Frogs vs fungus: the emergence of amphibian chytridiomycosis
Rebecca J. Webb A B * and Anthony W. Waddle AA One Health Research Group, Melbourne Veterinary School, Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Werribee, Vic., Australia.
B College of Public Health Medical and Veterinary Sciences, James Cook University, Douglas, Qld, Australia.

Rebecca Webb is a Research Fellow with the One Health Research Group, at the University of Melbourne. Rebecca is passionate about amphibian conservation, especially in regards to their biggest threat; the chytrid fungus. Her research aims to increase understanding of this fungal pathogen and identify its weaknesses, and use this information to inform potential control mechanisms. Currently her research focusses on using gene silencing techniques to characterise important fungal genes, and as a possible tool to reduce fungal virulence. |

Anthony Waddle is a Schmidt Science Fellow and postdoctoral scholar working at Macquarie University in Applied BioSciences. His research focuses on developing conservation interventions for amphibian species that have been impacted by chytrid fungus. Prior to his current position, Anthony worked on developing vaccines against chytrid, determining the mechanisms by which frog populations can persist despite the presence of chytrid, and developing habitat manipulations which can buffer against chytrid. He is now developing genome resources and synthetic biology techniques, which can be used to understand and promote host resistance to disease. |
Microbiology Australia 43(4) 169-172 https://doi.org/10.1071/MA22056
Submitted: 2 September 2022 Accepted: 17 November 2022 Published: 13 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
By the late 1980s, widespread dramatic declines in amphibian populations were causing alarm. The culprit was identified as Batrachochytrium dendrobatidis (Bd), a chytrid fungus that infects the skin of various amphibian hosts, particularly anurans (frogs), and the first example of a chytridiomycete parasitising vertebrates. The disease, chytridiomycosis, has spread globally and is linked to the decline and extinction of many amphibian species. This review summarises the discovery of Bd, its emergence as a panzootic pathogen, and some current mitigation strategies to conserve amphibians.
Keywords: amphibian, Australia, Batrachochytrium dendrobatidis, chytridiomycosis, conservation, disease, frog, fungal, wildlife.
Introduction
The emergence of chytridiomycosis in amphibians has changed the way we think of wildlife diseases. Never has a fungal disease had such a profound impact on biodiversity.1 The organism responsible, Batrachochytrium dendrobatidis (Bd),2 can infect an unusually broad range of amphibian hosts, with a propensity to cause severe population declines and extinctions.1 The discovery of chytridiomycosis,3 and subsequent attempts to control the pathogen, presents unique challenges and highlights the need for cross discipline collaboration.4
Discovery of chytridiomycosis
Like many organisms, amphibian populations are declining due to human activities such as deforestation and pollution. When frogs and toads started disappearing from pristine protected areas, however, scientists were puzzled. Throughout the 1980s, reports of amphibian declines were coming from the cloud forests of Costa Rica, the alpine Sierra Nevadas in the USA, and the Atlantic forests of Brazil. Frogs decompose quickly in the forest, hampering efforts to determine the cause of death. In Australia, two species of the unique gastric brooding frog (the northern and southern gastric brooding frogs, Rheobatrachus vitellinus and R. silus), and two species of day frog (the southern and Eungella day frogs Taudactylus diurnus and T. eungellensis) went missing from the rainforest of Central and Southern Queensland. Concern was mounting for the North Queensland frogs, and particularly for a related species of day frog,the sharp snouted day frog (T. acutirostris). Intense monitoring began at ‘Big Tableland’, one of the last remaining T. acutirostris populations. It was not long before fears were realised and a mass die-off was observed in real time. In addition to T. acutirostris, multiple species of stream breeding frogs simultaneously vanished. At the Big Tablelands upland site, the co-occurring common mist frogs (Litoria rheocola), Australian lace-lids (Litoria dayi), and waterfall frogs (Litoria nannotis), disappeared over the space of a few months.5 Although devastating, this was an opportunity to collect enough freshly dead frogs for pathology to determine the cause of death. A tiny skin parasite in the outer layers of skin, originally dismissed as a secondary infection, was the only common factor. Although such a superficial skin infection seemed unlikely to cause such catastrophic declines, experiments demonstrated that skin from dead frogs could transmit disease to healthy frogs. Electron microscopy and sequencing showed it to be a novel chytrid fungus.3 When mass die-offs were detected in Panama, an extraordinary international collaborative effort suggested it was the same fungal species causing disease across continents.3 A parallel investigation into captive frog mortalities in the United States led to the taxonomic description of the culprit, a chytrid fungus: Batrachochytrium dendrobatidis (Bd).2 This was the first report of a chytrid parasitising a vertebrate host.
Like other chytrid fungi, Bd produces motile zoospores that swim via flagella. But unlike the majority of chytrids, the zoospore is infective, penetrating the hosts skin via germ tube, before developing into a zoosporangium.6 The zoosporangium asexually produces more zoospores, which can infect the same host or spread to a new one. The resulting disease, named chytridiomycosis,3 presents as lethargy, with characteristic reddened and shedding skin. Death can often occur rapidly after the onset of clinical signs. Such severe outcomes from a seemingly mild, non-systemic skin infection were contrary to conventional wisdom, and it was thought that the fungus must produce a toxin. Thorough investigation revealed that instead, Bd infection destroys the integrity of the skin, leading to electrolyte imbalance and cardiac arrest.7 Supportive supplementation with electrolytes can slow disease progression but cannot keep up with the damage caused by the fungus as it continues to multiply in the skin.7 Early histological analysis revealed a lack of immune response in infected hosts, with poor infiltration of professional immune cells (e.g. macrophages) to the site of infection and minimal inflammation.8 The down regulation of immune genes during infection suggested that Bd dampens the host immune capabilities.9 This was further supported by the discovery that Bd cells produce a suite of metabolites that can paralyse amphibian immune cells10,11 and prevent fungal clearance, eventually leading to death of the host. By targeting the skin, an organ universally important for amphibians, the fungus can cause disease in an incredibly broad range of hosts. Bd infects all three orders of amphibians, predominantly the frogs and toads, but also salamanders and caecilians, and at least 500 amphibian species have suffered population declines due to chytridiomycosis.1 In addition, the fungus appears to infect freshwater invertebrates as well, making it especially difficult to eradicate from the environment.
Emergence of chytridiomycosis
Histological analysis of museum specimens and a newly developed PCR diagnostic assay provided evidence linking Bd to many more past and current amphibian declines globally.12,13 But where had Bd come from and what was behind the sudden emergence at so many sites globally? The pathogen is in fact an ancient, early diverging fungal species thought to have originated in Asia. Genetic analysis revealed that Bd is comprised of several distinct lineages, some of which appear to be endemic and not associated with amphibian declines.14–16 However, the hypervirulent global panzootic lineage ‘BdGPL’ emerged in the early 20th century, and spread most likely via the amphibian trade.15 The BdGPL lineage appears responsible for the dramatic amphibian declines observed in Australia, the Americas and Europe, which are estimated to have caused the extinction of at least 90 amphibian species.1 Chytridiomycosis has had flow on effects extending beyond amphibians, as seen in the loss of neotropical snakes once their prey disappeared.17 The fungus thrives in cool, wet environments, therefore patterns of chytridiomycosis emergence can be explained predominately by temperature and rainfall.18 However, in some cases, co-occurring species can have vastly different responses despite a shared niche. For example, the iconic corroboree frog (Pseudophryne corroboree) is incredibly susceptible to Bd and has declined to near extinction,19 whereas the co-occurring common froglet (Crinia signifera) appears tolerant20 (Fig. 1). Uncovering the mechanism behind differential susceptibility is an important area of future research.
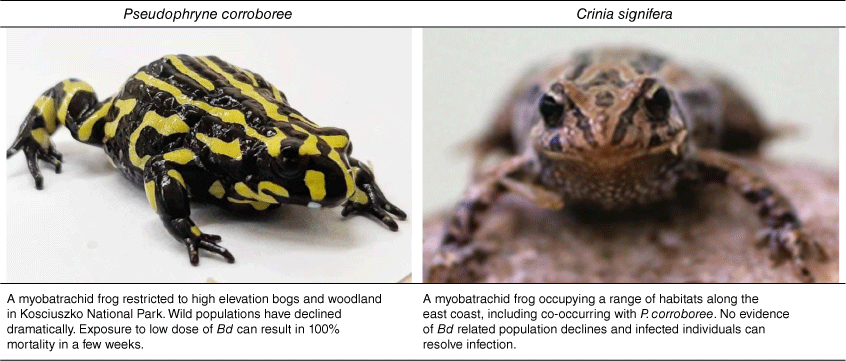
|
Combatting chytridiomycosis
Antifungal drugs borrowed from veterinary medicine are sufficient for the treatment of captive animals.21 Targeted antifungal treatment of wild amphibians can reduce infection and increase survival.22,23 However, as the fungus appears impossible to eradicate from the environment, antifungal treatment of wild amphibians only provides a short-term solution. Feasible, long-term solutions to combat chytridiomycosis are urgently required to prevent further loss of biodiversity.4 Potential mitigation strategies can be broadly divided into those that target the pathogen and those that aim to increase host resistance. Anti-pathogen strategies include modifying natural areas and creating unsuitable microhabitats, for example by increasing salt content24 or providing basking opportunities to help hosts overcome infection (Fig. 2). There has also been interest in developing viral biocontrol to reduce fungal virulence, as this technique has been successful in curing fungal diseases in wild plants.25 By reducing the virulence of Bd without eradicating it, these approaches could allow wild amphibians the opportunity to slowly develop natural resistance.

|
Alternatively, artificially increasing resistance of captive bred and released animals could create self-sustaining wild populations.4 Early attempts at vaccination were unsuccessful,26 which is not surprising given the suppressed host immune system. However, recent research suggests that vaccination might be protective in some species. Inoculations with low virulence strains or infection with highly virulent strains followed by antifungal treatment can bolster host defences to future infections.27,28 However, a vaccination strategy might still require ongoing intervention. Research into selective breeding of resistant individuals is underway, with the aim of using whole genome sequencing to identify alleles associated with survival (discussed in Kosch et al.4). Release of resistant animals could reduce the need for constant intervention. However, this is an expensive and long-term approach, which will likely only be feasible for a few select species. Therefore, a ‘silver bullet’ is unlikely, and a combination of approaches will be required for amphibian conservation.
Continued threat of chytridiomycosis
Although Bd has now spread around the globe, restricting pathogen movement remains a priority. Hybrid strains can display increased virulence,29 highlighting the importance of minimising opportunities for contact between divergent Bd lineages. In Australia, strict biosecurity and field hygiene protocols are necessary to prevent further Bd introductions, and to slow pathogen spread into uninfected areas such as parts of the Tasmanian Wilderness World Heritage Area and Cape York Peninsula, Queensland. These protocols are also essential to prevent the spread of other amphibian diseases. For example, another species of parasitic chytrid fungus; Batrachochytrium salamandrivorans or ‘Bsal’, was described in 2013 after causing fire salamander (Salamandra salamandra) mortality in Belgium.30 It is now reported in various locations around Europe, having likely emerged and spread from Asia in much the same way as Bd.31 There is mounting concern that the pathogen will reach North America and have devastating effects on the diverse salamander communities there.32 A collaborative taskforce is currently assessing the risk of Bsal invasion to North America using species susceptibility and environmental suitability modelling, as well as monitoring wild and imported salamanders. Although there are no native salamanders in Australia, Bsal may still pose a risk to our native amphibians. Preliminary laboratory experiments indicate that Bsal can infect frog species, highlighting the need for targeted monitoring and preventative measures.33 Australia has already lost six amphibian species due to chytridiomycosis.34 The good news is that some species, such as Fleay’s barred frog (Mixophyes fleayi), are showing signs of recovery and coexistence with Bd, but at least seven species are still in dire risk of extinction without intervention. Continued research and multidisciplinary collaboration are required to mitigate the current Bd epidemic, understand the mechanisms by which some species are developing resistance, and to prevent the introduction of a second amphibian chytrid species.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors acknowledge the contribution of their PhD supervisors, and the two reviewers of this manuscript.
References
[1] Scheele, BC et al.. (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463.| Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity.Crossref | GoogleScholarGoogle Scholar |
[2] Longcore, JE et al.. (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227.
| Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians.Crossref | GoogleScholarGoogle Scholar |
[3] Berger, L et al.. (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95, 9031–9036.
| Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America.Crossref | GoogleScholarGoogle Scholar |
[4] Kosch, TA et al.. (2022) Genetic approaches for increasing fitness in endangered species. Trends Ecol Evol 37, 332–345.
| Genetic approaches for increasing fitness in endangered species.Crossref | GoogleScholarGoogle Scholar |
[5] McDonald K, Alford R (1999) A review of declining frogs in northern Queensland. In Declines and Disappearances of Australian Frogs (Campbell A, ed.). pp. 14–22. Environment Australia, Canberra.
[6] Van Rooij, P et al.. (2012) Germ tube mediated invasion of Batrachochytrium dendrobatidis in amphibian skin is host dependent. PLoS One 7, e41481.
| Germ tube mediated invasion of Batrachochytrium dendrobatidis in amphibian skin is host dependent.Crossref | GoogleScholarGoogle Scholar |
[7] Voyles, J et al.. (2009) Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326, 582–585.
| Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines.Crossref | GoogleScholarGoogle Scholar |
[8] Berger, L et al.. (2005) Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis Aquat Organ 68, 65–70.
| Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis.Crossref | GoogleScholarGoogle Scholar |
[9] Ellison, AR et al.. (2014) Fighting a losing battle: vigorous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3 4, 1275–1289.
| Fighting a losing battle: vigorous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki.Crossref | GoogleScholarGoogle Scholar |
[10] Fites, JS et al.. (2013) The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342, 366–369.
| The invasive chytrid fungus of amphibians paralyzes lymphocyte responses.Crossref | GoogleScholarGoogle Scholar |
[11] Rollins-Smith, LA et al.. (2015) Immunomodulatory metabolites released by the frog-killing fungus Batrachochytrium dendrobatidis. Infect Immun 83, 4565–4570.
| Immunomodulatory metabolites released by the frog-killing fungus Batrachochytrium dendrobatidis.Crossref | GoogleScholarGoogle Scholar |
[12] Boyle, DG et al.. (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60, 141–148.
| Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay.Crossref | GoogleScholarGoogle Scholar |
[13] Cheng, TL et al.. (2011) Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA 108, 9502–9507.
| Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis.Crossref | GoogleScholarGoogle Scholar |
[14] Rosenblum, EB et al.. (2013) Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc Natl Acad Sci USA 110, 9385–9390.
| Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data.Crossref | GoogleScholarGoogle Scholar |
[15] O’Hanlon, SJ et al.. (2018) Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360, 621–627.
| Recent Asian origin of chytrid fungi causing global amphibian declines.Crossref | GoogleScholarGoogle Scholar |
[16] Byrne, AQ et al.. (2019) Cryptic diversity of a widespread global pathogen reveals expanded threats to amphibian conservation. Proc Natl Acad Sci USA 116, 20382–20387.
| Cryptic diversity of a widespread global pathogen reveals expanded threats to amphibian conservation.Crossref | GoogleScholarGoogle Scholar |
[17] Zipkin, EF et al.. (2020) Tropical snake diversity collapses after widespread amphibian loss. Science 367, 814–816.
| Tropical snake diversity collapses after widespread amphibian loss.Crossref | GoogleScholarGoogle Scholar |
[18] Murray, K et al.. (2010) The distribution and host range of the pandemic disease chytridiomycosis in Australia, spanning surveys from 1956–2007. Ecology 91, 1557–1558.
| The distribution and host range of the pandemic disease chytridiomycosis in Australia, spanning surveys from 1956–2007.Crossref | GoogleScholarGoogle Scholar |
[19] Kosch, TA et al.. (2017) Characterization of MHC class IA in the endangered southern corroboree frog. Immunogenetics 69, 165–174.
| Characterization of MHC class IA in the endangered southern corroboree frog.Crossref | GoogleScholarGoogle Scholar |
[20] Brannelly, LA et al.. (2018) Non-declining amphibians can be important reservoir hosts for amphibian chytrid fungus. Anim Conserv 21, 91–101.
| Non-declining amphibians can be important reservoir hosts for amphibian chytrid fungus.Crossref | GoogleScholarGoogle Scholar |
[21] Martel, A et al.. (2011) Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians. Med Mycol 49, 143–149.
| Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians.Crossref | GoogleScholarGoogle Scholar |
[22] Bosch, J et al.. (2015) Successful elimination of a lethal wildlife infectious disease in nature. Biol Lett 11, 20150874.
| Successful elimination of a lethal wildlife infectious disease in nature.Crossref | GoogleScholarGoogle Scholar |
[23] Cook, K et al.. (2022) In situ treatment of juvenile frogs for disease can reverse population declines. Conserv Sci Pract 4, e12762.
| In situ treatment of juvenile frogs for disease can reverse population declines.Crossref | GoogleScholarGoogle Scholar |
[24] Stockwell, MP et al.. (2015) Evidence of a salt refuge: chytrid infection loads are suppressed in hosts exposed to salt. Oecologia 177, 901–910.
| Evidence of a salt refuge: chytrid infection loads are suppressed in hosts exposed to salt.Crossref | GoogleScholarGoogle Scholar |
[25] Webb, RJ et al.. (2022) Non-detection of mycoviruses in amphibian chytrid fungus (Batrachochytrium dendrobatidis) from Australia. Fungal Biol 126, 75–81.
| Non-detection of mycoviruses in amphibian chytrid fungus (Batrachochytrium dendrobatidis) from Australia.Crossref | GoogleScholarGoogle Scholar |
[26] Cashins, SD et al.. (2013) Prior infection does not improve survival against the amphibian disease chytridiomycosis. PLoS One 8, e56747.
| Prior infection does not improve survival against the amphibian disease chytridiomycosis.Crossref | GoogleScholarGoogle Scholar |
[27] McMahon, TA et al.. (2014) Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511, 224–227.
| Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression.Crossref | GoogleScholarGoogle Scholar |
[28] Waddle, AW et al.. (2021) Amphibian resistance to chytridiomycosis increases following low-virulence chytrid fungal infection or drug-mediated clearance. J Appl Ecol 58, 2053–2064.
| Amphibian resistance to chytridiomycosis increases following low-virulence chytrid fungal infection or drug-mediated clearance.Crossref | GoogleScholarGoogle Scholar |
[29] Greenspan, SE et al.. (2018) Hybrids of amphibian chytrid show high virulence in native hosts. Sci Rep 8, 9600.
| Hybrids of amphibian chytrid show high virulence in native hosts.Crossref | GoogleScholarGoogle Scholar |
[30] Martel, A et al.. (2013) Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci USA 110, 15325–15329.
| Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians.Crossref | GoogleScholarGoogle Scholar |
[31] Yap, TA et al.. (2017) Batrachochytrium salamandrivorans and the risk of a second amphibian pandemic. Ecohealth 14, 851–864.
| Batrachochytrium salamandrivorans and the risk of a second amphibian pandemic.Crossref | GoogleScholarGoogle Scholar |
[32] Gray, MJ et al.. (2015) Batrachochytrium salamandrivorans: the north American response and a call for action. PLoS Pathog 11, e1005251.
| Batrachochytrium salamandrivorans: the north American response and a call for action.Crossref | GoogleScholarGoogle Scholar |
[33] Towe, AE et al.. (2021) Batrachochytrium salamandrivorans can devour more than salamanders. J Wildl Dis 57, 942–948.
| Batrachochytrium salamandrivorans can devour more than salamanders.Crossref | GoogleScholarGoogle Scholar |
[34] Skerratt, LF et al.. (2016) Priorities for management of chytridiomycosis in Australia: saving frogs from extinction. Wildl Res 43, 105–120.
| Priorities for management of chytridiomycosis in Australia: saving frogs from extinction.Crossref | GoogleScholarGoogle Scholar |


