On the use of probiotics to improve dairy cattle health and productivity
Divya Krishnan A , Hulayyil Al-harbi A , Justine Gibson A , Timothy Olchowy A B and John Alawneh A CA Good Clinical Practice Research Group, School of Veterinary Science, University of Queensland, Gatton, Qld 4343, Australia
B Faculty of Veterinary Medicine, Department of Production Animal Health, University of Calgary, Calgary, Alberta T2N4Z6, Canada
C Tel.: +61 7 5460 1992, Email: j.alawneh@uq.edu.au
Microbiology Australia 41(2) 86-90 https://doi.org/10.1071/MA20022
Published: 5 May 2020
Abstract
Probiotics are genetically identifiable, live microorganisms that when administered in adequate amounts, confer appropriately sized health benefit (e.g. correcting dysbiosis, immunomodulatory effect) on a target host. In cattle, probiotics have shown promising results and long-term benefits in productivity when used on animals under stress. The health and production benefits of probiotics were attributed to improvement in fermentation in rumen and intestine, the stabilisation of rumen pH, and improvements in the intestinal barriers. In the bovine udder, a dysbiosis of the commensal intramammary microbiota and the presence of mastitis causing-bacteria has been linked to increased intramammary infections. Probiotic bacteria capable of biofilm formation inside the udder either serve as a barrier against pathogens or disrupt and replace biofilms formed by pathogens. Over the past two decades, several types of probiotics have been used as feed additives; however, the effect of probiotic use on disease prevention and cattle health and performance indicators, and characterisation of the immunomodulatory association between probiotic microbiota and host target system microbiota are yet to be quantified or documented.
The advent of the ban on the use of the antibiotics in agriculture in 1986 in Sweden, followed in 1999 by The Netherlands1. In 2003, the United Nations tripartite (World Health Organization, Food and Agriculture Organisation and World Organisation for Animal Health) released a joint report titled ‘Non-human antimicrobial usage and antimicrobial resistance: scientific assessment report’, which recommended strict surveillance and monitoring, and moderation of antimicrobial usage in the food-producing animal industry, specifically due to the public health implications entailed by zoonotic transmission of bacteria such as Escherichia coli, Salmonella spp., Campylobacter spp., and Enterococcus spp. and antimicrobial usage or resistance risks2.
The report raises many issues related to antimicrobial use patterns and their implications on animal and human health. The most important question arising from the animal health perspective was how health and productivity standards, on both animal and herd levels, can be maintained while reducing (or eliminating) the needs for antimicrobials? Noting that the definition of animal- and herd-level health indicator metrics such as mortality/morbidity rates, disease incidence, immune response and feed conversion efficiency vary between animal production systems3. Therefore, quantifying the effect of antimicrobial usage reduction on animal health and productivity across production systems remains a challenging task4–6.
Probiotics are defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’7,8, the microorganisms must be must be alive in an adequate number when administered, strains must be identified genetically and appropriately tested on target conditions and hosts8. The probiotic interaction with host’s system microbiota (e.g. udder, rumen, intestine) results in correcting system dysbiosis9 and controlling several infectious inflammatory conditions through antagonism and immunomodulation10. Lactic acid bacteria (LAB) that are well known antibacterial producers and generally recognised as safe in the food industry offer a possible alternative to conventional antimicrobials11.
The mammary gland contains unique microbiota12,13. The presence of bacteria not associated with mastitis in the healthy udder reinforces the concept of commensal mammary microbiota, and the ecological structure of the healthy udder microbiota may provide an understanding of the pathogenesis of intramammary infections (IMI) and offer opportunities for developing therapeutic or prophylactic products as an alternative to antimicrobials14. A dysbiosis of the commensal intramammary microbiota and the presence of mastitis causing-bacteria has been linked to IMI in dairy cattle12. The use of probiotics is proposed to correct the dysbiosis9. Studies have been conducted using viable cultures of LAB as intramammary infusions to successfully treat mastitis pathogens with the same efficiency as conventional antimicrobials15. Direct infusion with Lactococcus lactis in the udder has been shown to induce a rapid and considerable innate immune response with the greatest increase in immune gene expression coinciding with peaks in somatic cell counts (SCC)10.
In 2015, a study was conducted to determine the effect of an intramammary infusion with a LAB-based probiotic mix in healthy lactating dairy cows16. The mix successfully elicited a massive inflammatory/immune response in the infused quarters16. The magnitude of the response is particularly noteworthy as the LAB-based mix did not colonise within the udder and bacterial counts recovered from milk decreased to zero 48 h post infusion. All animals experienced an increase in SCC and swollen udder quarters. The immune response was short-lived and SCC returned to pre-infusion levels within five days. It was hypothesised that the immune profile elicited by the LAB-mix was different from a pathogen assault and may prove to be a successful non-antibiotic treatment for mastitis because of the LAB-mix’s ability to produce a bacteriocin with broad-spectrum antibacterial activity against gram-positive pathogens and elicit a rapid and substantial innate immune response. The 2015 study findings compare very favourably with other therapies recently investigated to treat mastitis10.
Probiotic bacteria capable of biofilm formation have also shown promising results in the prevention of mastitis. The biofilm formation inside the udder either serves as a barrier against pathogens17 or disrupts and replaces biofilms formed by pathogens18. The latter could have been driven by interspecies interactions: high growth rates and dominance of probiotic organisms over other biofilm formers19 and substrate competition20,21.
A controlled, crossover study was conducted in 2018 to evaluate the safety and efficacy of LAB-based probiotic applied as a teat spray in improving SCC of lactating dairy cattle22. On average, milk SCC in the control group was 66% higher (1.66, 95% confidence interval (CI) 1.08–2.56, P = 0.02) compared with the probiotics group (Figure 1)22. The study concluded that the probiotic bacteria may have produced a biofilm which could have hindered the colonisation of other bacterial isolates resulting in reduced bacterial counts on the teats. Our results compare favourably with the literature17. More work is needed to better understand the exact mode of action of the probiotic product tested. The successful identification of inflammatory modulators (pro/pre), antibacterial peptides and development of a new biological mastitis therapy could significantly reduce the substantial economic losses incurred by the dairy industry worldwide and improve animal health, productivity and welfare while increasing food safety23.

|
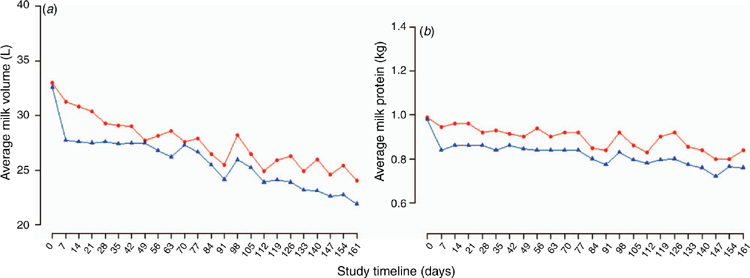
In cattle, probiotics used as feed additives have shown health and productivity benefits when used when animals are assumed to be under stress24. In lactating dairy cattle, after controlling for the effect of days in milk, and cow parity, cows ingesting probiotics have been reported to produce an average of 1.21 L/day more milk (95% CI 0.34–2.08 L/cow per day; Figure 2a), more milk protein (0.03 kg/day; 95% CI 0.01–0.05 kg/day; Figure 2b), numerically lower average SCC and fewer clinical cases of lameness and mastitis than the control cows25. Similar effects on calf health and productivity were also reported26. Calves on probiotics were heavier at weaning, and on average, rumen and intestinal organs’ folding and crypts were more developed and more adapted compared with control calves26. These benefits were hypothesised to be linked to improvement in the ruminal and intestinal fermentation27. The current known mechanisms of action of probiotics in ruminants appears to be through a shift in the microbiota of rumen and rear-gut (small and large intestine), an improvement in fermentation or volatile fatty acids, the stabilisation of rumen pH, and improvements in the intestinal mucosal barriers through the probiotics competitively excluding pathogens and improving the local and systemic immune response28,29.
|
Probiotic bacteria have also been isolated from soil23, fermented green tea30, the gastro-intestinal tract of various animals including poultry31 and cattle32. The most common genera of bacteria used include Lactobacillus spp., Bacillus spp., Bifidobacterium spp., Streptococcus spp. and Enterococcus spp. The intended application of probiotic bacteria varies. Studies that use bacteria like Dietzia spp., and Megasphaera spp., are more focused on their prophylactic uses. The choice of the interventions was based on the presumed mechanism of action of the probiotic strains, e.g. bacteriocin production, lactic acid production, oxygen scavenging, immune-modulation and more generally, their ability to establish a healthier microbial composition in the gastrointestinal tract. The general intent of probiotics is to replace the need for antimicrobials, but the combination of the two have been explored by few: Click (2011) used tetracycline with Dietzia spp. to prevent the development of Johne’s disease symptoms in calf neonates33, and Timmerman et al. (2005) used a prophylactic antibiotic and probiotic mixture to improve the health and growth of veal calves34. In more recent years, probiotic development has shifted from bacteria to using other organisms like yeast (Saccharomyces spp., Candida spp.) and mould (Aspergillus spp.). It has been identified that a mixture of organisms is more effective and are generally better for prophylactic therapy34. Some commercial probiotics are combined with other naturally isolated compounds such as allicin (e.g. Enteroguard®- Romvac Company) and medicinal plant mixes35, which enhance the beneficial effects of the probiotic bacteria by acting synergistically. The most commonly observed or hypothesised mechanisms include the production of inhibitory substances like bacteriocins, organic acids and hydrogen peroxide, production of biofilms by changing bacterial population of gastrointestinal tract; ‘stimulating faecal shedding of coliforms, decreasing concentration of stress hormones like cortisol, increasing in number of CD3+, CD4+, CD45R+, CD8+, T cells, WC1+, CD282+, detoxification of blood from heavy metals like zinc, cadmium and lead’36,37. There is a consensus in the literature further investigations into the exact mechanism of action of probiotics is required in order to maximise the outcome benefits that may be derived from probiotics bacteria.
Conclusions
Probiotics have been proposed as a viable alternative to antimicrobials to enhance animal health and productivity. Over the past two decades, several types of probiotics have been used as feed additives, however, the effect of probiotics use on disease prevention on cattle health and performance indicators (e.g. rumen health and development, growth rate, feed conversion), and characterisation of the immunomodulatory association between probiotic microbiota and host target system microbiota are yet to be quantified or documented.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Mr Jacopo Milazzo for providing feedback on this paper. This research did not receive any specific funding.
References
[1] Landers, T.F. et al. (2012) A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 127, 4–22.| A review of antibiotic use in food animals: perspective, policy, and potential.Crossref | GoogleScholarGoogle Scholar | 22298919PubMed |
[2] World Health Organization (2004) Joint FAO/OIE/WHO Expert Workshop on Non-Human Antimicrobial Usage and Antimicrobial Resistance: scientific assessment: Geneva, December 1–5, 2003. https://apps.who.int/iris/handle/10665/68883
[3] Ivemeyer, S. et al. (2012) Impact of animal health and welfare planning on medicine use, herd health and production in European organic dairy farms. Livest. Sci. 145, 63–72.
| Impact of animal health and welfare planning on medicine use, herd health and production in European organic dairy farms.Crossref | GoogleScholarGoogle Scholar |
[4] Molina, A. (2019) Probiotics and their mechanism of action in animal feed. Agron. Mesoam. 30, 601–611.
| Probiotics and their mechanism of action in animal feed.Crossref | GoogleScholarGoogle Scholar |
[5] Shakoor, M. et al. (2017) Comprehensive overview on the applications of probiotics for healthy animal farming. Indo. Am. J. Pharm. Sci. 4, 1487–1496.
[6] de Magalhaes, J.T. et al. (2015) Chapter 31: the use of probiotics to enhance animal performance. In Probiotics and Prebiotics: Current Research and Future Trends. pp. 459–468.
[7] FAO/WHO (2002). Guidelines for the evaluation of probiotics in food. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf
[8] Hill, C. et al. (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514.
| The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic.Crossref | GoogleScholarGoogle Scholar | 24912386PubMed |
[9] Rainard, P. and Foucras, G. (2018) A critical appraisal of probiotics for mastitis control. Front. Vet. Sci. 5, 251.
| A critical appraisal of probiotics for mastitis control.Crossref | GoogleScholarGoogle Scholar | 30364110PubMed |
[10] Beecher, C. et al. (2009) Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1β and IL-8 gene expression. J. Dairy Res. 76, 340–348.
| Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1β and IL-8 gene expression.Crossref | GoogleScholarGoogle Scholar | 19445831PubMed |
[11] Athanasiou, L.V. et al. (2019) Serum protein electrophoretic profile in diarrheic neonatal calves. Comp. Clin. Path. 28, 685–688.
| Serum protein electrophoretic profile in diarrheic neonatal calves.Crossref | GoogleScholarGoogle Scholar | 32214978PubMed |
[12] Oikonomou, G. et al. (2014) Microbiota of cow’s milk; distinguishing healthy, sub-clinically and clinically diseased quarters. PLoS One 9, e85904.
| Microbiota of cow’s milk; distinguishing healthy, sub-clinically and clinically diseased quarters.Crossref | GoogleScholarGoogle Scholar | 24638139PubMed |
[13] Taponen, S. et al. (2019) Bovine milk microbiome: a more complex issue than expected. Vet. Res. 50, 44.
| Bovine milk microbiome: a more complex issue than expected.Crossref | GoogleScholarGoogle Scholar | 31171032PubMed |
[14] Derakhshani, H. et al. (2018) Invited review: microbiota of the bovine udder: contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 101, 10605–10625.
| Invited review: microbiota of the bovine udder: contributing factors and potential implications for udder health and mastitis susceptibility.Crossref | GoogleScholarGoogle Scholar | 30292553PubMed |
[15] Bouchard, D.S. et al. (2013) Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei. Appl. Environ. Microbiol. 79, 877–885.
| Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei.Crossref | GoogleScholarGoogle Scholar | 23183972PubMed |
[16] Alawneh, J. et al. (2015) Exploratory safety and milk residue study of a biologic intramammary infusion product administered to lactating dairy cows. Good Clinical Practice Research Group, School of Veterinary Science, University of Queensland. p. 87.
[17] Frola, I.D. et al. (2012) Effects of intramammary inoculation of Lactobacillus perolens CRL1724 in lactating cows’ udders. J. Dairy Res. 79, 84–92.
| Effects of intramammary inoculation of Lactobacillus perolens CRL1724 in lactating cows’ udders.Crossref | GoogleScholarGoogle Scholar | 22077995PubMed |
[18] Wallis, J.K. et al. (2019) Biofilm challenge: lactic acid bacteria isolated from bovine udders versus Staphylococci. Foods 8, 79.
| Biofilm challenge: lactic acid bacteria isolated from bovine udders versus Staphylococci.Crossref | GoogleScholarGoogle Scholar |
[19] James, G.A. et al. (1995) Interspecies bacterial interactions in biofilms. J. Ind. Microbiol. 15, 257–262.
| Interspecies bacterial interactions in biofilms.Crossref | GoogleScholarGoogle Scholar |
[20] Kim, H.J. et al. (2008) Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc. Natl. Acad. Sci. USA 105, 18188–18193.
| Defined spatial structure stabilizes a synthetic multispecies bacterial community.Crossref | GoogleScholarGoogle Scholar | 19011107PubMed |
[21] Liu, W. et al. (2016) Interspecific bacterial interactions are reflected in multispecies biofilm spatial organization. Front. Microbiol. 7, 1366.
| Interspecific bacterial interactions are reflected in multispecies biofilm spatial organization.Crossref | GoogleScholarGoogle Scholar | 27630624PubMed |
[22] Alawneh, J. et al. (2018) Discovery level udder safety study of a biologic-based, teat conditioner product intended for use in lactating dairy cows. Good Clinical Practice Research Group, School of Veterinary Science, University of Queensland. p. 80.
[23] Siraj, N.M. et al. (2017) Isolation and identificaction of potential probiotic bacteria from cattle farm soil in Dibrugarh District. Adv. Microbiol. 7, 265–279.
| Isolation and identificaction of potential probiotic bacteria from cattle farm soil in Dibrugarh District.Crossref | GoogleScholarGoogle Scholar |
[24] Renaud, D.L. et al. (2019) Evaluation of a multispecies probiotic as a supportive treatment for diarrhea in dairy calves: a randomized clinical trial. J. Dairy Sci. 102, 4498–4505.
| Evaluation of a multispecies probiotic as a supportive treatment for diarrhea in dairy calves: a randomized clinical trial.Crossref | GoogleScholarGoogle Scholar | 30852016PubMed |
[25] Olchowy, T.W.J. et al. (2019) The effect of a commercial probiotic product on the milk quality of dairy cows. J. Dairy Sci. 102, 2188–2195.
| The effect of a commercial probiotic product on the milk quality of dairy cows.Crossref | GoogleScholarGoogle Scholar |
[26] Alawneh, J. et al. (2019) Discovery level study on the use of biological based milk supplement to improve the health and productivity of dairy calves throughout the pre-weaning period. Good Clinical Practice Research Group, School of Veterinary Science, University of Queensland.
[27] Zeineldin, M. et al. (2018) Synergetic action between the rumen microbiota and bovine health. Microb. Pathog. 124, 106–115.
| Synergetic action between the rumen microbiota and bovine health.Crossref | GoogleScholarGoogle Scholar | 30138752PubMed |
[28] Aikman, P.C. et al. (2011) Rumen pH and fermentation characteristics in dairy cows supplemented with Megasphaera elsdenii NCIMB 41125 in early lactation. J. Dairy Sci. 94, 2840–2849.
| Rumen pH and fermentation characteristics in dairy cows supplemented with Megasphaera elsdenii NCIMB 41125 in early lactation.Crossref | GoogleScholarGoogle Scholar | 21605754PubMed |
[29] Qadis, A.Q. et al. (2014) Effects of a bacteria-based probiotic on ruminal pH, volatile fatty acids and bacterial flora of Holstein calves. J. Vet. Med. Sci. 76, 877–885.
| Effects of a bacteria-based probiotic on ruminal pH, volatile fatty acids and bacterial flora of Holstein calves.Crossref | GoogleScholarGoogle Scholar | 24614603PubMed |
[30] Sarker, M.S. et al. (2010) Effect of different feed additives on growth performance and blood profiles of Korean Hanwoo calves. Asian-Australas. J. Anim. Sci. 23, 52–60.
| Effect of different feed additives on growth performance and blood profiles of Korean Hanwoo calves.Crossref | GoogleScholarGoogle Scholar |
[31] Bujnakova, D. et al. (2014) In vitro evaluation of the safety and probiotic properties of Lactobacilli isolated from chicken and calves. Anaerobe 29, 118–127.
| In vitro evaluation of the safety and probiotic properties of Lactobacilli isolated from chicken and calves.Crossref | GoogleScholarGoogle Scholar | 24291759PubMed |
[32] Zhao, T. et al. (1998) Reduction of carriage of enterohemorrhagic Escherichia coli O157: H7 in cattle by inoculation with probiotic bacteria. J. Clin. Microbiol. 36, 641–647.
| Reduction of carriage of enterohemorrhagic Escherichia coli O157: H7 in cattle by inoculation with probiotic bacteria.Crossref | GoogleScholarGoogle Scholar | 9508288PubMed |
[33] Click, R.E. (2011) A 60-day probiotic protocol with Dietzia subsp. C79793-74 prevents development of Johne’s disease parameters after in utero and/or neonatal MAP infection. Virulence 2, 337–347.
| A 60-day probiotic protocol with Dietzia subsp. C79793-74 prevents development of Johne’s disease parameters after in utero and/or neonatal MAP infection.Crossref | GoogleScholarGoogle Scholar | 21701254PubMed |
[34] Timmerman, H.M. et al. (2005) Health and growth of veal calves fed milk replacers with or without probiotics. J. Dairy Sci. 88, 2154–2165.
| Health and growth of veal calves fed milk replacers with or without probiotics.Crossref | GoogleScholarGoogle Scholar | 15905445PubMed |
[35] Seifzadeh, S. et al. (2017) The effects of a medical plant mix and probiotic on performance and health status of suckling Holstein calves. Ital. J. Anim. Sci. 16, 44–51.
| The effects of a medical plant mix and probiotic on performance and health status of suckling Holstein calves.Crossref | GoogleScholarGoogle Scholar |
[36] Zhang, R. et al. (2016) Effect of oral administration of probiotics on growth performance, apparent nutrient digestibility and stress‐related indicators in Holstein calves. J. Anim. Physiol. Anim. Nutr. (Berl.) 100, 33–38.
| Effect of oral administration of probiotics on growth performance, apparent nutrient digestibility and stress‐related indicators in Holstein calves.Crossref | GoogleScholarGoogle Scholar |
[37] Seo, J. et al. (2010) Direct-fed microbials for ruminant animals. Asian-Australas. J. Anim. Sci. 23, 1657–1667.
| Direct-fed microbials for ruminant animals.Crossref | GoogleScholarGoogle Scholar |
Biographies
Ms Divya Krishnan, BSc, is currently a student of veterinary medicine at the University of Queensland. She graduated in 2017 with her Bachelor of Science degree, having completed it with three majors in Biotechnology, Chemistry and Zoology. Her research interests include epidemiology, wildlife medicine and rehabilitative medicine.
Hulayyil Alharbi, BSc, is a PhD candidate in the group of Dr John Alawneh, in the School of Veterinary Science at the University of Queensland Gatton campus. He completed his MSc in Veterinary Public Health at the University of Glasgow and is now pursuing a PhD project in bovine udder microbiome research focusing on intramammary infection and its influence on udder microbiota.


