonomically and ecologically distant, being a predatory megalopteran (Archichauliodes sp.) and a filter-feeding dipteran (Corynoneura sp.), so it was unlikely that they would interact with salt-tolerant invertebrates in the same way.
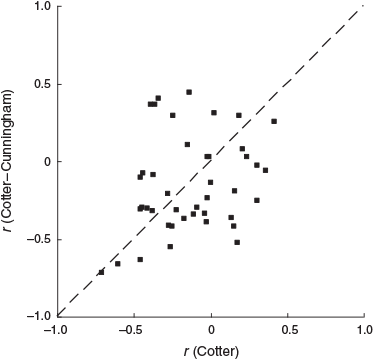
|
Conclusion
This analysis provides no support for the hypothesis that salt-tolerant invertebrates alter the salinity response of salt-sensitive invertebrates. Relationships of EC to densities of purportedly salt-sensitive taxa differed no more between mesocosms with more or fewer salt-tolerant invertebrates than expected from stochastic variation. Moreover, even if a significant difference of this kind had been found, it would have been difficult to interpret because it could have been caused by either variation in exposure to salt-tolerant invertebrates or variation in the initial assemblage of salt-sensitive invertebrates. It seems that the original study findings were misguided by unappreciated confounding of the experimental design, inadequate statistical hypothesis testing and accepting ostensible effects without considering their plausibility given the biology and ecology of the species concerned.
Conflicts of interest
The work reported by Bray et al. (2019) was partly funded by Australian Research Council Linkage Project LP160100093. The present author is listed as one of six chief investigators in the grant application for that project, but was not involved in the work described by Bray et al. (2019).
Declaration of funding
The present research did not receive any funding.
Acknowledgements
The author thanks the authors of the original study and the Dryad Digital Repository for making the data available for further analysis. The author also thanks the Editor and anonymous referees for comments on the manuscript.
References
Bray, J. P., Reich, J., Nichols, S. J., Kon Kam King, G., Mac Nally, R., Thompson, R., O’Reilly-Nugent, A., and Kefford, B. J. (2019). Biological interactions mediate context and species-specific sensitivities to salinity. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 374, 20180020.| Biological interactions mediate context and species-specific sensitivities to salinity.Crossref | GoogleScholarGoogle Scholar |
Brooks, A. J., Haeusler, T., Reinfelds, I., and Williams, S. (2005). Hydraulic microhabitats and the distribution of macroinvertebrate assemblages in riffles. Freshwater Biology 50, 331–344.
| Hydraulic microhabitats and the distribution of macroinvertebrate assemblages in riffles.Crossref | GoogleScholarGoogle Scholar |
Cañedo-Argüelles, M., Kefford, B. J., Piscart, C., Prat, N., Schäfer, R. B., and Schulz, C.-J. (2013). Salinisation of rivers: an urgent ecological issue. Environmental Pollution 173, 157–167.
| Salinisation of rivers: an urgent ecological issue.Crossref | GoogleScholarGoogle Scholar | 23202646PubMed |
Cañedo-Argüelles Iglesias, M. (2020). A review of recent advances and future challenges in freshwater salinization. Limnetica 39, 185–211.
Downes, B. J., Lake, P. S., and Schreiber, E. S. G. (1993). Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization. Freshwater Biology 30, 119–132.
| Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization.Crossref | GoogleScholarGoogle Scholar |
Everall, N. C., Johnson, M. F., Wood, P., Farmer, A., Wilby, R. L., and Measham, N. (2017). Comparability of macroinvertebrate biomonitoring indices of river health derived from semi-quantitative and quantitative methodologies. Ecological Indicators 78, 437–448.
| Comparability of macroinvertebrate biomonitoring indices of river health derived from semi-quantitative and quantitative methodologies.Crossref | GoogleScholarGoogle Scholar |
Fairchild, J. F., La Point, T. W., Zajicek, J. L., Nelson, M. K., Dwyer, F. J., and Lovely, P. A. (1992). Population-, community- and ecosystem-level responses of aquatic mesocosms to pulsed doses of a pyrethroid insecticide. Environmental Toxicology and Chemistry 11, 115–129.
| Population-, community- and ecosystem-level responses of aquatic mesocosms to pulsed doses of a pyrethroid insecticide.Crossref | GoogleScholarGoogle Scholar |
Friberg-Jensen, U., Wendt-Rasch, L., Woin, P., and Christoffersen, K. (2003). Effects of the pyrethroid insecticide, cypermethrin, on a freshwater community studied under field conditions. I. Direct and indirect effects on abundance measures of organisms at different trophic levels. Aquatic Toxicology 63, 357–371.
| Effects of the pyrethroid insecticide, cypermethrin, on a freshwater community studied under field conditions. I. Direct and indirect effects on abundance measures of organisms at different trophic levels.Crossref | GoogleScholarGoogle Scholar | 12758002PubMed |
Giri, M. L., Chester, E. T., and Robson, B. J. (2010). Does sampling method or microhabitat type determine patterns of macroinvertebrate assemblage structure detected across spatial scales in rivers? Marine and Freshwater Research 61, 1313–1317.
| Does sampling method or microhabitat type determine patterns of macroinvertebrate assemblage structure detected across spatial scales in rivers?Crossref | GoogleScholarGoogle Scholar |
Heino, J., Louhi, P., and Muotka, T. (2004). Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshwater Biology 49, 1230–1239.
| Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure.Crossref | GoogleScholarGoogle Scholar |
Hughes, B. D. (1975). A comparison of four samplers for benthic macro-invertebrates inhabiting coarse river deposits. Water Research 9, 61–69.
| A comparison of four samplers for benthic macro-invertebrates inhabiting coarse river deposits.Crossref | GoogleScholarGoogle Scholar |
James, K. R., Cant, B., and Ryan, T. (2003). Response of freshwater biota to rising salinity levels and implications for saline water management: a review. Australian Journal of Botany 51, 703–713.
| Response of freshwater biota to rising salinity levels and implications for saline water management: a review.Crossref | GoogleScholarGoogle Scholar |
Kesavaraju, B., Alto, B., Afify, A., and Gaugler, R. (2010). Malathion influences competition between Aedes albopictus and Aedes japonicus. Journal of Medical Entomology 47, 1011–1018.
| Malathion influences competition between Aedes albopictus and Aedes japonicus.Crossref | GoogleScholarGoogle Scholar | 21175048PubMed |


