Amplification of probiotic bacteria in the skin microbiome to combat Staphylococcus aureus infection
Tristan Yusho Huang A B , Deron Raymond Herr C , Chun-Ming Huang A D and Yong Jiang E FA Department of Dermatology, School of Medicine, University of California, San Diego, CA 92093, USA
B Canyon Crest Academy, San Diego, CA 92130, USA
C Department of Pharmacology, National University of Singapore, 117600, Singapore
D Department of Biomedical Sciences and Engineering, National Central University, Taoyuan, 32001, Taiwan
E America Diagnosis, Inc., San Diego, CA 92121, USA
F Tel.: +858-207-6226, Email: yJiang@ameridx.com
Microbiology Australia 41(2) 61-64 https://doi.org/10.1071/MA20018
Published: 5 May 2020
Abstract
Staphylococcus aureus (S. aureus) is a Gram-positive bacterium. When pathogenic S. aureus colonises onto a skin wound or diabetic ulcer, it can cause a serious infection and lead to amputation or death. The current solutions (e.g. antibiotics and probiotics) are not sufficient enough to be a cure for this infection. To worsen the situation, the S. aureus bacteria continue to develop greater resistance towards antibiotics and are becoming more commonplace. An effective solution is to amplify the activity of probiotic bacteria in the skin microbiome by using selective fermentation initiators (SFIs) to induce fermentation. Our data demonstrated that the numbers of Cutibacterium acnes (C. acnes) and Staphylococcus epidermidis (S. epidermidis), two major bacteria in skin microbiome, on human skin did not vary significantly over the span of seven days. This stimulates probiotic bacteria such as S. epidermidis to produce sufficient short-chain fatty acids (SCFAs) to suppress the growth of S. aureus. The development of this new cure to S. aureus may reduce hospitalisation greatly as S. aureus accounts for the hospitalisation of more than five thousand people per year. Besides antibiotic, probiotics and bacteriophages, SFIs may become novel agents for treatment of infection.
Skin microbiome and dysbiosis
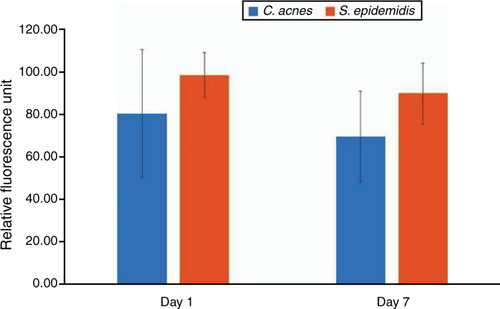
The skin microbiome comprises the microbiota in skin that is home to millions of bacteria, fungi and viruses1. Skin dysbiosis refers to a condition in which microbial imbalances occur in the skin microbiome2,3. Mounting evidence indicates that the probiotic microbes in the human microbiome can employ bacterial interference4 to rein in the overgrowth of opportunistic pathogens5,6. However, little is known about the interactions among probiotic bacteria within the human microbiome for maintaining homeostasis of the microbiome. Bacterial interference, used by probiotic Staphylococcus epidermidis, prevents growth of pathogens and has shown to be a promising modality for preventing and/or treating infections. Literature has demonstrated that Cutibacterium acnes and S. epidermidis, two major bacteria in the skin microbiome7–9, can fermentatively metabolise glycerol, a naturally occurring metabolite found in human skin10, to repel the over-growth of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA). Our results showed the abundances of both C. acnes and S. epidermidis on the skin surface of the same person have no significant changes from Day 1 to Day 7 (Figure 1), indicating the stability of commensal bacteria in skin. The stability of abundances of commensal bacteria in skin will make it possible to apply a fixed dose of prebiotic to induce fermentation. SCFAs are one of metabolites of glycerol fermentation of C. acnes and S. epidermidis. Several SCFAs have been approved by the U.S. Environmental Protection Agency (EPA) or the Food and Drug Administration (FDA) as active compounds for use as antimicrobials11–13. It has been illustrated that a specific SCFA, butyric acid, can diminish inflammation via inhibition of histone deacetylase (HDAC) in host cells14, suggesting the dual antimicrobial and anti-inflammatory abilities of SCFAs.
|
S. aureus infection in diabetic wounds
Infection of the skin by S. aureus is a major cause of hospitalisation and can cause death and organ failure. It is estimated to account for the outpatient visits of 12 million people per year, worldwide, and the problem continues to grow. Furthermore, doctors consistently rely on the use of antibiotics, resulting in the development of MRSA. MRSA is a major issue among people with diabetic ulcers15. Diabetic ulcers occur in 15% of people with diabetes, creating wounds that permit pathogens to enter the body, with one of the most common pathogens being MRSA. Already in a frail state, due to poor blood flow in the ulcer, a pathogenic infection impedes the healing of diabetic ulcers, and the spread of such infections to soft tissue or bony structures often results in the need for amputation. Considering these possible outcomes, the estimated 30% of diabetic ulcers that are colonised with MRSA means that MRSA is among the most common causes of amputation. S. aureus poses a potent threat not only to diabetic patients, but to healthy, normally functioning people as well. Not only can S. aureus enter diabetic ulcers, but also into traumatic skin wounds, which can lead to persistent tissue infection that occasionally progresses to systemic infection and death. Furthermore, MRSA is easily transferred. A mere touch of the infected skin or a touch of even an object that has come in contact with the infected skin can spread this infection. As antibiotics can only serve to be a temporary solution to this problem, scientists continue to propose new solutions to the ongoing issue.
Possible problems of antibiotic, probiotic and bacteriophage for treatment of S. aureus skin infection
The use of antibiotics has provided an accessible and successful solution to almost all bacterial infections. However, antibiotics, if overused, can result in the development of antibiotic-resistant bacteria, which deems antibiotics to be undesirable for long-term management of bacterial infections. The emergence of MRSA provides a clear example of the shortcoming of this approach. The problems of antimicrobial resistance are discussed in the May 2019 issue of Microbiology Australia, while ‘S. aureus’ drug resistance was part of the theme in September 2008. The use of probiotics represents a potential solution to this problem. Probiotics are essentially symbiotic microorganisms that outcompete pathogenic bacteria16. Adding probiotic bacteria to human skin will shift the course of infection leading to the balanced ratio of bacteria. As addressed earlier, S. aureus is an infection on the skin. However, the FDA prohibits the application of probiotics on the skin because probiotics are live bacteria and entrance of live bacteria into the bloodstream can cause other infections leading to death. Thus, probiotics can only be present in edible items such as yogurt and currently, does not represent a viable treatment for S. aureus infection. The last of the current solutions to combat dysbiosis would be the use of bacteriophage. Bacteriophage are viruses that selectively kill certain bacterial species17. Although this represents a creative approach to replace antibiotics, it has been reported that there are certain limitations inherent in bacteriophage therapy18.
Prebiotic as a bacteria-specific carbon source for fermentation
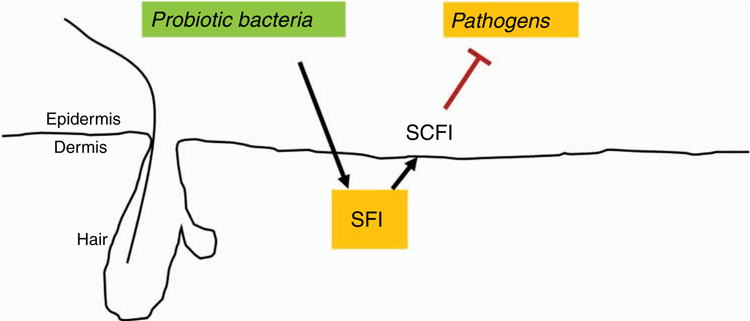
The use of prebiotics represents a potential solution to the existing problems facing the management of MRSA infection. This approach essentially consists of assisting the beneficial or probiotic bacteria, while weakening pathological or undesirable bacteria. The fact that not all people who come in contact with S. aureus get an infection implies the existence of endogenous mechanisms preventing infection. In general, commensal bacteria use a carbon source derived from human cells (e.g. fibre or glucose) to make SCFAs such acetic acid and butyric acid via fermentation19,20. Among other things, these SCFAs can serve as ‘microbial weapons’ by which certain bacterial strains can inhibit the growth of competing species. If harmful bacteria overwhelm the probiotic bacteria, this may result in an infection or injury from pathogens. If the probiotic bacteria overwhelm the pathogens, the person would be safe from injury. The imbalance of bacteria in the microbiome is referred to as dysbiosis, resulting in pathologic infection. As current treatments proved ineffective against S. aureus, a new solution (Figure 2) to this problem would be to provide a defined prebiotic as a carbon source, also named a selective fermentation initiator (SFI), to selectively induce fermentation of probiotic bacteria. Pathogens and the probiotic bacteria in humans each have different enzymes to yield different SCFAs. This results from the fact that there are certain carbon sources that only the probiotic bacteria can ferment to combat pathogens. Due to differences in the enzymes of probiotics and pathogens, there are certain sources in which only the probiotics can utilise to ferment and produce SCFAs. Such carbon sources would be SFIs.
Different bacterial species make different enzymes that ferment specific carbon sources. All S. aureus, S. epidermidis and C. acnes can ferment glucose to SCFAs21–23. To gain maximum survival advantage, S. aureus and S. epidermidis/C. acnes that co-exist within a diabetic ulcer24,25 exclude each other via production of SCFAs by fermentation of glucose. When S. aureus survives after competitive bacterial interference the infection will proceed to continue to damage the host. However, polyethylene glycol dimethacrylate (PEG-DMA) has been developed as a SFI that can specifically intensify fermentation activity of S. epidermidis, but not S. aureus26,27. The exclusive induction of the fermentation of S. epidermidis by PEG-DMA amplified the probiotic activity of S. epidermidis against S. aureus.
In a skin wound or diabetic ulcer, the microbiome is comprised of probiotic bacteria and S. aureus where probiotic bacteria act to inhibit the proliferation of S. aureus. The prebiotic strategy would result in the cultivation of fermentation specifically in probiotic bacteria such as S. epidermidis, amplifying their activity against S. aureus within diabetic ulcers. The probiotic bacteria metabolising these SFIs will create SCFAs via fermentation that prevent pathogens from entering skin wounds. SFIs do not eliminate all bacteria like antibiotics, therefore it would not leave the wound susceptible to opportunistic pathogens. Furthermore, since SFIs do not kill the pathogens directly, pathogens cannot develop resistance. SFIs also represent a more feasible solution compared to probiotics, since SFIs are not live entities, would not cause infection and therefore could be applied on the skin. Therefore, SFIs could be the most plausible solution to MRSA infections in diabetic ulcers. SFIs can potentially reduce hospitalisation, the need for amputations, and delays for healing diabetic ulcers.
Conclusion
The technology of bacterial fermentation has been widely employed in the development of various products including yogurt, wine, and vinegar. The concept of using SFI to activate the fermenting probiotic bacteria against S. aureus and restore the dysbiotic skin microbiome not only may inspire the next generation probiotic/prebiotic-based medicine but also defines novel roles of probiotic bacteria and their associated prebiotics in the innate immunity of the skin against S. aureus infections.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank Christopher M Bates, an Assistant Professor at Materials Department, University of California, Santa Barbara, for discussing the feasibility of using PEG-based compounds as a skin probiotic. This research did not receive any specific funding.
References
[1] Chen, Y.E. et al. (2018) Skin microbiota-host interactions. Nature 553, 427–436.| Skin microbiota-host interactions.Crossref | GoogleScholarGoogle Scholar | 29364286PubMed |
[2] Kaur, N. et al. (2011) Intestinal dysbiosis in inflammatory bowel disease. Gut Microbes 2, 211–216.
| Intestinal dysbiosis in inflammatory bowel disease.Crossref | GoogleScholarGoogle Scholar | 21983063PubMed |
[3] Grice, E.A. et al. (2012) The human microbiome: our second genome. Annu. Rev. Genomics Hum. Genet. 13, 151–170.
| The human microbiome: our second genome.Crossref | GoogleScholarGoogle Scholar | 22703178PubMed |
[4] Ren, T. et al. (2013) 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids. Environ. Microbiol. 15, 535–547.
| 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids.Crossref | GoogleScholarGoogle Scholar | 23113966PubMed |
[5] Iwase, T. et al. (2010) Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465, 346–349.
| Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization.Crossref | GoogleScholarGoogle Scholar | 20485435PubMed |
[6] Naik, S. et al. (2012) Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119.
| Compartmentalized control of skin immunity by resident commensals.Crossref | GoogleScholarGoogle Scholar | 22837383PubMed |
[7] Grice, E.A. et al. (2011) The skin microbiome. Nat. Rev. Microbiol. 9, 244–253.
| The skin microbiome.Crossref | GoogleScholarGoogle Scholar | 21407241PubMed |
[8] Ahn, C. et al. (1996) Microbial evaluation: 139 implants removed from symptomatic patients. Plast. Reconstr. Surg. 98, 1225–1229.
| Microbial evaluation: 139 implants removed from symptomatic patients.Crossref | GoogleScholarGoogle Scholar | 8942908PubMed |
[9] Cogen, A.L. et al. (2008) Skin microbiota: a source of disease or defence? Br. J. Dermatol. 158, 442–455.
| Skin microbiota: a source of disease or defence?Crossref | GoogleScholarGoogle Scholar | 18275522PubMed |
[10] Fluhr, J.W. et al. (2008) Glycerol and the skin: holistic approach to its origin and functions. Br. J. Dermatol. 159, 23–34.
| Glycerol and the skin: holistic approach to its origin and functions.Crossref | GoogleScholarGoogle Scholar | 18510666PubMed |
[11] Ushijima, T. et al. (1984) Acetic, propionic, and oleic acid as the possible factors influencing the predominant residence of some species of Propionibacterium and coagulase-negative Staphylococcus on normal human skin. Can. J. Microbiol. 30, 647–652.
| Acetic, propionic, and oleic acid as the possible factors influencing the predominant residence of some species of Propionibacterium and coagulase-negative Staphylococcus on normal human skin.Crossref | GoogleScholarGoogle Scholar | 6744125PubMed |
[12] Ryssel, H. et al. (2009) The antimicrobial effect of acetic acid--an alternative to common local antiseptics? Burns 35, 695–700.
| The antimicrobial effect of acetic acid--an alternative to common local antiseptics?Crossref | GoogleScholarGoogle Scholar | 19286325PubMed |
[13] Sebastian, S. et al. (1996) Comparative assessment of bacterial inoculation and propionic acid treatment of aerobic stability and microbial populations of ensiled high-moisture ear corn. J. Anim. Sci. 74, 447–456.
| Comparative assessment of bacterial inoculation and propionic acid treatment of aerobic stability and microbial populations of ensiled high-moisture ear corn.Crossref | GoogleScholarGoogle Scholar | 8690682PubMed |
[14] Tong, X. et al. (2004) Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem. Biophys. Res. Commun. 317, 463–471.
| Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition.Crossref | GoogleScholarGoogle Scholar | 15063780PubMed |
[15] Hassan, M.A. et al. (2019) Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers. Diabetes Metab. Syndr. 13, 1261–1270.
| Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers.Crossref | GoogleScholarGoogle Scholar | 31336475PubMed |
[16] Norouzi, H. et al. (2018) Marine actinomycetes with probiotic potential and bioactivity against multidrug-resistant bacteria. Int. J. Mol. Cell. Med. 7, 44–52.
| Marine actinomycetes with probiotic potential and bioactivity against multidrug-resistant bacteria.Crossref | GoogleScholarGoogle Scholar | 30234072PubMed |
[17] Prazak, J. et al. (2019) Bacteriophages improve outcomes in experimental Staphylococcus aureus ventilator associated pneumonia. Am. J. Respir. Crit. Care Med. 200, 1126–1133.
| Bacteriophages improve outcomes in experimental Staphylococcus aureus ventilator associated pneumonia.Crossref | GoogleScholarGoogle Scholar | 31260638PubMed |
[18] Carlton, R.M. (1999) Phage therapy: past history and future prospects. Arch. Immunol. Ther. Exp. (Warsz.) 47, 267–274.
| 10604231PubMed |
[19] Ding, Y. et al. (2019) In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 125, 751–760.
| In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum.Crossref | GoogleScholarGoogle Scholar | 30552927PubMed |
[20] Tsitko, I. et al. (2019) A small in vitro fermentation model for screening the gut microbiota effects of different fiber preparations. Int. J. Mol. Sci. 20, 1925.
| A small in vitro fermentation model for screening the gut microbiota effects of different fiber preparations.Crossref | GoogleScholarGoogle Scholar |
[21] Barbirato, F. et al. (1997) Propionic acid fermentation from glycerol: comparison with conventional substrates. Appl. Microbiol. Biotechnol. 47, 441–446.
| Propionic acid fermentation from glycerol: comparison with conventional substrates.Crossref | GoogleScholarGoogle Scholar |
[22] Robbins, G.B. et al. (1940) Fermentation of sugar acids by bacteria. J. Bacteriol. 39, 399–404.
| Fermentation of sugar acids by bacteria.Crossref | GoogleScholarGoogle Scholar | 16560301PubMed |
[23] Safonova, T.B. et al. (1978) Importance of carbohydrate tests for interspecies differentiation of staphylococci. Zh. Mikrobiol. Epidemiol. Immunobiol. 9, 98–101.
[24] Louie, T.J. et al. (1976) Aerobic and anaerobic bacteria in diabetic foot ulcers. Ann. Intern. Med. 85, 461–463.
| Aerobic and anaerobic bacteria in diabetic foot ulcers.Crossref | GoogleScholarGoogle Scholar | 970773PubMed |
[25] Dowd, S.E. (2008) Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS One 3, e3326.
| Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP).Crossref | GoogleScholarGoogle Scholar | 18833331PubMed |
[26] Kao, M.S. et al. (2017) Microbiome precision editing: using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. 12, .
| Microbiome precision editing: using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar | 27982519PubMed |
[27] Wang, Y. et al. (2016) A precision microbiome approach using sucrose for selective augmentation of Staphylococcus epidermidis fermentation against Propionibacterium acnes. Int. J. Mol. Sci. 17, 1870.
| A precision microbiome approach using sucrose for selective augmentation of Staphylococcus epidermidis fermentation against Propionibacterium acnes.Crossref | GoogleScholarGoogle Scholar |
Biographies
Tristan Yusho Huang is a rising senior student at Canyon Crest Academy and conducting research as an intern at Department of Dermatology, University of California, San Diego. His project is working on the use of prebiotic as a carbon source for bacterial fermentation and electricity production.
Deron Raymond Herr is an Assistant Professor at Department of Pharmacology, National University of Singapore. His research focused on the metabolism and signal transduction of bioactive lipids, specifically, sphingosine 1-phosphate and lysophosphatidic acid. He is working on validation of lipids as a skin prebiotic.
Chun-Ming Huang is a Professor at Department of Biomedical Sciences and Engineering, National Central University and Department of Dermatology, University of California, San Diego. His research is centering on the skin microbiome and its association with skin diseases such as acne vulgaris. He has isolated various skin probiotic bacteria for establishment of a skin probiotic bank, which is used as a platform to screen bacteria-specific prebiotic.
Yong Jiang is a CEO at America Diagnosis, Inc., San Diego. Dr Jiang has developed a DNA chip for high-throughput screening the relative abundance of microbes in the human microbiome.