Xylella: the greatest threat to Australian agriculture?
Philip Taylor A *A CABI E-UK, Bakeham Lane, Egham, Surrey, TW20 9TY, UK.

Philip Taylor gained a first-class honours degree in Plant Sciences from Wye College, University of London, in 1982. He then pursued an academic career path with a PhD at John Innes Institute on the Downy mildew of pea and these studies led to two post-docs, one in Illinois and one in Durham, UK. Subsequently, he became a lecturer in molecular plant pathology at the University of Hull, UK. He then gave up the academic life to become a farmer, successfully running a large commercial farm taking it into organic, and GM production. His interest remained in science and during this time he took an MSc in science communication at Imperial College and represented the National Farmers Union on biotechnical matters as part of their working party. Years later another change of tack took him to CABI as part of the international development group and he became the training manager for Plantwise; a large donor funded programme designed to bolster extension services in developing countries. He has travelled extensively in this role. |
Microbiology Australia 43(4) 165-168 https://doi.org/10.1071/MA22055
Submitted: 5 October 2022 Accepted: 4 November 2022 Published: 29 November 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The realities of climate change and global world trade could be playing into the hands of plant pathogens, none more so than Xylella fastidiosa. A relatively unimportant and parochial pathogen 50 years ago, it has become one of the most important plant diseases in the world threatening crop production in a wide variety of tree crops all over the globe. It moves within a region within insect vectors analogous to virus transmission but long-distance spread is through traded, often asymptomatic, plants. On arrival in a new region many of the local sap feeding insect population are candidates for its spread and this uncertainty coupled with the potential for the range of these as yet unidentified vectors to enlarge is heaping uncertainty on uncertainty. In addition to crop plants, many amenity trees species are susceptible, infection is often fatal and there is no cure once infection has occurred. Phytosanitation officers around the globe are deeply concerned about this new threat, the likes of which have never been seen previously.
Keywords: biosecurity, climate change, devastating, economic impact, insect vectors, olive quick decline syndrome, sharp shooter, Xylella fastidiosa.
Xylella fastidiosa is a bacterial plant pathogen like no other and if not already could become one of the world’s most important bacterial plant pathogens in history. Distantly related to the more familiar Xanthomonas group of plant pathogens it was voted in the top ten most important plant pathogenic bacteria by the international community in 20121 and that was before it made the 4500 mile journey from America to Europe. It is now firmly established in Italy where it has become a major threat to the olive industry and there are numerous other well documented reports of it being elsewhere in Europe, namely: France, Spain, Germany, the Czech Republic, Kosovo and further afield in Israel, Taiwan, and Iran.2
|
Despite this recent surge in interest the pathogen is nothing new. The diseases it causes were originally studied by the world’s first professional plant pathologist Newton B. Pierce who stated that he was going to work on the problem (originally called Anaheim disease after the town in California where it was first observed) and not move onto any other disease until he had cracked it.3 This pathogen was a particularly poor choice for Pierce’s career: unlike almost every other plant pathogenic bacterium it cannot be directly inoculated from one plant to another and as it requires extremely exacting culture media, it was first cultured in the early 1980s and was awarded the name Xylella fastidiosa in 1987.4,5
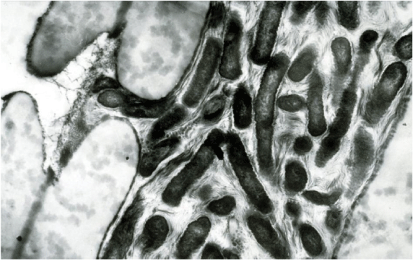
X. fastidiosa proliferates only in xylem vessels, of roots, stems and leaves (Fig. 1). The vessels are ultimately blocked by bacterial aggregates and by tyloses and gums formed by the plant, although disease induction may not be due simply to water blockage.6 It appears to be able to move against the transpiration stream moving down into the roots as well as up into the leaves in many hosts whereas in others it will multiply but not leave the site of infection.7 These findings have considerable implications for plants where grafting between scions and rootstocks is commonplace.
Over a century since Pierce’s death we still have not ‘cracked it’, and it is cruel irony that one of the most environmentally important, but least understood plant diseases, bears his name; ‘Pierce’s disease of grapevine’.
However, Pierce’s disease is not the only named disease caused by X. fastidiosa. Using molecular means, the species has been split into at least five subspecies, which have evolved in distinct geographical regions:8
X. fastidiosa subsp. fastidiosa, thought to be native to southern Central America,9 is associated primarily with Pierce’s disease of grapevines and almond leaf scorch.
X. fastidiosa subsp. multiplex, thought to be native to temperate and subtropical North America, is associated with scorch disease in a wide range of trees, including phony peach disease and plum leaf scorch.10
X. fastidiosa subsp. pauca, thought to be native to South America, is associated with strains causing disease in citrus and coffee.11
X. fastidiosa subsp. sandyi, believed to originate from the southern region of the USA, is associated with oleander leaf scorch.12
X. fastidiosa subsp. taiwanensis believe to be isolated in Taiwan and is mostly affecting pear trees.13
The cases of X. fastidiosa within Europe are not all of one subspecies, which strongly indicates several recent introductions rather than one introduction followed by subsequent spread within Europe especially so as there is considerable specificity between sub-species and vector.8,14
Unlike almost all other bacterial plant pathogens X. fastidiosa requires an insect vector to transmit the disease in a manner analogous to many insect vectored viruses. The glassy-winged sharpshooter, Homalodisca vitripennis (Fig. 2) is the most common vector of Pierce’s disease of grapevine but this species is only found in North America and other xylem-feeding insects including froghoppers and spittlebugs are vectors of other subspecies in other regions.15

|
On arrival in a new region it is difficult to predict which potential vectors (which will never have encountered the bacterium previously) will spread the recently arrived subspecies. Additionally, the spectre of climate change which may increase the range of potential vectors make predictions as to the pathogen spread very difficult. Bosso et al. using modelling methods, predicts that the disease could spread much further than its current location in Italy.16
The success of X. fastidiosa is reflected in its host range. Many hosts are woody trees and bushes both ornamental and fruit crops. However, other hosts include fodder crops (clover, lucerne and ryegrass), vegetable crops such as brassicas, herbs (e.g. rosemary) and weeds such as dandelion, wild oat and chickweed.2
Due to continued observation in the field and laboratory research the number of recorded hosts is rising on an almost weekly basis, 11 new species were added to the list of susceptible hosts between May 2021 and the end of that year taking the total number of hosts to 664 plant species, 299 genera and 88 families.17
What makes the long range spread of X. fastidiosa particularly insidious is that many hosts are asymptomatic. Perfectly ‘healthy’ plants could be imported in good faith while bringing the pathogen into a new area. The tests for Xylella are not cheap and often it is not feasible to test every plant.18 To avoid testing, hot water treatment to kill the pathogen has been used in some cases.
The most well documented attempts to contain spread within a region is that of the outbreak in Italy. Believed to have arrived on coffee plants in 2008 the pathogen has wiped out vast swathes of olives.19 In desperate attempts to save their trees farmers have come up with their own control measures: sprays containing zinc, copper and citric acid are claimed to reduce the severity of the disease,20 but these claims are disputed by the scientific community.21 The only effective way to limit spread of the disease is through vector control, coupled with a cordon sanitaire whereby all known hosts of the pathogen are removed around the affected area – something not easy due to the vast host range. Despite the best efforts of the Italian authorities the pathogen is spreading at approximately 10 km per year.22
Each of the subspecies causes different symptoms on their respective hosts; however, the most familiar and dramatic symptom is leaf scorch. On grapes particularly, an early sign is sudden drying of part of a green leaf, which then turns brown while adjacent tissues turn yellow or red reminiscent of an autumnal display (Fig. 3). The desiccation spreads and the whole leaf may shrivel and drop, leaving only the petiole attached. Once infected, grapevines rarely survive more than 2–3 years.23

|
The disease of citrus takes a different course; initially trees show interveinal chlorosis on young leaves as they mature. This variegation spreads throughout the canopy and to mature leaves with time. As the leaves mature, small, light-brown, slightly raised gummy lesions (becoming dark-brown or even necrotic) appear on the underside, directly opposite the yellow chlorotic areas on the upper side. Affected trees show stunting and slow growth rate; twigs and branches die back and the canopy thins, but affected trees do not die.24 Timing of blossom and fruit set are unaffected, but natural fruit thinning does not occur. Therefore, on infected trees the fruits remain small; however, as more fruits remain on the tree, total production is not hugely reduced.
The threat to Australian native flora and crops is significant. Hafi et al. make the point that the economic impact would depend on which subspecies arrived25 (the arrival of X. fastidiosa subsp. fastidiosa would be by far the most economically damaging subspecies but in combination with other subspecies the situation would be worse).
Crops under threat include high value Australian horticultural crops; avocados, citrus, grapes, nuts, olives and stone fruits are all susceptible. Together these crops contribute to around 9% of the Australia’s total agricultural GVP.26 The effects on the downstream processing industries would also be significant. Victoria and South Australia would suffer the greatest economic losses were the pathogen to become established.
The pathogen affects amenity trees and shrubs, native Australian shrubs are affected in North America and North American and European amenity trees common in Australia are similarly affected.
The Department of Agriculture, Fisheries and Forestry has estimated that if a single subspecies were to establish in Australia it would cost between $1.2 billion and $8.9 billion in 2017–18 over 50 years at a 3% discount rate, and of course more if multiple subspecies established.25
Departments of Agriculture from all over the world are deeply troubled by this relatively new threat of an old pathogen, as can be seen by the size of the conferences dedicated to this pathogen. In 2015, 100 plant health specialists met in Brussels, whereas in April 2021, 900 interested stakeholders from more than 60 countries met online to discuss this threat to world agriculture.27 Australia has world class biosecurity and is taking the threat very seriously. In 2019, the Department of Agriculture, Fisheries and Forestry produced the National Xylella Action Plan, detailing prevention, detection, response, cross-cutting issues such as coordination of stakeholders.28 In addition to this document the Inspector-General of Biosecurity commissioned a document on the effectiveness of the biosecurity arrangements already in place.29 While the threat is real, no other country is doing more to prevent the arrival of this pest or deal with it should it arrive.
It is ironic that after all this time, the first pathogen studied by the first professional plant pathologist is still causing the world the most headaches.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The author declares that he has no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
Thanks to Fiona Taylor for help with proofreading and assistance with reference collation.
References
[1] Mansfield, J et al.. (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13, 614–629.| Top 10 plant pathogenic bacteria in molecular plant pathology.Crossref | GoogleScholarGoogle Scholar |
[2] CABI (2022) Crop protection compendium. https://www.cabi.org/cpc
[3] Campbell C et al. (1999) The formative years of plant pathology in the United States. American Phytopathological Society, APS Press.
[4] Wells, J et al.. (1981) Medium for isolation and growth of bacteria associated with plum leaf scald and phony peach diseases. Appl Environ Microbiol 42, 357–363.
| Medium for isolation and growth of bacteria associated with plum leaf scald and phony peach diseases.Crossref | GoogleScholarGoogle Scholar |
[5] Wells, J et al.. (1987) Xylella fastidiosa gen. nov., sp. nov: gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int J Syst Evol Microbiol 37, 136–143.
| Xylella fastidiosa gen. nov., sp. nov: gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp.Crossref | GoogleScholarGoogle Scholar |
[6] Goodwin, P et al.. (1988) Physiological responses of Vitis vinifera cv. “Chardonnay” to infection by the Pierce’s disease bacterium. Physiol Mol Plant Pathol 32, 17–32.
| Physiological responses of Vitis vinifera cv. “Chardonnay” to infection by the Pierce’s disease bacterium.Crossref | GoogleScholarGoogle Scholar |
[7] Meng, Y et al.. (2005) Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J Bacteriol 187, 5560–5567.
| Upstream migration of Xylella fastidiosa via pilus-driven twitching motility.Crossref | GoogleScholarGoogle Scholar |
[8] Almeida, R et al.. (2015) How do plant diseases caused by Xylella fastidiosa emerge? Plant Dis 99, 1457–1467.
| How do plant diseases caused by Xylella fastidiosa emerge?Crossref | GoogleScholarGoogle Scholar |
[9] Nunney, L et al.. (2010) Population genomic analysis of a bacterial plant pathogen: novel insight into the origin of Pierce’s disease of grapevine in the US. PLoS One 5, e15488.
| Population genomic analysis of a bacterial plant pathogen: novel insight into the origin of Pierce’s disease of grapevine in the US.Crossref | GoogleScholarGoogle Scholar |
[10] Nunney, L et al.. (2014) Intersubspecific recombination in Xylella fastidiosa strains native to the United States: infection of novel hosts associated with an unsuccessful invasion. Appl Environ Microbiol 80, 1159–1169.
| Intersubspecific recombination in Xylella fastidiosa strains native to the United States: infection of novel hosts associated with an unsuccessful invasion.Crossref | GoogleScholarGoogle Scholar |
[11] Nunney, L et al.. (2012) Detecting genetic introgression: high levels of intersubspecific recombination found in Xylella fastidiosa in Brazil. Appl Environ Microbiol 78, 4702–4714.
| Detecting genetic introgression: high levels of intersubspecific recombination found in Xylella fastidiosa in Brazil.Crossref | GoogleScholarGoogle Scholar |
[12] Yuan, X et al.. (2010) Multilocus sequence typing of Xylella fastidiosa causing Pierce’s disease and oleander leaf scorch in the United States. Phytopathology 100, 601–611.
| Multilocus sequence typing of Xylella fastidiosa causing Pierce’s disease and oleander leaf scorch in the United States.Crossref | GoogleScholarGoogle Scholar |
[13] Su, C et al.. (2016) Xylella taiwanensis sp. nov. causing pear leaf scorch disease. Int J Syst Evol Microbiol 66, 4766–4771.
| Xylella taiwanensis sp. nov. causing pear leaf scorch disease.Crossref | GoogleScholarGoogle Scholar |
[14] Landa, B et al.. (2020) Emergence of a plant pathogen in Europe associated with multiple intercontinental introductions. Appl Environ Microbiol 86, e01521-19.
| Emergence of a plant pathogen in Europe associated with multiple intercontinental introductions.Crossref | GoogleScholarGoogle Scholar |
[15] Cornara, D et al.. (2019) An overview on the worldwide vectors of Xylella fastidiosa. Entomol Gen 39, 157–181.
| An overview on the worldwide vectors of Xylella fastidiosa.Crossref | GoogleScholarGoogle Scholar |
[16] Bosso, L et al.. (2016) Shedding light on the effects of climate change on the potential distribution of Xylella fastidiosa in the Mediterranean basin. Biol Invasions 18, 1759–1768.
| Shedding light on the effects of climate change on the potential distribution of Xylella fastidiosa in the Mediterranean basin.Crossref | GoogleScholarGoogle Scholar |
[17] Delbianco, A et al.. (2022) Scientific report on the update of the Xylella spp. host plant database–systematic literature search up to 31 December 2021. EFSA J 20, 7356.
[18] Baldi, P and La Porta, N (2017) Xylella fastidiosa: host range and advance in molecular identification techniques. Front Plant Sci 8, 9.
| Xylella fastidiosa: host range and advance in molecular identification techniques.Crossref | GoogleScholarGoogle Scholar |
[19] Sicard, A et al.. (2021) Introduction and adaptation of an emerging pathogen to olive trees in Italy. Microb Genom 7, 000735.
| Introduction and adaptation of an emerging pathogen to olive trees in Italy.Crossref | GoogleScholarGoogle Scholar |
[20] DeAndreis P (2021) Treatment to mitigate the impact of Xylella fastidiosa shows promise in Italy. https://www.oliveoiltimes.com/production/treatment-to-mitigate-impact-xylella-fastidiosa-italy/89888
[21] DeAndreis P (2021) Puglia warns farmers of ineffective Xylella fastidiosa cures. https://www.oliveoiltimes.com/business/puglia-warns-farmers-of-ineffective-xylella-fastidiosa-cures/99881
[22] Kottelenberg, D et al.. (2021) Shape and rate of movement of the invasion front of Xylella fastidiosa spp. pauca in Puglia. Sci Rep 11, 1061.
| Shape and rate of movement of the invasion front of Xylella fastidiosa spp. pauca in Puglia.Crossref | GoogleScholarGoogle Scholar |
[23] Smith R et al. (2021) University of California IPM Pest Management Guidelines: Grape UC ANR Publication 3448.
[24] Coletta-Filho, H et al.. (2020) Citrus variegated chlorosis: an overview of 30 years of research and disease management. Trop Plant Pathol 45, 175–191.
| Citrus variegated chlorosis: an overview of 30 years of research and disease management.Crossref | GoogleScholarGoogle Scholar |
[25] Hafi A et al. (2021) Protecting Australia’s horticultural industries from disease: the impacts of Xylella fastidiosa on Australian horticulture and the environment.
[26] Australian bureau of statistics (2020) Agricultural Commodities, Australia. Cat. no. 7121.0. Australian Bureau of Statistics, Canberra, ACT, Australia.
[27] ESFA (2021) 3rd European Conference on Xylella fastidiosa and XF-ACTORS final meeting. https://www.efsa.europa.eu/en/events/event/3rd-european-conference-xylella-fastidiosa-and-xf-actors-final-meeting
[28] Department of Agriculture (2019) National Xylella Action Plan 2019–2029. Canberra.
[29] Inspector-General of Biosecurity (2022) Effectiveness of preventative biosecurity arrangements to mitigate the risk of entry into Australia of the serious plant pest Xylella fastidiosa. Review Report No. 2021–22/03. Inspector-General of Biosecurity, Canberra.


