Rethinking Coxiella infections in Australia
Charlotte Oskam A B , Jadyn Owens A , Annachiara Codello A , Alexander Gofton A and Telleasha Greay AA Vector and Waterborne Pathogens Research Group, School of Veterinary and Life Sciences, Murdoch University, Murdoch, WA 6150, Australia
B Tel: +61 8 9360 6349, Email: c.oskam@murdoch.edu.au
Microbiology Australia 39(4) 223-225 https://doi.org/10.1071/MA18069
Published: 8 November 2018
Coxiellaburnetii is the causative agent of coxiellosis in animals and Q fever in humans. Despite being a vaccine preventable disease, Q fever remains a frequently reported zoonotic infection in Australia. Recently, a Coxiella species was identified in brown dog ticks (Rhipicephalus sanguineus) in urban and rural regions of Australia. Further molecular characterisation revealed that it is genetically identical to ‘Candidatus Coxiella massiliensis’ (KM079627) described in R. sanguineus ticks removed from humans with eschars in France and serologic cross-reactivity among ‘Ca. Coxiella massiliensis’ and C.burnetii may occur. This report highlights the need for molecular testing of seropositive companion animals and humans to determine which species of Coxiella they are infected with, in order to further assess Coxiella species associated with Coxiella infections in Australia.
Coxiella burnetii is a small, obligate intracellular, Gram-negative coccobacillus found worldwide (except in New Zealand) and has a sylvatic lifecycle involving wildlife and domestic mammals, birds, and arthropods1,2. Coxiella burnetii was first described in the 1930s as the causative agent of Q (query) fever in abattoir workers in Brisbane, Queensland, Australia3. Coxiella burnetii is also the known cause of coxiellosis in animals and is persistently shed by infected animals in secretions and parturient by-products. Transmission occurs predominantly through direct or indirect contact with infected tissues from domestic ruminants and companion animals, rather than as a consequence of tick bite4. Clinical presentations of Q fever range from acute to chronic, and can lead to post-Q fever fatigue syndrome, although asymptomatic Q fever represents >54–60% of infections3. High annual reports of human Q fever in Australia persist despite a readily available vaccine5; over 4800 cases were reported between 2007 and 2017, with 716 notifications of Q fever in the past 18 months6.
Australian serological surveys have reported the number of infected dogs with C. burnetii has increased over 26 years to nearly 22%7, with free-roaming dogs within Indigenous communities having the highest seroprevalence compared with breeding, pet, or shelter dogs, in a most recent study8. It has been proposed that dogs become infected with C. burnetii through consumption of infected raw meat, hunting, and scavenging wildlife, or due to heavy tick infestations8, most commonly with Rhipicephalus sanguineus ticks9. While our knowledge about the epidemiology of C. burnetii in companion animals continues to increase, it is unclear whether the high C. burnetii-seropositivity observed in these animals contributes to increasing reports of Q fever cases in humans.
In addition to C. burnetii, several other Coxiella species and subtypes of the genus have been identified in a range of different hosts, including C. cheraxi, the cause of mass mortalities in Australian redclaw crayfish, (Cherax quadricarinatus)10; Coxiella spp. endosymbionts of ticks11; and more recently, ‘Candidatus Coxiella massiliensis’, associated with ticks removed from humans with eschars12. Molecular evidence suggests that C. burnetii originated from an inherited symbiont in soft ticks and acquired virulence factors enabling it to infect vertebrate cells11. To date, over 40 tick species have been associated with C. burnetii and Coxiella spp. Amblyomma, Dermacentor,Ixodes, and Rhipicephalus species are the most frequently implicated vectors11,13.
Tick-associated Coxiella spp. have a role in maintaining tick health and influence the vertical transmission of other tick-borne pathogens14. Due to their symbiotic role in ticks, Coxiella spp. endosymbionts of ticks are considered non-pathogenic to vertebrates, however, the dogma of what is considered an endosymbiont versus a pathogen has been challenged recently though the observation of serological reactions to a number of tick-associated endosymbionts in people following a tick bite14,15. Furthermore, a retrospective study identified Coxiella sp. (‘Ca. Coxiella massiliensis’) in several tick species, including R. sanguineus ticks removed from patients presenting with scalp eschars, cervical lymphadenopathy, fever, increased C-reactive protein and thrombocytopenia11,12. Following the recent molecular characterisation of a Coxiella sp. in R. sanguineus ticks in Australia16, this present study screened 41 R. sanguineus ticks with a Coxiella-specific GroEL PCR assay to determine the genetic relatedness to ‘Ca. Coxiella massiliensis’.
A Coxiella-specific PCR assay, targeting a 659 bp region of the GroEL gene was performed using the primers Cox-660f (GGCGCICARATGGTTAARGA) and Cox-1320r (AACATCGCTTTACGACGA) according to Angelakis et al.12, with the following modifications: each 25 μL PCR reaction contained 1× Perfect Taq buffer (5 Prime, Germany), 1 mg/mL BSA (Fisher Biotech, Australia), 2.5 mM MgCl2, 1 mM dNTPs, 400 nM of each primer, 1.25 U Perfect Taq polymerase (5 Prime, Germany) and 2 μL of undiluted DNA. All samples were performed under the following thermal conditions: initial denaturation at 95°C for 5 min, 40 cycles of denaturation at 95°C for 30s, annealing at 52°C for 30 s, extension at 72°C for 1 min, and a final extension at 72°C for 5 min. A phylogenetic tree was constructed with a 547 bp trimmed alignment of all known Coxiella GroEL sequences, including those obtained in this study, with MrBayes 3.2.617.
DNA was successfully amplified in 80% (33/41) of the R. sanguineus ticks and Sanger sequencing was conducted on 10 positive samples according to Oskam et al.16. All 10 sequences were identical to each other (MK119208), and 100% similar to ‘Ca. Coxiella massiliensis’ isolated from R. sanguineus in France (KM079627). Phylogenetic analysis revealed the ‘Ca. Coxiella massiliensis’ identified in this study had high support (posterior probability 1.0) to ‘Ca. Coxiella massiliensis’ found within other R. sanguineus ticks (Figure 1)12. The prevalence of ‘Ca. Coxiella massiliensis’ in this study was higher than the ‘Ca. Coxiella massiliensis’ prevalence of 35% (7/20) reported by Angelakis et al. in R. sanguineus12.
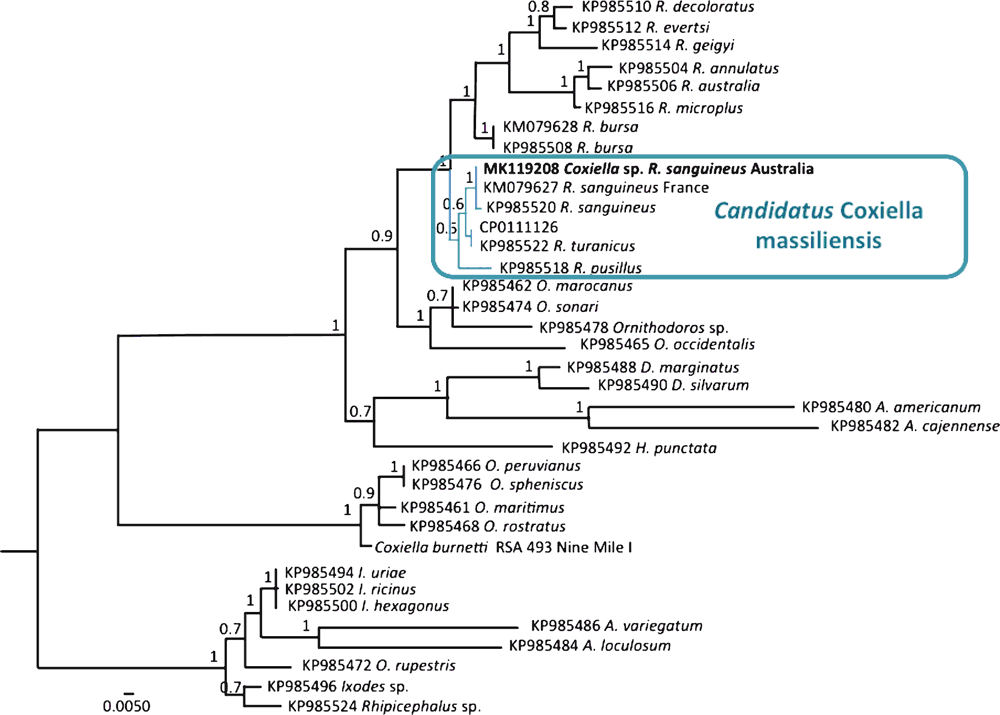
|
It is still unknown whether ‘Ca. Coxiella massiliensis’ can be transmitted to humans via tick bite or aerosol inhalation in Australia, however it prompts further investigation to determine if cross-reactions can occur among other Coxiella sp. in Q fever serological tests. This study highlights the need for molecular testing of companion animals and humans that are seropositive for C. burnetii to determine which species of Coxiella they are infected with and to comprehensively assess all species of Coxiella in Australia for health risks.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Shapiro, A.J. et al. (2015) Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia. Vet. Microbiol. 177, 154–161.| Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
[2] Traub, R.J. et al. (2005) Canine gastrointestinal parasitic zoonoses in India. Trends Parasitol. 21, 42–48.
| Canine gastrointestinal parasitic zoonoses in India.Crossref | GoogleScholarGoogle Scholar |
[3] Maurin, M. and Raoult, D. (1999) Q fever. Clin. Microbiol. Rev. 12, 518–553.
| Q fever.Crossref | GoogleScholarGoogle Scholar |
[4] Kopecny, L. et al. (2013) Investigating Coxiella burnetii infection in a breeding cattery at the centre of a Q fever outbreak. J. Feline Med. Surg. 15, 1037–1045.
| Investigating Coxiella burnetii infection in a breeding cattery at the centre of a Q fever outbreak.Crossref | GoogleScholarGoogle Scholar |
[5] Tozer, S.J. et al. (2014) Potential animal and environmental sources of Q fever infection for humans in Queensland. Zoonoses Public Health 61, 105–112.
| Potential animal and environmental sources of Q fever infection for humans in Queensland.Crossref | GoogleScholarGoogle Scholar |
[6] Department of Health (2018) Number of notifications of Q fever, Australia. National Notifiable Diseases Surveillance System. http://www9.health.gov.au/cda/source/rpt_3.cfm (accessed 14 August 2018).
[7] Cooper, A. et al. (2011) Serological evidence of Coxiella burnetii infection in dogs in a regional centre. Aust. Vet. J. 89, 385–387.
| Serological evidence of Coxiella burnetii infection in dogs in a regional centre.Crossref | GoogleScholarGoogle Scholar |
[8] Shapiro, A.J. et al. (2016) Seroprevalence of Coxiella burnetii in Australian dogs. Zoonoses Public Health , .
| Seroprevalence of Coxiella burnetii in Australian dogs.Crossref | GoogleScholarGoogle Scholar |
[9] Greay, T.L. et al. (2016) A survey of ticks (Acari: Ixodidae) of companion animals in Australia. Parasit. Vectors 9, 207.
| A survey of ticks (Acari: Ixodidae) of companion animals in Australia.Crossref | GoogleScholarGoogle Scholar |
[10] Tan, C.K. and Owens, L. (2000) Infectivity, transmission and 16S rRNA sequencing of a rickettsia, Coxiella cheraxi sp. nov., from the freshwater crayfish Cherax quadricarinatus. Dis. Aquat. Organ. 41, 115–122.
| Infectivity, transmission and 16S rRNA sequencing of a rickettsia, Coxiella cheraxi sp. nov., from the freshwater crayfish Cherax quadricarinatus.Crossref | GoogleScholarGoogle Scholar |
[11] Duron, O. et al. (2015) The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 11, e1004892.
| The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii.Crossref | GoogleScholarGoogle Scholar |
[12] Angelakis, E. et al. (2016) Candidatus Coxiella massiliensis infection. Emerg. Infect. Dis. 22, 285–288.
| Candidatus Coxiella massiliensis infection.Crossref | GoogleScholarGoogle Scholar |
[13] Parola, P. and Raoult, D. (2001) Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32, 897–928.
| Ticks and tickborne bacterial diseases in humans: an emerging infectious threat.Crossref | GoogleScholarGoogle Scholar |
[14] Ahantarig, A. et al. (2013) Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol. (Praha) 58, 419–428.
| Hard ticks and their bacterial endosymbionts (or would be pathogens).Crossref | GoogleScholarGoogle Scholar |
[15] Mariconti, M. et al. (2012) Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: Is Midichloria a novel pathogen, or just a marker of tick bite? Pathog. Glob. Health 106, 391–396.
| Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: Is Midichloria a novel pathogen, or just a marker of tick bite?Crossref | GoogleScholarGoogle Scholar |
[16] Oskam, C.L. et al. (2017) Molecular investigation into the presence of a Coxiella sp. in Rhipicephalus sanguineus ticks in Australia. Vet. Microbiol. 201, 141–145.
| Molecular investigation into the presence of a Coxiella sp. in Rhipicephalus sanguineus ticks in Australia.Crossref | GoogleScholarGoogle Scholar |
[17] Ronquist, F. et al. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542.
Biographies
Dr Charlotte Oskam is a senior lecturer and team leader in the Vector and Waterborne Pathogens Research Group at Murdoch University. Her research interests extend from ancient DNA, microbiomes, ticks, to zoonoses.
Jadyn Owens is a Murdoch University graduate in Molecular Biology and completed an independent study contract supervised by Dr Oskam in the Vector and Waterborne Pathogens Research Group at Murdoch University.
Annachiara Codello was a research assistant during this project in the Vector and Waterborne Pathogens Research Group at Murdoch University.
Alexander Gofton is a PhD student in the Vector and Waterborne Pathogens Research Group at Murdoch University. His research interests are in tick microbiomes and tick-borne pathogens of animals and humans.
Telleasha Greay is a PhD student in the Vector and Waterborne Pathogens Research Group at Murdoch University. Her research interests are in tick microbiomes and tick-borne pathogens of companion animals.


