The future of faecal microbiota transplantation in gastrointestinal illness
Hayley Reed A B C and Jakob Begun A B DA Mater Research Institute University of Queensland Brisbane, Qld, Australia
B Faculty of Medicine The University of Queensland Qld, Australia
C Email: hayley.reed1@uq.net.au
D Email: jakob.begun@mater.uq.edu.au
Microbiology Australia 41(2) 70-74 https://doi.org/10.1071/MA20027
Published: 19 May 2020
Abstract
The gut microbiome is made up of hundreds of trillions of microorganisms that reside in a state of homeostatic balance within the healthy individual. Next generation sequencing has provided insight into the diversity of these microorganisms that reside within our gastrointestinal tract; despite developments in metabolomics and culturing techniques, the functions of many of these bacteria remain largely elusive. As such, research into the capacity of the gut microbiome to regulate immune homeostasis has revealed the importance of bacteria in human health, with the potential for exploiting these bacteria only now coming into focus.
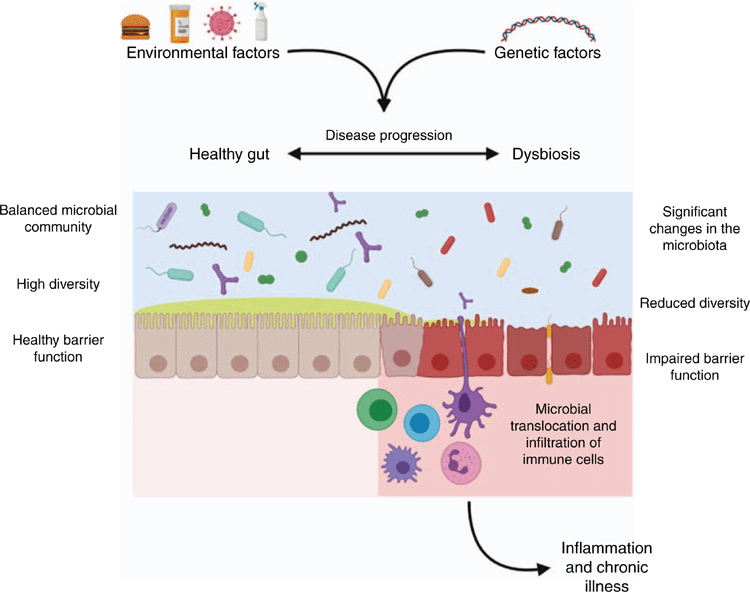
A number of diseases have been associated with ‘dysbiosis’, a term that denotes shifts in the relative abundance of the microbial communities in individuals with a disease relative to healthy individuals1,2 (Fig. 1). This is generally characterised by a significant reduction in microbial diversity, and frequently a reduction in the abundance of beneficial commensals and an increase in pathogenic or pathobiont-like species. However, the characterisation of dysbiosis based on taxonomy is challenging, given the significant inter-individual variability at the microbial species level and the effect of environmental factors, such as diet and medications, on microbiome composition3. Additionally, the bioactive capacity of bacteria is not always phylogenetically conserved, with closely related microbes displaying variable immunomodulatory activity4.
Faecal microbiota transplantation
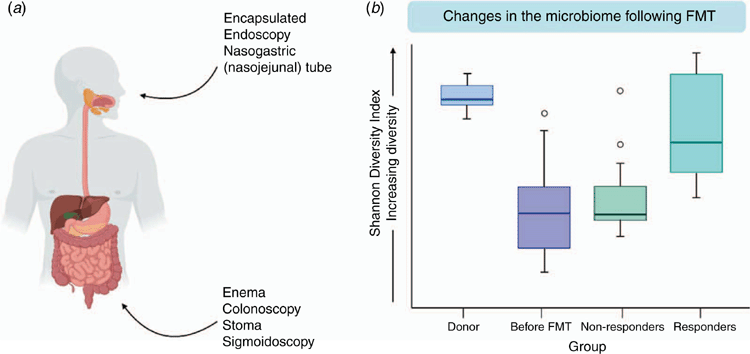
Faecal microbiota transplantation (FMT) involves the infusion of healthy human donor faeces into the bowel of a patient most commonly via colonoscopy or enema, though oral routes have also been used (Fig. 2a). In administering FMT, the central hypothesis is that the contribution of the dysbiotic microbiome to disease can be overcome through restoration to one that resembles that of a healthy individual. The basis of this hypothesis is supported by increases in the Shannon diversity index that occur in responders versus non-responders following FMT in a number of diseases6 (Fig. 2b).
|
Due to a plethora of successful research in the area, FMT is currently the recommended treatment method for recurrent Clostridium difficile infection (rCDI), with a cure rate of greater than 80–85%7. For the treatment of rCDI, FMT is effective regardless of the route of delivery, though lower GI delivery has demonstrated higher efficacy and less associated aspiration events; current consensus statements suggest that this should be individualized based on patient and disease characteristics, with careful consideration of the benefits and risks of each route of administration8.
While antibiotics can be successful in eliminating the C. difficile bacterium, they also reduce the overall diversity of protective bacteria in the gut, creating an environment that encourages spore formation, vegetative growth, and toxin production. It is postulated that the reintroduction of a diverse array of bacteria through FMT restores the colonisation resistance potential of the microbiome, in which resident microbes able to out-compete C. difficile, thus preventing recurrent infection9.
FMT has strong clinical evidence of efficacy for the treatment of rCDI, and emerging evidence for the treatment of a range of other pathologies.
FMT in inflammatory bowel diseases
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract, of which the two main manifestations are ulcerative colitis (UC) and Crohn’s disease (CD). In 2017, IBD was estimated to effect 6.8 million people globally10.
IBD is underpinned by inappropriate immune responses to the commensal intestinal microbiome, in genetically susceptible hosts who are exposed to environmental factors that may trigger disease onset11. Current treatment paradigms for IBD rely on a variety of approaches including dietary therapy, the administration of corticosteroids, immunomodulatory drugs, and biologic antibody-based therapies, as well as surgery for resection of the affected area of the gut. Despite the success of these approaches there still remains a therapeutic gap, with 10–30% of IBD patients being recalcitrant to medical treatment12.
The microbiome of IBD patients is notably different to that of healthy individuals. IBD patients maintain significantly reduced taxonomic richness and a shift in abundance of key phyla, with general reductions in the abundance of members of bacterial families including Erysipelotrichales, Bacteriodales, and Clostridiales and increases in the abundance of Veillonellaceae, Enterobacteriaceae, Pasteurellaceae, and Fusobacteriaceae13. In addition, evidence supports the association of specific bacteria, including adherent-invasive Escherichia coli14 and Mycobacterium avium subspecies paratuberculosis15 with IBD, although it remains unclear if these organisms directly drive disease pathogenesis or are merely more abundant in the presence of underlying gut inflammation.
This taxonomic dysbiosis is coupled with functional dysbiosis, which has been increasingly explored in the literature. Non-targeted metabolomic analyses have revealed increases in metabolites including primary bile acids, amino acids and sphingolipids, and reductions in tetrapyrrole, triacylglycerol, cholesterol, and long chain fatty acids, in IBD patients when compared with non-IBD controls16. Changes in many of these metabolites are believed to be related to bacterial processes17. Therefore alternative treatment approaches including FMT, aimed at restoring an ‘anti-inflammatory’ gut microbiome, are gaining traction.
FMT in ulcerative colitis
UC presents as continuous, superficial inflammation and ulceration of the colon and rectum, in which symptoms occur intermittently as the disease flares and remits. Complications of UC can include toxic megacolon, colorectal cancer, and extraintestinal manifestations in the liver, eyes, skin, and joints18. Research into the use of FMT in UC has been promising despite disease heterogeneity. To date, four double-blinded placebo-controlled RCTs have been conducted in the area19–22, accompanied by a large number of case reports, case series, and cohort studies. These studies generally involve multiple FMT treatments, up to five enemas per week over two months. These studies have demonstrated that FMT is efficacious in inducing remission in mild-moderately active UC, with primary remission rates following FMT reported in a meta-analysis to be approximately 30%7, which is similar to that of many biologic agents studied in UC.
Generally these clinical trials have reported increased microbial diversity and altered composition in UC patients that achieve remission following FMT, when compared with pre-FMT samples or patients that do not respond23. Following a double-blind trial of 81 patients with active UC, Paramsothy et al. (2019) reported that patients in remission after FMT had increased abundance of Eubacterium hallii and Roseburia inulivorans, which contrasted with the higher abundance of Fusobacterium gonidiaformans, Sutterella wadsworthensis, and Escherichia spp. in patients that did not respond to FMT23. Significant changes in the functional capacity of the microbiome have also been reported to co-occur with the taxonomic shifts following FMT; Paramsothy et al. (2019) also reported that UC patients who achieved remission after FMT had higher levels of short chain fatty acid biosynthesis and secondary bile acids when compared with non-responders, who maintained increased heme and lipopolysaccharide biosynthesis profiles23.
FMT in Crohn’s disease
Research is ongoing into the use of FMT in CD, the subtype of IBD that exhibits transmural, discontinuous inflammation throughout the gastrointestinal tract. As of April 2020, five placebo- or sham-controlled RCTs were listed on the U.S. National Library of Medicine Clinical Trials website as being in the pre-recruitment or recruitment phases of study. The results of these studies will be informative; however, there is insufficient evidence at present to support FMT for treatment of CD7.
The efficacy of FMT in IBD appears much lower than in rCDI, potentially reflecting the multifactorial aetiology of IBD, and the likelihood that bacterial species within this dysbiotic microbiome have well developed niches and therefore difficult to displace24. The higher variability of response seen in IBD studies when compared with rCDI is likely reflective of differences in study methodologies examined in IBD, and highlights the potential of donor and/or patient dependent effects.
FMT in irritable bowel syndrome
As with IBD, research into the use of FMT in other illnesses associated with gut dysbiosis is emerging. Despite affecting up to 1 in 5 individuals, the aetiology of irritable bowel syndrome (IBS) is poorly understood and treatment options are limited. Data collected through RCTs has been mixed, and when administration routes were analysed together, FMT did not consistently improve symptoms in patients despite positive results in some individual trials25. Many studies pool subtypes of IBS, which include constipation predominant, diarrhoea predominant, and mixed subtype, despite the potential for different underlying pathophysiologies, which may impact the analysis of efficacy in these trials. Available RCT data appears to show more success in the diarrhoea predominant subtype; however, further studies are required to understand the characteristics of patients.
FMT for extra-intestinal illnesses
Changes in the gut microbiota have also been reported to co-occur with progression of chronic liver disorders such as non-alcoholic fatty liver disease (NAFLD)26, non-alcoholic steatohepatitis (NASH)2, cirrhosis27, alcoholic liver disease28, and hepatocellular carcinoma (HCC)29. In NAFLD for example, gut dysbiosis and increased gut permeability are associated with chronic and systemic liver inflammation that can increase the risk for developing HCC; the gut microbiota is therefore a potential target for managing this disease. As a result, clinical trials are currently underway to assess the efficacy of FMT in the context of liver disease (NCT02496390, NCT02469272).
As in other emerging clinical areas, there has been some preliminary success in the use of FMT in recurrent hepatic encephalopathy (HE), a complication of cirrhosis that manifests as an altered mental status. When compared with standard of care treatments, including lactulose and rifaximin treatment, those who received FMT had reduced HE recurrence and liver-related hospitalisation events, as well as improved cognition, demonstrating the promise of FMT in this setting30.
Beyond faecal microbiota transplantation
Ongoing study in the emerging area of FMT therapy is clearly needed. Despite its preliminary successes, practical difficulties associated with FMT including donor recruitment and screening, manipulation of faeces, choice of delivery route, and lack of regulation, have encouraged research into the development of more defined therapies to overcome these barriers.
Research on products that contain either single microbial species, or a defined consortia of microbes, in an attempt to harness those bacteria with specific beneficial immunomodulatory capacities, is gaining traction. These products may contain live or dead bacteria, or their secreted bioactive products, and are designed to target specific pathways. In the case of IBD, these products may be developed to modulate aberrant immune responses or increase mucosal barrier integrity.
Examples of specific bacterial bioactive compounds include the microbial anti-inflammatory molecule (MAM) peptide produced by Faecalibacterium prausnitzii, which inhibits pro-inflammatory signalling in epithelial cells and reduces inflammation in murine models of chemically induced colitis31, and polysaccharide A (PSA) from Bacteroides fragilis, which was found to suppress pro-inflammatory cytokines32. There is the potential for products such as these to be developed into single formulation ‘probiotics’ that can be taken orally to treat disease.
Probiotics and consortia products also offer the potential for regulated standardised treatments, though thus far these therapies have had limited success and require further trial in a clinical setting. Products such as SER-287 by Seres™ Therapeutics33, and Rebiotix product RBX266034, are currently in the clinical trial phases for IBD and rCDI respectively.
Conclusion
Harnessing the power of the microbiome is an attractive therapeutic option for a number of diseases. However, as treatment approaches shift towards personalisation the use of FMT to manage disease may appear archaic. Nevertheless, its success in the treatment of rCDI and emerging successes in other clinical areas demonstrates its value within the treatment armamentarium. Regulatory pressures and a need for greater safety and reporting are resulting in a preference for FMT products originating from stool banks or commercial facilities. There is likely to be further evolution of microbial directed therapies; however, whether this will be single bacteria or consortia products remains to be seen, and whether these products will be superior to FMT depends on their success in clinical trials in the years to come.
Conflicts of interest
JB has received consulting fees from Ferring Pharmaceuticals.
Acknowledgements
This research did not receive any specific funding.
References
[1] Human Microbiome Project (2012) Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214.| Structure, function and diversity of the healthy human microbiome.Crossref | GoogleScholarGoogle Scholar | 22699609PubMed |
[2] Boursier, J. et al. (2016) The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63, 764–775.
| The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota.Crossref | GoogleScholarGoogle Scholar | 26600078PubMed |
[3] Lloyd-Price, J. et al. (2019) Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662.
| Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases.Crossref | GoogleScholarGoogle Scholar | 31142855PubMed |
[4] Zhai, R. et al. (2019) Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front. Cell. Infect. Microbiol. 9, 239.
| Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice.Crossref | GoogleScholarGoogle Scholar | 31334133PubMed |
[5] Kao, D. et al. (2017) Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 318, 1985–1993.
| Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 29183074PubMed |
[6] Vaughn, B.P. et al. (2016) Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm. Bowel Dis. 22, 2182–2190.
| Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease.Crossref | GoogleScholarGoogle Scholar | 27542133PubMed |
[7] Paramsothy, S. et al. (2017) Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 11, 1180–1199.
| Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 28486648PubMed |
[8] Haifer, C. et al. (2020) Australian consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice. Gut , .
| Australian consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice.Crossref | GoogleScholarGoogle Scholar | 32047093PubMed |
[9] Britton, R.A. and Young, V.B. (2014) Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 146, 1547–1553.
| Role of the intestinal microbiota in resistance to colonization by Clostridium difficile.Crossref | GoogleScholarGoogle Scholar | 24503131PubMed |
[10] GBD 2017 Inflammatory Bowel Disease Collaborators (2020) The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. , .
| The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017.Crossref | GoogleScholarGoogle Scholar | 32416863PubMed |
[11] Baumgart, D.C. and Carding, S.R. (2007) Inflammatory bowel disease: cause and immunobiology. Lancet 369, 1627–1640.
| Inflammatory bowel disease: cause and immunobiology.Crossref | GoogleScholarGoogle Scholar | 17499605PubMed |
[12] Roda, G. et al. (2016) Loss of response to anti-TNFs: definition, epidemiology, and management. Clin. Transl. Gastroenterol. 7, e135.
| Loss of response to anti-TNFs: definition, epidemiology, and management.Crossref | GoogleScholarGoogle Scholar | 26938479PubMed |
[13] Gevers, D. et al. (2014) The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15, 382–392.
| The treatment-naive microbiome in new-onset Crohn’s disease.Crossref | GoogleScholarGoogle Scholar | 24629344PubMed |
[14] Palmela, C. et al. (2018) Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 67, 574–587.
| Adherent-invasive Escherichia coli in inflammatory bowel disease.Crossref | GoogleScholarGoogle Scholar | 29141957PubMed |
[15] Zamani, S. et al. (2017) Mycobacterium avium subsp. paratuberculosis and associated risk factors for inflammatory bowel disease in Iranian patients. Gut Pathog. 9, 1.
| Mycobacterium avium subsp. paratuberculosis and associated risk factors for inflammatory bowel disease in Iranian patients.Crossref | GoogleScholarGoogle Scholar | 28053669PubMed |
[16] Lavelle, A. and Sokol, H. (2020) Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. , .
| Gut microbiota-derived metabolites as key actors in inflammatory bowel disease.Crossref | GoogleScholarGoogle Scholar | 32076145PubMed |
[17] Franzosa, E.A. et al. (2019) Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 4, 293–305.
| Gut microbiome structure and metabolic activity in inflammatory bowel disease.Crossref | GoogleScholarGoogle Scholar | 30531976PubMed |
[18] Vavricka, S.R. et al. (2015) Extraintestinal manifestations of inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1982–1992.
| Extraintestinal manifestations of inflammatory bowel disease.Crossref | GoogleScholarGoogle Scholar | 26154136PubMed |
[19] Costello, S.P. et al. (2019) Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 321, 156–164.
| Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 30644982PubMed |
[20] Pai, N. and Popov, J. (2017) Protocol for a randomised, placebo-controlled pilot study for assessing feasibility and efficacy of faecal microbiota transplantation in a paediatric ulcerative colitis population: PediFETCh trial. BMJ Open 7, e016698.
| Protocol for a randomised, placebo-controlled pilot study for assessing feasibility and efficacy of faecal microbiota transplantation in a paediatric ulcerative colitis population: PediFETCh trial.Crossref | GoogleScholarGoogle Scholar | 29101138PubMed |
[21] Paramsothy, S. et al. (2017) Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228.
| Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial.Crossref | GoogleScholarGoogle Scholar | 28214091PubMed |
[22] Rossen, N. G. et al. (2015) Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149, 110–118.e4.
| Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis.Crossref | GoogleScholarGoogle Scholar | 25836986PubMed |
[23] Paramsothy, S. et al. (2019) Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 156, 1440–1454.e2.
| Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis.Crossref | GoogleScholarGoogle Scholar | 30529583PubMed |
[24] Zmora, N. et al. (2018) Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174, 1388–1405.e21.
| Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features.Crossref | GoogleScholarGoogle Scholar | 30193112PubMed |
[25] Ianiro, G. et al. (2019) Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 50, 240–248.
| Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome.Crossref | GoogleScholarGoogle Scholar | 31136009PubMed |
[26] Michail, S. et al. (2015) Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 91, 1–9.
| Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease.Crossref | GoogleScholarGoogle Scholar | 25764541PubMed |
[27] Qin, N. et al. (2014) Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64.
| Alterations of the human gut microbiome in liver cirrhosis.Crossref | GoogleScholarGoogle Scholar | 25079328PubMed |
[28] Kirpich, I.A. et al. (2008) Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42, 675–682.
| Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study.Crossref | GoogleScholarGoogle Scholar | 19038698PubMed |
[29] Yu, L.X. and Schwabe, R.F. (2017) The gut microbiome and liver cancer: mechanisms and clinical translation. Nat. Rev. Gastroenterol. Hepatol. 14, 527–539.
| The gut microbiome and liver cancer: mechanisms and clinical translation.Crossref | GoogleScholarGoogle Scholar | 28676707PubMed |
[30] Bajaj, J.S. et al. (2017) Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 66, 1727–1738.
| Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 28586116PubMed |
[31] Sokol, H. et al. (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 105, 16731–16736.
| Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients.Crossref | GoogleScholarGoogle Scholar | 18936492PubMed |
[32] Mazmanian, S.K. et al. (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625.
| A microbial symbiosis factor prevents intestinal inflammatory disease.Crossref | GoogleScholarGoogle Scholar | 18509436PubMed |
[33] Simmons, S. et al. (2018) Engraftment of Ser-287, an investigational microbiome therapeutic, is related to clinical remission in a placebo-controlled, double-blind randomized trial (Seres-101) in patients with active mild to moderate ulcerative colitis (UC). Gastroenterology 154, S1371–S1372.
| Engraftment of Ser-287, an investigational microbiome therapeutic, is related to clinical remission in a placebo-controlled, double-blind randomized trial (Seres-101) in patients with active mild to moderate ulcerative colitis (UC).Crossref | GoogleScholarGoogle Scholar |
[34] Blount, K.F. et al. (2019) Restoration of bacterial microbiome composition and diversity among treatment responders in a phase 2 trial of RBX2660: an investigational microbiome restoration therapeutic. Open Forum Infect. Dis. 6, ofz095.
| Restoration of bacterial microbiome composition and diversity among treatment responders in a phase 2 trial of RBX2660: an investigational microbiome restoration therapeutic.Crossref | GoogleScholarGoogle Scholar | 31024971PubMed |
Biographies
Hayley Reed is a second year PhD candidate at the Mater Research Institute within The University of Queensland. Her research focuses on the anti-inflammatory capacity of healthy gut bacteria in the context of Inflammatory Bowel Disease.
Dr Jakob Begun completed his MPhil in Biochemistry at Cambridge University, and his MD and PhD in genetics at Harvard Medical School. He completed his advanced training in Gastroenterology and Inflammatory Bowel Disease (IBD) at Massachusetts General Hospital. He returned to Australia in 2014 to pursue his interests in clinical and translational IBD research and gut health. He is the Director of IBD at the Mater Hospital in Brisbane, IBD Group leader at the Mater Research Institute, and an Associate Professor at the School of Medicine, The University of Queensland. He leads a basic and translational laboratory at the Translational Research Institute investigating the interaction between the innate immune system and the gut microbiome, as well as genetic contributions to disease. He also performs clinical research examining predictors of response to therapy, minimising barriers of care for adolescents and young adults with IBD, improving outcomes in pregnancy and IBD, and the use of intestinal ultrasound in IBD. He is the chair of the Gastroenterology Society of Australia-IBD Faculty and of the president of the Gastroenterology Network of Intestinal Ultrasound (GENIUS).